Abstract
Introduction:
Obesity is defined as an excess of accumulation of fat that can be harmful to health. Storage of excess fat in the adipose tissue triggers an inflammatory process, which makes obesity a low-grade chronic inflammatory disease. Obesity is considered a complex and multifactorial disease; hence, no intervention strategy appears to be an ideal treatment for all individuals. Therefore, new therapeutic alternatives are often studied for the treatment of this disease. Currently, herbal medicines are gaining ground in the treatment of obesity and its comorbidities. In this context, much attention is being paid to Cannabis sativa derivatives, and their therapeutic functions are being widely studied, including in treating obesity.
Objective:
Highlight the pharmacological properties of Δ9-tetrahydrocannabivarin (THCV), Δ9-tetrahydrocannabidinol (THC), and cannabidiol (CBD), the predominant isolated components of Cannabis sativa, as well as its therapeutic potential in the treatment of obesity.
Methods:
This is a narrative review that shows the existing scientific evidence on the clinical application of Cannabis sativa as a possible treatment for obesity. Data collection was performed in the PubMed electronic database. The following word combinations were used: Cannabis and obesity, Cannabis sativa and obesity, THCV and obesity, THC and obesity, CBD and obesity, and Cannabis sativa and inflammation.
Results:
Evidence shows that Cannabis sativa derivatives have therapeutic potential due to their anti-inflammatory properties. In addition, people who use cannabis have a lower body mass index than those who do not, making the plant an option to reduce and reverse inflammation and comorbidities in obesity.
Conclusion:
It is concluded that phytocannabinoids derived from Cannabis sativa have therapeutic potential due to its anti-inflammatory, antioxidant, and neuroprotective properties, making the plant a study option to reduce and reverse inflammation and comorbidities associated with obesity.
Keywords: obesity, inflammation, Cannabis sativa, Δ9-tetrahydrocannabivarin, Δ9-tetrahydrocannabinol, cannabidiol
Introduction
Obesity has become one of the greatest public health challenges, warranting a state of alert, as the prevalence of this disease in all age groups continues to increase.1,2 Updated global data from the World Health Organization (WHO) reveal a rapid increase in the prevalence of obesity.3 These data are very worrying, considering the global economic impact of this disease. In addition to excessive expenditure on health care, obesity also imposes costs in the form of lost productivity at work, mortality, and permanent disability.4
According to the WHO, obesity is an excess of accumulation of fat that can be harmful to health.3 This accumulation occurs due to an imbalance between high caloric consumption and low energy expenditure.5 Excess fat stored in adipose tissue triggers an initially local and subsequently systemic inflammatory process, which makes obesity a low-grade chronic inflammatory disease.6
Seen as an inflammatory disease and a trigger for other comorbidities, obesity exhibits the need for specific treatment. The first line of treatment for this disease is a change in lifestyle, which consists of reducing caloric consumption plus increasing physical exercise. Pharmacological therapy plays a minor role, and in addition to having a less lasting effect, it has shown therapies developed to date have shown numerous side effects, even leading to the withdrawal of drugs from the market as they present an unfavorable risk-benefit ratio.7–9
In addition, the development of obesity depends on a diversity of genetic, biological, psychological, sociocultural, and environmental factors.10 In view of these facts, no intervention strategy appears as an ideal treatment for all individuals. Great variability in response to treatment for weight loss is a common theme among intervention studies for this purpose, demonstrating that different treatments may be effective for some individuals, but result in little or no benefit in others.11
Therefore, new therapeutic alternatives are often studied for the treatment of obesity. Currently, much attention is being paid to Cannabis sativa derivatives that interact with constituents of the endocannabinoid system (ECS), such as Δ9-Tetrahydrocannabivarin (THCV), THC, and CBD, due to their anti-inflammatory properties, antioxidants, anorectic and thermogenesis, which propose its use in the treatment of obesity.12,13
Therefore, the objective of this narrative review was to summarize the existing scientific evidence on the clinical application of Cannabis sativa as a possible treatment for obesity. This review was carried out in the electronic database PubMed, collecting articles related to the topic. The following word combinations were used: cannabis and obesity, Cannabis sativa and obesity, THCV and obesity, Δ9-tetrahydrocannabidinol and obesity, CBD and obesity, e Cannabis sativa and inflammation.
The Extent of the Obesity Epidemic Problem
Lifestyle and diet in most modern and developing societies has undergone significant changes in the past decades. Sedentary lifestyle, as well as the excessive consumption of calorie-dense foods, has established an “obesogenic” environment and contributed to a serious obesity epidemic.14 Thus, obesity is defined as the excessive accumulation of fat that results mainly from the imbalance between high caloric intake and low energy expenditure that compromises the health of individuals.15
Obesity not only increases the risk of cardiovascular disease and certain types of cancer, but is also characterized as a key factor in the development of other components of metabolic syndromes. These components include insulin resistance, hyperglycemia with prediabetes or type 2 diabetes mellitus (DM), dyslipidemia, and hypertension, points that constitute a systemic metabolic dysfunction.16–18 In view of this, obesity has become a rapidly growing public health problem and affects several countries around the world, due to its prevalence, costs, and damage to health.19
Obesity has had its incidence numbers tripled since 1975, in 2016 reaching >1.9 billion adults >18 years being overweight (39%), and 650 million (13%) with some degree of obesity. Furthermore, WHO data reveal that 340 million children and adolescents between 5 and 19 years were overweight or obese. In 2019, 38 million children <5 years were diagnosed as overweight or obese.3 With this, and being recognized as a pathology that has affected the population at increasingly early ages, obesity has been gaining prominence in the worldwide epidemiological scenario, reaching the WHO classification of an epidemic.20 However, an increase in adiposity is a chronic and potentially reversible process, with adequate diagnosis and treatment.21
The main means of assessment for obesity adopted by the WHO is the body mass index (BMI), which consists of the division of weight in kilograms by the square of height in meters (kg/m2). This method is used for the diagnosis of overweight and obese individuals, in addition to associating them with the risk of developing other comorbidities.15 However, in general, BMI is not sufficient for the assessment of nutritional status, and should be associated with other measures, such as skinfolds and segment circumferences, bioimpedance, magnetic resonance, computed tomography, and dual energy X-ray densitometry.22–24 The early diagnosis of obesity is essential due to factors related to pathophysiology, as well as to avoid worsening of the disease and an increase in comorbidities associated with obesity.25
The Pathophysiological Mechanism of Obesity and Its Relationship with the Inflammatory Response
Obesity is an inflammatory disease influenced by several genetic and environmental factors. However, its main cause is the imbalance between high caloric consumption through food and low energy expenditure, providing a positive balance of energy. This excess energy is stored in the form of triglycerides in the cells of the white adipose tissue (WAT) called adipocytes.26 WAT is an important endocrine and regulatory organ,27,28 its impairment reflects the homeostatic imbalance of substances important for energy storage and metabolism, such as leptin, insulin, and ghrelin.29,30
Leptin is an anorexigenic substance, produced by WAT, and acts on its receptors present in the arcuate nucleus of the hypothalamus. This substance is able to suppress neurons that produce neuropeptide Y (NPY) and agouti-related protein peptide, which exert an orexigenic effect (stimulate appetite), increase the activity of pro-opiomelanocortin and cocaine and amphetamine-regulated transcript, which are anorexigenic (inhibit appetite), regulating food when stored energy levels are sufficient. Its production is proportional to the increase in adipose mass; therefore, in obesity, its serum levels are high,31–33 and a resistance of its receptors is also observed at the hypothalamic level, causing an important imbalance in its interpretation in relation to satiety and energy stocks.34
Obesity also influences the development of peripheral insulin resistance. Insulin is a hormone secreted by β-pancreatic cells that binds to specific receptors that provide the entry of serum glucose into cells, serving as a substrate for cellular respiration and formation of adenosine triphosphate. Resistance to this hormone leads to an accumulation of glucose in the bloodstream, contributing to the development of metabolic diseases, especially type 2 DM.35
Concomitant with leptin and insulin, ghrelin has its activity compromised in obesity, since its secretion is increased. It is naturally secreted by stomach cells and acts on the arcuate nucleus of the hypothalamus to signal the need for nutrients, especially in the premeal and prolonged fasting states. It works by activating orexigenic neurons, mainly NPY producers, inducing hunger, with the opposite effect to leptin. When increased, ghrelin can be an important factor in the imbalance of the reward system.36,37
In addition, the high fat content stimulates adipocyte hypertrophy, a situation in which the adipocytes increase in size, thus, the lipid storage capacity can be expanded giving rise to additional adipocytes (adipogenesis) in a process called “hyperplasia.”38,39
The adipose expansion derived from obesity can lead to a narrowing of the extracellular space, compressing the blood vessels in the region, impairing blood supply and oxygen distribution, which favors the state of hypoxia.40,41 The condition of low oxygenation in the adipose tissue induces the infiltration of macrophages that act by eliminating necrotic adipocytes, which subsequently amplify a local inflammatory response, producing interleukin (IL)-6 and tumor necrosis factor (TNF)-α42,43 and reducing the production of adiponectin, promoting inflammation and oxidative stress in adipose tissue.44 The high concentration of proinflammatory factors secreted at the site reaches the bloodstream, causing systemic chronic inflammation characterized by low intensity.45
It is known that the macrophages residing in the adipose tissue of eutrophic individuals belong to the alternatively activated type M2, which are fundamental in the adipose tissue homeostasis. In contrast, the number of classically activated M1 macrophages increases in the adipose tissue of obese individuals, favoring the processes of inflammation and insulin resistance (IR).46,47
Elevated levels of proinflammatory cytokines, free fatty acids, and immune cells present in the bloodstream in obese individuals can generate brain inflammation. These substances in question can access the central nervous system (CNS) through fissures present in the blood–brain barrier and activate Toll-like receptors, thus stimulating an even greater production of proinflammatory factors in the hypothalamus, inducing neuroinflammation, microglial proliferation, and affecting other brain structures.48
In addition, the impairment of energy metabolism, resulting from hormonal dysregulation, associated with inflammation installed in obesity, promotes an increase in glucose and serum lipids. This increase can generate an excessive supply of energetic substrates to the metabolic pathways in the cells, which may increase the production of reactive oxygen species (ROS), and reflect on the damage of proteins, lipids, and DNA of tissues.49 Among the affected tissues, brain structures are severely impaired, due to the high amount of lipid in its composition, in addition to the use of glucose for its metabolism.50 Considering that obesity and its comorbidities are not only peripheral disorders, but also imply neurological changes, effective therapies are certainly needed to minimize such damage.
