Abstract
Background
Despite implementation of enhanced recovery after surgery (ERAS) in lung surgery, potential barriers for improvements should be identified. The aim of this single-centre, prospective ERAS cohort study was to explore reasons for delayed patient discharge after video-assisted thoracoscopic surgery (VATS) lobectomy with a median length of hospital stay (LOS) of 2 days.
Methods
Consecutive patients referred for VATS lobectomy were consulted twice daily by an investigator for the primary reasons for continued hospitalization. The secondary outcomes were risk factors for delayed recovery using univariate and multivariate regression analyses.
Results
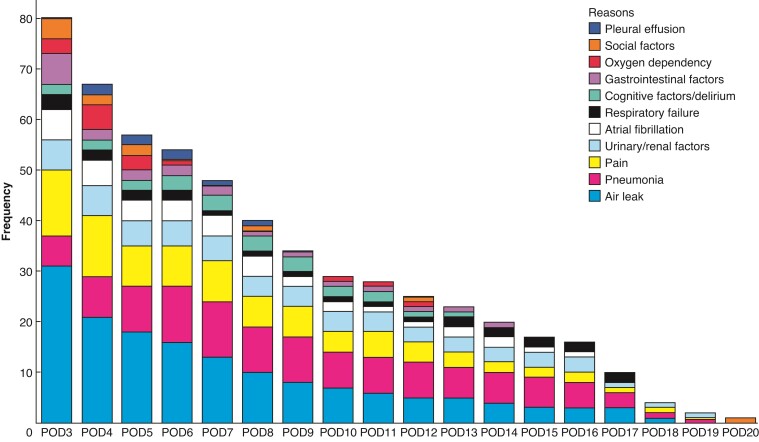
A total of 147 patients were included (69 with LOS more than 2 days and 78 with LOS of 2 days or less) from April 2020 to December 2020. Air leak (27.7 per cent), pneumonia (20.2 per cent), pain (15.3 per cent), urinary/renal factors (11.0 per cent), atrial fibrillation (7.0 per cent), respiratory failure (4.5 per cent), cognitive factors/delirium (4.3 per cent), gastrointestinal factors (3.8 per cent), oxygen dependency (2.7 per cent), social factors (2.0 per cent), and pleural effusion (1.4 per cent) were important factors for discharge more than 2 days after surgery. The 30-day readmission rate after discharge was 21 per cent for LOS of 2 days or less and 22 per cent for LOS more than 2 days (P = 0.856). On a multivariate regression model, age (per 5-year increase, odds ratio (OR) 1.29, 95 per cent c.i. 1.01 to 1.66, P = 0.043) and forced expiratory volume in 1 s (FEV1) per cent (per 5 per cent increase, OR 0.89, 95 per cent c.i. 0.81 to 0.98, P = 0.021) were significantly related to discharge after more than 2 days.
Conclusion
Despite a short median LOS of 2 days, air leak, pneumonia, and pain remain the most important challenges for further improvement of the ERAS programme. Age and FEV1 per cent were statistically significant risk factors for LOS longer than 2 days.
Despite a short median LOS of 2 days after VATS lobectomy, air leak, pneumonia, and pain were the most important challenges for further improvement of the ERAS programme.
Introduction
Video-assisted thoracoscopic surgery (VATS) has become standard of care for pulmonary lobectomy. Advantages have been demonstrated in large cohort studies and a recent randomized clinical trial with less pain, faster return to daily activities, better shoulder function, fewer complications, better tolerance of adjuvant chemotherapy, and shorter length of stay (LOS)1,2 in hospital. Enhanced recovery after surgery (ERAS) protocols have successfully been adopted in most surgical procedures3 and recently, the ERAS® Society and the European Society of Thoracic Surgeons published their guidelines for lung surgery, recommending 45 items for enhancing recovery4. Although fewer elements may be required5, increased compliance with an ERAS pathway has been shown to be beneficial and to reduce LOS as well as opioid use without increasing postoperative adverse events and costs6.
Nevertheless, several challenges remain for further improvement3,5, including an analysis of ‘Why do patients stay in hospital after surgery?’ as conducted for colonic and orthopaedic procedures7,8. However, little is known about specific reasons for similar questions after VATS lobectomy9,10 despite adoption of an ERAS programme.
The primary aim of this prospective consecutive cohort study was to explore reasons for delayed patient discharge after VATS lobectomy following an established ERAS protocol with a median LOS of 2 days. The secondary aim was to identify other perioperative (preoperative and intraoperative) risk factors for LOS longer than 2 days.
Materials and methods
The study was reported complying with STROBE Guidelines11.
Study design, setting, and participants
The study was approved by the Danish Regional Ethics Committee (H-20014489) and the Danish Data Protection Agency, with a single-centre, prospective, observational design. The study was preregistered with an analysis plan at www.clinicaltrials.gov (registration no. NCT04294108).
