Abstract
During the COVID-19 pandemic, all countries implemented lockdown to prevent transmission of coronavirus. The prolonged stay-at-home process created some unfavourable effects like unhealthy lifestyle, physical inactivity and sedentary behaviour especially in patients with cardiovascular risk. Hypertensive individuals are also affected in the pandemic because of limited access to healthcare services, screening, and altered lifestyles. We aimed to investigate physical activity (PA) level, sedentary behaviour, mental health and healthy lifestyle behaviours in patients with hypertension and compare these parameters with healthy controls. This prospective, cross-sectional study included 40 hypertensive and 40 age-sex matched healthy controls. We assessed PA with the International Physical Activity Questionnaire long-form, quality of life with Short-Form 36 (SF-36) questionnaire, anxiety and depression with Hospital Anxiety and Depression Scale (HADS) and lifestyle behaviours with Health-Promoting Lifestyle Profile Scale-II (HPLP-II). Moderate and vigorous PA levels of hypertensives’ were statistically lower than healthy controls (P = 0.001; P = 0.003, respectively). Hypertensive patients exhibited lower SF-36 physical function (P = 0.001), energy/vitality (P = 0.042), body pain scores than those of healthy controls (P = 0.007). Although HADS-anxiety, depression scores were similar (P > 0.05), the depression ratio (45%) was more common in the hypertensive group during the lockdown. The main findings are that hypertensive patients have lower PA levels and worse quality of life than healthy controls during the pandemic. In addition, the presence of depression is more common among hypertensive patients. Considering unhealthy lifestyles, governments, and health professionals should take some precautions and plan interventions against physical inactivity. As known, providing regular physical activity is a keystone to fighting against cardiovascular disease.
Keywords: COVID-19, hypertension, pandemics, physical activity
Introduction
Early in 2020, China declared a new Coronavirus type named Coronavirus Disease 2019 (COVID-19) that leads to severe acute respiratory disease. While the transmission rate is very high and transmission way is human to human with droplets or other body fluids, COVID-19 symptoms and effects vary among humans [1]. After WHO declared the situation as a pandemic in March 2020, various restrictions such as self-isolation, ‘stay at home’ order, travel restrictions and shutting down of public places were implemented.
Pandemic had many different effects on individuals’ lifestyles. To prevent transmission and infection, people tended to stay at home and interrupted social interactions and events all around the world. These altered situations caused some undesirable results like less physical activity (PA), increased emotional problems and altered eating habits. Behaviours pandemic period, many studies examining various health conditions’ behaviours, and they reported worse mental health and higher anxiety and depression levels, less PA, increased sedentary behaviours, unhealthy eating habits and lifestyle behaviours [2–5]. Ghosh et al., [2] investigated type 2 diabetic patients’ lifestyles before and during the lockdown. They reported 42% of their population had a reduction in PA, 87% of their population was psychologically affected as a result of lockdown and 19% stated that they gained 5–10% weight during lockdown. In another previous study, Chavel et al., [3] analyzed PA, depressive symptoms, perceived global mental-physical health and their relationships in the healthy population. They reported that whereas sedentary time increased, time spent in PA decreased, and increased sedentary time was associated with poorer physical and mental health [3].
Because PA, healthy diet and emotional control are key components of hypertension management, analyzing patients’ status during the pandemic period is vital. Although many studies reported decreased PA levels during the pandemic period, there is no study that compares hypertensive and healthy individuals’ parameters related to cardiovascular health during the pandemic in the literature [2]. Therefore, this study aimed to compare PA level, healthy lifestyle behaviours, mental health and quality of life of hypertensive patients and healthy individuals.
Methods
This cross-sectional study was carried out between June 2020 and September 2020 at the Hacettepe University, Faculty of Medicine, Department of Cardiology and Hacettepe University, Faculty of Physical Therapy and Rehabilitation in Ankara, Turkey. The study was accepted by the Ethical Committee of Hacettepe University with the 2020/12-31 decision number on June 2020. Hypertensive individuals and healthy subjects who decided to participate in the study signed an online informed consent form.
We included 40 stage 1–2 hypertensive patients who visited the Cardiology Department and 40 volunteer healthy individuals who were reached by social media posts of researchers’ during this period. Participants’ phone number was recorded with permission and researchers conducted phone calls to ask about PA level, anxiety and depression, quality of life and healthy lifestyle behaviours. Researchers first explained the aim of the study in this phone call and verbal consent was taken for the study. It took approximately 30 min to complete questionnaires for each individual.