Treatment for Obesity: From Lifestyle Changes to a Pharmacotherapeutic Approach
The first line of treatment and prevention of obesity consists of changing lifestyle to include frequent physical exercise and the adoption of a healthy diet with adequate distribution of macronutrients, as well as the regular consumption of fruits, vegetables, and micronutrients. These are considered as good strategies to reduce the prevalence of obesity and associated comorbidities.51 In this way, it is possible to rebalance the consumption and expenditure of calories, culminating in the burning of stored fat and weight loss.9,52
However, treatment should not focus only on weight loss, but also on the prevention and reversal of associated metabolic comorbidities; therefore, pharmacological therapies are often used when caloric restriction and physical exercise are not sufficient in the weight loss process.53 The use of pharmacotherapy for weight control is consistent with the treatment of obesity as a chronic disease that requires a multifaceted approach, including dietary modification, behavioral intervention, and appropriate medical intervention. Current guidelines suggest that individuals who do not respond to lifestyle interventions after 6 months of treatment and who have a BMI >30 kg/m2, or a BMI >27 kg/m2 with a comorbidity associated with obesity can be considered in drug treatment for weight loss.54
Some drugs approved by the Food and Drug Administration (FDA) are used in the treatment of obesity only for a short period, due to the associated risks, whereas some others are approved for use in the long term.53 Although some pharmacological therapies assist in the control of obesity, the majority of commonly used drugs are associated with adverse health effects51 that include cardiovascular complications, possibly due to the formation of thrombi, insomnia, constipation, dry mouth, dizziness, dysgeusia, headache, fatigue, gastrointestinal symptoms, among others. As a result, many drugs have been withdrawn from the market due to unwanted side effects.9,53,55
Bariatric surgery is increasingly being performed in the medically complicated obese population, not only to achieve significant weight loss, but also to correct obesity-related diseases.56 The criteria for considering bariatric surgery include the presence of obesity (BMI >30), history of multiple attempts to reduce weight by O'Brien57 nonsurgical means, awareness of potential risks, and commitment to participate in the follow-up program. It is worth emphasizing that bariatric procedures can be associated with complications that include leaks, strictures, bleeding, and venous thromboembolic events.58
In view of this, herbal medicines are gaining ground in the treatment of obesity and its comorbidities. Several plants and their derivatives have brought positive results, mainly in weight reduction, waist circumference, and suppression of inflammatory pathways.59–68 In this context, drugs derived from the Cannabis sativa plant, called phytocannabinoids, have been studied as a treatment option for several pathologies. This plant and its derivatives bring important results in the literature associated with anti-inflammatory, antioxidant, and anticonvulsant effects, among others,12,69–73 and can be an alternative for the metabolic re-establishment in obesity, given the complexity and incidence of adverse events related to the currently available therapeutic options.
Cannabis sativa, ECS, and Its Anti-Inflammatory Potential as Indirect Support in the Treatment of Obesity
The cannabis genus includes three species, Cannabis sativa, Cannabis indica, and Cannabis ruderalis.74 Cannabis sativa, popularly known as marijuana,74,75 has been widely studied for its varied therapeutic potential,71,72 since it produces substances capable of interacting with receptors of a homeostasis system called the ECS.78 This system was discovered in 1988, during in-depth studies of the effects of Cannabis sativa.79
The ECS consists of two members of the family of receptors coupled to protein G, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), as well as endogenous ligands, among these, the most studied are anandamide and 2-arachidonoylglycerol (2-AG).80,81 This signaling system occurs within the CNS and in various peripheral organs.82 CB1 is present mainly in the CNS, modulating mood, appetite, memory, and pain.78,83 It is also expressed in several peripheral tissues, such as adipose tissue, liver, skeletal muscle, gastrointestinal tract, and pancreas.84,85 He is involved in studies evaluating the effects of Cannabis sativa on neuropsychiatric and neurodegenerative diseases.80
In addition, CB1 is a primary mediator for energy capture, storage, and conservation. It works by enhancing the absorption and conservation of energy through various mechanisms. Stimulation of CB1 modulates the taste and smell pathways to increase the palatability of food. It also stimulates the brain's appetite centers, promoting hyperphagia and contributing to the accumulation of fat in adipose tissue.86–94 CB2 acts on the peripheral nervous system, is expressed in the spleen and tonsils, as well as in B cells, monocytes and T cells, and is associated with the balance of the immune system.78,83,95,96 Therefore, the interaction with CB2 is the cause of the anti-inflammatory and immunomodulatory effects of Cannabis sativa.97
In addition to endogenous substances, phytocannabinoids, which are substances produced in plant trichomes in the genus Cannabis sativa, are also capable of interacting with ECS receptors.83,98 Cannabis sativa produces >400 compounds, 66 of which are classified as phytocannabinoids.99 Among the phytocannabinoids present in Cannabis sativa, the ones that are most relevant to the studies are THCV, THC, and CBD.13,100
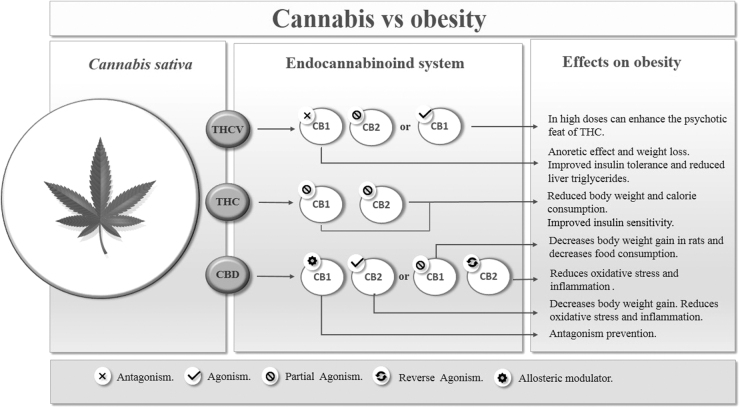
THCV primarily works by antagonizing the CB1 receptor; this compound can, therefore, have anorectic effects, inhibiting appetite, controlling food, and consequently decreasing body weight.101 However, at high dosages, THCV can demonstrate CB1 agonism and partial CB2 agonism.102 Therefore, even though it is a THC analogue, THCV behaves differently in relation to receptors, which may potentiate (in high doses), or antagonize the psychoactive or nonpsychoactive effects of THC (Fig. 1).101
FIG. 1.
Cannabis versus obesity. THCV, Δ9-tetrahydrocannabivarin.
THC is the most abundant phytocannabinoid in the Cannabis sativa plant, being the main psychoactive constituent of the plant. It is a highly fat-soluble compound, a partial agonist of both CB1 and CB2 receptors, with greater affinity for CB1.98 Therefore, this compound is responsible for the known psychoactive effects of Cannabis sativa, such as euphoria, sedation, and hallucination.83,102 This agonist effect on CB1 explains the use of this substance for pain relief in several clinical conditions,103 such as neuropathic pain,104 for nausea and vomiting associated with chemotherapy,103 and for the treatment of psychiatric diseases such as anxiety and depression.105 However, this compound used alone can cause several side effects (Fig. 1).97
CBD, in contrast, acts as a weak antagonist (inhibits the effects) of CB1 and CB2 receptors, and an important inverse agonist (decreases the effect, but does not inhibit) of CB2 receptors.97,98 The anti-inflammatory potential of CBD can primarily be explained by the effect on CB2, since by softening the effect of receptors on immune cells, it automatically softens the activity of these cells (Fig. 1).97
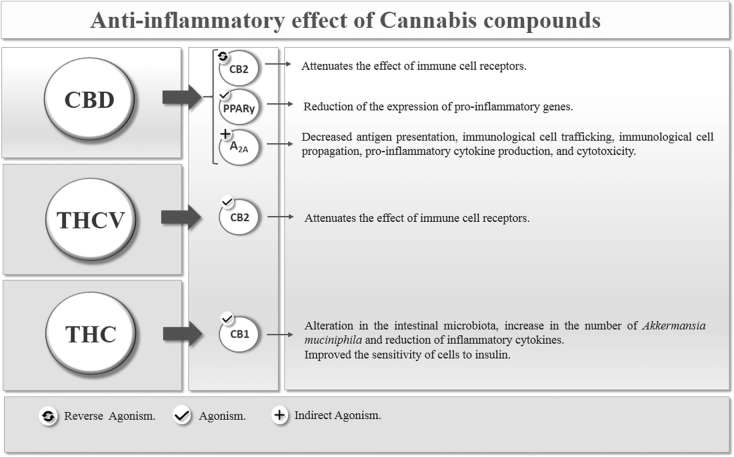
In addition to CB receptors, it has been suggested that CBD is also associated with other pharmacological substrates. In this context, CBD also showed an affinity for peroxisome proliferation-activated receptors (PPARs), which are a family of transcription factors inducible by ligands belonging to the nuclear hormone receptor superfamily. In humans, there are three PPAR PPARα, PPARβ/δ, and PPARγ isoforms that are encoded by distinct genes and are expressed in different ways in organs and tissues.106,107 CBD appears to activate the transcriptional activity of PPARγ, which plays an essential role in controlling adipocyte formation, insulin sensitivity, and activation of the inflammatory response. Thus, CBD activates PPARγ receptors causing less expression of proinflammatory genes (Fig. 2).108–110
FIG. 2.
