Abstract
Introduction:
Given the increasing availability and use of cannabis among individuals with post-traumatic stress disorder (PTSD) and the addition of PTSD as an eligible diagnosis in several U.S. medical cannabis programs, the efficacy of dispensary-obtained cannabis needs to be thoroughly examined.
Materials and Methods:
This prospective study assessed PTSD symptoms and functioning every 3 months over the course of a year in two samples of participants diagnosed with PTSD: (1) those with PTSD using dispensary-obtained cannabis (cannabis users) and (2) those with PTSD, who do not use cannabis (controls). Linear mixed-effects models and generalized estimating equations tested whether trajectories of symptoms differed between the two subsamples.
Results:
A total of 150 participants (mean [standard deviation] age, 50.67 [15.26] years; 73% male) were enrolled in the study. Over the course of 1 year, the cannabis users reported a greater decrease in PTSD symptom severity over time compared to controls [group×time interaction=−0.32 (95% confidence interval [CI]=−0.59 to −0.05, R2=0.13; t=−2.35, p=0.02). Participants who used cannabis were 2.57 times more likely to no longer meet DSM-5 criteria for PTSD at the end of the study observation period compared to participants who did not use cannabis (95% CI=1.12–6.07; p=0.03).
Conclusions:
This study provides evidence that the types of cannabis available in recreational and medical cannabis dispensaries might hold promise as an alternative treatment for PTSD. Randomized placebo-controlled trials are needed to assess safety and determine how different preparations of cannabis impact PTSD and functioning.
Keywords: cannabis, control, longitudinal, post-traumatic stress disorder, PTSD
Introduction
As of 2020, 24 U.S. states have legalized medical cannabis as a treatment for post-traumatic stress disorder (PTSD), a condition that affects an estimated 13.8–30.9% of U.S. military veterans.1,2 However, the majority of physicians do not currently recommend cannabis to their patients with PTSD,3 despite the availability of cannabis products for PTSD through state-regulated dispensaries. This reluctance is likely due to the general lack of rigorous prospective epidemiological studies and well-controlled clinical trials demonstrating effectiveness of cannabis as a treatment for PTSD. Accordingly, in order for policy makers, providers, and consumers to make evidence-based decisions, studies are needed to determine whether (1) cannabis can help alleviate PTSD symptoms, (2) any medical benefit from cannabis is sustained over time, (3) PTSD patients who use cannabis through these programs lead more productive lives, and (4) cannabis is safe and well tolerated in this patient population.
A growing body of research suggests that the endocannabinoid (eCB) system, which includes the primary receptors activated by cannabis' phytocannabinoids, CB1 and CB2, might be directly involved in development and maintenance of PTSD (see Hill et al. for review),4 suggesting that augmentation of the eCB system might hold therapeutic potential. PTSD's primary neurological hallmarks include hyperactivity of the amygdala and hypoactivity of the medial prefrontal cortex (mPFC),5 features that can also be produced by deficiencies in eCB signaling.6 Hyperactivity of the amygdala and hypoactivity of the mPFC account for many of PTSD's symptoms, including overall heightened anxiety and startle,7 attentional bias to fear and trauma cues,8 as well as impairments in extinguishing traumatic memories.9
While primarily at the pre-clinical stage, this work suggests that at least two of the cannabinoids found in the cannabis plant, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), either individually or in combination, could modulate fear-based learning and fear extinction, either directly or indirectly through activation of the eCB.10 Specifically, administering THC or CBD before extinction learning trials in rats and mice reduces cue-elicited fear,11,12 blocks reconsolidation of fear memory,13,14 and facilitates faster extinction of fear memory in animal models of PTSD.15,16 These findings provide preliminary evidence of cannabis' therapeutic potential for PTSD, as impaired extinction learning is one of the primary mechanisms of PTSD pathophysiology.17
Early-stage, open-label clinical studies generally uphold pre-clinical findings and offer support for developing cannabinoid medications for PTSD.18–22 While the results of these small-scale clinical studies appear promising (e.g., reduced PTSD symptoms, improvements in sleep, nightmares, and hyperarousal), these studies have only tested single-molecule preparations of cannabinoids (i.e., CBD, THC, and synthetic THC). There are no published randomized clinical trials or matched observational studies that specifically test the short- and long-term impact of the types of cannabis being used by medical cannabis users (i.e., dispensary-obtained cannabis) on PTSD symptoms.
Two types of studies are needed to determine whether the types of cannabis currently used by medical cannabis users are an effective treatment for PTSD: (1) experimental and (2) observational. Both methods have a number of advantages and disadvantages, and together would provide the knowledge missing from this literature. A controlled observational study of the impact of dispensary-obtained cannabis on PTSD symptoms was chosen for the current investigation because such a study could provide important information regarding “real-world” and long-term patient use behaviors and the impact of such use on patient symptoms. Moreover, a clinical trial that tests administration of the cannabis products currently commercially available to cannabis users in states with medical and recreational cannabis laws would not be legally permitted in the United States and most other countries, as these products remain illegal under the U.S. controlled substance act and international drug control treaties. The aim of this study was to assess PTSD symptoms and functioning using structured assessments in a sample of 150 Colorado residents (military veterans and nonveterans) with PTSD (half non-cannabis users and half medical or recreational users) over the course of a year.
