Abstract
Background:
Opioid prescribing practices contribute to opioid misuse, dependency, and diversion. There are currently no comprehensive and quantitative evidence-based guidelines that give procedure-specific recommendations regarding opioid prescribing in plastic surgery.
Methods:
A retrospective review of 479 plastic surgery patients encompassing 23 different plastic surgery procedure categories was performed. Opioid prescribing patterns and patient-reported opioid use at 1 and 3 months postoperatively are reported.
Results:
Opioid overprescribing was common, averaging an excess of 13 pills per patient across all procedure categories (prescribed versus consumed, 25.4 ± 23.1 versus 12.1 ± 19.7; p = 3.0 × 10−19), with a total excess of 5895 pills (30,967 oral morphine equivalents) for the study’s sample. Fifty-two percent of all opioid pills prescribed went unused. Opioid consumption ranged between four and 37 pills across procedure categories. A greater proportion of patients who reported a history of preoperative opioid use were still using opioids at the time of their 1-month and 3-month follow-up appointments (62 percent versus 9 percent at 1 month, and 31 percent versus 1 percent at 3 months). Most patients (83 percent) did not store opioids in a locked location, and 64 percent did not dispose of opioids at 1 month.
Conclusions:
Opioids are commonly overprescribed by plastic surgery providers. This study determined procedure-specific opioid consumption patterns, which can help providers reduce opioid waste. In addition, patients do not properly store or dispose of opioids, demonstrating the need for better patient education.
The Centers for Disease Control and Prevention estimates that there are 58 opioid prescriptions for every 100 Americans and that 46 people die every day from prescription opioid overdose.1 Previous prescription opioid exposure has been identified as a major risk factor for opioid use disorder, and higher doses of prescribed opioids have been linked to increased risk of opioid overdose deaths.2,3 In addition, of people who misuse opioids, more than half obtained opioids from friends or family who had legitimate opioid prescriptions, termed opioid “diversion.”4 Opioid prescribing practices, therefore, are a contributor to this major public health problem.
Plastic and reconstructive surgery patients often receive opioid medications for postoperative pain management. Indeed, the most common reason for new opioid prescriptions in previously opioid-naive patients is acute postoperative pain.5 To define the risk of opioid dependence associated with postoperative opioid prescribing, multiple studies have determined rates of “new persistent use,” defined as the filling of an opioid prescription 60 to 180 days after surgery in opioid-naive patients. Among plastic and reconstructive surgery patients, the rates of new persistent use have been reproducibly shown to range from 6 to 13 percent,6–9 highlighting the substantial risk for new opioid dependence for a large number of postoperative patients.
One solution to mitigating the risk of new persistent use, and also opioid prescription diversion, is to examine and modify prescribing practices. Expanded use of enhanced recovery after surgery protocols with multimodal analgesia allow for superior pain management with a decreased dependence on historically prevalent opioid-predominant regimens.10–14 However, a major limitation to improving prescribing practices in plastic surgery is a lack of granular data describing opioid consumption to inform procedure-specific prescribing guidelines. Overprescribing by plastic surgery providers has been identified in upper extremity operations by Rodgers et al. using a sample of 250 patients, and two other studies, of 95 and 170 patients, on outpatient breast procedures.15–17 There exists a pressing need to expand this line of investigation to provide robust, evidence-based prescribing guidance across the spectrum of plastic surgery procedures.
This study presents a review of 479 patients across 23 different procedural categories for both major and minor, inpatient and outpatient plastic surgery procedures. Study aims were to determine the scope of opioid overprescribing in a large cohort of patients and to measure granular opioid consumption patterns. Further analysis highlighted risks associated with prior opioid consumption and a gap in patient education regarding opioid storage and disposal. The results of this study can be used to inform opioid prescribing practices in plastic surgery.
PATIENTS AND METHODS
Study Design and Patient Selection
After institutional review board approval (The Ohio State University Medical Center Institutional Review Board no. 2019H0263), a retrospective review was completed for 479 patients who underwent any surgical procedure performed through a university hospital–based plastic surgery department from March of 2018 to September of 2019. Information regarding opioid and nonopioid medication use, medication storage, medication disposal, and prior opioid use was collected by means of surveys that were administered as part of a quality initiative for opioid waste. Patients completed these surveys in the clinic at 1 month (range, 3 to 5 weeks) and 3 months (range, 2 to 4 months) postoperatively. We identified 979 eligible patients based on these criteria from approximately 2500 department-wide operations performed during the same year. Of these, 479 completed 1-month surveys (response rate, 49 percent). Only patients who completed a 1-month survey and had an appointment in the 3-month follow-up period (n = 200) were eligible to receive a 3-month survey (n = 124; response rate, 62 percent). Demographic information, procedure, procedure date, disposition, and pain medications prescribed at discharge were collected by means of chart review.
Survey Questions
The 1-month survey encompassed postoperative opioid and nonopioid medication use, refill requests, medication storage, medication disposal, prior opioid use, and satisfaction with medication communication and pain control. The 3-month survey inquired about continued opioid and nonopioid medication use, refill requests, and medication disposal. (See Figure, Supplemental Digital Content 1, which shows surveys administered at 4-week and 3-month postoperative follow-up appointments, http://links.lww.com/PRS/E400.)
Statistical Analysis
Statistical analyses were performed in Microsoft Excel (Microsoft Corp., Redmond, Wash.). Descriptive statistics; two-sample, two-tailed t tests; or chi-square analyses for proportions were performed where applicable, with p < 0.05 as the threshold for significance.
RESULTS
Study Population
The average age for 1-month survey respondents was 53 years (range, 18 to 91 years; 95 percent CI, 51 to 54 years) (Fig. 1). Seventy-seven percent of respondents were female and 23 percent were male. Breast procedures were most common, with 186 respondents (38.8 percent) undergoing breast reconstruction–related procedures and 33 patients (6.9 percent) undergoing breast reductions or mastopexies unrelated to breast reconstruction. The next most common procedural category was abdominoplasty/ panniculectomy, with 43 patients (9.0 percent). Four uncategorized patients underwent vascularized lymph node transfer, lower extremity rotationplasty, augmentation mammaplasty, and nerve decompression for migraine treatment. Nonrespondents were similar to respondents in terms of age and sex but had a slightly higher rate of outpatient procedures (37 percent versus 30 percent; p = 0.031). [See Figure, Supplemental Digital Content 2, which shows demographic and procedure comparisons of nonrespondents versus respondents. Data are presented as mean (95 percent CI) where appropriate; p values for age were calculated using two-sample, two-tailed t tests; p values for sex and disposition after surgery were calculated using Pearson chi-square tests for independence, http://links.lww.com/PRS/E401.]