Anti-inflammatory effect of cannabis compounds.
The anti-inflammatory effect of CBD is also mediated by the adenosine A 2A (A 2A) receptor, whose activation attenuates the immune system, causing a decrease in antigen presentation, immune cell trafficking, immune cell propagation, proinflammatory cytokine production, and cytotoxicity.111 CBD has been shown to intensify A 2A receptor signaling by inhibiting cell renewal of an adenosine transporter, generating anti-inflammatory and antioxidant effects (Fig. 2).112
Along with the effects on the immune system, CBD demonstrates poorly understood antioxidant effects, which may have neuroprotective potential113 and can modulate serotonin receptors.102 Therefore, CBD can have a therapeutic function in several conditions involving inflammation and oxidative stress, such as Parkinson's disease,114,115 rheumatoid arthritis,116 Alzheimer's disease,117 ischemia-reperfusion injury,118 metabolic diseases,119,120 among others. In addition, CBD demonstrates opposite effects to THC on CB1 receptors, which allows control of adverse effects when used in combination.98,121
Considering the properties mentioned (anti-inflammatory, antioxidant, and antiobesity), CBD appears to be a potential therapeutic agent that could be used in the treatment of obesity, type 2 DM and its complications, ischemia, and neurodegenerative diseases, in addition to pain relief and depression. Although the anti-inflammatory and neuroprotective properties of CBD are well determined, there are few studies that have investigated the antiobesity effects of this compound.12,87,122–128
Based on the data presented, the hypothesis arises that Cannabis sativa and its derivatives can be potentially effective in treating and reversing the damage caused by inflammation in obesity.
Cannabinoids as a promising metabolic re-establishment target in obesity
The therapeutic benefits of Cannabis sativa plant extracts and their subspecies have been widely studied. THCV, THC, and CBD are the predominant isolated components of Cannabis sativa and have been extensively studied in the modern literature.76,129
Some components of Cannabis sativa are known to stimulate appetite, whereas others are promising for the treatment of obesity. In view of this, there was an interest in investigating the role of the ECS in the control of obesity and in the associated metabolic syndrome130,131 (Table 1). This interest subsequently led to the development of an effective therapeutic strategy for obesity, which consisted of blocking CB1 cannabinoid receptors using ligands such as Rimonabant.130 A synthetic CB1 receptor antagonist that performs its function in the ECS selectively blocking CB1 receptors, thereby decreasing appetite, leading to hypophagia and improving the metabolic profile.132–134
Table 1.
Use of Cannabinoids in Obesity
| References in chronological order | Substance | Dose route of administration | Object of study | Results |
|---|---|---|---|---|
| Van Gaal et al., 2005132 | Rimonabant | 5 and 20 mg daily for 12 months | Obese humans with comorbidities | There was weight loss, reduced waist circumference, increased HDL cholesterol, and the insulin resistance marker (mainly on 20 mg). But the adverse effects were also greater. |
| Després et al., 2005133 | Rimonabant | 5 and 20 mg daily for 12 months | Obese humans with comorbidities | There was weight loss, reduced waist circumference, increased HDL cholesterol, reduction in triglycerides, and increase in plasma adiponectin levels (mainly on 20 mg). |
| Järbe and DiPatrizio, 2005155 | THC e Rimonabant | Study 1: 0.1, 0.3, 0.56, 1.8 mg/kg THC I.P., twice weekly. 0.03, 0.3 mg/kg Rimonabant. I.P. Study 2: 0.56, 1.0, 1.8 mg/kg of THC I.P. |
Male Sprague-Dawley rats | There was a dose-related increase in high-fat diet intake, peaking at 0.56–1 mg/kg D9-THC. SR-141716 alone suppressed the high-fat diet intake below control levels. A combination of 0.3 mg/kg SR 141716 and 0.56 mg/kg D9-THC counteracted the effects on consumption of either drug alone. In study 2, attenuation of the hyperphagia (high-fat diet) was evident after the second injection Increasing doses of D9-THC (1 and 1.8 mg/kg, for two and three consecutive days, respectively) did not reinstate the initial hyperphagia. |
| Weiss et al., 2006122 | CBD | 5 mg/kg per day. 10 to 20 injections (five times a week). | Female nonobese diabetic NOD/LtJ mice | Reduction in diabetes incidence from 86% to 30%. Reduction of plasma levels of proinflammatory cytokines (INF-g and TNF-a) and production of T cells associated with the TH1 profile. Increase in cytokines associated with the th2 profile and reduced insulitis. |
| Durst et al., 2007124 | CBD | 5 mg/kg per day, I.P. | Male Sprague-Dawley rats | In vivo studies showed preservation of shortening fraction in CBD-treated animals. Infarct size was reduced by 66% in CBD-treated animals. Infarcts in CBD-treated animals were associated with reduced myocardial inflammation and reduced IL-6 levels. |
| Nissen et al., 2008136 | Rimonabant | 20 mg daily for 3, 6, 12, and 18 months | Obese humans with comorbidities | In the rimonabant vs. placebo groups, increase in percent atheroma volume and decrease in total atheroma volume. There was weight loss, reduced waist circumference, increased HDL cholesterol, and reduction in triglycerides. Rimonabant-treated patients had greater decreases in high-sensitivity C-reactive protein. Psychiatric adverse effects were more common in the rimonabant group. |
| Weiss et al., 2008123 | CBD | Dose of 5 mg/kg per day, in 0.1 mL, I.P. | Nonobese female NOD/LtJ mice and BALB/c female mice | It softened the manifestation of diabetes mellitus from 86 to 100% in untreated groups with CBD to 32% in treated groups. In addition, it reduced IL-12 (proinflammatory) and increased IL-10 (anti-inflammatory). Pancreatic islets were much more intact in animals treated with CBD. |
| Riedel et al., 2009101 | AM251 and THCV | 10 mg/kg AM251; 0.48, 0.96, 1, 1.44, 3, 10, 30 mg/kg THCV; 10 mg/kg CBD, I.P. | Male C57 BL6 mice | AM251 suppressed food intake and weight gain in fasted and nonfasted animals. Pure THCV also induced hypophagia and weight reduction at doses as low as 3 mg/kg. However, a THCV-rich cannabis-extract failed to suppress food intake and weight gain, possibly due to residual THC. This THC effect was overcome by the co-administration of CBD. |
| Gallant et al., 2009168 | THC and a lipophilic cannabis extract | Cannabis extract containing THC levels ranging from 2.5–10 mg/mL | In vitro | Insulin-induced glucose uptake increased, whereas the rate of adipogenesis decreased with increasing THC concentration. Insulin resistance was induced using TNF-a, exposed to the extract and insulin-induced glucose uptake measured. Insulin-induced glucose was increased in these cell safer exposures to the extract. There was a significant increase in IRS-1, IRS-2, and GLUT-4 genes expression for THC. |
| Klein et al., 2011172 | CBD and THC | 1, 3, and 10 mg/kg THC I.P.; 1, 3, and 10 mg/kg CBD I.P. for 21 days | Male Australian Albino Wistar rats | CBD potentiated an inhibition of body weight gain caused by chronic THC. |
| Ignatowska-Jankowska et al., 2011147 | CBD and AM630 | Study 1: 2.5 and 5 mg/kg per day CBD I.P. for 14 consecutive days. Study 2: CBD+vehicle (5 mg/kg) and vehicle+vehicle, vehicle+AM630, CBD+AM630 (1 mg/kg) |
Male Wistar rats | Both doses of CBD produced significant decrease in body weight gain, with the effect produced by 5 mg/kg being more pronounced. The CB2 receptor selective antagonist, AM630, blocked the decrease in body weight gain. AM630 alone did not affect body weight gain. The results suggest that CBD has the ability to alter body weight gain, possibly through the CB2 receptor. |
| Meye et al., 2013140 | DNQX; Bicuculline; Tetrodotoxin, WIN55,212–2; URB597; URB602; O-2050; AM251; NESS0327; Rimonabant | 1 mL/kg−1 I.P. | Electrophysiological exp.: C57Bl/6 mice. Behavioral exp.: Wistar rats | NESS0327 does not suppress CB1R constitutive activity, unlike Rimonabant. Therefore, it promotes the reduction of body weight and food intake with safer effects than Rimonabant. |
| Wargent et al., 2013143 | THCV | Study 1: 0.3, 1, 2.5, 5 and 12.5 mg/kg, orally twice a day for 30 days. Study 2: 0.1, 0.5, 2.5 and 12.5 mg/kg, orally once a day for 45 days. Study 3: 0.3 and 3 mg/kg, orally once daily. Study 4: 0.1, 0.5, 2.5 and 12.5 mg/kg, oral once daily in ob/ob for 30 days. |
For studies 1 and 2, female C57Bl/6 mice DIO. For studies 3 and 4, C57Bl/6 female ob/ob mice. |