Materials and Methods
Study design
The study was a 1-year, longitudinal, prospective assessment of PTSD symptoms among Colorado residents with PTSD, which compared PTSD symptoms and functioning among cannabis-using participants and nonusing controls. Structured assessments were conducted at the Denver Veterans Affairs (VA) Medical Center and the de-identified data were analyzed at the San Diego VA Medical Center. The study was approved by the Denver VA, University of Colorado, and San Diego VA Institutional Review Boards. All participants gave written informed consent before enrollment. Participants completed clinician-administered standardized outcome assessments at baseline and then again at 3-month intervals for the subsequent calendar year (i.e., 3, 6, 9, and 12 months). Wrist actigraphs (Philips Respironics Actiwatch) were worn for 1 week after each in-person structured assessment was completed. Actigraphy data were scored based on behavioral sleep medicine guidelines.23 Participants were compensated up to $300 for completing all assessments and actigraphy.
Participants
Participants were included in the study if they were 18 years of age or older, met DSM-5 criteria for PTSD, and either (1) reported using cannabis at least once per week from a licensed medical or recreational dispensary in Colorado (cannabis group), or (2) reported no cannabis use in the prior 6 months. Cannabis use was assessed using a timeline follow-back diary, which collected information on past 3-month (1) frequency of cannabis use in days, (2) preparation of cannabis (i.e., flower, concentrate, edible, and tincture), (3) quantity of use per day (in grams for flower and concentrates and milligrams for edibles and tinctures), and (4) THC concentration of cannabis products used. Cannabis use was also verified by urine toxicology. Potential participants reporting symptoms of psychosis and those who were pregnant or breastfeeding were excluded.
After enrollment, all participants were tracked for 1 year to determine whether cannabis users would reduce their use over time or controls would initiate use. Only participants who continued to use (cannabis users) or did not initiate regular use (controls) were included in the final analysis of symptom outcomes.
Outcome assessments
The primary outcome measure was change in total severity score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5)24 across all time points. Secondary outcome measures included the following:
-
1.
Rate of change in PTSD diagnosis based on diagnosis of PTSD on the CAPS-5. PTSD diagnostic status was based on whether the participant met DSM-5 criteria at the time of assessment. Individual symptom severity scores were dichotomized into present or absent based on whether the severity score was rated ≥2 (moderate/threshold). To meet DSM-5 criteria for PTSD, participants had to report the presence of at least one criterion B symptom (intrusions), one criterion C symptom (avoidance), two criterion D symptoms (mood/cognitions), and two criterion E symptoms (hyperarousal) over the past 30 days.
-
2.
Change in overall psychosocial functioning based on Inventory of Psychosocial Functioning (IPF)25 total score
-
3.
Change in patient reports of sleep, as measured by total insomnia symptoms on the Insomnia Severity Index (ISI),26 and overall sleep quality on the Pittsburgh Sleep Quality Index (PSQI)27
-
4.
Change in objective indicators of sleep quality, as measured by wrist actigraph.28 Actigraphy parameters included the following: change in sleep-onset latency (SOL), sleep efficiency (SE), wake after sleep onset (WASO), number of awakenings (NWAK), and total sleep time (TST).
-
5.
Change in physical activity level on the International Physical Activity Questionnaire (IPAQ).29
Study power
Total enrollment was 150 participants (n=75 per group) to allow for the detection of a small to medium (d>0.40) between-group effect in change in total CAPS-5 scores with 80% power.
Statistical analyses
Initial analyses compared patient characteristics across control and cannabis groups with t-tests and analysis of variances for continuous measures and chi-square tests for categorical measures. The analysis of group differences was conducted to identify any demographic characteristics that might be potential nuisance variables in the primary and secondary analyses.
Subsequent longitudinal analyses evaluated changes in primary and secondary outcomes across groups over time. Continuous outcomes (CAPS-5, PSQI, ISI, IPF, SOL, SE, WASO, NWAK, and TST) were analyzed using linear mixed-effects models and ordinal outcomes (IPAQ) were analyzed using generalized linear mixed-effects models with a cumulative log link. Mixed-effects models included random intercepts and slopes, and fixed effects of group, time, and their interaction. The models were fit using restricted maximum likelihood estimation. A survival analysis was conducted to evaluate group differences in time to PTSD remission. Owing to the small number of measurement occasions, the survival analysis used a discrete-time hazard model with a complementary log-log link.
Mixed-effects and hazard models were conducted in R version 3.6.130 using the lme4,31 ordinal,32 and survival33 packages. Primary and secondary outcomes were analyzed using more conservative two-tailed tests for significance. A priori alpha for all primary and secondary outcome analyses was set at p<0.05.
Post-hoc analysis
Post-hoc analysis was conducted to describe the observed result of the primary outcome. Linear mixed-effects models tested whether cannabis users showed a rate of symptom decline compared to controls for each PTSD subscale score on the CAPS-5 (e.g., Intrusive memories [CAPS-5 Subscale B], Avoidance [CAPS-5 Subscale C], Negative alterations in mood/cognitions [CAPS-5 Subscale D], and Hyperarousal [CAPS-5 Subscale E]).