Consecutive patients scheduled for VATS lobectomy in the Department of Cardiothoracic Surgery, Copenhagen University Hospital, Rigshospitalet were recruited for the study. The standard perioperative care pathway was applied for every patient referred for pulmonary resection, containing all components of the ERAS guidelines4,5. All patients were operated by way of a standardized three-port anterior approach as previously described12. A multimodal opioid-sparing regimen consisting of paracetamol, ibuprofen, and gabapentin was used. A paravertebral single-shot block was applied intraoperatively by the surgeon for more than five thoracic levels with a total of 20 ml 0.5 per cent bupivacaine. At the end of surgery an intercostal catheter was inserted at the drain site with continuous bupivacaine 0.25 per cent, 6 ml/h and remained until chest drain removal13. Routinely, only one chest drain was applied and connected to a digital drainage system Thopaz+ (Medela, Switzerland) with a standard suction of −2 cm H2O14. Only adults speaking Danish were included in the study. Patients who received any other procedure than VATS lobectomy were excluded. Written informed consent was obtained from all patients.
Routinely, patients were admitted on the morning of surgery. The discharge criteria were self-mobilization, normal gastrointestinal function, chest drain and all intravenous lines removed, and no need for opioids. The criteria for chest drain removal was air leak 20 ml/min or less for more than 12 h, whereas there was no upper threshold for serous fluid unless with chyle or blood14. A chest X-ray was performed 2 h after chest drain removal. Another chest X-ray was performed when the patient was seen in the outpatient clinic 2 weeks after surgery. Patients with prolonged air leak are not sent home with a chest drain in this setting. All patients in this cohort were discharged to their homes. If complications occurred, they were re-admitted to the department, but complete follow-up was secured by the electronic record system in Easter Denmark.
Variables and data measurement
LOS was counted as the number of nights hospitalized after surgery. Duration of chest drainage was defined from the day of placement to the day of removal. All participants were consulted to assess reasons for staying in hospital twice daily (08:00 hours and 16:00 hours) by an investigator (L.H.) asking ‘why do you stay in hospital now?’ and double checking the reasons using data from the Thopaz+, reviewing the medical record, and consulting with clinically responsible surgeons and nurses. R.H.P. and H.K. supervised the process, and any discrepancies were discussed together. To decrease bias, the consultation at 08:00 hours aimed not only to interactively secure factors to be collected at 16:00 hours, but also to supplement reasons occurring after 16:00 hours the day before. Each reason for non-discharge was individually assessed and analysed each day.
Air leak was defined as continuous air flow of more than 20 ml/min on the Thopaz+, recurrent pneumothorax requiring another chest drain or expanding subcutaneous emphysema. Postoperative pain was considered a barrier for discharge if the patient noted this in the daily interviews. Pneumonia was defined as the need for treatment with antibiotics for a respiratory infection and at least one of the following criteria: new or changed purulent sputum; new or changed lung opacity on a clinically indicated chest X-ray; temperature greater than 38.3°C; leucocyte count greater than 12 000/µl. Atrial fibrillation was verified by an ECG. Urinary/renal factors covered all complications associated with the urinary tract, for instance urinary tract infection and renal insufficiency, diagnosed by blood sample or microbiological examination. Respiratory failure was verified by blood gas analysis. Oxygen dependency was defined as the need for oxygen therapy without symptoms of breathlessness. Cognitive factors/delirium covered any cognitive disorder and delirium, for example hallucination, forgetting appointments, and dates, forgetting recent conversations and events, and becoming more impulsive or apathetic. Gastrointestinal factors were defined as any complication associated with any symptom in the gastrointestinal system, for instance nausea, vomiting, diarrhoea, or constipation. Pleural effusion was defined as needing reinsertion of a chest drain due to pleural fluid without air leak. Other medical diagnoses as reasons for delaying discharge were adjusted in accordance with the ICD-10. Social factors were defined as any factor without association to health, for example living alone, or waiting for a home transportation.
Demographic data (age, sex, BMI, smoking, and alcohol status, activity, and living status, surgical history, and distance of living from hospital) were recorded as well as clinical parameters (percentage of forced expiratory volume in 1 s (FEV1 per cent), FEV1/forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), co-morbidity, weight loss, chronic pain, duration of surgery, blood loss, type of lobectomy, surgeon experience, and time point of ending surgery). Smoking status was classified as ‘non-smoker’, ‘former smoker’, and ‘current smoker’. Alcohol status was classified as ‘no and limited alcohol use’, and ‘excess alcohol use’. Excess alcohol use means more than 14 units/week for men, more than 7 units/week for women, or any patient with a history of alcohol abuse. Normal activity was defined as walking without any assistance (such as roller and wheelchair), except for a limp. Surgical history was classified as any invasive surgery less than 3 months before the operation. All co-morbidities were defined in accordance with ICD-10. Chronic pain was defined as ongoing pain before surgery. A senior surgeon was defined as a consultant with more than 10 years of experience. Readmission was defined as admission to any hospital within 30 days after the index discharge. All data were collected via an electronic healthcare software (Epic, Madison, Wisconsin).