THCV did not significantly affect food intake or body weight gain in any of the studies, but produced an early and transient increase in energy expenditure. It dose dependently reduced glucose intolerance in ob/ob mice and improved glucose tolerance and increased insulin sensitivity in DIO mice, without consistently affecting plasma lipids. THCV also restored insulin signalling in insulin-resistant hepatocytes and myotubes. |
| Cluny et al., 2015163 | THC | 2 mg/kg for 3 weeks and 4 mg/kg for 1 additional week | Adult male DIO and lean mice | THC reduced weight gain, fat mass gain, and energy intake in DIO but not lean mice. DIO induced changes in select gut microbiota were prevented in mice chronically administered THC. THC had no effect on locomotor activity or whole gut transit in either lean or DIO mice. |
| Jadoon et al., 2016144 | THCV and CBD | 100 mg CBD; 5 mg THCV; 1:1 ratio (5 mg/5 mg) of CBD and THCV; 20:1 ratio (100 mg/5 mg) of CBD and THCV, twice daily for 13 weeks. | Humans with type 2 diabetes | THCV significantly reduced fasting glycemia with parallel improvement in β cell function, as well as increased APO A, and adiponectin. CBD decreased resistin and increased levels of glucose-dependent insulinotropic peptide. CBD and THCV were well tolerated. |
| Parray and Yun, 2016184 | CBD | 1, 5, and 10 μm for 72 h | In vitro | CBD enhanced expression of a core set of brown fat-specific marker genes (Ucp1, Cited1, Tmem26, Prdm16, Cidea, Tbx1, Fgf21, and Pgc-1a) and proteins (UCP1, PRDM16, and PGC-1a). Increased expression of UCP1 and other brown fat-specific markers contributed to the browning of 3T3-L1 adipocytes possibly through activation of PPARc and PI3K. In addition, CBD increased protein expression levels of CPT1, ACSL, SIRT1, and PLIN, whereas downregulating JNK2, SREBP1, and LPL. These data suggest possible roles for CBD in browning of white adipocytes, augmentation of lipolysis, thermogenesis, and reduction of lipogenesis. |
AM251, CB1 receptor antagonist drug; AM630, selective CB2 agonist drug; APO A, apolipoprotein A; BDS, botanical drug substance; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; CBC, cannabichromene; CBDA, cannabidiolic acid; CBDV, cannabidivarin; CBDVA, cannabidivarinic acid; CBG, cannabigerol; CBGA, cannabigerolic acid; CBGV, cannabigerivarin; CBN, cannabinol; DIO, diet-induced obesity; DPCPX, cyclopentyl-1,3-dipropylxanthine; HDL, high-density lipoprotein; IL, interleukin; I.P., intraperitoneally; IRS-1, insulin receptor substrate 1; IRS-2, insulin receptor substrate 2; PPAR, peroxisome proliferation-activated receptor; Rimonabant, CB1 receptor antagonist drug; THCA, tetrahydrocannabinolic acid; THCV, Δ9-tetrahydrocannabivarin; TNF, tumor necrosis factor.
In addition, it has been suggested that the clinical effects of rimonabant may be associated with antagonism of CB1 receptors not only in the brain, but also in the liver and adipose tissue, leading to reduced lipid storage and increased glucose sensitivity.95
Animal and human studies have shown that CB1 receptor antagonism has increased O2 consumption and energy expenditure at rest. In comparison with the animals in the control group, rats treated with 3 and 10 mg/kg of rimonabant had an increase in O2 consumption of 18% and 49%, respectively, after 3 h.135 In humans, blocking CB1 with rimonabant 20 mg, combined with a low-calorie diet for 1 year, promoted a significant decrease in body weight and waist circumference, and improved cardiovascular risk factors.132 Although effective, Rimonabant has been linked to increased rates of depression, anxiety, and even suicide; it has, therefore, been withdrawn from the market.133,134,136
However, ongoing studies suggest that restricted peripheral CB1 antagonists, such as URB447 and AM6545, may provide therapeutic benefits in obesity without these psychiatric side effects.137–139
THCV is an inverse agonist of the CB1 receptor, similar to Rimonabant; however, it does not present the adverse effects of this medication.77,140,141 THCV is a natural analogue of THC. Although THCV has a structure similar to THC (differentiated only by the length of its lipophilic alkyl chain), it has several molecular targets and pharmacological profiles. Compared with THC, which expresses its effects through the weak partial agonist activity of CB1 and CB2 endocannabinoid receptors, THCV acts as a neutral CB1 antagonist and partial CB2 agonist.142
Neutral antagonists have been suggested as a potentially safer alternative to Rimonabant in the treatment of obesity. It is suggested that THCV avoids the psychological effects of THC, which include anxiety, depression, cognitive impairment, and the potential for abuse and dependence. However, the mechanism by which THCV antagonizes the effect of THC is unknown.13,140
Animal studies have shown that neutral antagonists appear to decrease food consumption and weight gain similar to Rimonabant, but unlike this drug they do not inhibit activity in areas of emotion regulation, such as the ventral tegmental area and the basolateral amygdala.140 However, the mechanism by which THCV interacts with the reward and aversion systems in the human brain is still unknown.141
In this way, Tudge et al.141 examined the effects of THCV using a reward and aversion processing model in the human brain. Since THCV is thought to be free of the depressogenic side effects seen with Rimonabant, it would consequently not alter the responses in areas that have been shown to be involved in reward processing. Twenty healthy volunteers received a single dose of THCV (10 mg) and placebo in random order on two separate occasions. The neural responses to gratifying stimuli (vision and/or chocolate flavor) and aversive stimuli (image of moldy strawberries and/or less pleasant strawberry flavor) were measured using functional magnetic resonance images. The volunteers assessed pleasure, intensity, and desire for each stimulus.
In conclusion, the findings showed that treatment with THCV increased responses to chocolate stimuli in the midbrain, anterior cingulate cortex, caudate, and putamen. In addition, it increased responses to aversive stimuli in the amygdala, insula, middle orbitofrontal cortex, caudate, and putamen, that is, it did not reduce the processing of rewards, but improved activations in the main areas of rewards and aversion processing. This may indicate that THCV does not compromise motivational processes, probably because does not cause depressive symptom, since it does not mitigate the activation of stimuli with positive or negative values in key areas of brain motivation. This effect profile suggests therapeutic activity in obesity, perhaps with a lower risk of depressive side effects.141
Wargent et al.143 evaluated the effect of THCV on metabolic disorders that accompany obesity, such as hyperglycemia, dyslipidemia, and fatty liver using diet-induced obese (DIO) and genetically obese (ob/ob) mice. Thus, they performed two studies of dose variation in DIO mice; study 1: 0.3, 1, 2.5, 5 and 12.5 mg/kg, orally twice a day for 30 days and study 2: 0.1, 0.5, 2.5 and 12.5 mg/kg, orally once a day for 45 days. A pilot study (study 3: 0.3 and 3 mg/kg, orally once daily) and a complete varied dose study (study 4: 0.1, 0.5, 2.5 and 12.5 mg/kg, oral once daily) in ob/ob for 30 days. The results were compared with a potent CB1 inverse agonist (AM251) administered orally at 10 mg/kg once daily or 5 mg/kg twice daily as a positive control.
According to the results, THCV did not significantly affect food intake or body weight gain, but it did produce an early and transient increase in energy expenditure. Depending on the dose, glucose intolerance decreased in ob/ob mice, improved glucose tolerance, and increased insulin sensitivity in DIO mice; in addition, the higher doses of THCV (2.5 and 12.5 mg/kg) led to a reduction in the concentration of hepatic triglycerides in ob/ob mice. In conclusion, the authors suggest that THCV may be useful for the treatment of metabolic syndrome and/or type 2 DM associated with obesity, alone or in combination with existing treatments.
Riedel et al.101 evaluated the effects of antagonists of synthetic cannabinoid receptors and THCV in fasting and nonfasting mice. A standard fasting protocol and a long-term domestic cage observation system with free-feeding animals were used to assess the feeding behavior of mice treated with the CB1 AM251 antagonist. Likewise, the effects of THCV were also determined in animals with feed released. AM251 suppressed food intake and weight gain in fasting and nonfasting animals. THCV also induced a reduction in weight and hypophagia at doses as low as 3 mg/kg. In conclusion, the authors suggested that THCV is a new compound with hypophagic properties and a potential treatment for obesity.