We included individuals whose daily routines (work, socialization, etc) were limited because of the COVID-19 pandemic for both groups, and our exclusion criteria for the healthy group were having COVID-19 infection in the last 1 month, any physical impairments for PA, having COVID-19 symptoms or any suspicion of COVID-19. The healthy control group included volunteers with no chronic diseases (diabetes, hypertension, hyperlipidemia, any cardiac, neurological and pulmonary disease) and similar characteristics to the patients in terms of age and gender.
Patients’ body weight, height, BMI, smoking status, concomitant diseases, marital status, working status and quarantine compliance were recorded. We categorized COVID-19 compliance as; complete compliance, complete compliance except for urgent status, complete compliance except for working hours and partial compliance. Patients selected compliance status according to their routines.
The number of cardiovascular diseases (CVDs) risk factors was recorded according to the chart previously reported by Kwong et al., [6].
Physical activity level
PA level was assessed with long-form of International Physical Activity Questionnaire (IPAQ). The IPAQ is a widely used PA questionnaire that is valid for the Turkish population. The long-form of IPAQ contains eight domains of PA; vigorous PA, moderate PA, walking PA, occupational PA, transportation PA, house-yard PA, leisure-time PA and sitting time. Total scores were presented as metabolic equivalent level MET × min/week and every domain has a MET value. Every vigorous activity is calculated with eight MET × min × day (gardening equals 5.5 MET × min × day), every walking activity is calculated with 3.3 MET × min × day and every moderate-intensity activity is calculated with a 4 MET × min × day formula [7]. The sitting time has no point for the total score but gives information about sedentary behaviour. Participants reported the frequency and duration of all activities during the last week. The patients were categorized as sedentary, minimally active and active according to IPAQ categorization for PA level [8].
Quality of life
The Short Form-36 (SF-36) questionnaire was used for quality of life assessment. The SF-36 is a self-reported generic health status measurement and includes 36 items assessing eight domains of health. These dimensions are physical functioning, role limitations due to physical health problems, pain, general health, vitality, social functioning, role limitations due to emotional problems and mental health. The scores range between 0 and 100 and higher scores are related to better health status [9,10].
Psychosocial status
The psychosocial status of patients was evaluated with Hospital Anxiety and Depression Scale (HADS). The HADS assesses anxiety and depression levels [11]. HADS has two subscales such as HADS-depression (HADS-D) and HADS-anxiety (HADS-A) and each subscale contains seven items. The HADS score between 0 and 7 is normal, scores between 8 and 10 mild, 11–14 moderate and 15–21 means severe anxiety or depression [12].
Healthy lifestyle behaviours
Lifestyle behaviours were evaluated with the Health Promoting Lifestyle Profile Scale-II (HPLP). The HPLP-II consists of six subscales: health responsibility, PA, nutrition, stress management, interpersonal relationship and spiritual growth. The HPLP-II is a self-reported, Likert-type scale and contains 52 items. The total score is between 52 and 208 and patients are categorized as; 52–104 points inappropriate healthy lifestyle, 104–156 intermediate healthy lifestyle and 156–208 proper healthy lifestyle according to total score [13].
Statistical analysis
The statistical analysis was performed using the SPSS for Windows (Version 20.0, IBM Inc., Armonk, New York, USA). Descriptive statistics were expressed as mean ± SD, minimum and maximum values. Normality was tested using histograms and Kolmogorov–Smirnov test. Parametric variables were compared with Student’s t-test under parametric conditions and Mann–Whitney U test under nonparametric variables. The probability of error was taken as P < 0.05. According to the sample size calculation made by G*Power statistical program (G*Power ver.3.1, Henrich-Heine-University, Dusseldorf, Germany), a minimum of 16 individuals was required for each group to provide 80% power and 5% type 1 error based on Page et al’s study [14].