Fig. 1.
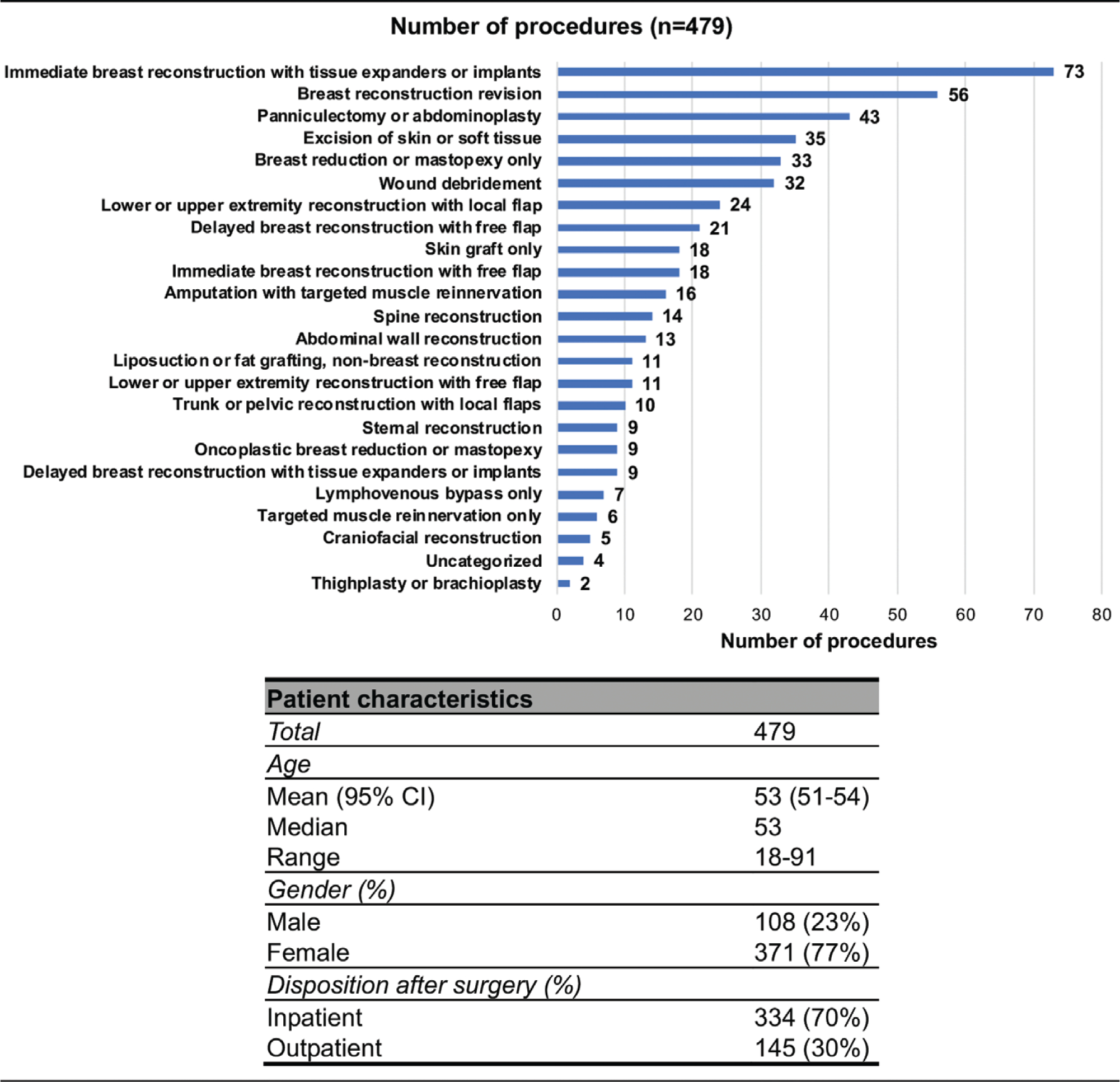
Patient demographics and procedure categories.
Opioid Consumption Patterns and Nonopioid Medication Use
At 1 month postoperatively, patients were asked how many opioid pills they consumed for pain control after surgery (Table 1). Not surprisingly, wide ranges of pills were consumed based predominately on procedure type. Patients undergoing amputation with targeted muscle reinnervation consumed the most opioid pills [mean (SD), 37.1 (36.3) pills], whereas patients undergoing liposuction/fat grafting consumed the fewest [4.7 (6.8) pills]. For breast reconstruction procedures, opioid consumption varied from 7.2 (9.7) pills for patients undergoing secondary breast reconstruction procedures to 14.6 (9.2) pills for patients undergoing delayed reconstruction with implants or tissue expanders. Breast reduction/mastopexy patients consumed 9.5 (8.9) pills and panniculectomy/abdominoplasty patients consumed 14.9 (11.3) pills.
Table 1.