Data were captured by L.H. with REDCap (Research Electronic Data Capture tool)15.
Sample size
The power calculation was based on LOS of patients scheduled for VATS lobectomy in the department in 2019 (unpublished, n = 340, mean(s.d.) 4.76(5.20) days, median 3 days, percentage of LOS of 2 days or less, 45 per cent). LOS of 2 days or less was the goal for the ERAS programme. Consequently, a sample of 160 patients was estimated to be sufficient for a 90 per cent chance of detecting a difference between the goal and the non-goal groups at the 5 per cent level of significance with a two-sided Wilcoxon–Mann–Whitney U test (two groups) and allowing a 10 per cent non-completion rate. The calculation was made via G*Power version 3.116.
Statistical analysis
Continuous variables without normal distribution were identified by Kolmogorov–Smirnov and Shapiro–Wilk test and presented by median and interquartile range (i.q.r.). Categorical variables were presented as frequencies (percentage). The patients were separated into one group with LOS of 2 days or less and another with LOS more than 2 days. Comparison of continuous variables was conducted with a Wilcoxon–Mann–Whitney U test, whereas categorical variables were compared with a Pearson chi-squared or Fisher’s exact test. Independent factors of delayed discharge, as defined by P ≤ 0.1 on univariate logistic regression analysis (unadjusted), were tested via multivariate logistic regression model (adjusted) with backward stepwise analysis. A 2.2 per cent missing data frequency in the original data set was found to be acceptable17.
The statistical analyses were conducted with statistical software SPSS® (version 25.0, IBM, Armonk, New York, USA) and R Software (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria). Tableau Software (version 2020.4, Salesforce, San Francisco, California, USA) was applied for data visualization. A P value <0.05 (two-sided) was considered statistically significant.
Results
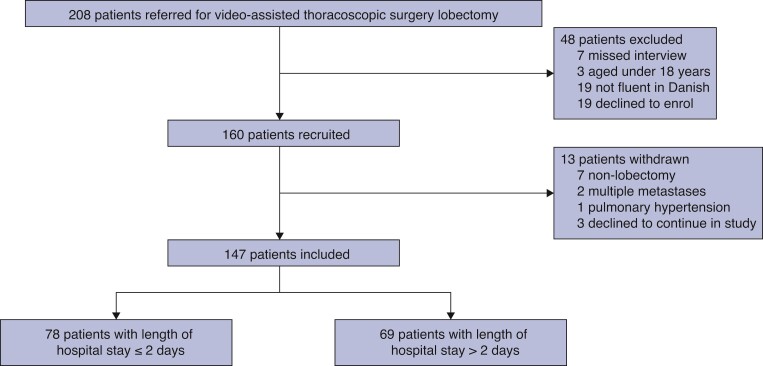
From April 2020 to December 2020, 160 eligible patients who met the inclusion criteria were identified (Fig. 1). Of these, 10 did not receive a VATS lobectomy and 3 declined to continue participation, leaving 147 for analysis. Demographics are shown in Table 1. Median age was 71 (i.q.r. 66–75) years and 51.0 per cent were male. Patients with LOS of more than 2 days were older than patients with LOS of 2 days or less (median 73.0 (i.q.r. 69.0–76.0) years versus 69.5 (i.q.r. 63.0–74.0) years, P = 0.001), and had a lower FEV1 per cent (median 79.0 (i.q.r. 68.0–95.5) versus 93.5 (i.q.r. 78.0–106.3), P = 0.001), whereas FEV1/FVC and DLCO were not significantly lower. Smoking and alcohol status, distance of living from hospital, surgical history, and type of lobectomy were not significantly different between the two groups.
Fig. 1.
Flow chart of patients enrolled and included.
Table 1.