Results
Sample characteristics
One hundred fifty-nine individuals were screened and consented for a final sample of 150 eligible participants. The overall attrition rate was 33%. One hundred fifty participants (100%) completed the baseline assessment, 117 (78%) completed the 3-month assessment, 103 (69%) completed the 6-month assessment, 97 (65%) completed the 9-month assessment, and 101 (67%) completed the final 12-month assessment. Attrition did not significantly differ by group [χ2 (1)=0.16, p=0.68].
Sample demographics appear in Table 1. At baseline, groups significantly differed by number of prescription medications (Δ=2.03, t=4.32, p<0.01), age (Δ=12.55, t=5.51, p<0.01), marital status [χ2 (1)=11.04, p=0.03], ratio of veterans to civilians [χ2 (1)=17.56, p<0.01], and primary trauma type identified at screening [χ2 (2)=11.84, p<0.01]. Given the strong correlations between ratio of primary trauma type and veteran status, and age and marital status, only veteran status and age were included in the adjusted models to limit overfitting.
Table 1.
Demographic Characteristics of Sample by Group (N=150)
| Baseline characteristics | Controls |
Cannabis users |
Total |
p-Value |
|---|---|---|---|---|
| (n=75) | (n=75) | (N=150) | ||
| Age, mean (SD) | 57.49 (15.32) | 44.36 (12.64) | 50.67 (15.26) | <0.01 |
| Sex, no. (%) | 0.27 | |||
| Male | 58 (77%) | 52 (69%) | 110 (73%) | |
| Female | 17 (23%) | 23 (31%) | 40 (27%) | |
| Veteran status, no. (%) | <0.01 | |||
| Veteran | 71 (95%) | 51 (68%) | 122 (81%) | |
| Non-veteran | 4 (5%) | 24 (32%) | 28 (19%) | |
| Marital status, no. (%) | 0.03 | |||
| Married | 40 (53%) | 24 (32%) | 64 (43%) | |
| Single | 14 (19%) | 25 (33%) | 39 (26%) | |
| Cohabitating | 1 (1%) | 6 (8%) | 7 (5%) | |
| Widowed | 5 (7%) | 4 (5%) | 9 (6%) | |
| Divorced/separated | 15 (20%) | 16 (21%) | 31 (21%) | |
| Ethnicity, no. (%) | 0.49 | |||
| Non-Hispanic | 62 (84%) | 64 (85%) | 126 (85%) | |
| Hispanic | 12 (16%) | 11 (15%) | 23 (15%) | |
| Race, no. (%) | 0.32 | |||
| Caucasian/white | 52 (69%) | 51 (68%) | 103 (69%) | |
| Black or African American | 10 (13%) | 13 (17%) | 23 (15%) | |
| Native American/Alaskan Native | 2 (3%) | 6 (8%) | 8 (5%) | |
| Multiracial | 2 (3%) | 1 (1%) | 3 (2%) | |
| Other | 9 (12%) | 4 (5%) | 13 (9%) | |
| Trauma exposure, no. (%) | ||||
| Transportation accident | 64 (85%) | 67 (89%) | 131 (87%) | 0.46 |
| Fire or explosion | 59 (79%) | 55 (73%) | 114 (76%) | 0.44 |
| Natural disaster | 65 (87%) | 60 (80%) | 125 (83%) | 0.27 |
| Serious accident at work, home, or recreational activity | 45 (60%) | 48 (64%) | 93 (62%) | 0.61 |
| Exposure to toxic substance | 55 (73%) | 39 (52%) | 94 (63%) | <0.01 |
| Physical assault | 66 (88%) | 67 (89%) | 133 (89%) | 0.80 |
| Sexual assault | 61 (81%) | 64 (85%) | 125 (83%) | 0.51 |
| Other unwanted sexual experience | 39 (52%) | 40 (53%) | 79 (53%) | 0.87 |
| Combat exposure | 37 (49%) | 39 (52%) | 76 (51%) | 0.75 |
| Captivity | 61 (81%) | 49 (65%) | 110 (73%) | 0.03 |
| Life-threatening illness or injury | 60 (80%) | 54 (72%) | 114 (76%) | 0.25 |
| Severe human suffering | 54 (72%) | 53 (71%) | 107 (71%) | 0.86 |
| Sudden violent death | 54 (72%) | 48 (64%) | 102 (68%) | 0.29 |
| Sudden accidental death | 60 (80%) | 58 (77%) | 118 (79%) | 0.69 |
| Perpetrated serious injury/harm/death | 57 (76%) | 55 (73%) | 112 (75%) | 0.71 |
| Other | 37 (49%) | 39 (52%) | 76 (51%) | 0.74 |
| Screened trauma type, no. (%) | <0.01 | |||
| Combat trauma | 44 (59%) | 30 (40%) | 74 (49%) | |
| Sexual trauma | 15 (20%) | 9 (12%) | 24 (16%) | |
| Other | 16 (21%) | 36 (48%) | 52 (35%) | |
| Concurrent medications, mean (SD), no. | 5.64 (3.04) | 3.61 (2.70) | 4.63 (3.04) | <0.01 |