Results
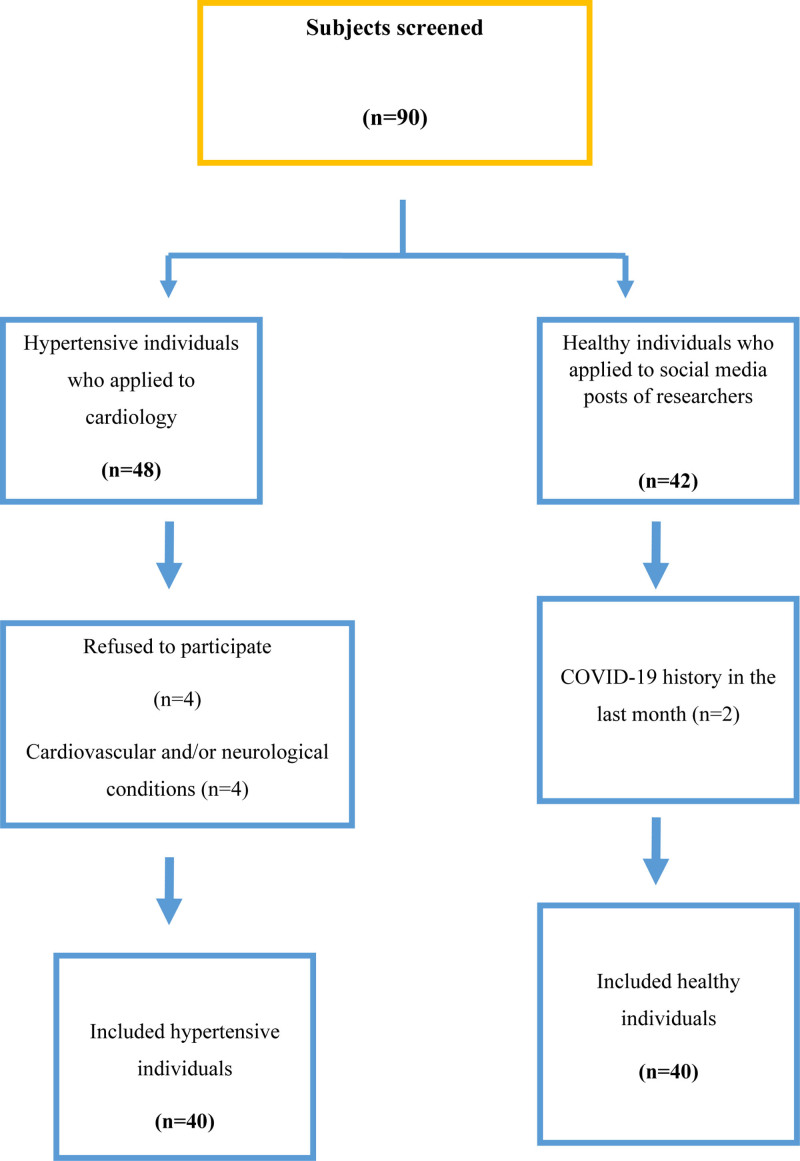
A total of 48 hypertensive patients were informed about the study and 40 patients accepted to participate in the study who fulfilled inclusion criteria. In total 42 healthy individuals agreed to participate in the study one refused to participate in the study after detailed information and one was excluded because of COVID-19 history (Fig. 1). We completed the study with 40 hypertensive patients and 40 healthy controls and patients’ characteristics are presented in Table 1. Age, sex, quarantine compliance and working status of two groups during the COVID-19 pandemic were similar between groups (P > 0.05, Table 1). BMI of the hypertensive group was significantly higher and the educational level was statistically different between groups (P < 0.05). In the hypertensive group, 29 (72.5%) had only diagnosis of hypertension, 8 (20%) had concomitant hyperlipidemia, 4 (10%) had concomitant type 2 diabetes and 2 (5%) had concomitant asthma. The numbers of CVD risk factors in the hypertensive group were statistically higher than the healthy group. Results of patients’ work status during the COVID-19 pandemic were like that 25 (62.5%) had any work, 10 (25%) worked full-time, 5 (12.5%) worked part-time in the hypertensive group and 23 (57.5%) had any work, 10 (25%) worked full-time, 3 (7.5%) had part-time and 4 (10%) worked from home in the healthy group and the working status during the pandemic period were similar between two groups (P = 0.205).
Fig. 1.
Flow chart of the study.
Table 1.
Characteristics of patients
| Variables | Hypertensive group (n = 40) | Healthy group (n = 40) | P value |
|---|---|---|---|
| Age | 51.1 ± 6.9 | 49.7 ± 8.4 | 0.420 |
| Sex (F/M) | 22/18 | 20/20 | 0.654 |
| BMI (kg/m2) | 28.4 ± 4.1 | 26.6 ± 2.8 | 0.025* |
| Duration of disease (months) | 19.8 ± 9.6 | Non-applicable | |
| Number of CAD risk factors | 2.5 ± 1.0 | 1.2 ± 0.9 | <0.001** |
| Comorbidities; n (%) | |||
| Hyperlipidemia | 5 (12.5%) | ||
| T2DM | 5 (2.5%) | ||
| Asthma | 2 (5%) | ||
| Hyperlipidemia | 3 (7.5%) | ||
| Medications | |||
| None | 2 (5%) | ||
| Angiotensin-converting enzyme inhibitors | 10 (25%) | ||
| Angiotensin-converting enzyme inhibitors+diuretics | 12 (30%) | ||
| Calcium channel blocker | 6 (15%) | ||
| Beta-blocker | 2 (5%) | ||
| Angiotensin-converting enzyme inhibitors+calcium channel blocker | 8 (20%) | ||
| Oral antidiabetic agent | 3 (7.5%) | ||
*P < 0.05.