In a randomized double-blind placebo-controlled clinical trial, 62 subjects with type 2 DM not treated with insulin were randomized to five treatment groups: CBD (100 mg twice daily), THCV (5 mg twice daily), 1: 1 ratio of CBD and THCV (5 mg/5 mg, twice daily), 20: 1 ratio of CBD and THCV (100 mg/5 mg twice daily) or combined placebo for 13 weeks. This study showed that THCV significantly reduced fasting glycemia with parallel improvement in β cell function, as well as increased apolipoprotein A (APO A), and adiponectin compared with placebo, CBD decreased resistin and increased levels of glucose-dependent insulinotropic peptide. CBD and THCV were well tolerated.144
APO A is an important structural protein and an indispensable component of high-density lipoprotein, which plays an essential role in the reverse transport of cholesterol and in the homeostasis of cellular cholesterol. It also plays a multifunctional role in immunity, inflammation, apoptosis, viral, bacterial infection, and so on.145
Adiponectin is the most abundant peptide secreted by adipocytes, the reduction of which plays a central role in diseases associated with obesity, such as insulin resistance/type 2 DM and cardiovascular diseases. Evidence has shown that the administration of adiponectin in humans and rodents has insulin sensitizing, antiatherogenic, and anti-inflammatory effects, and, in some situations, also reduces body weight.146
Resistin, in turn, was initially known as a hormone secreted by adipocytes (adipokine) associated with obesity and insulin resistance in rodents. Currently, it is known that it is primarily expressed and secreted by macrophages in humans. Circulating resistin levels are correlated with inflammatory markers, such as C-reactive protein, TNF-α, and IL-6 in the general population and in individuals with DM2, coronary atherosclerosis, rheumatoid arthritis, and/or sepsis.147–152
It has been suggested that the antiobesity characteristic of THCV can be attributed to its ability to interact with other receptor sites. Evidence has shown that THCV interacts with different transient receptor potential channels (TRP), including TRP vanilloid 1 (TRPV1) and TRP vanilloid 2 (TRPV2).142,153 TRP channels are a group of membrane proteins involved in the transduction of a multitude of chemical and physical stimuli. These channels coordinate the entry of ions, mediating several neural signaling processes involved in the sensations of temperature, pressure, and pH, in addition to smell, taste, vision, and pain perception. Several diseases are associated with TRP channel dysfunction, including neuropathic pain, inflammation, and respiratory disorders.154
The stimulating effects of cannabinoids on TRPV1 and TRPV2 channels result in desensitization of these channels. Desensitization can have important consequences for the potential use of cannabinoids as therapeutic agents, especially in those disorders in which these channels have been shown to be involved and play a permissive role, such as inflammation, and may contribute to the effects anti-inflammatory, analgesic, and anticancer.142,153
It is well established that the ECS is associated with both the control of energy balance and the development of obesity. Cannabinoid receptor antagonists and neutral antagonists are understood to inhibit food intake.140 Whereas, conversely, the activation of the CB1 receptor by agonists stimulates short-term feeding when administered acutely.155 The main psychoactive compound of Cannabis sativa, THC, increases food intake and synthetic THC, Dronabinol®, is prescribed as an appetite stimulant, as well as for the improvement of chemotherapy-induced nausea and vomiting.156 However, contrary to what might be expected, the BMI of regular cannabis smokers was lower than that of nonusers.157
There is ample evidence that exposure to cannabis and/or THC causes negative regulation of CB1. Continued use of cannabis is associated with desensitization and negative regulation of CB1, and CB1 levels remain low for 3–4 weeks after stopping use.158–162 CB1 is known to play an essential role in the assimilation, storage, and conservation of energy, and this reduced regulation leads to a decrease in endocannabinoid tone.82 Therefore, BMI is reduced in cannabis users and is expected to decrease further when users stop using cannabis, as CB1 remains under reduced regulation for many weeks after chronic cannabis use.158–162
Recently, abstinent users would experience reduced appetite and increased metabolic rates during this period. However, they will no longer have short-term stimulation of appetite, energy intake, and storage and decreased metabolic rates during each episode of acute cannabis use. Therefore, weight loss will increase as energy intake and storage remain reduced and metabolism stimulated, until CB1 returns to levels before cannabis use.82
In view of this, Cluny et al.163 investigated the effect of chronic THC administration on body weight and intestinal microbiota in DIO and lean mice. DIO adult males and lean mice were treated daily with vehicle or THC (2 mg/kg for 3 weeks and 4 mg/kg for an additional week). The results showed that THC reduced weight gain, fat mass gain, and energy intake in DIO, but not in lean mice. This effect may be associated with the fact that THC is a partial agonist of the CB1 and CB2 receptors. Thus, it does not produce maximum stimulation of the aforementioned receptors and may prevent complete endogenous agonists, such as anandamide, from binding to CB1 receptors in situations of high endocannabinoid tone, as observed in obesity. They also showed that DIO-induced changes in the selected intestinal microbiota were prevented in mice administered chronically with THC.
The intestinal microbiota alters endocannabinoid signaling in obesity; increasing intestinal permeability and favoring low-grade inflammation associated with obesity and influencing adipogenesis.164 This study demonstrates that THC-induced weight gain prevention is associated with changes in the microbiota. In the same study, the authors observed an increase in the abundance of Akkermansia muciniphila in DIO mice treated with chronic THC. The abundance of A. muciniphila, a bacterium that deteriorates mucin, has been inversely associated with body fat mass and glucose intolerance in rats.165
A. muciniphila has been characterized as a beneficial participant in body metabolism and has great prospects for the treatment of obesity-related metabolic disorders, in addition to being considered as a state-of-the-art therapeutic agent. It can reduce the serum level of inflammatory cytokines, such as IL-2, interferon-gamma, subunit beta of interleukin 12 (IL-12p40), and monocyte chemoattractant protein-1 (MCP-1) (Fig. 2).166,167
In addition, it has previously been indicated that THC has a beneficial effect on the regulation of insulin sensitivity in adipocytes resistant to this hormone. In a study by Gallant et al.168 was evaluated the biological effects of a lipophilic Cannabis sativa extract containing THC levels ranging from 2.5 to 10 mg/mL, in normal and insulin-resistant 3T3-L1 adipocytes.
The results demonstrated that the extract considerably improved the gene expression of the glucose transporter isotype 4 (GLUT4), and the insulin receptor substrate 1 and 2 (IRS-1 and IRS-2), in addition to decreasing adipogenesis in an in vitro model, thus optimizing the ability of adipocytes to respond to insulin stimulation. In the same study, it was observed that the level of TNF-α in 3T3-L1 cells was significantly reduced in the presence of the extract containing THC, which also improved the sensitivity of cells to insulin.
In a study by Ngueta et al.,169 the relationship between cannabis use, obesity, and insulin resistance was analyzed. Data from 786 Inuit adults from the Nunavik Inuit Health Survey (2004) were analyzed. Information on cannabis use was obtained from a self-administered questionnaire.
The results showed that cannabis use was highly prevalent in the study population (57.4%) and was statistically associated with lower BMI (p<0.001), lower% of fat mass (p<0.001), lower fasting insulin (p=0.04), and lower homeostasis model assessment of insulin resistance (HOMA-IR) (p=0.01), after adjusting for several confounding variables. The authors concluded that the use of cannabis was associated with a lower BMI, and this association did not occur through the glucose metabolic process or related inflammatory markers. The association between cannabis use and insulin resistance was mediated by its influence on weight.
Rajavashisth et al.170 conducted a cross-sectional study to determine the association between DM and cannabis use. The study included participants from NHANES III, a nationally representative sample of the U.S. population. The total analytical sample was 10,896 adults. The study included four groups (n=10,896): nonusers of cannabis (61.0%), ex-users of cannabis (30.7%), light (once to four times/month; 5.0%), and heavy (more than five times/month) current cannabis users (3.3%). DM was defined based on self-report or abnormal glycemic parameters. The results showed that cannabis users had a lower prevalence of age-adjusted DM compared with non-cannabis users (odds ratio [OR] 0.42, 95% confidence interval [CI] 0.33–0.55; p<0.0001).
A meta-analysis, conducted by Alshaarawy and Anthony, showed epidemiological estimates of eight independent replicates (1) of the National Health and Nutrition Examination Surveys and (2) of the National Drug and Health Surveys (2005–12). For each participant in the national survey, computer-assisted self-interviews assessed cannabis smoking (CS) and DM diagnosed by doctors. The results showed that recently active CS and DM are inversely associated. The OR of the meta-analytical summary is 0.7 (95% CI 0.6–0.8).171
Klein et al.172 examined the interactions between THC and CBD during chronic treatment and at equivalent doses. Adolescent rats were treated with increasing daily doses of THC for 21 days (1 and 3 and 10 mg/kg). Some rats received equivalent doses of CBD 20 min before each THC injection to allow examination of possible antagonistic effects of CBD. The findings showed that CBD potentiated an inhibition of body weight gain caused by chronic THC.
A publication by Le Foll et al.130 suggested that chronic treatment with THC, as well as administration of combined THC/CBD medications in carefully controlled circumstances, can lead to weight loss and possibly improvement of metabolic symptoms in individuals with obesity. It is possible for THC to maintain its effectiveness in combination with CBD, but this has not yet been determined.