| PTSD medications, no. (%) | 7 (9%) | 4 (5%) | 11 (7%) | 0.35 |
| Comorbid psychiatric conditions, mean (SD), no. | 1.58 (1.66) | 1.58 (1.24) | 1.58 (1.46) | 0.99 |
| PTSD severity, CAPS-5 total score, mean (SD) | 36.33 (8.46) | 36.88 (8.52) | 36.61 (8.46) | 0.69 |
| Interpersonal functioning, IPF total score, mean (SD) | 3.44 (0.83) | 3.16 (1.04) | 3.30 (0.95) | 0.08 |
| Sleep quality, PSQI total score, mean (SD) | 11.65 (4.11) | 10.25 (3.77) | 10.95 (3.99) | 0.03 |
| Insomnia, ISI total score, mean (SD) | 14.30 (6.03) | 14.60 (6.67) | 14.45 (6.39) | 0.77 |
| Physical activity, IPAQ, no. (%) | ||||
| Low | 28 (38%) | 13 (18%) | 41 (28%) | |
| Moderate | 25 (34%) | 26 (35%) | 51 (35%) | |
| High | 21 (28%) | 35 (47%) | 56 (38%) | |
Concurrent PTSD medications represent those medications reported by participants as being prescribed specifically for PTSD. Medications included Latuda, Valium, Prazosin, Prozac, Bupropion, Fluoxetine, Sertraline, Trazodone, Citalopram, Wellbutrin, and Duloxetine
CAPS-5, Clinician-Administered PTSD Scale for DSM-5; IPAQ, International Physical Activity Questionnaire; ISI, Insomnia Severity Index; PTSD, post-traumatic stress disorder; SD, standard deviation.
Cannabis use frequency, quantity, and concentration (THC %) are summarized by assessment and appear in Table 2. At baseline, participants in the cannabis group reported that they primarily smoked flower cannabis and that they primarily used THC-dominant cannabis (n=63/69 reporting; 91%); three participants reported primarily using CBD-dominant cannabis (4%), and three participants reported primarily using a balanced composition of THC:CBD (4%). This ratio of primary type of cannabis used over the past 90 days did not significantly change across assessment time points [χ2 (8)=1.57, p=0.99]. Over the course of the study period, one participant stopped using cannabis and two initiated cannabis use. The two participants in the control condition who initiated and maintained cannabis use during the course of the observation year were excluded from analyses. Patterns of significance were not dependent on whether participants were excluded or included in the models.
Table 2.
Frequency, Quantity, and Concentration of Cannabis Use by Assessment
| Timeline follow-back assessment (past 3 months) |
|||||
|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | |
| Frequency (days of use), mean (SD) | |||||
| Flower | 75.55 (25.97) | 75.49 (27.39) | 67.96 (32.82) | 75.10 (26.32) | 67.60 (30.82) |
| Concentrates | 65.50 (33.20) | 58.17 (36.35) | 53.71 (36.27) | 73.22 (27.10) | 65.27 (34.15) |
| Edibles | 29.00 (29.32) | 33.85 (36.84) | 33.42 (34.63) | 48.41 (38.85) | 90.00 (31.84) |
| Tincture | 29.00 (38.97) | 49.00 (—) | 0 | 0 | 2.00 (—) |
| Quantity, mean (SD) | |||||
| Flower (g) | 1.75 (1.86) | 1.84 (2.15) | 1.95 (2.81) | 1.66 (1.44) | 1.41 (1.31) |
| Concentrates (g) | .29 (.37) | 1.56 (6.20) | 0.22 (0.14) | 0.28 (0.23) | 1.67 (5.08) |
| Edibles (mg) | 72.19 (75.12) | 59.58 (68.23) | 52.94 (80.85) | 47.93 (50.59) | 84.88 (157.49) |
| Tincture (mg) | 47.35 (32.30) | (—) | (—) | (—) | (—) |
| Concentration of THC, mean (SD) | |||||
| Flower | 23.71 (4.83) | 23.21 (4.76) | 23.60 (3.95) | 23.64 (3.37) | 23.11 (3.99) |
| Concentrates | 72.29 (20.11) | 70.72 (22.70) | 78.00 (5.37) | 77.00 (20.95) | 82.17 (3.49) |
| Edibles | (—) | (—) | (—) | 30.00 (33.08) | (—) |
| Tincture | (—) | (—) | (—) | (—) | (—) |
Frequency, quantity, and concentration of past 3-month cannabis use assessed using the TLFB Diary Assessment.
(—)=value not available due to too few cases reporting.
THC, tetrahydrocannabinol; TLFB, timeline follow-back.