Demographics and clinical characteristics
| Characteristics | Total (n = 147) | LOS ≤2 days (n = 78) | LOS >2 days (n = 69) | P |
|---|---|---|---|---|
| Age, year * | 71 (66–75) | 69.5 (63–74) | 73 (69–76) | 0.001 |
| Male sex † | 75 (51.0) | 42 (54) | 33 (48) | 0.511 |
| BMI, kg/m2 * | 26.2 (22.6–29.0) | 26.6 (22.9–29.0) | 25.9 (22.4–29.1) | 0.458 |
| FEV1% * | 86.5 (73.0–103.3) | 93.5 (78.0–106.3) | 79.0 (68.0–95.5) | 0.001 |
| FEV1/FVC, % * | 69.0 (63.0–76.0) | 71.5 (64.3–77.0) | 67.5 (62.0–73.8) | 0.045 |
| DLCO, % * | 73.0 (62.0–88.3) | 76.0 (63.0–93.8) | 71.0 (55.8–85.3) | 0.055 |
| Smoking status † | 0.529 | |||
| Non-smoker | 23 (15.6) | 14 (18) | 9 (13) | |
| Former smoker | 89 (60.5) | 44 (56) | 45 (65) | |
| Current smoker | 35 (23.8) | 20 (26) | 15 (22) | |
| Alcohol status † | 0.650 | |||
| No and limited alcohol use | 120 (81.6) | 65 (83) | 55 (80) | |
| Excess alcohol use | 27 (18.4) | 13 (17) | 14 (20) | |
| Normal activity † | 131 (89.1) | 74 (95) | 57 (83) | 0.031 |
| Live alone † | 60 (40.8) | 25 (32) | 35 (51) | 0.029 |
| Distance of living from hospital, km * | 10 (3–26) | 9 (3–25) | 11 (4–30) | 0.264 |
| Surgical history † | 108 (73.5) | 59 (76) | 49 (71) | 0.502 |
| Charlson co-morbidity index * | 2 (1–3) | 1 (1–3) | 2 (1–3) | 0.501 |
| Pulmonary co-morbidity † | 43 (29.3) | 19 (24) | 24 (35) | 0.204 |
| Arrhythmia required treatment † | 17 (11.6) | 6 (8) | 11 (16) | 0.130 |
| Chronic pain † | 36 (24.5) | 17 (22) | 19 (28) | 0.154 |
| Diabetes † | 16 (10.9) | 7 (9) | 9 (13) | 0.447 |
| Hypertension † | 64 (43.5) | 27 (35) | 37 (54) | 0.031 |
| Weight loss † | 28 (19.0) | 9 (12) | 19 (28) | 0.024 |
| Type of lobectomy † | 0.927 | |||
| Left upper lobectomy | 25 (17.0) | 14 (18) | 11 (16) | |
| Left lower lobectomy | 26 (17.7) | 14 (18) | 12 (17) | |
| Right upper lobectomy | 47 (32.0) | 23 (30) | 24 (35) | |
| Right middle lobectomy | 6 (4.1) | 4 (5) | 2 (3) | |
| Right lower lobectomy | 37 (25.2) | 19 (24) | 18 (26) | |
| Bi-lobectomy | 6 (4.1) | 4 (5) | 2 (3) | |
| Duration of surgery, min * | 99 (83–119) | 93 (81–112) | 106 (88–123) | 0.012 |
| Blood loss, ml * | 25 (5–50) | 20 (0–50) | 25 (10–95) | 0.016 |
| Ending of surgery before 12:00 hours † | 73 (49.7) | 40 (51) | 33 (48) | 0.742 |
| Senior surgeon † | 54 (36.7) | 25 (32) | 29 (42) | 0.234 |
median (interquartile range).
frequency (proportion).
DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LOS, length of hospital stay.
Patients with LOS of more than 2 days had a lower activity level (P = 0.03) and more patients in this group lived alone (P = 0.03), had more hypertension (P = 0.03), and weight loss (P = 0.02), but otherwise there was no difference in co-morbidity. The group with LOS of more than 2 days had longer duration of surgery (median 13 min, P = 0.01) and more blood loss (median 5 ml, P = 0.02) than patients discharged earlier. Completion of surgery before noon and surgeon experience were not different between groups (P = 0.74 and P = 0.23 respectively). In this cohort, there were three patients (2.0 per cent) needing another chest drain insertion due to pneumothorax. Additionally, three patients (2.0 per cent) underwent re-operation, all due to postoperative bleeding, and were treated with a VATS procedure.
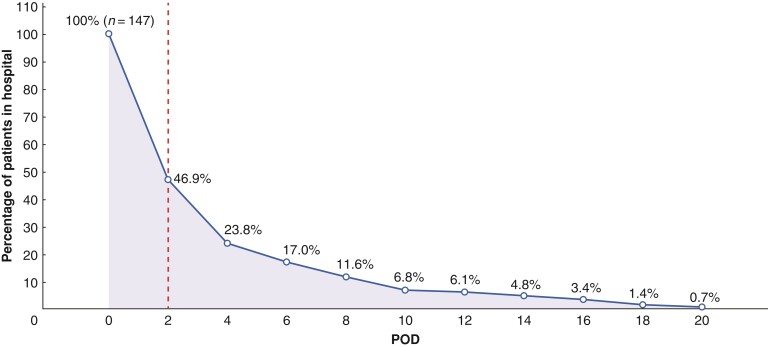
The number and proportion of hospitalized patients are shown in Fig. 2. Seventy-eight patients (53.1 per cent) were discharged on or before 2 days, whereas 69 (46.9 per cent) stayed for more than 2 days. More than 75 per cent of patients were discharged within 4 days. The median LOS was 2 (i.q.r. 2–4) days, and the median duration of chest drainage was 1 (i.q.r. 1–2) day.