Opioid Consumption Patterns and Opioid Waste Generated by Major Plastic Surgery Procedures*
| Procedure Category | No. of Patients | Average No. of Pills Prescribed (SD) | Average No. of Pills Consumed (SD) | p (Pills Consumed vs. Pills Prescribed) | Average No. of Pills in Excess | Total No. of Pills in Excess |
|---|---|---|---|---|---|---|
|
| ||||||
| Immediate breast reconstruction with tissue expanders or implants | 65 | 35.4 (15.5) | 11.9 (14.9) | 7.2 × 10−15 | 23.5 | 1528 |
| Breast reconstruction revision | 55 | 18.6 (9.2) | 7.2 (9.7) | 5.9 × 10−9 | 11.4 | 627 |
| Panniculectomy or abdominoplasty | 43 | 24.4 (7.9) | 14.9 (11.3) | 1.8 × 10−5 | 9.6 | 412 |
| Excision of skin or soft tissue | 34 | 17.2 (20.7) | 7.4 (8.3) | 0.013 | 9.9 | 336 |
| Wound débridement | 32 | 24.0 (20.1) | 12.3 (15.4) | 0.011 | 11.8 | 376 |
| Breast reduction or mastopexy only | 31 | 19.3 (7.4) | 9.5 (8.9) | 1.5 × 10−5 | 9.8 | 305 |
| Lower extremity or upper extremity reconstruction with local flap | 23 | 30.3 (27.2) | 13.2 (19.3) | 0.018 | 17.1 | 393 |
| Delayed breast reconstruction with free flap | 20 | 19.2 (7.7) | 7.3 (9.0) | 6.6 × 10−5 | 12.0 | 239 |
| Immediate breast reconstruction with free flap | 17 | 23.1 (12.5) | 9.5 (11.3) | 0.0023 | 13.6 | 231 |
| Skin graft only | 15 | 17.3 (12.3) | 11.8 (12.5) | 0.23 | 5.5 | 83 |
| Amputation with targeted muscle reinnervation | 12 | 45.3 (32.2) | 37.1 (36.3) | 0.57 | 8.2 | 98 |
| Spine reconstruction | 12 | 46.0 (83.0) | 36.8 (85.5) | 0.79 | 9.2 | 110 |
| Lower extremity or upper extremity reconstruction with free flap | 11 | 30.4 (22.1) | 19.2 (19.3) | 0.22 | 11.2 | 123 |
| Abdominal wall reconstruction | 11 | 34.7 (35.3) | 12.7 (13.9) | 0.076 | 22.0 | 242 |
| Liposuction or fat grafting, nonbreast reconstruction | 10 | 17.1 (6.6) | 4.7 (6.8) | 5.9 × 10−4 | 12.4 | 124 |
| Oncoplastic breast reduction or mastopexy | 9 | 25.9 (7.2) | 8.0 (6.1) | 3.6 × 10−5 | 17.9 | 161 |
| Trunk or pelvic reconstruction with local flaps | 9 | 46.6 (49.1) | 17.4 (29.3) | 0.15 | 29.1 | 262 |
| Delayed breast reconstruction with tissue expanders or implants | 8 | 21.4 (4.9) | 14.6 (9.2) | 0.10 | 6.8 | 54 |
| Sternal wound reconstruction | 8 | 13.3 (12.3) | 7.6 (8.7) | 0.31 | 5.6 | 45 |
| Lymphovenous bypass only | 7 | 12.6 (13.9) | 5.3 (11.2) | 0.30 | 7.3 | 51 |
| Targeted muscle reinnervation only | 6 | 21.7 (15.6) | 11.3 (10.8) | 0.21 | 10.3 | 62 |
| Craniofacial reconstruction | 5 | 13.6 (10.1) | 11.8 (10.5) | 0.79 | 1.8 | 9 |
| Thighplasty or brachioplasty | 1 | 28 (N/A) | 4 (N/A) | N/A | 24.0 | 24 |
| Total sample size | 444 | 25.4 (23.1) | 12.1 (19.7) | 3.0 × 10−19 | 13.3 | 5895 |
Data are presented as mean (SD)
p values were calculated using two-sample, two-tailed t test.
Most patients (81 percent) used at least one nonopioid medication, and 19 percent of patients used no nonopioid medication (Fig. 2). Acetaminophen was the most common nonopioid, with 64 percent of 389 respondents using this medication. Nonsteroidal antiinflammatory drugs were the next most common, with 60 percent reporting use. Flexeril, a common adjunct prescribed for our tissue expander breast reconstruction patients, was used by 20 percent of patients.
Fig. 2.
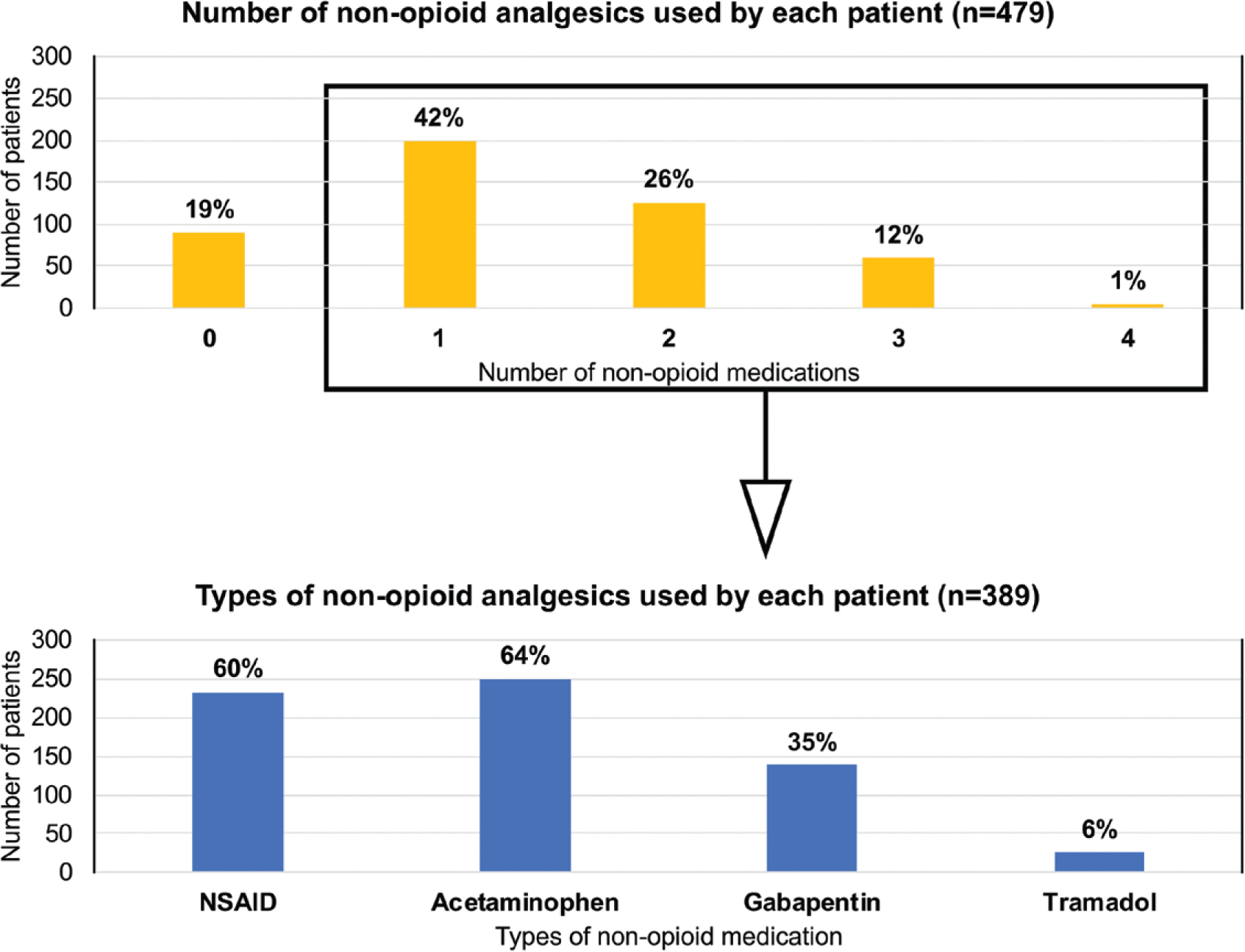
Use of nonopioid modalities for pain control. Although most patients used at least one nonopioid pain medication, 19 percent of patients used no nonopioid pain medication. Of patients who used a nonopioid pain medication, most patients used nonsteroidal antiinflammatory drugs (NSAID) or acetaminophen.