Primary outcome
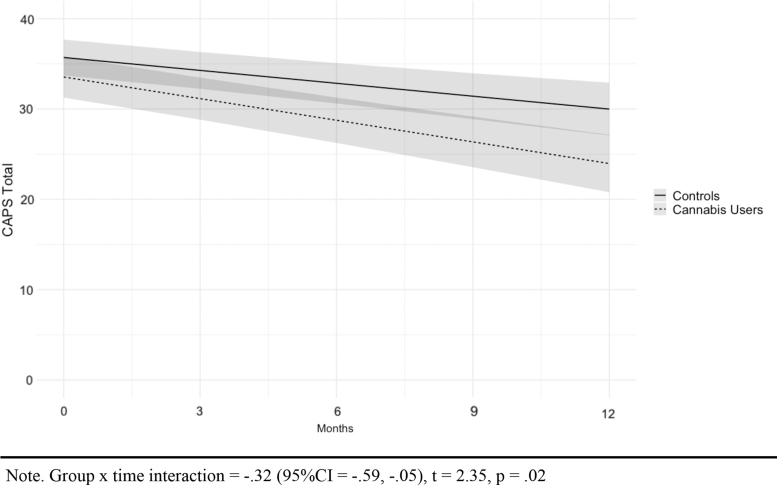
Results of the mixed-effects model for CAPS-5 total scores are displayed in Table 3 and Figure 1 (see Supplementary Figure 1 for estimated marginal means). After controlling for age and veteran status, total PTSD symptom severity decreased in both groups over time, with cannabis users reporting a significantly greater rate of decline over time compared to controls (group×time interaction=−0.32 [95% confidence interval, CI=−0.59 to −0.05], R2=0.13; t=−2.35 p=0.02).
Table 3.
Mixed-Effects Models for Primary and Secondary Outcomes
| Outcome variable | Model estimates adjusting for age and veteran status (95% CI) |
|||
|---|---|---|---|---|
| Intercept | Group | Time | Group×time | |
| PTSD severity (CAPS-5 total score) | 35.32 (31.39–39.26) | −2.16 (−5.18 to 0.86) | −0.48* (−0.66 to −0.29) | −0.32 (−0.59 to −0.05) |
| Sleep quality (PSQI total) | 11.11 (9.42–12.80) | −1.59* (−2.94 to −0.23) | −0.11* (−0.18 to −0.03) | −0.01 (−0.12 to 0.10) |
| Insomnia (ISI total) | 15.62 (12.91–18.33) | −0.55 (−2.68 to 1.57) | −0.14* (−0.25 to −0.04) | −0.12 (−0.27 to 0.03) |
| Psychosocial functioning (IPF total) | 3.50 (3.09–3.91) | −0.34* (−0.67 to −0.02) | −0.01 (−0.03 to 0) | 0 (−0.02 to 0.02) |
| SOL, min | 27.94 (15.92–39.95) | 4.73 (−5.16 to 14.61) | −0.25 (−0.95 to 0.44) | 0.30 (−0.68 to 1.28) |
| SE, % | 79.50 (75.28–83.73) | −3.19 (−6.68 to 0.29) | 0.07 (−0.12 to 0.26) | 0 (−0.26 to 0.27) |
| WASO, min | 51.17 (41.72–60.62) | 7.84 (−0.12 to 15.81) | 0.31 (−0.18 to 0.80) | −0.38 (−1.07 to 0.31) |
| NWAK, n | 49.51 (40.86–58.16) | −1.96 (−9.38 to 5.46) | −0.73* (−1.15 to −0.30) | 0.73* (0.13–1.33) |
| TST, min | 401.48 (364.15–438.82) | −30.94* (−60.48 to −1.39) | 0.78 (−0.88 to 2.44) | −0.10 (−2.45 to 2.24) |
| IPAQ (low, moderate, and high) | — | −1.29 (0.38–1.22) | 0.88* (0.79–0.99) | 1.05 (0.92–1.31) |
p<0.05.
Intercept=estimated baseline mean for control group.
Group=group difference in estimated baseline mean, control as reference.
Time=slope of control group.
Group×time=difference in slope by group, control as reference.
PSQI, Pittsburgh Sleep Quality Index; ISI, Insomnia Severity Index; IPF, Inventory of Psychosocial Functioning; SE, sleep efficiency; WASO, wake after sleep onset; NWAK, number of awakenings; CI, confidence interval.
FIG. 1.
Estimated PTSD symptom severity scores by group, adjusted by age and veteran status. PTSD, post-traumatic stress disorder.
Secondary outcomes
Twenty-six participants reported PTSD symptom severity patterns that fluctuated between meeting and not meeting current DSM-5 criteria for PTSD at each assessment time point, with remission followed by recurrence and possible subsequent remission. We classified these participants according to the first instance of their final PTSD diagnostic status in the survival analysis. For example, a participant who reported no longer meeting criteria for a PTSD diagnosis at 3 months, PTSD recurrence at 6 months, and again not meeting criteria for PTSD at 9 and 12 months was classified as no longer meeting PTSD criteria at month 9.