Fig. 2.
The percentage of patients in hospital after video-assisted thoracoscopic surgery lobectomy.
The dotted line represents the cut-off for dividing participants into with length of hospital stay greater than 2 days or with LOS of 2 days or less. POD, postoperative day.
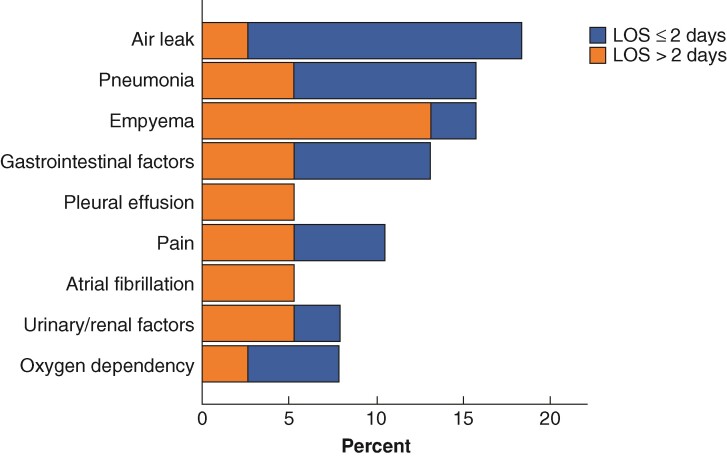
The distribution and time course of reasons for discharge after 2 days is shown in Fig. 3. The most prevalent reasons were air leak (27.7 per cent), pneumonia (20.2 per cent), and pain (15.3 per cent), whereas social reasons only accounted for 2.0 per cent, including patients awaiting home transportation. From postoperative day (POD)3 to POD19, air leak, pneumonia, postoperative pain, and urinary/renal factors remained the most common, with atrial fibrillation, and respiratory failure being additional contributing factors. The occurrence of the different reasons for LOS greater than 2 days gradually decreased from POD3, except cognitive confusion/delirium, oxygen dependency, pneumonia, and respiratory failure. The incidence of readmission within 30 days of discharge was 21 per cent (n = 16) in LOS of 2 days or less and 22 per cent (n = 15) in LOS more than 2 days (P = 0.856 between groups). Air leak was the most important reason for 30-day readmission (18 per cent), followed by pneumonia (16 per cent), empyema (16 per cent), gastrointestinal factors (13 per cent), and pain (11 per cent). In LOS of 2 days or less, the dominant reason of 30-day readmission was air leak, whereas empyema was dominant in LOS more than 2 days. (Fig. 4)
Fig. 3.
Reasons for length of hospital stay greater than 2 days.
POD. postoperative day.
Fig. 4.
The reasons for 30-day readmissions.
In the univariate regression analysis (Table 2), preoperative risk factors for a LOS greater than 2 days included a lower FEV1 per cent, lower activity level, and living alone. In the multivariate regression model, higher age (per 5-year increase, OR 1.29, 95 per cent c.i. 1.01 to 1.66, P = 0.043) and lower FEV1 per cent (per 5 per cent increase, OR 0.89, 95 per cent c.i. 0.81 to 0.98, P = 0.021) were associated with LOS greater than 2 days.
Table 2.
Preoperative and intraoperative factors associated with length of hospital stay greater than 2 days in univariate (unadjusted) and multivariate regression analysis (adjusted)
| Characteristics | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% c.i.) | P | OR (95% c.i.) | P | |
| Age, per 5-year increase | 1.43 (1.14–1.80) | 0.002 | 1.29 (1.01–1.66) | 0.043 |
| Sex | ||||
| Female | Ref. | |||
| Male | 0.79 (0.41–1.50) | 0.466 | ||
| BMI, per 1 kg/m2 increase | 1.00 (0.94–1.06) | 0.987 | ||
| FEV1%, per 5% increase | 0.86 (0.79–0.95) | 0.001 | 0.89 (0.81–0.98) | 0.021 |
| FEV1/FVC, per 5% increase | 0.96 (0.85–1.09) | 0.521 | ||
| DLCO, per 5% increase | 0.93 (0.86–1.00) | 0.064 | 0.97 (0.89–1.06) | 0.470 |
| Smoking status | ||||
| Non-smoker | Ref. | |||
| Former smoker | 1.52 (0.60–3.86) | 0.375 | ||
| Current smoker | 1.26 (0.42–3.84) | 0.680 | ||
| Alcohol status | ||||
| No and limited alcohol use | Ref. | |||
| Excess alcohol use | 1.27 (0.55–2.94) | 0.572 | ||
| Normal activity | ||||
| No | Ref. | |||
| Yes | 0.26 (0.08–0.84) | 0.024 | 2.06 (0.52–8.11) | 0.301 |
| Live alone | ||||
| No | Ref. | |||
| Yes | 2.18 (1.12–4.27) | 0.022 | 1.92 (0.87–4.22) | 0.107 |
| Distance of living from hospital, per 1 km increase | 1.00 (0.99–1.00) | 0.950 | ||
| Surgical history | ||||
| No | Ref. | |||
| Yes | 1.27 (0.61–2.64) | 0.526 | ||
| Charlson co-morbidity index, per 1 increase | 1.03 (0.87–1.23) | 0.722 | ||
| Pulmonary co-morbidity | ||||
| No | Ref. | |||
| Yes | 1.66 (0.81–3.39) | 0.167 | ||
| Arrhythmia requiring treatment | ||||
| No | Ref. | |||
| Yes | 2.28 (0.79–6.52) | 0.126 | ||
| Chronic pain | ||||
| No | Ref. | |||
| Yes | 1.36 (0.64–2.90) | 0.420 | ||