According to Clark et al.,82 an increase in the ratio of omega-6 to omega-3 fatty acids contributes to obesity rates by increasing the levels of endocannabinoid N-arachidonoylethanolamide (or anandamide) and 2-AG, overstimulating CB1 and leading to increased caloric intake, reduced metabolic rates, and weight gain. Cannabis, or THC, also stimulates CB1 and increases caloric intake during acute exposures. Thus, the authors conducted a meta-analysis to establish a relationship between the use of cannabis and the BMI and provide a theoretical explanation for this relationship. BMI data for cannabis users and nonusers, or studies reporting adjusted ORs for obese or overweight cannabis users, were obtained from the literature.
Studies addressing the health impact of cannabis use have been identified using database searches and citation lists. The present meta-analysis revealed that the BMI and obesity rates are significantly reduced in cannabis users, together with the increase in caloric intake. The authors provide, for the first time, a causal explanation for this paradox, in which the rapid and long-lasting regulation of CB1 after acute cannabis consumption reduces energy storage and increases metabolic rates, thus reversing the impact of the BMI.
CBD is one of the most common nonpsychotropic constituents of the Cannabis sativa plant. It was revealed that CBD generates a series of pharmacological effects through several mechanisms. It works as a negative allosteric modulator of the CB1 receptor; allosteric modulators of CB1 receptors have the ability to treat the CNS and peripheral disorders, preventing the adverse effects associated with the orthostatic agonism or antagonism of these receptors.173 Therefore, it has therapeutic potential in the treatment of CNS diseases such as neurodegenerative diseases, epilepsy, anxiety, and depression, without simultaneous psychotic adverse effects.174–176 In addition, it has been indicated that CBD is able to block the CB1 receptor, thus producing antiobesity effects. Conversely, CBD unexpectedly exhibited a high affinity for the CB2 receptor, for which it could act as an agonist.177 An interesting result of studies is the fact that CBD has a greater affinity for several receptors, including 5-HT1A, TRPV, and PPARγ channels.110,153,178 Activation of the 5-HT1A receptor can act as an antioxidant by capturing ROS preventing membrane oxidation.179 Therefore, through the activation of 5-HT1A, CBD can neutralize the peroxidation of phospholipids and thus participate in the protection of biomembranes against oxidative changes.180
CBD activates TRPV, thereby directly or indirectly increasing the level of endogenous anandamide, which is one of the endogenous TRPV1 agonists. Modulation of these channels by cannabinoids, such as CBD, is complex and the relationship between channels and their activation in response to cannabinoids can be further explored for various therapeutic uses, including chronic pain and inflammation.154
Studies by Hegde et al.181 and by Esposito et al.109 indicated that CBD considerably induced PPARγ transcriptional activity. It is known that PPARγ is a potential therapeutic target for the treatment of inflammatory disorders due to its important role in regulating adipogenesis and lipid storage, as well as in glucose homeostasis, lipoprotein metabolism, and inflammation. In addition, it is a central participant in thermogenesis and is an active modulator of lipid metabolism and insulin sensitivity.182,183
Parray and Yun184 investigated the effects of CBD in inducing the darkening of 3T3-L1 adipocytes. For CBD treatment, 3T3-L1 preadipocytes were incubated with different doses of CBD (1, 5, and 10 μM) during differentiation and until mature adipocyte formation. According to the results, CBD improved the expression of a main set of specific marker genes (Ucp1, Prdm16, Cidea, Fgf21, and Pgc-1α) and proteins (UCP1, PRDM16, and PGC-1α) for brown fat. The increased expression of UCP1 and other specific brown fat markers contributed to the darkening of 3T3-L1 adipocytes, possibly through activation of PPARγ and PI3K.
In addition, CBD increased the levels of protein expression of CPT1, ACSL, SIRT1, and PLIN, while regulating JNK2, SREBP1, and LPL. These data suggest the possible roles of CBD in the darkening of white adipocytes, increased lipolysis, thermogenesis, and reduced lipogenesis.184
In a study by Ignatowska-Jankowska et al.,177 the effects of repeated administration of CBD on body weight gain in rats were investigated. The animals received intraperitoneal injections of CBD at doses of 2.5 and 5 mg/kg per day for 14 consecutive days and body weight gain was monitored. Both doses of CBD produced a significant decrease in body weight gain, with the effect produced by treatment with 5 mg/kg being more pronounced.
These results suggest that CBD has the ability to alter body weight gain, possibly by acting on the CB2 receptor. CB2 receptors can play a role in regulating body weight. Furthermore, it is important to note that the activation of CB2 leads to a decrease in the levels of ROS and TNF-α, which reduces oxidative stress and inflammation (Fig. 2).185
In conclusion, the data suggest that CBD plays dual modulating roles in the induction of the brown phenotype, in addition to promoting lipid metabolism. Thus, CBD can be explored as a potentially promising therapeutic agent for the prevention of obesity.184
Conclusion
From the data presented, it can be concluded that the derivatives of Cannabis sativa can produce an increase in lipolysis, thermogenesis, and a reduction in lipogenesis, playing a role in the regulation of body weight, through action on several receptors. In addition, studies have shown that derivatives of Cannabis sativa can reduce levels of ROS and proinflammatory cytokines such as TNF-α, causing a reduction in oxidative stress and inflammation.
Given the aforementioned, it is clear that phytocannabinoids derived from Cannabis sativa have therapeutic potential due to their anti-inflammatory, antioxidant, and neuroprotective properties, making the plant a study option for reducing and reversing inflammation and comorbidities associated with obesity.
Abbreviations Used
- 2-AG
2-arachidonoylglycerol
- A 2A
adenosine A 2A
- APO A
apolipoprotein A
- BDS
botanical drug substance
- BMI
body mass index
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CBC
cannabichromene
- CBD
cannabidiol
- CBDA
cannabidiolic acid
- CBDV
cannabidivarin
- CBDVA
cannabidivarinic acid
- CBG
cannabigerol
- CBGA
cannabigerolic acid
- CBGV
cannabigerivarin
- CBN
cannabinol
- CI
confidence interval
- CNS
central nervous system
- CS
Cannabis smoking
- DIO
diet-induced obese
- DM
diabetes mellitus
- DPCPX
cyclopentyl-1,3-dipropylxanthine
- ECS
endocannabinoid system
- GLUT4
glucose transporter isotype 4
- HDL
high-density lipoprotein
- I.P.
intraperitoneally
- IL
interleukin
- IRS-1
insulin receptor substrate 1
- IRS-2
insulin receptor substrate 2
- MCP-1
monocyte chemoattractant protein-1
- NPY
neuropeptide Y
- OR
odds ratio
- PPAR
peroxisome proliferation-activated receptor
- ROS
reactive oxygen species
- THC
Δ9-tetrahydrocannabinol
- THCV
Δ9-tetrahydrocannabivarin
- THCVA
tetrahydrocannabivarin acid
- TRP
transient receptor potential channels
- TRPV1
TRP vanilloid 1
- TRPV2
TRP vanilloid 2
- WAT
white adipose tissue
- WHO
World Health Organization
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research has not received any specific grants from funding agencies in the public, commercial or non-profit sectors.
Cite this article as: Cavalheiro EKFF, Costa AB, Salla DH, da Silva MR, Mendes TF, da Silva LE, da Rosa Turatti C, de Bitencourt RM, Rezin GT (2022) Cannabis sativa as a treatment for obesity: from anti-inflammatory indirect support to a promising metabolic re-establishment target, Cannabis and Cannabinoid Research 7:2, 135–151, DOI: 10.1089/can.2021.0016.