Opioid Prescribing Practices
Overall, patients were prescribed an average of 25.4 (23.1) pills and used an average of 12.1 (19.7) pills, resulting in an average excess of 13.3 pills (p = 3.0 × 10−19) across all procedure categories (Table 1). Furthermore, the State of Ohio limits opioid prescribing for episodes of acute pain to 30 oral morphine equivalents per day for up to 7 days unless documentation exists for a medical exception.18 We found that 22 percent of our opioid prescriptions were over the recommended limit. [See Table, Supplemental Digital Content 3, for percentage of patients prescribed over state limits by procedure. Average oral morphine equivalents (OMEs) prescribed per patient and frequency of prescribing over Ohio’s opioid prescribing limit for acute pain (30 oral morphine equivalents per day for 7 days). Data are presented as mean (SD), where appropriate, http://links.lww.com/PRS/E402.] Most procedure categories had a significantly greater number of pills prescribed in comparison to pills actually consumed, including all breast procedures, except delayed implant-based breast reconstruction. Overall, 5895 pills (30,967 oral morphine equivalents) were prescribed in excess for 444 respondents. The greatest contributor to opioid excess in this study was immediate implant-based reconstruction, accounting for 1528 excess pills (11,510 oral morphine equivalents), independently. Forty-seven percent of these patients received prescriptions over the state-recommended limit (see Table, Supplemental Digital Content 3, http://links.lww.com/PRS/E402).
Inpatient procedures, overall, had higher opioid prescribing and higher opioid consumption, resulting in five more pills in excess in comparison to outpatient procedures (15 pills versus 10 pills; p = 1.12 × 10−4) (Table 2). In addition, patients who had an inpatient stay were more likely to have been prescribed more than Ohio’s state-recommended limit for opioid prescribing (28 percent of inpatients versus 4 percent of outpatients; p = 1.66 × 10−8).
Table 2.
Comparison of Opioid Consumption and Opioid Waste for Inpatient and Outpatient Plastic Surgery Procedures*
| Procedure Category | No. of Patients | Average No. of Pills Prescribed (SD) | p (Inpatient vs. Outpatient Pills Prescribed) | Average No. of Pills Consumed (SD) | p (Inpatient vs. Outpatient Pills Consumed) | Average No. of Pills in Excess | p (Inpatient vs. Outpatient Pills in Excess) | p (Pills Prescribed vs. Pills Consumed) | Percentage of Patients Prescribed over State-Recommended Limit |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Breast reconstruction revision | |||||||||
| Inpatient | 14 | 26 (12.2) | 11 (15.3) | 15 | 6.68 × 10−4 | 14 | |||
| Outpatient | 41 | 16 (6.1) | 0.008 | 6 (6.7) | 0.228 | 10 | 0.182 | 8.32 × 10−11 | 0 |
| Delayed breast reconstruction with tissue expanders or implants | |||||||||
| Inpatient | 4 | 21 (5.4) | 13 (9.8) | 8 | 0.0839 | 0 | |||
| Outpatient | 4 | 22 (5.2) | 0.749 | 17 (9.6) | 0.605 | 6 | 0.585 | 0.163 | 0 |
| Breast reduction or mastopexy only | |||||||||
| Inpatient | 4 | 31 (6.5) | 11 (15.1) | 20 | 0.0239 | 25 | |||
| Outpatient | 27 | 18 (6.0) | 0.0208 | 9 (8.1) | 0.885 | 8 | 0.0810 | 3.49 × 10−6 | 0 |
| Panniculectomy | |||||||||
| Inpatient | 37 | 24 (8.1) | 15 (11.7) | 9 | 2.20 × 10−5 | 16 | |||
| Outpatient | 6 | 27 (5.9) | 0.340 | 12 (7.9) | 0.401 | 15 | 0.225 | 0.017 | 50 |
| Skin graft only | |||||||||
| Inpatient | 11 | 18 (14.0) | 13 (14.4) | 5 | 0.167 | 18 | |||
| Outpatient | 4 | 16 (6.9) | 0.792 | 9 (4.7) | 0.495 | 7 | 0.741 | 0.235 | 0 |
| Lower extremity or upper extremity reconstruction with local flap | |||||||||
| Inpatient | 20 | 32 (28.6) | 14 (20.1) | 19 | 0.00453 | 35 | |||
| Outpatient | 3 | 17 (9.2) | 0.105 | 10 (15.6) | 0.740 | 7 | 0.123 | 0.187 | 33 |
| Wound debridement | |||||||||
| Inpatient | 22 | 28 (22.4) | 15 (16.8) | 13 | 0.015 | 36 | |||
| Outpatient | 10 | 16 (9.7) | 0.0375 | 6 (8.5) | 0.0366 | 10 | 0.6420 | 0.0046 | 0 |
| Liposuction or fat grafting, nonbreast reconstruction | |||||||||
| Inpatient | 6 | 19 (7.4) | 5 (8.6) | 14 | 0.0429 | 0 | |||
| Outpatient | 4 | 14 (4.0) | 0.194 | 4 (3.3) | 0.697 | 10 | 0.577 | 0.058 | 0 |
| Excision of skin or soft tissue | |||||||||
| Inpatient | 8 | 20 (9.7) | 13 (12.3) | 7 | 0.095 | 13 | |||
| Outpatient | 26 | 17 (23.1) | 0.607 | 6 (6.0) | 0.139 | 11 | 0.446 | 0.0265 | 4 |
| Overall | |||||||||
| Inpatient | 309 | 29 (20.5) | 14 (15.2) | 15 | 6.22 × 10−26 | 28 | |||
| Outpatient | 139 | 17 (11.9) | 1.65 × 1012 | 7 (7.7) | 4.29 × 10−6 | 10 | 1.12 × 10−4 | 1.37 × 10−15 | 4 1.66 × 10−8 |
Data are presented as mean (SD)
p values were calculated using two-sample, two-tailed t tests, except when comparing proportions of patients prescribed over state-recommended limits, which were calculated using Pearson χ2 tests for independence. Procedure categories were selected for analysis if they had at least three patients each for inpatient and outpatient categories.