| Diabetes | ||||
| No | Ref. | |||
| Yes | 1.45 (0.53–3.99) | 0.467 | ||
| Hypertension | ||||
| No | Ref. | |||
| Yes | 2.18 (1.12–4.24) | 0.021 | 1.63 (0.75–3.55) | 0.221 |
| Weight loss | ||||
| No | Ref. | |||
| Yes | 2.91 (1.22–6.97) | 0.016 | 2.20 (0.84–5.77) | 0.110 |
| Type of lobectomy | ||||
| Bi-lobectomy | Ref. | |||
| Left upper lobectomy | 1.71 (0.27–11.06) | 0.571 | ||
| Left lower lobectomy | 1.57 (0.24–10.22) | 0.636 | ||
| Right upper lobectomy | 2.09 (0.35–2.51) | 0.421 | ||
| Right middle lobectomy | 1.00 (0.09–11.03) | 1.000 | ||
| Right lower lobectomy | 1.90 (0.31–11.64) | 0.490 | ||
| Duration of surgery, per 10 min increase | 0.93 (0.86–1.00) | 0.064 | 0.97 (0.89–1.06) | 0.470 |
| Blood loss, per 10 ml increase | 1.06 (0.96–1.17) | 0.259 | ||
| Ending of surgery before 12:00 hours | ||||
| No | Ref. | |||
| Yes | 1.15 (0.60–2.20) | 0.676 | ||
| Senior surgeon | ||||
| No | Ref. | |||
| Yes | 0.65 (0.33–1.28) | 0.212 | ||
OR, odds ratio; DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Discussion
Implementation of an ERAS protocol reduces LOS18. Even though 53.1 per cent of unselected patients undergoing VATS lobectomy within an effective ERAS programme were discharged on or before 2 days, as previously demonstrated in selected patients19,20, the current results clearly demonstrate the challenges for further improvement in the remaining hospitalized patients. Although compliance with elements of an ERAS protocol apparently was considered high (median LOS 2 days), several somatic, organizational, and preoperative co-morbidity factors were responsible for LOS greater than 2 days. Consequently, the challenge to improve implementation and design of a future optimized ERAS protocol should focus on the undesirable pathophysiological responses and organ dysfunctions3 as well as the overall compliance with the ERAS protocol21,22
Air leak was the most dominating factor for LOS greater than 2 days. To reduce air leak, numerous prediction, and interventional models have been proposed including sex, pulmonary function, weight, smoking status, surgical history, activity, surgeon expertise, operational position, and type of surgery23,24. Although intuitively prehabilitation seems rational, the outcomes regarding pulmonary function and complications are still debatable25,26. A water test with sterile water is mandatory at completion of surgery to detect and repair a potential air leak27. Gentle handling of the pulmonary tissue and the application of fissure-less techniques (the fissure is left untouched, and the hilar structures are divided before a complete stapling of the fissure) have been demonstrated to reduce air leak28,29. Application of sealants may be considered, although the evidence for the efficacy is sparse30. Additionally, the postoperative chest drain placement has little impact on air leak, although a low-suction programme may be helpful14.
Despite early discharge with an effective ERAS programme being potentially beneficial1,2,19–21, it apparently does not eliminate the recovery problem as postoperative complications may prolong LOS. Patient-reported pain was associated with prolonged LOS, and was probably related to chest drain placement. Thus, pain and chest drain placement make early mobilization more difficult, increasing the rate of hypostatic pneumonia and alveolar atelectasis. Thus, for expedited rehabilitation, multimodal opioid-sparing analgesia treatment is important, following the procedure-specific evidence4. Moreover, as air leak increases the incidence of pulmonary and other complications31, the future focus should be on air leak, as well as pain, and early mobilization. The risk of respiratory failure with continuous oxygen dependency further emphasizes this problem; however, intensified mobilization may be helpful3,32.