**P < 0.01.
BMI, body mass index; CAD, coronary artery disease; F/M, female/male; T2DM, type 2 diabetes mellitus.
METs of every category, PA classification and activity categories according to intensity were presented in Table 2. Vigorous occupational activity scores, transportation-related walking scores, total transportation activity scores, leisure-time vigorous activity scores, house-yard vigorous and moderate activity scores, total PA scores were statistically different and hypertensive individuals were less active in all domains (P < 0.05, Table 2). According to classification 12.5% and 32.5% were active, 47.5% and 50% minimally active, 40% and 17.5% were sedentary in hypertensive and healthy groups, respectively and activity classifications were different between groups (P = 0.029). Moderate and vigorous-intensity activity levels were lower in hypertensives’ but walking domains of all activities were similar between groups (Table 2).
Table 2.
Physical activity status and healthy lifestyle behaviours
| Variables | Hypertension group (n = 40) | Healthy group (n = 40) | P value |
|---|---|---|---|
| IPAQ occupational (METXmin/week) | |||
| Vigorous | 62.5 ± 175.1 | 233 ± 556.7 | 0.008* |
| Walking | 101.4 ± 202.1 | 251.2 ± 524.2 | 0.527 |
| Total | 226.9 ± 474.2 | 633.2 ± 1064.9 | 0.177 |
| IPAQ transportation (METXmin/week) | |||
| Walking | 134.4 ± 176.2 | 221.1 ± 168.5 | 0.001* |
| Cycling | Non-applicable | 24.7 ± 120.5 | 0.155 |
| Total | 134.4 ± 176.2 | 245.8 ± 199.3 | <0.001** |
| IPAQ house yard (METXmin/week) | |||
| Gardening, vigorous | 291.1 ± 303.0 | 583.0 ± 458.0 | 0.003* |
| Gardening, moderate | 57 ± 120.7 | 219.5 ± 181.7 | <0.001** |
| House-Yard, moderate | 199.1 ± 271.5 | 246 ± 272.2 | 0.182 |
| Total | 291.1 ± 303.0 | 583.0 ± 458 | 0.003* |
| IPAQ leisure (METXmin/week) | |||
| Walking | 363 ± 421.6 | 254.1 ± 266.0 | 0.842 |
| Moderate | 103 ± 215.6 | 120 ± 205.1 | 0.345 |
| Vigorous | Non-applicable | 129 ± 282.9 | 0.002* |
| Total score | 466 ± 498.0 | 503.1 ± 526.5 | 0.486 |
| Total physical activity Score | 1118.6 ± 863.4 | 1965.2 ± 1461.7 | 0.002* |
| Activity intensity (total score) (METXmin/week) | |||
| Vigorous | 98.0 ± 344.2 | 395.5 ± 619.4 | 0.001* |
| Moderate | 421.6 ± 465.3 | 818.5 ± 745.5 | 0.003* |
| Walking | 598.9 ± 564.3 | 726.4 ± 645.7 | 0.276 |
| Sitting time (minutes in 24 h) | 401.7 ± 240.8 | 299 ± 181.7 | 0.066 |
| Activity status | |||
| Activity | 12.5% | 32.5% | 0.029* |
| Minimally active | 32.5% | 50% | |
| Inactive | 40% | 17.5% | |
| Healthy lifestyle behaviours | |||
| Health responsibility | 24.7 ± 4.4 | 22.4 ± 3.9 | 0.015* |
| Physical activity | 16.4 ± 5.8 | 17.7 ± 6.0 | 0.315 |
| Nutrition | 23.2 ± 4.0 | 23.1 ± 4.2 | 0.957 |
| Stress management | 19.6 ± 3.5 | 20.0 ± 5.1 | 0.896 |
| Interpersonal relationship | 26.7 ± 3.6 | 28 ± 3.9 | 0.137 |
| Spiritual growth | 26.7 ± 4.1 | 27 ± 4.4 | 0.795 |
| Total score | 137.5 ± 19.2 | 138.3 ± 24.0 | 0.762 |
*P < 0.05.