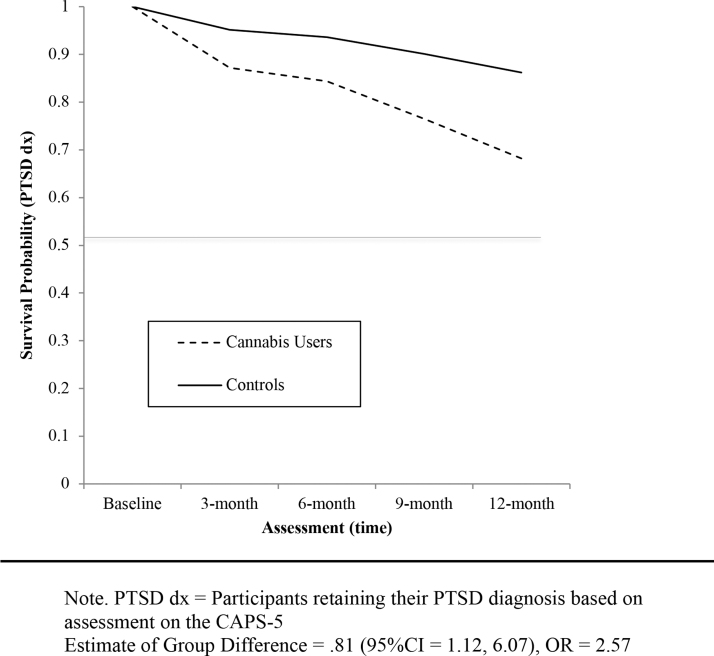
Results of the survival analysis for PTSD diagnosis found a significant difference by group in likelihood of meeting DSM-5 criteria for PTSD over time [χ2 (1)=2.21 hazard ratio=2.57 (95% CI=1.12–6.07), p=0.03]. Adjusted for age and veteran status, cannabis users were 2.57 times more likely to no longer meet DSM-5 criteria for PTSD at each assessment point after baseline compared to controls (See Fig. 2 for estimated rate of PTSD diagnosis over time).
FIG. 2.
Fitted survival probability of retaining PTSD diagnosis (dx) by group, adjusted for age and veteran status.
Analysis of between-group differences over time in rate of change on the patient-reported continuous secondary outcome measures (PSQI, ISI, and IPF) failed to reach significance (Table 3). Analysis of group differences in rate of change between physical activity level (low, moderate, and high on the IPAQ) over time also failed to reach significance (Z=1.05 [95% CI=0.92–1.31], p=0.29). Mixed-effects models for the objective sleep parameters revealed one significant between-group difference over time for NWAK (Table 3), where cannabis users recorded a lower rate of reduction in number of awakenings after adjusting for age and veteran status (group×time interaction=0.73 [95% CI=0.13–1.33], R2=0.19; t=2.38, p=0.02).
Post-hoc outcomes
Results of the post-hoc analysis of CAPS-5 subscale scores appear in Table 4. Adjusting for age and veteran status, cannabis users showed a significantly greater rate of decline for hyperarousal symptoms compared to controls. While not statistically significant, there was a trend for group differences in avoidance. Rate of change in intrusive thoughts and negative alterations in mood and cognitions failed to reach significance.
Table 4.
Post-Hoc Mixed-Effects Models for CAPS-5 Subscale Scores
| |
Model estimates adjusting for age and veteran status (95% CI) |
|||
|---|---|---|---|---|
| Outcome variable | Intercept | Group | Time | Group×time |
| Intrusions subscale | 9.54 (8.24–10.84) | −0.47 (−1.47 to 0.53) | −0.14* (−0.2 to −0.07) | −0.07 (−0.16 to 0.03) |
| Avoidance subscale | 4.06 (3.47–4.64) | 0.42 (−0.06 to 0.90) | −0.05* (−0.09 to −0.02) | −0.04 (−0.04 to 0.01) |
| Mood/cognitions subscale | 11.93 (10.06–13.80) | −1.31 (−2.80 to 0.18) | −0.23* (−0.32 to −0.14) | −0.1 (−0.24 to 0.03) |
| Hyperarousal subscale | 9.80 (8.56–11.03) | −0.7 (−1.69 to 0.29) | −0.07* (−0.13 to −0.01) | −0.11* (−0.21 to −0.02) |
p<0.05.
Intercept=estimated baseline mean for control group.
Group=group difference in estimated baseline mean, control as reference.
Time=slope of control group.
Group×time=difference in slope by group, control as reference.
Discussion
This study found significant group differences in PTSD symptom severity (primary outcome) and rate of PTSD diagnosis over time, which were driven by greater observed reductions in PTSD hyperarousal symptoms in cannabis users relative to nonusers.
Despite cannabis users and controls showing significant differentiation on the primary outcome, this study found no group difference in any secondary measure of functioning, including overall psychosocial functioning, subjective and objective measures of sleep, and physical activity. It is surprising that the cannabis group reported significantly greater sustained improvements in PTSD symptoms on the CAPS compared to controls, including greater reductions in hyperarousal symptoms, when they did not show differentiation from controls with respect to sleep and insomnia on sleep-specific measures. This effect might be attributable to cannabis differentially impacting symptoms even within the same subscale (e.g., having a larger effect on difficulty relaxing than insomnia). Moreover, there are many precipitating factors for PTSD-related insomnia and not all are due to hyperarousal. Patients' sleep difficulties might be due to nightmares (which would fall within the intrusive subscale) or depression (mood subscale).