Opioid Persistent Use
In both 1-month and 3-month surveys, patients were asked whether they still used opioids at the time of survey completion, in addition to the number of refills requested for opioid medications (Fig. 3). At 1-month, 18 percent of patients reported taking opioid medications. When subdivided, patients with preexisting opioid use constituted most of this category. Of 358 opioid-naive respondents, 9 percent reported continued use of opioids, whereas 62 percent of the 84 patients reporting previous opioid use reported continual use (p = 8.5 × 10−24). Overall, 14.4 percent of respondents reported requesting a refill of opioid medication within 1 month of surgery, where 53 percent of these were opioid naive, 44 percent had a history of prior opioid use, and 3 percent had an unknown prior opioid use status.
Fig. 3.
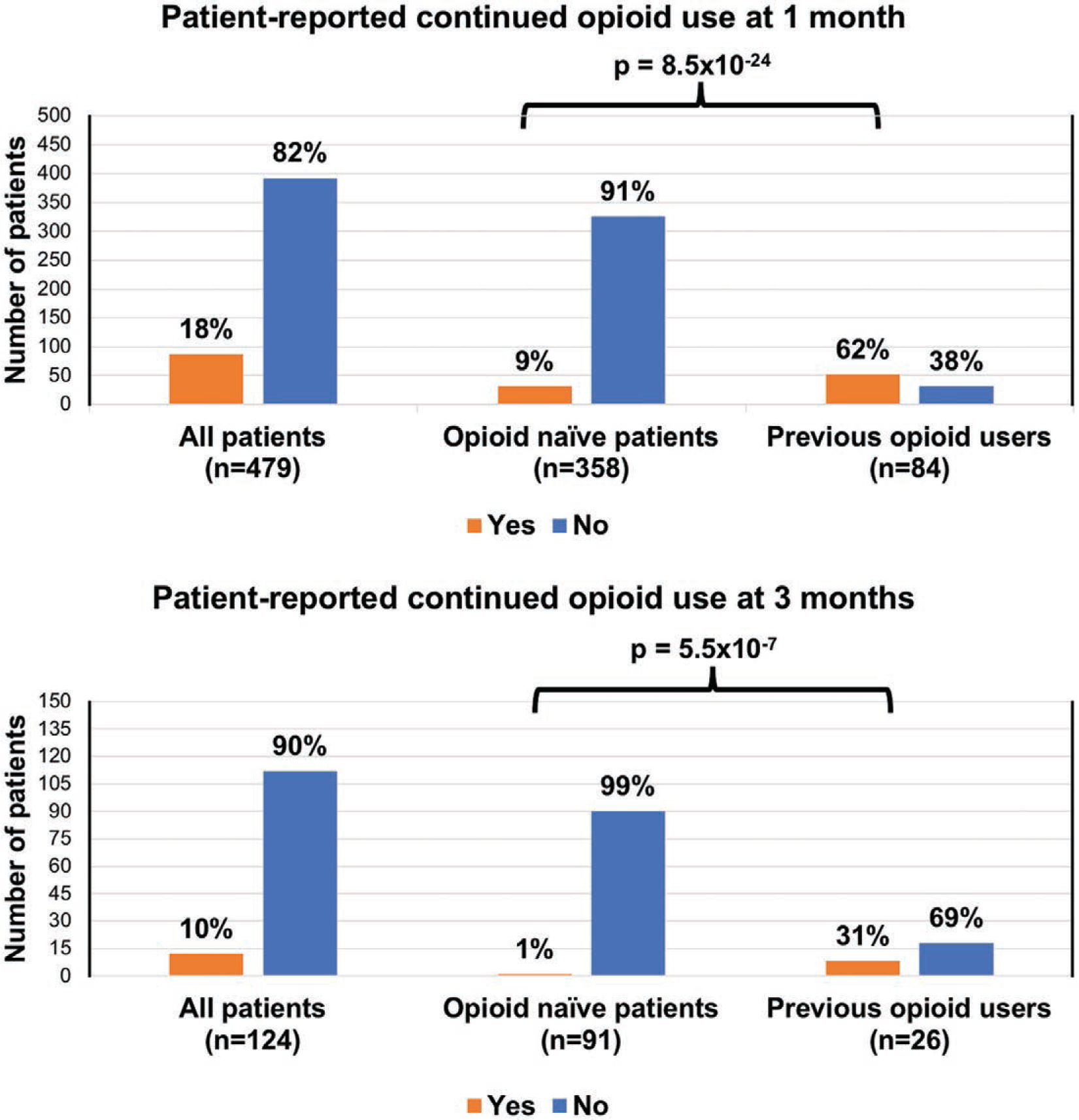
Persistent opioid use. Patients who were opioid-naive had a significantly lower rate of persistent opioid use at 4 weeks and at 3 months. Data are presented as proportions; p values were calculated using the Pearson chi-square test for independence.
At 3 months, 10 percent of patients overall reported persistent use of opioid medications. There was a significant difference in persistent use between opioid-naive patients and previous opioid users, with 1 percent of opioid-naive patients and 31 percent of previous opioid users reporting persistent use (p = 5.5 × 10−7).
Opioid Storage and Disposal
Of 410 respondents, 30 percent of patients stored opioid medications on a bedside table and 48 percent of patients stored their medications in their bathroom or kitchen cabinets (Fig. 4). Notably, 83 percent of patients stored their medication in an unlocked location. In addition, at 1 month, 64 percent of 394 respondents had not disposed of their opioid medications, and 2 percent reported returning their medications to a medication disposal site.