**P < 0.01.
IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent.
Quality of life, anxiety and depression status were presented in Table 3. Despite, similar anxiety and depression scores of groups, depression was more common in the hypertensive group (P = 0.033). In the hypertensive group, 20% of individuals had mild anxiety, 10% had moderate anxiety and 22% had mild depression, 12.5% had moderate depression, 5% had severe depression. When considering healthy groups’ anxiety and depression; 5% had mild anxiety, 12% had moderate anxiety, 2.5% had severe anxiety and 17.5% had mild depression, 7.5% had moderate, 5% had severe depression (P = 0.706 and P = 0.787, respectively).
Table 3.
Quality of life and psychosocial status
| Variables | Hypertension group (n = 40) | Healthy group (n = 40) | P value |
|---|---|---|---|
| SF-36 physical health component | |||
| Physical function | 71.6 ± 14.6 | 77.6 ± 13.0 | 0.001* |
| Role physical | 75 ± 36.6 | 81.1 ± 8.8 | 0.015* |
| Bodily pain | 64.7 ± 24.6 | 78.3 ± 25.7 | 0.007* |
| General health | 60.5 ± 21.0 | 67.7 ± 17.7 | 0.147 |
| SF-36 mental health | |||
| Component | 51.7 ± 19.6 | 61.5 ± 20.3 | 0.042* |
| Energy/vitality | 65.3 ± 28.5 | 65.3 ± 26.8 | 0.957 |
| Social function | 60.9 ± 30.1 | 64.1 ± 38.1 | 0.460 |
| Role emotional mental health | 66.8 ± 15.2 | 67.5 ± 15.1 | 0.996 |
| Psychosocial status | |||
| HADS-anxiety score | 6.4 ± 2.7 | 6.1 ± 3.9 | 0.381 |
| HADS-depression score | 6.5 ± 4.0 | 5.4 ± 4.6 | 0.147 |
| HADS total score | 13.0 ± 6.0 | 11.5 ± 7.3 | 0.224 |
| Anxiety and depression status | (%) | (%) | |
| Anxiety | |||
| Mild | 20 | 5 | 0.706 |
| Moderate | 10 | 12 | |
| Severe | Non-applicable | 2.5 | |
| Depression | |||
| Mild | 22 | 17.5 | |
| Moderate | 12.5 | 7.5 | 0.787 |
| Severe | 5 | 5 | |
*P < 0.05.
**P < 0.01.
HADS, Hospital Anxiety and Depression Scale; SF-36, Short-Form 36.
Physical functioning, pain, role limitations due to physical health problems, and vitality scores were statistically different and hypertensives’ functions were worse than healthy individuals (P < 0.05). SF-36 social functioning, mental health, role limitations due to emotional problems, and general health scores were similar between groups (P > 0.05). According to HPLP-II results, although the health responsibilities of groups were different and hypertensive individuals had higher health responsibility, PA, nutrition, stress management, interpersonal relationship and spiritual growth subdimension scores were similar between groups (P > 0.05). According to HPLP-II cutoff points, 7.5% of hypertensive patients had inappropriate, 82.5% had intermediate and 10% had a proper lifestyle, 2.5% of healthy individuals had inappropriate, 75% had intermediate and 22.5% had a proper lifestyle and there was not any statistically significant difference between groups (P > 0.05).
Discussion
To the best of our knowledge, this is the first study investigating PA, quality of life, depression and anxiety and healthy lifestyle behaviours in hypertensive and healthy individuals during COVID-19 restrictions. The main findings of our study were that hypertensive patients were less active and showed more sedentary behaviours compared to healthy controls during the COVID-19 pandemic. In addition, hypertensive patients had worse physical functioning, role limitations due to physical health, energy/vitality and body pain. Moreover, depression was more common in the hypertensive group when compared to age and sex-matched healthy controls.
COVID-19 is a life-threatening condition but its psychosocial and physiologic burden became vital for the whole world. Pandemic and quarantines affect patients’ lifestyles in many different ways. Decreased outdoor activities and prolonged staying at home cause more sedentary time and less PA [15]. On the other hand, difficulties in reaching healthcare services can cause complications and coronavirus infection and related conditions create panic, anxiety and depression [16].