This study's discrepancy between primary and secondary results could also be attributable to heterogeneity in participant dosing, as the study was entirely observational. Previous studies on cannabis and sleep report mixed results for improving sleep and insomnia, although the impact of cannabis on sleep appears largely dependent on cannabinoid ratio and dose (for review see Babson et al.34). The two primary active cannabinoids within the cannabis plant, THC and CBD, vary widely in content across plant types,35 and are associated with very different psychoactive profiles.36,37 While data on cannabis type were collected as a possible post-hoc predictor, variability in type of cannabis that the sample reported using was too limited to include as an independent variable. Indeed, the overwhelming majority of the sample reported using primarily THC-dominant cannabis. This is consistent with previous data showing that military veterans who use cannabis to self-treat PTSD overwhelmingly choose THC-dominant cannabis, even when barriers to access a range of cannabis types are reduced.38
Nevertheless, the major limitation of this study is that condition was not randomly assigned. While attempts were made to adjust for demographic differences between groups in the models, there might be other unaccounted for variables that could also predict the observed group differences (e.g., concurrent psychotherapy and changes in non-PTSD medication use throughout the observation period). The cannabis using group was also already using cannabis at the time that observation began; the drop in symptoms among the cannabis group might be attributable to an observer effect. Moreover, because all participants knowingly and intentionally were using or not using cannabis when they entered the study, observed group differences include expectations of cannabis' effects.
An experimental study of cannabis for the treatment of PTSD could theoretically include double-blind administration, and provide more control of factors such as cannabis administration and potency. However, given that cannabis is classified as a Schedule I substance at the federal level, such a study would require the administration of cannabis obtained from the National Institute on Drug Abuse (NIDA). The types of cannabis available through the NIDA Drug Supply System are not representative of the variety or potency of the cannabis used by patients in Colorado or other medical cannabis states.39 Thus, there is substantial value and ecological validity in the results of this prospective, observational study, which was able to assess a wider range of drug type. Moreover, the long-term assessment period of this study allowed for evaluation of whether cannabis use was associated with not only short-term but also sustained remission of PTSD symptoms, which would be difficult to achieve in a standard randomized control trial. Nevertheless, while potentially hampered by access, randomized control trials of the types of cannabis used by this population are certainly warranted to determine how different preparations of cannabis impact PTSD and functioning.
Conclusions
Despite limitations, this study's primary outcome supports the theory that cannabis should be trialed as a potential therapeutic for PTSD. Participants who used primarily THC-dominant cannabis reported a greater reduction in PTSD symptom severity over time compared to controls. Cannabis users also showed a greater than two-fold rate of remission from their PTSD diagnosis (defined by no longer meeting criteria for a PTSD diagnosis on the CAPS-5) compared to controls by the 1-year follow-up assessment. Post-hoc follow-up indicated that group differences in PTSD symptom severity were likely driven by greater improvements in avoidance and hyperarousal symptoms among the cannabis-using participants.
Additional work is needed to determine how differences in cannabis preparations, route of administration, and dosing regimens impact PTSD symptomology, and whether the results of this study are upheld in randomized and blinded clinical trials. Finally, future studies are needed to determine safety, including risk of side effects and potential impact on suicidality, associated with using cannabis as a treatment for PTSD.
Disclaimer
The information contained in this article is solely that of the author(s). No confidential or proprietary information is contained in this article. Nothing in this article represents an official policy or procedure of any of the authors' current or past affiliations.
Supplementary Material
Abbreviations Used
- CAPS-5
Clinician-Administered PTSD Scale for DSM-5
- CBD
cannabidiol
- CI
confidence interval
- eCB
endocannabinoid
- IPAQ
International Physical Activity Questionnaire
- IPF
Inventory of Psychosocial Functioning
- ISI
Insomnia Severity Index
- mPFC
medial prefrontal cortex
- NIDA
National Institute on Drug Abuse
- NWAK
number of awakenings
- PSQI
Pittsburgh Sleep Quality Index
- PTSD
post-traumatic stress disorder
- SD
standard deviation
- SE
sleep efficiency
- SOL
sleep-onset latency
- THC
tetrahydrocannabinol
- TLFB
timeline follow-back
- TST
total sleep time
- WASO
wake after sleep onset
Author Disclosure Statement
Author M.O.B.-M. is an employee of Canopy Growth Corporation, during which time he has received stock options, serves on the Board of Directors for AusCann Group Holdings Limited, was a prior employee of Zynerba Pharmaceuticals, and has received consulting fees from Tilray, Inc. Author M.B. is an employee of Canopy Growth Corporation who, in the course of her employment, has received stock options. Author M.J.L. is on the scientific advisory board for FSD Pharma and has received consulting fees from Greenwich Biosciences in the past 2 years. Author R.V. is on the scientific advisory board for FSD Pharma and receives consulting fees from Canopy Growth Corporation and Zynerba Pharmaceuticals. Author K.A.B. is an employee of Jazz Pharmaceuticals who, in the course of her employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Authors A.S. and H.W. declare no conflicts to report.