References
- 1. Inoue Y, Qin B, Poti J, et al. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Obesity and overweight. April 1, 2020. Accessed June 12, 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 4. Tremmel M, Gerdtham UG, Nilsson PM, et al. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun X, Li P, Yang X, et al. From genetics and epigenetics to the future of precision treatment for obesity. Gastroenterol Rep. 2017;5:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease Find the latest version: inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merra G, Gratteri S, De Lorenzo A, et al. Effects of very-low-calorie diet on body composition, metabolic state, and genes expression: a randomized double-blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2017;21:329–345. [PubMed] [Google Scholar]
- 8. Turner M, Jannah N, Kahan S, et al. Current knowledge of obesity treatment guidelines by health care professionals. Obesity. 2018;26:665–671. [DOI] [PubMed] [Google Scholar]
- 9. Ruban A, Stoenchev K, Ashrafian H, et al. Current treatments for obesity. Clin Med (Lond). 2019;19:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39:79–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bielawiec P, Harasim-Symbor E, Chabowski A. Phytocannabinoids: useful drugs for the treatment of obesity? Special focus on cannabidiol. Front Endocrinol (Lausanne). 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abioye A, Ayodele O, Marinkovic A, et al. Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. J Cannabis Res. 2020;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xi L, Chow C-M, Kong X. Role of tissue and systemic hypoxia in obesity and type 2 diabetes. J Diabetes Res. 2016;2016:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Obesity. Accessed June 12, 2020. Available from: https://www.who.int/topics/obesity/en/
- 16. O'Brien PD, Hinder LM, Callaghan BC, et al. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marseglia L, Manti S, D'Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2015;16:378–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Savas M, Wester VL, Visser JA, et al. Extensive phenotyping for potential weight-inducing factors in an outpatient population with obesity. Obes Facts. 2019;12:369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agha M, Agha R. The rising prevalence of obesity: part B—public health policy solutions. Int J Surg Oncol. 2017;2:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Controlling the global obesity epidemic. Accessed June 12, 2020. Available from: https://www.who.int/nutrition/topics/obesity/en/
- 21. De Lorenzo A, Romano L, Di Renzo L, et al. Obesity: a preventable, treatable, but relapsing disease. Nutrition. 2020;71:110615. [DOI] [PubMed] [Google Scholar]
- 22. de-Mateo-Silleras B, De-la-Cruz-Marcos S, Alonso-Izquierdo L, et al. Bioelectrical impedance vector analysis in obese and overweight children. PLoS One. 2019;14:e0211148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu HH, Branca RT, Hernando D, et al. Magnetic resonance imaging of obesity and metabolic disorders: summary from the 2019 ISMRM Workshop. Magn Reson Med. 2020;83:1565–1576. [DOI] [PubMed] [Google Scholar]
- 24. Marra M, Sammarco R, Lorenzo A, et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy X-ray absorptiometry (DXA): a critical overview. Contrast Media Mol Imaging. 2019;2019:3548284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Lorenzo A, Gratteri S, Gualtieri P, et al. Why primary obesity is a disease? J Transl Med. 2019;17:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohashi K, Shibata R, Murohara T, et al. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25:348–355. [DOI] [PubMed] [Google Scholar]
- 27. Cohen P, Spiegelman BM. Cell biology of fat storage. Mol Biol Cell. 2016;27:2523–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Q, Scherer PE. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat Rev Nephrol. 2018;14:105–120. [DOI] [PubMed] [Google Scholar]
- 29. Crujeiras AB, Carreira MC, Cabia B, et al. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015;140:57–63. [DOI] [PubMed] [Google Scholar]
- 30. Cheung CK, Wu JC-Y. Role of ghrelin in the pathophysiology of gastrointestinal disease. Gut Liver. 2013;7:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. [DOI] [PubMed] [Google Scholar]
- 32. de Git KCG, Peterse C, Beerens S, et al. Is leptin resistance the cause or the consequence of diet-induced obesity? Int J Obes. 2018;42:1445–1457. [DOI] [PubMed] [Google Scholar]
- 33. Robertson SA, Leinninger GM, Myers MG. Molecular and neural mediators of leptin action. Physiol Behav. 2008;94:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajkovic N, Zamaklar M, Lalic K, et al. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: relevance for cardiovascular risk prevention. Int J Environ Res Public Health. 2014;11:4049–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parvaresh Rizi E, Loh TP, Baig S, et al. A high carbohydrate, but not fat or protein meal attenuates postprandial ghrelin, PYY and GLP-1 responses in Chinese men. PLoS One. 2018;13:e0191609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato T, Ida T, Nakamura Y, et al. Physiological roles of ghrelin on obesity. Obes Res Clin Pract. 2014;8:e405–e413. [DOI] [PubMed] [Google Scholar]
- 38. Haczeyni F, Bell-Anderson KS, Farrell GC. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev. 2018;19:406–420. [DOI] [PubMed] [Google Scholar]
- 39. Verboven K, Wouters K, Gaens K, et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep. 2018;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michailidou Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr Opin Physiol. 2019;12:39–43. [Google Scholar]
- 41. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. [DOI] [PubMed] [Google Scholar]
- 42. O'Rourke RW, White AE, Metcalf MD, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujisaka S, Usui I, Ikutani M, et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia. 2013;56:1403–1412. [DOI] [PubMed] [Google Scholar]
- 44. Ellulu MS, Patimah I, Khaza'ai H, et al. Obesity & inflammation: the linking mechanism & the complications. Arch Med Sci. 2017;13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saltiel AR, Olefsky JM. Inflammatory linking obesity and metabolic disease and metabolic disease. J Clin Invest. 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Castoldi A, Naffah de Souza C, Câmara NOS, et al. The macrophage switch in obesity development. Front Immunol. 2016;6:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chylikova J, Dvorackova J, Tauber Z, et al. M1/M2 macrophage polarization in human obese adipose tissue. Biomed Pap. 2018;162:79–82. [DOI] [PubMed] [Google Scholar]
- 48. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. [DOI] [PubMed] [Google Scholar]
- 49. McMurray F, Patten DA, Harper M-E. Reactive oxygen species and oxidative stress in obesity-recent findings and empirical approaches. Obesity. 2016;24:2301–2310. [DOI] [PubMed] [Google Scholar]
- 50. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jakicic JM, Rogers RJ, Davis KK, et al. Role of physical activity and exercise in treating patients with overweight and obesity. Clin Chem. 2018;64:99–107. [DOI] [PubMed] [Google Scholar]
- 53. Joo JK, Lee KS. Pharmacotherapy for obesity. J Menopausal Med. 2014;20:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tchang BG, Kumar RB, Aronne LJ. Pharmacologic treatment of overweight and obesity in adults. [Updated 2020 Oct 7]. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc., 2000. [Google Scholar]
- 55. LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2018;320:1172–1191. [DOI] [PubMed] [Google Scholar]
- 56. Ma IT, Madura JA. Gastrointestinal complications after bariatric surgery. Gastroenterol Hepatol (N Y). 2015;11:526–535. [PMC free article] [PubMed] [Google Scholar]
- 57. O'Brien P. Surgical treatment of obesity. [Updated 2016 Jan 19]. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc., 2000. [Google Scholar]
- 58. Lim R, Beekley A, Johnson DC, et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018;3:e000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Sun M, Yao H, et al. Herbal medicine for the treatment of obesity: an overview of scientific evidence from 2007 to 2017. Evid Based Complement Altern Med. 2017;2017:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karri S, Sharma S, Hatware K, et al. Natural anti-obesity agents and their therapeutic role in management of obesity: a future trend perspective. Biomed Pharmacother. 2019;110:224–238. [DOI] [PubMed] [Google Scholar]
- 61. Payab M, Hasani-Ranjbar S, Shahbal N, et al. Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phyther Res. 2020;34:526–545. [DOI] [PubMed] [Google Scholar]
- 62. Yang CS, Wang H, Sheridan ZP. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J Food Drug Anal. 2018;26:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang J, Wang Y, Xie Z, et al. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 201430;68:1075–1087. [DOI] [PubMed] [Google Scholar]
- 64. Maia-Landim A, Ramírez JM, Lancho C, et al. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC Complement Altern Med. 2018;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haber SL, Awwad O, Phillips A, et al. Garcinia cambogia for weight loss. Am J Health Syst Pharm. 2018;75:17–22. [DOI] [PubMed] [Google Scholar]
- 66. Namazi N, Larijani B, Ayati MH, et al. The effects of Nigella sativa L. on obesity: a systematic review and meta-analysis. J Ethnopharmacol. 2018;219:173–181. [DOI] [PubMed] [Google Scholar]
- 67. Onakpoya I, Davies L, Posadzki P, et al. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: a systematic review of randomized controlled trials. J Diet Suppl. 2013;10:29–38. [DOI] [PubMed] [Google Scholar]
- 68. Astell KJ, Mathai ML, McAinch AJ, et al. A pilot study investigating the effect of Caralluma fimbriata extract on the risk factors of metabolic syndrome in overweight and obese subjects: a randomised controlled clinical trial. Complement Ther Med. 2013;21:180–189. [DOI] [PubMed] [Google Scholar]
- 69. Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants (Basel). 2019;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gallily R, Yekhtin Z, Hanuš LO. The anti-inflammatory properties of terpenoids from cannabis. Cannabis Cannabinoid Res. 2018;3:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373:1048–1058. [DOI] [PubMed] [Google Scholar]
- 74. Pollio A. The name of cannabis: a short guide for nonbotanists. Cannabis Cannabinoid Res. 2016;1:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hua T, Vemuri K, Nikas SP, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547:468–471. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76. Chakrabarti B, Persico A, Battista N, et al. Endocannabinoid signaling in autism. Neurotherapeutics. 2015;12:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McPartland JM, Duncan M, Di Marzo V, et al. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reekie TA, Scott MP, Kassiou M. The evolving science of phytocannabinoids. Nat Rev Chem. 2018;2:0101. [Google Scholar]
- 79. Nazarenus C. The discovery of the endocannabinoid system. In: Medical cannabis handbook. New York, NY: Springer Publishing Company, 2019, 29–35. [Google Scholar]
- 80. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Donvito G, Nass SR, Wilkerson JL, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43:52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clark TM, Jones JM, Hall AG, et al. Theoretical explanation for reduced body mass index and obesity rates in cannabis users. Cannabis Cannabinoid Res. 2018;3:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fonseca B, Costa M, Almada M, et al. The endocannabinoid system—a therapeutic perspective [in Portuguese]. Acta Farm Port. 2013;2:37–44. [Google Scholar]