In the literature, many studies are analyzing PA behaviour of individuals and predictors of physical inactivity during the pandemic period but there is no study comparing PA levels of hypertensives with healthy controls. We analyzed all components of PA and according to our results, almost all domains of PA were lower (except walking) in the hypertensive group than healthy controls. It may be the impact of more strict rules and a more pronounced fear of infection [16]. In Stockwell et al.’s [17] systematic review, they analyzed 66 studies that compare PA levels in prelockdown and lockdown periods in different populations (eating disorders, healthy individuals, chronic medical conditions, obesity, heart failure and neuromuscular disease). The majority of these studies reported decreased PA levels, increased sedentary behaviours and these led to higher anxiety and depression. These findings were comparable with our population’s results. Our all study groups had extended sitting periods and these were longer in the hypertensive group. Spence et al. examined determinants of PA during pandemic [18]. This study included 1521 individuals and the majority of participants (57%) reported the comparable or increased PA according to the prelockdown period. But authors stated that sedentary-related behaviours during work and leisure time increased during the lockdown period and that was parallel to our results. Additionally, there may be another issue explaining physical inactivity for hypertensive ones. Hypertension is considered an important mortality predictor of COVID-19 infection and hypertensives’ disease severity is higher according to healthy controls [19,20]. Not only hypertension itself but also treatment regimes (such as medications) increase disease severity and inflammation [19,21]. Patients are informed about these factors and these may increase their self-isolation.
Considering pandemic situations, the domain and place of PA gained importance. Yang et al., [22] investigated PA domains with more than 10 000 youth participants. During the pandemic period, activity patterns of youths’ have changed and while moderate and vigorous activity levels decreased, sedentary behaviour increased. Stockwell et al., [17] also emphasized altered activity patterns. Although some studies reported increased housework and gardening activity levels, total PA was reduced during the pandemic period [17]. Dobler et al. compared patients with pulmonary artery hypertension (PAH) and age-matched healthy individuals’ PA level during the COVID-19 pandemic. All domains’ of PA were lower in the PAH group when compared to healthy controls [23]. We found similar light intensity and household PA levels between groups but total PA level, moderate and vigorous activity levels were lower in hypertensive individuals in line with previous results. In the light of these results, physical inactivity is an important topic for the pandemic period. Similar to prelockdown, WHO recommends at least 150 min/week moderate PA or 75 min/week vigorous PA and breaking up prolonged sedentary periods (for example every 30 min sitting can be broken up with 2 min walking) for lockdown period. Despite many websites, clinics, organizations conducted online PA programs and gave PA advices to eliminate physical inactivity, previous studies and our study showed that people did not attend PA programs and could not meet suggested PA levels during lockdown [17,24,25].
There are many studies reporting worse eating habits and health-related behaviours during the lockdown period [23,26]. Worrying about health, sitting, limited social and work-related activities may lead to binge eating and consumption of unhealthy foods [16]. Hypertensives and healthy individuals exhibited similar dietary patterns and health-related behaviours. Cancello et al., [27] examined determinants of lifestyle changes during the COVID-19 pandemic in 490 adults. Forty-two percent of participants increased food intake, 38% increased smoking and chronic disease status, BMI and gender were not determinants of lifestyle changes. In a review, Marçal et al. underline that negative behaviours (physical inactivity, sedentary behaviour and unhealthy food consumption) disrupt metabolic and cardiovascular parameters including blood glucose, body fat, blood pressure, etc. during the COVID-19 outbreak [28].
Together with these undesirable behaviours, anxiety and depression, poor mental health were other common problems for the pandemic period. Many studies stated higher anxiety and depression level in all groups during coronavirus outbreak and similar to our results, Özdin et al., [29] reported higher anxiety and depression scores in patients with concomitant chronic diseases. Similar to this finding, Salari et al. [30] reported in their systematic review and meta-analysis that COVID-19 was not only a physical health problem but also a psychological problem and concomitant chronic illnesses increased psychiatric distress levels among the general population. Our study also demonstrated that although anxiety and depression scores of hypertensive and healthy groups were comparable, depression presence was more common and many participants had elevated anxiety and depression levels.
Outbreaks negatively affect individuals’ physical, social and psychological functions and as a natural result, societies’ and individuals’ quality of life decrease. A trial conducted with 647 individuals reported gradual worsening in physical function during the pandemic period [31]. Hypertensive patients’ physical functions and physical role limitations were worse during the lockdown period in this study. As known, higher PA levels had a relationship with higher emotional and physical status so inactivity and sedentary behaviour may have led to worse physical functions in our hypertensive group [17,32]. In addition to worse physical function, hypertensive individuals had more bodily pain during the lockdown. Increased sedentary behaviour, health-related anxiety, change in working status, the concomitant disease may change somatic sensation and increase body pain [33,34]. Considering all results, hypertensive patients’ longer sedentary times, lower PA levels, medication use, concomitant diseases may explain their worse body pain, physical functioning and role limitation and energy/vitality scores.