Funding Information
This study was funded by the Colorado Department of Public Health and Environment (CDPHE) (Grant number: 16-FHHA 82488, COMIRB: 15-0346).
Supplementary Material
Cite this article as: Bonn-Miller MO, Brunstetter M, Simonian A, Loflin MJ, Vandrey R, Babson KA, Wortzel H (2022) The long-term, prospective, therapeutic impact of cannabis on posttraumatic stress disorder, Cannabis and Cannabinoid Research 7:2, 214–223, DOI: 10.1089/can.2020.0056.
References
- 1. Atwoli L, Stein DJ, Koenen KC, et al. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: https://www.ptsd.va.gov/professional/treat/essentials/epidemiology.asp (accessed March 31, 2020).
- 3. Rosenberg J, Loflin MJE, Hurd YL, et al. Prescribing health care providers' attitudes, experiences, and practices surrounding cannabis use in patients with anxiety disorders and post-traumatic stress disorder. Cannabis Cannabinoid Res. 2019;4:124–130. [Google Scholar]
- 4. Hill MN, Campolongo P, Yehuda R, et al. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2017;43:80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. [DOI] [PubMed] [Google Scholar]
- 6. McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42:116–131. [DOI] [PubMed] [Google Scholar]
- 7. Andrewes DG, Jenkins LM. The role of the amygdala and the ventromedial prefrontal cortex in emotional regulation: implications for post-traumatic stress disorder. Neuropsychol Rev. 2019;29:220–243. [DOI] [PubMed] [Google Scholar]
- 8. El Khoury-Malhame M, Reynaud E, Soriano A, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. [DOI] [PubMed] [Google Scholar]
- 9. Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. [DOI] [PubMed] [Google Scholar]
- 10. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol Clin Exp. 2009;24:515–523. [DOI] [PubMed] [Google Scholar]
- 11. Gomes F V, Reis DG, Alves FHF, et al. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J Psychopharmacol. 2010;26:104–113. [DOI] [PubMed] [Google Scholar]
- 12. Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. 2010;207:105–111. [DOI] [PubMed] [Google Scholar]
- 13. Stern CAJ, Gazarini L, Takahashi RN, et al. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37:2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stern CAJ, Gazarini L, Vanvossen AC, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25:958–965. [DOI] [PubMed] [Google Scholar]
- 15. Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. [DOI] [PubMed] [Google Scholar]
- 16. Do Monte FH, Souza RR, Bitencourt RM, et al. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav Brain Res. 2013;250:23–27. [DOI] [PubMed] [Google Scholar]
- 17. Milad MR, Orr SP, Lasko NB, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13:953–960. Available at: https://ingentaconnect.com/content/ben/cnsnddt/2014/00000013/00000006/art00007 (accessed March 18, 2019). [DOI] [PubMed]
- 19. Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. [DOI] [PubMed] [Google Scholar]
- 20. Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–588. [DOI] [PubMed] [Google Scholar]
- 21. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roitman P, Mechoulam R, Cooper-Kazaz R, et al. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34:587–591. [DOI] [PubMed] [Google Scholar]
- 23. Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. [DOI] [PubMed] [Google Scholar]
- 24. Weathers FW, Bovin MJ, Lee DJ, et al. The clinician-administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schnurr PP, Lunney CA, Bovin MJ, et al. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29:727–735. [DOI] [PubMed] [Google Scholar]
- 26. Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 28. Sadeh A, Hauri PJ, Kripke DF, et al. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. [DOI] [PubMed] [Google Scholar]
- 29. Fogelholm M, Malmberg J, Suni J, et al. International Physical Activity Questionnaire: validity against fitness. Med Sci Sports Exerc. 2006;38:753–760. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team. R: a language and environment for statistical computing. 2017. https://r-project.org/ (accessed March 31, 2020).
- 31. Bates D, Mächler M, Bolker BM, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 32. Christensen R. Ordinal—regression models for ordinal data. R Packag version 201912-10. 2019. https://cran.r-project.org/package=ordinal (accessed March 31, 2020).
- 33. Therneau T. A package for survival analysis in R. 2015. https://cran.r-project.org/package=survival (accessed March 31, 2020).
- 34. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:23. [DOI] [PubMed] [Google Scholar]
- 35. Fischedick JT, Hazekamp A, Erkelens T, et al. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71:2058–2073. [DOI] [PubMed] [Google Scholar]
- 36. Martin-Santos R, A. Crippa J, Batalla A, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18:4966–4979. [DOI] [PubMed] [Google Scholar]
- 37. Batalla A, Crippa JA, Busatto GF, et al. Neuroimaging studies of acute effects of THC and CBD in humans and animals: a systematic review. Curr Pharm Des. 2014;20:2168–2185. [DOI] [PubMed] [Google Scholar]
- 38. Loflin M, Babson K, Sottile J, et al. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019;45:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwabe AL, Hansen CJ, Hyslop RM, et al. Research grade marijuana supplied by the National Institute on Drug Abuse is genetically divergent from commercially available cannabis. bioRxiv. 2019;592725. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.