Cognitive confusion/delirium could also limit patient ambulation and should be possible to reduce with improved ERAS protocols3. Although the use of a urinary catheter is not recommended in ERAS protocols unless there is gross renal dysfunction before surgery, it needs attention with early removal and following updated evidence-based re-catheterization principles33. Additionally, an emphasis on care to reduce renal morbidity (euvolemia, avoidance of non-steroidal anti-inflammatory drugs (NSAIDs) in preoperative kidney insufficiency), and gastrointestinal morbidity (such as NSAIDs versus COX-2 inhibitors and laxatives) should be instituted3. Finally, in some communities, social factors may play a more important role for delayed discharge, a topic that has been discussed from the very beginning of enhanced recovery protocols with increased patient information and to make the patients ‘better before faster’, thereby limiting the specific role of social factors.
In the final model, FEV1 per cent, and age were associations for delayed discharge. The findings of a lower preoperative FEV1, low activity level, and living alone are similar to the findings by Pompili and colleagues9, demonstrating an increasing incidence of prolonged duration of hospital stay after VATS lobectomy; however, in that study the median length of stay was 4 days and prolonged LOS was defined as greater than 7 days. Interestingly, smoking status, distance of living from the hospital, and experience of the surgeon were not significant risk factors for prolonged LOS.
The 30-day readmission rate is rather high, and it may be speculated whether this was due to the very early discharge. However, there was no significant difference between patients discharged early or later in this study, which correlates with another recent study34.
Despite implementation of an effective ERAS programme with a median LOS of 2 days, this study has important limitations. First, the sample size was limited, which introduces potential reporting bias into outcomes with lack of power for subgroup analysis in the longer LOS group. Second, it was a single-centre study, which limits generalizability; however, the strength of the study is the well described and effective ERAS programme, the consecutive unselected VATS lobectomy cohort (in the inclusion criteria, patients with central tumours, involvement of N1 or single N2 categories, or induction radiochemotherapy were allowed) and the complete follow-up.
Acknowledgements
The authors thank J. Madsen for her assistance in patient enrolment, the staff at the Department of Cardiothoracic Surgery for supporting the flow of the trial, and the Section of Biostatistics, University of Copenhagen, for assistance in relation to statistical methodology.
Disclosure. R.H.P. has received speaker fees from Medtronic, AstraZeneca and AMBU, and acts as an advisory board member for AstraZeneca, MSD and Roche. The authors declare no other conflict of interest.
Contributor Information
Lin Huang, Department of Cardiothoracic Surgery, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Henrik Kehlet, Section of Surgical Pathophysiology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
René Horsleben Petersen, Department of Cardiothoracic Surgery, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Funding
This work was supported by the China Scholarship Council (no. 201908430204 to L.H.).
Data availability
Data are available on request from the authors.
References
- 1. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early-stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–844 [DOI] [PubMed] [Google Scholar]
- 2. Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hürtgen M, Petersen RH et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2016;49:602–609 [DOI] [PubMed] [Google Scholar]
- 3. Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia 2020;75:e54–e61 [DOI] [PubMed] [Google Scholar]
- 4. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91–115 [DOI] [PubMed] [Google Scholar]
- 5. Petersen RH, Huang L, Kehlet H. Guidelines for enhanced recovery after lung surgery: need for re-analysis. Eur J Cardiothorac Surg 2021;59:291–292 [DOI] [PubMed] [Google Scholar]
- 6. Haro GJ, Sheu B, Marcus SG, Sarin A, Campbell L, Jablons DM et al. Perioperative lung resection outcomes after implementation of a multidisciplinary, evidence-based thoracic ERAS program. Ann Surg 2021;274:e1008–e1013 [DOI] [PubMed] [Google Scholar]
- 7. Munk-Madsen P, Eriksen JR, Kehlet H, Gögenur I. Why still in hospital after laparoscopic colorectal surgery within an enhanced recovery programme? Colorectal Dis 2019;21:1438–1444 [DOI] [PubMed] [Google Scholar]
- 8. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop 2011;82:679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pompili C, McLennan Battleday F, Chia WL, Chaudhuri N, Kefaloyannis E, Milton R et al. Poor preoperative quality of life predicts prolonged hospital stay after VATS lobectomy for lung cancer. Eur J Cardiothorac Surg 2021;59:116–121 [DOI] [PubMed] [Google Scholar]