Our study has some strength. We used long IPAQ for PA assessment and it assesses all domains of PA because assessing outdoor activities does not give appropriate information on PA behaviour. We assessed PA in the same periods of the year and in this way, we eliminate seasonal alterations and weather conditions. In the literature, current studies generally used online forms and surveys and it can cause misunderstanding of patients but we eliminated this with phone calls. Moreover, many studies analyzing PA and health-related behaviours used nonstandardized questionnaires and yes/no questions; all questionnaires that we used in our study were valid and reliable.
Also, we have some limitations in that study. The main limitation of this study was sample size but because of the pandemic condition we could have reached limited number of patients and we selected patients during their admissions to the Cardiology Department. Another limitation was the PA assessment method. We used a subjective method because of pandemic and device limitation. Lastly, we did not assess the same patients’ PA levels before the pandemic so we did not analyze the difference in lifestyle and PA levels during prelockdown and lockdown periods.
Conclusion
In conclusion, we showed that despite their increased CVD risk, hypertensive individuals were more sedentary and less physically active, and they experienced the worse quality of life during the pandemic period. Unexpectedly, healthy individuals’ anxiety and depression status were similar to hypertensive ones.
The lockdown periods change the lifestyles of patients. Self-care issues including PA, healthy nutrition and lifestyle behaviours, maintaining and developing mental health are vital for all individuals but it is more crucial for patients with CVD risk factors. Otherwise, after the pandemic, noncommunicable diseases including hypertension, physical inactivity, and related health consequences could create serious public health problems for individuals and societies. Our results give an insight into interventions, management strategies during and after the pandemic. Considering these results, we suggest immediate precautions to prevent physical inactivity, unhealthy behaviours in hypertensive patients. Hypertensive and cardiovascular risky patients should be informed about the importance of PA, nutrition, stress management and awareness about lifestyle modifications should be created.
Highlights
-
(1)
This is the first study that compares health-related behaviours and PA of hypertensive and healthy individuals’ during COVID-19 restrictions.
-
(2)
Hypertensive individuals had less PA than healthy controls and they did not meet recommended PA levels.
-
(3)
Hypertensive individuals had common depression and worse quality of life during a pandemic.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Maugeri G, Castrogiovanni P, Battaglia G, Pippi R, D’Agata V, Palma A, et al. The impact of physical activity on psychological health during Covid-19 pandemic in Italy. Heliyon 2020; 6:e04315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh A, Arora B, Gupta R, Anoop S, Misra A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab Syndr 2020; 14:917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheval B, Sivaramakrishnan H, Maltagliati S, Fessler L, Forestier C, Sarrazin P, et al. Relationships between changes in self-reported physical activity, sedentary behaviour and health during the coronavirus (COVID-19) pandemic in France and Switzerland. J Sports Sci 2021; 39:699–704. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Henry BM, Bovo C, Sanchis-Gomar F. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID-19). Diagnosis (Berl) 2020; 7:85–90. [DOI] [PubMed] [Google Scholar]
- 5.Pieh C, Budimir S, Probst T. The effect of age, gender, income, work, and physical activity on mental health during coronavirus disease (COVID-19) lockdown in Austria. J Psychosom Res 2020; 136:110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation 2003; 107:531–537. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane D, Chan A, Cerin E. Examining the validity and reliability of the Chinese version of the International Physical Activity Questionnaire, long form (IPAQ-LC). Public Health Nutr 2011; 14:443–450. [DOI] [PubMed] [Google Scholar]
- 8.Saglam M, Arikan H, Savci S, Inal-Ince D, Bosnak-Guclu M, Karabulut E, et al. International physical activity questionnaire: reliability and validity of the Turkish version. Percept Mot Skills 2010; 111:278–284. [DOI] [PubMed] [Google Scholar]
- 9.Hou Y, Wu Q, Zhang D, Jin X, Wu W, Wang X. The differences in self-perceptions of aging, health-related quality of life and their association between urban and rural Chinese older hypertensive patients. Health Qual Life Outcomes 2020; 18:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koçyiğit H, Aydemir Ö, Fişek G, Ölmez N, Memiş A. The validity and reliability of Turkish version of the Short Form 36 (SF-36). Turk J Drugs Ther 1999; 12:102–106. [Google Scholar]