Fig. 4.
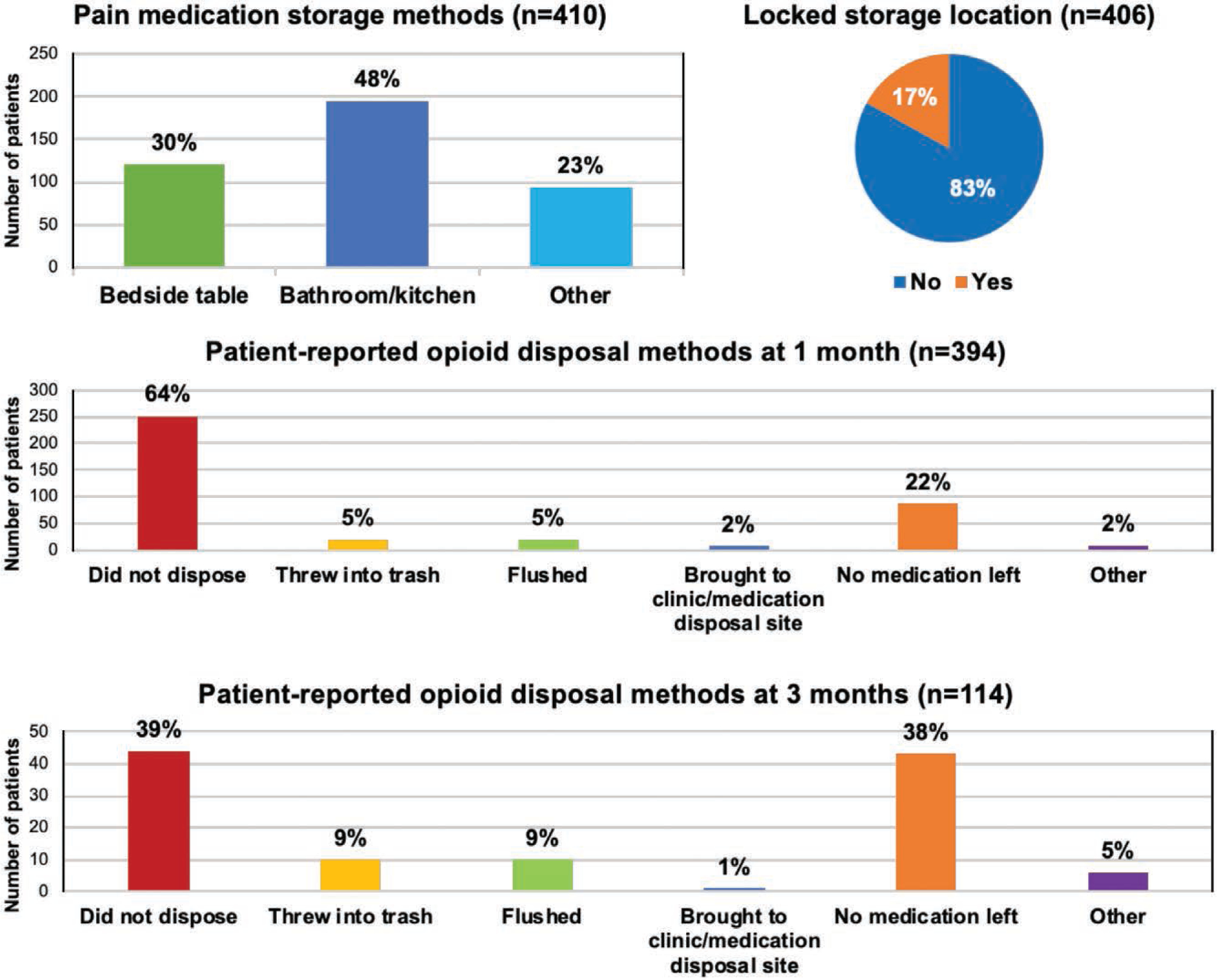
Opioid storage and disposal. A majority of patients (83 percent) did not store opioids in a locked location. At 3 months, almost 40 percent of patients had not disposed of leftover opioid medication.
At 3 months, patients were asked again about opioid medication disposal. Of 114 respondents, 39 percent of patients had not disposed of opioid medications, and 1 percent of patients had returned medications to a medication disposal site.
DISCUSSION
The nation is currently in the midst of an opioid epidemic, and medical providers’ prescribing practices are important contributors to opioid misuse, dependency, and diversion. At present, there are no clear evidence-based, procedure-specific guidelines regarding the appropriate quantity of opioids to prescribe for postoperative plastic surgery patients. This study contributes to the growing body of evidence highlighting the prevalence of opioid overprescribing and also provides granular numbers to aid in determining prescription quantities across a broad spectrum of procedure types in which most patients were also prescribed one or two adjunct nonopioid multimodal pain medications. In addition, this study demonstrates that patients are not securely storing or disposing of opioid medications, which holds implications for opioid misuse and diversion and demonstrates the need for improved perioperative education.
In this study, opioid overprescribing was universal across most procedure types. These findings included procedures where the plastic surgery team was the primary prescriber, and also for consultative operations where the plastic surgery team was not the primary prescriber. On average, 13.3 pills (69.7 oral morphine equivalents) of “leftover” opioid waste were generated per patient, meaning that 52 percent of all opioid medications prescribed went unused. To provide numerical context, 2531 plastic surgery procedures were performed in fiscal year 2018 for our institution. At the quantity of 13.3 excess pills per patient, a surplus of 33,662 pills (176,523 oral morphine equivalents) was distributed into the local community in a single year solely from operations involving plastic surgery. The consequences of having such excess are potentially enormous, given that most people who misuse opioids obtain them from friends or family members with legitimate prescriptions.
To our knowledge, this is the largest study to describe opioid prescribing and opioid consumption practices across a wide spectrum of procedure types in plastic and reconstructive surgery, and also one of the few to determine postdischarge average opioid needs in a procedure-specific manner. Hart et al. surveyed 95 patients and found that 11.4 pills were consumed for secondary breast reconstruction and 17.5 pills for breast reduction.16 Rose et al. (n = 170) reported 17 pills consumed for breast reduction, 16.5 pills for first-stage alloplastic breast reconstruction, 16.8 pills for second-stage breast reconstruction, 6.2 pills for breast reconstruction revisions, and 18.9 pills for abdominoplasty.15 These values are somewhat higher than what is reported in the present study; however, the reported rates of concurrent nonopioid medication use at 64 to 77.1 percent and 50 percent, respectively, were lower than what we observed (81 percent). In conjunction with prior work, the present study can serve as a baseline for providers to determine anticipated opioid consumption quantities to inform prescribing practices.