- 10. Forster C, Perentes JY, Ojanguren A, Abdelnour-Berchtold E, Zellweger M, Bouchaab H et al. Early discharge after thoracoscopic anatomical pulmonary resection for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2021;33:892–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gharaibeh A, Koppikar S, Bonilla-Escobar FJ. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) in the International Journal of Medical Students. Int J Med Stud 2014;2:36–37 [Google Scholar]
- 12. Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach: the Copenhagen experience. Ann Cardiothorac Surg 2012;1:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wildgaard K, Petersen RH, Hansen HJ, Møller-Sørensen H, Ringsted TK, Kehlet H. Multimodal analgesic treatment in video-assisted thoracic surgery lobectomy using an intraoperative intercostal catheter. Eur J Cardiothorac Surg 2012;41:1072–1077 [DOI] [PubMed] [Google Scholar]
- 14. Holbek BL, Christensen M, Hansen HJ, Kehlet H, Petersen RH. The effects of low suction on digital drainage devices after lobectomy using video-assisted thoracoscopic surgery: a randomized controlled trial. Eur J Cardiothorac Surg 2019;55:673–681 [DOI] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191 [DOI] [PubMed] [Google Scholar]
- 17. Dong Y, Peng C-YJ. Principled missing data methods for researchers. Springerplus 2013;2:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forster C, Doucet V, Perentes JY, Abdelnour-Berchtold E, Zellweger M, Faouzi M et al. Impact of an enhanced recovery after surgery pathway on thoracoscopic lobectomy outcomes in non-small cell lung cancer patients: a propensity score-matched study. Transl Lung Cancer Res 2021;10:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dumitra T-C, Molina J-C, Mouhanna J, Nicolau I, Renaud S, Aubin L et al. Feasibility analysis for the development of a video-assisted thoracoscopic (VATS) lobectomy 23-hour recovery pathway. Can J Surg 2020;63:E349–E358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linden PA, Perry Y, Worrell S, Wallace A, Argote-Greene L, Ho VP et al. Postoperative day 1 discharge after anatomic lung resection: a Society of Thoracic Surgeons database analysis. J Thorac Cardiovasc Surg 2020;159:667–678.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers LJ, Bleetman D, Messenger DE, Joshi NA, Wood L, Rasburn NJ et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843–1852 [DOI] [PubMed] [Google Scholar]
- 22. Forster C, Doucet V, Perentes JY, Abdelnour-Berchtold E, Zellweger M, Marcucci C et al. Impact of compliance with components of an ERAS pathway on the outcomes of anatomic VATS pulmonary resections. J Cardiothorac Vasc Anesth 2020;34:1858–1866 [DOI] [PubMed] [Google Scholar]
- 23. Pompili C, Falcoz PE, Salati M, Szanto Z, Brunelli A. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: an analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957–965 [DOI] [PubMed] [Google Scholar]
- 24. Attaar A, Winger DG, Luketich JD, Schuchert MJ, Sarkaria IS, Christie NA et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690–699.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg 2020;155:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Licker M, Karenovics W, Diaper J, Frésard I, Triponez F, Ellenberger C et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol 2017;12:323–333 [DOI] [PubMed] [Google Scholar]
- 27. Brunelli A, Salati M, Pompili C, Gentili P, Sabbatini A. Intraoperative air leak measured after lobectomy is associated with postoperative duration of air leak. Eur J Cardiothorac Surg 2017;52:963–968 [DOI] [PubMed] [Google Scholar]
- 28. Murakami K, Maehara S, Shimada Y, Makino Y, Hagiwara M, Kakihana M et al. The correlation between fissureless technique and prolonged air leak for patients undergoing video-assisted right upper lobectomy. World J Surg 2021;45:1569–1574 [DOI] [PubMed] [Google Scholar]
- 29. Li S-J, Zhou K, Li Y-J, Li P-F, Wu Y-M, Liu L-X et al. Efficacy of the fissureless technique on decreasing the incidence of prolonged air leak after pulmonary lobectomy: A systematic review and meta-analysis. Int J Surg 2017;42:1–10 [DOI] [PubMed] [Google Scholar]
- 30. Malapert G, Hanna HA, Pages PB, Bernard A. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. Ann Thorac Surg 2010;90:1779–1785 [DOI] [PubMed] [Google Scholar]
- 31. Attaar A, Luketich JD, Schuchert MJ, Winger DG, Sarkaria IS, Nason KS. Prolonged air leak after pulmonary resection increases risk of noncardiac complications, readmission, and delayed hospital discharge: a propensity score-adjusted analysis. Ann Surg 2021;273:163–172 [DOI] [PubMed] [Google Scholar]
- 32. Huang L, Kehlet H, Petersen RH. Effect of posture on pulmonary function and oxygenation after fast-tracking video-assisted thoracoscopic surgery (VATS) lobectomy: a prospective pilot study. Perioper Med (Lond) 2021;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bjerregaard LS, Hornum U, Troldborg C, Bogoe S, Bagi P, Kehlet H. Postoperative urinary catheterization thresholds of 500 versus 800 ml after fast-track total hip and knee arthroplasty: A randomized, open-label, controlled trial. Anesthesiology 2016;124:1256–1264 [DOI] [PubMed] [Google Scholar]
- 34. Patel DC, Leipzig M, Jeffrey Yang C-F et al. Early discharge after lobectomy for lung cancer does not equate to early readmission. Ann Thorac Surg 2021:S0003-4975(21)01012-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.