- 11.Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 2019; 39:E6–E11. [DOI] [PubMed] [Google Scholar]
- 12.Aydemir Ö, Guvenir T, Kuey L, Kultur S. Validity and reliability of Turkish version of hospital anxiety and depression scale. Turk Psikiyatri Dergisi 1997; 8:280–287. [Google Scholar]
- 13.Li J, Yu J, Chen X, Quan X, Zhou L. Correlations between health-promoting lifestyle and health-related quality of life among elderly people with hypertension in Hengyang, Hunan, China. Medicine 2018; 97:e10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page A, Cooper AR, Stamatakis E, Foster LJ, Crowne EC, Sabin M, et al. Physical activity patterns in nonobese and obese children assessed using minute-by-minute accelerometry. Int J Obes (Lond) 2005; 29:1070–1076. [DOI] [PubMed] [Google Scholar]
- 15.Petersen JM, Kemps E, Lewis LK, Prichard I. Promoting physical activity during the COVID-19 lockdown in Australia: the roles of psychological predictors and commercial physical activity apps. Psychol Sport Exerc 2021; 56:102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta SK, Lakshmi PVM, Kaur M, Rastogi A. Role of self-care in COVID-19 pandemic for people living with comorbidities of diabetes and hypertension. J Family Med Prim Care 2020; 9:5495–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockwell S, Trott M, Tully M, Shin J, Barnett Y, Butler L, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med 2021; 7:e000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spence JC, Rhodes RE, McCurdy A, Mangan A, Hopkins D, Mummery WK. Determinants of physical activity among adults in the United Kingdom during the COVID-19 pandemic: The DUK-COVID study. British Journal of Health Psychology 2021; 26:588–605. [DOI] [PubMed] [Google Scholar]
- 19.Trump S, Lukassen S, Anker MS, Chua RL, Liebig J, Thürmann L, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol 2021; 39:705–716. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have the more severe disease: a multicenter retrospective observational study. Hypertens Res 2020; 43:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Zhu J, Xu T. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients with hypertension on renin–angiotensin system inhibitors. Clin Exp Hypertens 2020; 42:656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S, Guo B, Ao L, Yang C, Zhang L, Zhou J, et al. Obesity and activity patterns before and during COVID-19 lockdown among youths in China. Clin Obes 2020; 10:e12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobler CL, Krüger B, Strahler J, Weyh C, Gebhardt K, Tello K, et al. Physical Activity and Mental Health of Patients with Pulmonary Hypertension during the COVID-19 Pandemic. J Clin Med 2020; 9:4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, et al. Effects of COVID-19 home confinement on eating behavior and physical activity: results of the ECLB-COVID19 international online survey. Nutrients 2020; 12:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto AJ, Dunstan DW, Owen N, Bonfá E, Gualano B. Combating physical inactivity during the COVID-19 pandemic. Nat Rev Rheumatol 2020; 16:347–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattioli AV, Ballerini Puviani M, Nasi M, Farinetti A. COVID-19 pandemic: the effects of quarantine on cardiovascular risk. Eur J Clin Nutr 2020; 74:852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancello R, Soranna D, Zambra G, Zambon A, Invitti C. Determinants of the lifestyle changes during COVID-19 pandemic in the residents of northern Italy. Int J Environ Res Public Health 2020; 17:E6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcal IR, Ciolac EG. The importance of promoting physical activity during the COVID-19 outbreak to control the worsening of old pandemics. Brazilian Journal of Motor Behavior 2021;15:20–6. [Google Scholar]
- 29.Özdin S, Bayrak Özdin Ş. Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: The importance of gender. Int J Soc Psychiatry 2020; 66:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health 2020; 16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Abascal EG, Martín-Díaz MD. Longitudinal study on affect, psychological well-being, depression, mental and physical health, prior to and during the COVID-19 pandemic in Spain. Pers Individ Dif 2021; 172:110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilani HA, Bataineh MF, Al-Nawayseh A, Atiyat K, Obeid O, Abu-Hilal MM, et al. Healthy lifestyle behaviors are major predictors of mental wellbeing during COVID-19 pandemic confinement: a study on adult Arabs in higher educational institutions. PLoS One 2020; 15:e0243524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iglesias-López E, García-Isidoro S, Castellanos-Sánchez VO. COVID-19 pandemic: pain, quality of life and impact on public health in the confinement in Spain. Ann Palliat Med 2021; 10:4338–4353. [DOI] [PubMed] [Google Scholar]
- 34.Baradaran Mahdavi S, Kelishadi R. Impact of Sedentary Behavior on Bodily Pain While Staying at Home in COVID-19 Pandemic and Potential Preventive Strategies. Asian J Sports Med 11:e103511. [Google Scholar]