In addition to directly reporting opioid consumption patterns, it is also possible to extrapolate opioid consumption quantities indirectly from studies reporting outcomes after the implementation of enhanced recovery after surgery protocols and multimodal analgesia regimens. This approach is limited by the relative paucity of procedural types for which enhanced recovery after surgery data are presently available and also variability in available postdischarge data, but it has the added benefit of controlling for standardized adjunct interventions and medications. Regardless of whether evidence is gleaned directly or indirectly, the key message is for providers to base prescription quantities on the amount anticipated to be consumed. It is no longer recommended to determine discharge opioid prescription quantities in a non–patient-specific, blanketed, and/or intentionally overprescribed manner. For example, through practice analysis of opioid consumption followed by tailored opioid prescribing practices, Jamieson et al. achieved reduction of opioid waste in hand and upper extremity surgery by 62 percent without negatively impacting patient satisfaction or increasing refill requests.19
We determined one key area for intervention, which is that prescribed opioid quantities should be continually reevaluated to reflect improvements in pain management with enhanced recovery after surgery protocol implementation. An example from this study is found in the abdominal wall reconstruction procedure group. We have recently implemented a robust abdominal wall reconstruction enhanced recovery after surgery protocol that has been highly successful for an operation with historically high morbidity and opioid consumption for pain management.20 Abdominal wall reconstruction demonstrated one of the highest discordances between prescribed and consumed opioid quantities (average 22 excess pills per patient), highlighting that in the era of enhanced recovery after surgery, opioid discharge prescription quantities should be continually reevaluated and adjusted. This can be specifically challenging in operations where the plastic surgeon may not be the “primary” prescriber, resulting in an added, yet important, layer of interdisciplinary coordination.
Another concerning pattern identified was the existence of imbalances in opioid waste generation between procedure types. We found specific “high-offender” procedural categories that disproportionally contributed to opioid waste. For example, immediate implant-based reconstruction, a single procedural category, accounted for 1528 excess pills of the 5895 excess pills observed for the entire sample size. This imbalance resulted from it being a common operation for our department and also having one of the highest discharge prescription quantities for an unknown systematic reason. Although we recognize that the high-offender procedure category may not be the same for every practice, these data highlight the importance of quality assessment for opioid prescribing to identify high-offender procedure types in individual practices. Those operations that systematically contribute to the largest opioid waste burden represent an ideal starting point for quality intervention.
We identified a notable imbalance in overprescribing between inpatient and outpatient procedures, even within the same procedure category. Patients receiving inpatient procedures were seven times more likely to receive an opioid quantity above the state-recommended limit for acute pain. However, patients with inpatient procedures did not necessarily consume more opioids, resulting in significantly more opioid waste for inpatient procedures versus outpatient procedures. These findings highlight the need for plastic surgery providers to reconsider prescribing bias that may exist for inpatient procedures when assessing postoperative opioid requirements.
The rate of persistent use in our study was 18 percent at 1 month and 10 percent at 3 months. However, the rate of new persistent use for opioid-naive patients was 1 percent at 3 months, and the vast majority of those who continued to use opioids in the long-term had a history of opioid exposure. This calls attention to the need for personalized approaches to preoperative counseling and pain management, especially for patients with preexisting opioid use. One percent is less than that reported in other plastic surgery populations for new persistent use, which have ranged from 6 to 13 percent,6–9 and may be reflective of the population sampling. Our population is skewed toward breast cancer operations, whereas others have evaluated body contouring or hand surgery cohorts that may have different risk factors for opioid dependence.
Most patients did not store opioids in a locked location and almost two-thirds of patients had not disposed of their opioid medications by 4 weeks. At 3 months, nearly 40 percent of patients still had opioids at home. Strikingly, almost no patients used a safe medication disposal site (1 to 2 percent). Similar rates are reflected in other studies for postoperative patients.21–23 This finding may be explained by patients being unaware of how to safely store and dispose of opioids and of the risks of unsafe storage and disposal. In fact, when given patient education regarding opioid disposal, multiple studies have demonstrated increased rates of disposal of leftover opioids.23–25
The present work examined a large number of patients with direct reporting of opioid consumption. This approach has the advantage of providing granular opioid consumption data versus a common surrogate methodology that uses electronic postoperative refill documentation to describe rates of opioid use. By examining many procedural categories, however, the sample size of each subgroup was reduced, decreasing the weight of conclusions that can be drawn from the lesser represented categories. Furthermore, although we quantified use of nonopioid medications, a limitation of our retrospective study was the ability to control for the frequency and duration of administration of multimodal adjuncts. An additional limitation is the potential risk of recall bias through a survey approach. This was addressed by prompting patients before surgery that their postoperative medication consumption quantities would be queried. Furthermore, selection bias may have occurred, given the 49 percent survey completion rate; there may have been confounding variables that impacted a patient’s ability or willingness to complete the survey. Lastly, we recognize that opioid prescribing practices may differ between regions, institutions, and individual practices. Therefore, the take-home message of this work is not intended to be a description of absolute values of opioid waste (prescribed minus consumed) for each procedure. Rather, it is our hope that “opioid consumed” values, in the setting of the majority of patients taking multimodal adjunct medications, can serve as a guidepost for prescription quantities that can then be adapted based on patient-specific and practice-specific factors.
At our institution, ongoing and future quality improvement work aims to address the findings presented. Toward this end, we are taking a two-prong approach. The first arm is to establish basic universal best practices that are applicable to any practice. These include the implementation of procedure-specific discharge prescription quantities based on the evidence presented and the implementation of improved patient education in both the preoperative and postoperative settings for opioid use, storage, and disposal. The second arm is to address systematic institutional issues identified through this quality initiative in a point-by-point approach in conjunction with multidisciplinary collaborators. These include addressing specific high-offender procedure categories, the bias toward inpatient overprescribing in our practice, and updating our enhanced recovery after surgery protocol discharge prescription quantities to reflect improvement in pain management.
CONCLUSIONS
Reduction of opioid waste is the responsibility of every provider. We have demonstrated that plastic surgery as a specialty within a larger organization contributes substantially to the number of unused pills that are dispensed into a community (33,662 excess pills in 1 year). We encourage all to examine opioid prescribing and to generate more thoughtful strategies for postoperative pain management. Reducing opioid waste reduces the opportunity for pill diversion. Given the correlation between prescription diversion and overdose deaths, prescribing in appropriate quantities can ultimately save lives. Key strategic points to reduce waste include the incorporation of evidence-based discharge prescription quantity protocols, reevaluation of discharge prescription needs in the post–enhanced recovery after surgery era, identification of high-offender operations that generate disproportionate opioid waste, and a concerted effort to improve patient education regarding opioid storage and disposal.
Supplementary Material
ACKNOWLEDGMENTS
Jenny C. Barker, M.D., Ph.D., was supported by National Institutes of Health grants T32AI106704 and F32HL144120 and The Ohio State University President’s Postdoctoral Scholar Program. The authors wish to thank Kanu Goyal, M.D., for insight and contribution to study implementation.
Footnotes
Disclosure: Dr. Janis receives royalties from Thieme Publishing and Springer Publishing. None of the other authors has a financial interest in any of the products, devices, or drugs mentioned in this article.
REFERENCES
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67:1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305:1315–1321. [DOI] [PubMed] [Google Scholar]
- 3.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: The role of opioid prescription. Clin J Pain 2014;30:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose J, Hedden SL, Lipari RN, Park-Lee E. 2017. NSDUH annual national report. Available at: https://www.sam-hsa.gov/data/report/2017-nsduh-annual-national-report. Accessed May 2, 2019.
- 5.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States: Are surgeons the gatekeepers? Ann Surg. 2017;265:728–730. [DOI] [PubMed] [Google Scholar]
- 6.Olds C, Spataro E, Li K, Kandathil C, Most SP. Assessment of persistent and prolonged postoperative opioid use among patients undergoing plastic and reconstructive surgery. JAMA Facial Plast Surg. 2019;21:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41:947–957.e3. [DOI] [PubMed] [Google Scholar]
- 8.Marcusa DP, Mann RA, Cron DC, et al. Prescription opioid use among opioid-naive women undergoing immediate breast reconstruction. Plast Reconstr Surg. 2017;140:1081–1090. [DOI] [PubMed] [Google Scholar]
- 9.Bennett KG, Kelley BP, Gunaseelan V, Waljee JF. Persistent opioid use in body contouring patients. Plast Reconstr Surg Glob Open 2018;6(Suppl):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker JC, DiBartola K, Wee C, et al. Preoperative multimodal analgesia decreases postanesthesia care unit narcotic use and pain scores in outpatient breast surgery. Plast Reconstr Surg. 2018;142:443e–450e. [DOI] [PubMed] [Google Scholar]
- 11.Offodile AC, Gu C, Boukovalas S, et al. Enhanced recovery after surgery (ERAS) pathways in breast reconstruction: Systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2019;173:65–77. [DOI] [PubMed] [Google Scholar]
- 12.Sebai ME, Siotos C, Payne RM, et al. Enhanced recovery after surgery pathway for microsurgical breast reconstruction: A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143:655–666. [DOI] [PubMed] [Google Scholar]
- 13.Joshi GP, Kehlet H. Postoperative pain management in the era of ERAS: An overview. Best Pract Res Clin Anaesthesiol. 2019;33:259–267. [DOI] [PubMed] [Google Scholar]
- 14.Joshi GP, Kehlet H. Enhanced recovery pathways: Looking into the future. Anesth Analg. 2019;128:5–7. [DOI] [PubMed] [Google Scholar]
- 15.Rose KR, Christie BM, Block LM, Rao VK, Michelotti BF. Opioid prescribing and consumption patterns following outpatient plastic surgery procedures. Plast Reconstr Surg. 2019;143:929–938. [DOI] [PubMed] [Google Scholar]
- 16.Hart AM, Broecker JS, Kao L, Losken A. Opioid use following outpatient breast surgery: Are physicians part of the problem? Plast Reconstr Surg. 2018;142:611–620. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37:645–650. [DOI] [PubMed] [Google Scholar]
- 18.State of Ohio Board of Pharmacy. For prescribers: New limits of prescription opioids for acute pain. Available at: https://www.pharmacy.ohio.gov/Documents/Pubs/Special/ControlledSubstances/For%20Prescribers%20-%20New%20Limits%20on%20Prescription%20Opioids%20for%20Acute%20Pain.pdf. Accessed February 26, 2021.
- 19.Jamieson MD, Everhart JS, Lin JS, Jain SA, Awan HM, Goyal KS. Reduction of opioid use after upper-extremity surgery through a predictive pain calculator and comprehensive pain plan. J Hand Surg Am. 2019;44:1050–1059.e4. [DOI] [PubMed] [Google Scholar]
- 20.Khansa I, Jefferson R, Khansa L, Janis JE. Optimal pain control in abdominal wall reconstruction. Plast Reconstr Surg 2018;142(Suppl):142S–148S. [DOI] [PubMed] [Google Scholar]
- 21.Cabo J, Hsi RS, Scarpato KR. Postoperative opiate use in urological patients: A quality improvement study aimed at improving opiate disposal practices. J Urol. 2019;201: 371–376. [DOI] [PubMed] [Google Scholar]
- 22.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: A systematic review. JAMA Surg. 2017;152: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasak JM, Roth Bettlach CL, Santosa KB, Larson EL, Stroud J, Mackinnon SE. Empowering post-surgical patients to improve opioid disposal: A before and after quality improvement study. J Am Coll Surg. 2018;226:235–240.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose P, Sakai J, Argue R, Froehlich K, Tang R. Opioid information pamphlet increases postoperative opioid disposal rates: A before versus after quality improvement study. Can J Anesth. 2016;63:31–37. [DOI] [PubMed] [Google Scholar]
- 25.C.S. Mott Children’s Hospital National Poll on Children’s Health. Narcotics in the medicine cabinet: Provider talk is key to lower risk. Available at: https://mottpoll.org/reports-surveys/narcotics-medicine-cabinet-provider-talk-key-lower-risk. Accessed May 2, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