- 84. Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res. 2008;36:135–145. [DOI] [PubMed] [Google Scholar]
- 85. Juan-Picó P, Fuentes E, Javier Bermúdez-Silva F, et al. Cannabinoid receptors regulate Ca2+ signals and insulin secretion in pancreatic β-cell. Cell Calcium. 2006;39:155–162. [DOI] [PubMed] [Google Scholar]
- 86. Khan SA, Ali A, Khan SA, et al. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediators Inflamm. 2014;2014:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schier A, Ribeiro N, Coutinho D, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13:953–960. [DOI] [PubMed] [Google Scholar]
- 88. Freitas HR, Isaac AR, Malcher-Lopes R, et al. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci. 2018;21:695–714. [DOI] [PubMed] [Google Scholar]
- 89. Mazier W, Saucisse N, Gatta-Cherifi B, et al. The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metab. 2015;26:524–537. [DOI] [PubMed] [Google Scholar]
- 90. Bisogno T, Maccarrone M. Endocannabinoid signaling and its regulation by nutrients. Biofactors. 2014;40:373–380. [DOI] [PubMed] [Google Scholar]
- 91. DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol. 2008;20:110–115. [DOI] [PubMed] [Google Scholar]
- 93. Cota D, Marsicano G, Lutz B, et al. Endogenous cannabinoid system as a modulator of food intake. Int J Obes. 2003;27:289–301. [DOI] [PubMed] [Google Scholar]
- 94. Cota D, Marsicano G, Tschöp M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ginsberg HN, Woods SC. The endocannabinoid system: potential for reducing cardiometabolic risk. Obesity. 2009;17:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. [DOI] [PubMed] [Google Scholar]
- 97. Cassano T, Calcagnini S, Pace L, et al. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Andre CM, Hausman J-F, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Katz-Talmor D, Katz I, Porat-Katz B-S, et al. Cannabinoids for the treatment of rheumatic diseases—where do we stand? Nat Rev Rheumatol. 2018;14:488–498. [DOI] [PubMed] [Google Scholar]
- 101. Riedel G, Fadda P, McKillop-Smith S, et al. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. Prog Chem Org Nat Prod. 2017;103:103–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacother J Hum Pharmacol Drug Ther. 2013;33:195–209. [DOI] [PubMed] [Google Scholar]
- 104. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20:E755–E796. [PubMed] [Google Scholar]
- 105. Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand. 2011;124:250–261. [DOI] [PubMed] [Google Scholar]
- 106. Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. [DOI] [PubMed] [Google Scholar]
- 107. Sun Y, Bennett A. Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007;2007:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Esposito G, Scuderi C, Savani C, et al. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br J Pharmacol. 2009;151:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Esposito G, Scuderi C, Valenza M, et al. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6:e28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. O'Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Magen I, Avraham Y, Ackerman Z, et al. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol. 2009;51:528–534. [DOI] [PubMed] [Google Scholar]
- 112. Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103:7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Campos AC, Fogaça MV, Sonego AB, et al. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127. [DOI] [PubMed] [Google Scholar]
- 114. Peres FF, Lima AC, Hallak JEC, et al. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–1098. [DOI] [PubMed] [Google Scholar]
- 116. Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Watt G, Karl T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer's disease. Front Pharmacol. 2017;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mukhopadhyay P, Rajesh M, Horváth B, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Di Marzo V, Piscitelli F, Mechoulam R.. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb Exp Pharmacol 2011:75–104. [DOI] [PubMed] [Google Scholar]
- 120. Santiago AN, Mori MA, Guimarães FS, et al. Effects of cannabidiol on diabetes outcomes and chronic cerebral hypoperfusion comorbidities in middle-aged rats. Neurotox Res. 2019;35:463–474. [DOI] [PubMed] [Google Scholar]
- 121. Niesink RJM, Van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Weiss L, Zeira M, Reich S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151. [DOI] [PubMed] [Google Scholar]
- 123. Weiss L, Zeira M, Reich S, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Durst R, Danenberg H, Gallily R, et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J Physiol Circ Physiol. 2007;293:H3602–H3607. [DOI] [PubMed] [Google Scholar]
- 125. Gonca E, Darıcı F. The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias. J Cardiovasc Pharmacol Ther. 2015;20:76–83. [DOI] [PubMed] [Google Scholar]
- 126. Fernández-Ruiz J, Sagredo O, Pazos MR, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cassano T, Villani R, Pace L, et al. From Cannabis sativa to cannabidiol: promising therapeutic candidate for the treatment of neurodegenerative diseases. Front Pharmacol. 2020;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Costa B, Trovato AE, Comelli F, et al. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. [DOI] [PubMed] [Google Scholar]
- 129. McPartland JM. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res. 2018;3:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Le Foll B, Trigo JM, Sharkey KA, et al. Cannabis and Δ9-tetrahydrocannabinol (THC) for weight loss? Med Hypotheses. 2013;80:564–567. [DOI] [PubMed] [Google Scholar]
- 131. Greenway FL, Kirwan JP. Medical marijuana—an obesity problem or opportunity? Int J Obes. 2019;43:761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Van Gaal LF, Rissanen AM, Scheen AJ, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet (London, England). 2005;365:1389–1397. [DOI] [PubMed] [Google Scholar]
- 133. Després J-P, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. [DOI] [PubMed] [Google Scholar]
- 134. Sam AH, Salem V, Ghatei MA. Rimonabant: from RIO to Ban. J Obes. 2011;2011:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kunz I, Meier MK, Bourson A, et al. Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes. 2008;32:863–870. [DOI] [PubMed] [Google Scholar]
- 136. Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease. JAMA. 2008;299:1547. [DOI] [PubMed] [Google Scholar]
- 137. Argueta DA, DiPatrizio NV. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol Behav. 2017;171:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Heinitz S, Basolo A, Piaggi P, et al. Peripheral endocannabinoids associated with energy expenditure in Native Americans of Southwestern Heritage. J Clin Endocrinol Metab. 2018;103:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Heinitz S, Basolo A, Piomelli D, et al. Endocannabinoid anandamide mediates the effect of skeletal muscle sphingomyelins on human energy expenditure. J Clin Endocrinol Metab. 2018;103:3757–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Meye FJ, Trezza V, Vanderschuren LJMJ, et al. Neutral antagonism at the cannabinoid 1 receptor: a safer treatment for obesity. Mol Psychiatry. 2013;18:1294–1301. [DOI] [PubMed] [Google Scholar]
- 141. Tudge L, Williams C, Cowen PJ, et al. Neural effects of cannabinoid CB1 neutral antagonist tetrahydrocannabivarin on food reward and aversion in healthy volunteers. Int J Neuropsychopharmacol. 2015;18:pyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. National Center for Biotechnology Information. PubChem Database. Tetrahydrocannabivarin, CID=93147. 2020. Accessed July 19, 2020. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Tetrahydrocannabivarin
- 143. Wargent ET, Zaibi MS, Silvestri C, et al. The cannabinoid Δ9-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr Diabetes. 2013;3:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39:1777–1786. [DOI] [PubMed] [Google Scholar]
- 145. Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: a molecule of diverse function. Indian J Clin Biochem. 2016;31:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Park HK, Kwak MK, Kim HJ, et al. Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med. 2017;32:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Su K, Li Y, Zhang D, et al. Relation of circulating resistin to insulin resistance in type 2 diabetes and obesity: a systematic review and meta-analysis. Front Physiol. 2019;10:1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Muse ED, Feldman DI, Blaha MJ, et al. The association of resistin with cardiovascular disease in the multi-ethnic study of atherosclerosis. Atherosclerosis. 2015;239:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fatel EC de S, Rosa FT, Simão ANC, et al. Adipokines in rheumatoid arthritis. Adv Rheumatol. 2018;58:25. [DOI] [PubMed] [Google Scholar]
- 151. Saboktakin L, Bilan N, Ghalehgolab Behbahan A, et al. Relationship between resistin levels and sepsis among children under 12 years of age: a case control study. Front Pediatr. 2019;7:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Karampela I, Christodoulatos G-S, Kandri E, et al. Resistin may predict severity and mortality in critically ill septic patients. Eur Respir J. 2018;52:PA2304. [Google Scholar]
- 153. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2019;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Järbe T, DiPatrizio NV. Delta9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav Pharmacol. 2005;16:373–380. [DOI] [PubMed] [Google Scholar]
- 156. Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc B Biol Sci. 2012;367:3353–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol. 2011;174:929–933. [DOI] [PubMed] [Google Scholar]
- 158. Ceccarini J, Kuepper R, Kemels D, et al. [18 F]MK-9470 PET measurement of cannabinoid CB 1 receptor availability in chronic cannabis users. Addict Biol. 2015;20:357–367. [DOI] [PubMed] [Google Scholar]
- 159. D'Souza DC, Cortes-Briones JA, Ranganathan M, et al. Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:60–67. [DOI] [PubMed] [Google Scholar]
- 160. Hirvonen J, Goodwin RS, Li C-T, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dudok B, Barna L, Ledri M, et al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci. 2015;18:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cluny NL, Keenan CM, Reimer RA, et al. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS One. 2015;10:e0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Muccioli GG, Naslain D, Bäckhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. [DOI] [PubMed] [Google Scholar]
- 166. Xu Y, Wang N, Tan H-Y, et al. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gallant M, Odei-Addo F, Frost CL, et al. Biological effects of THC and a lipophilic cannabis extract on normal and insulin resistant 3T3-L1 adipocytes. Phytomedicine. 2009;16:942–949. [DOI] [PubMed] [Google Scholar]
- 169. Ngueta G, Bélanger RE, Laouan-Sidi EA, et al. Cannabis use in relation to obesity and insulin resistance in the inuit population. Obesity. 2015;23:290–295. [DOI] [PubMed] [Google Scholar]
- 170. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Alshaarawy O, Anthony JC. Cannabis smoking and diabetes mellitus: results from meta-analysis with eight independent replication samples. Epidemiology. 2015;26:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Klein C, Karanges E, Spiro A, et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl). 2011;218:443–457. [DOI] [PubMed] [Google Scholar]
- 173. Laprairie RB, Bagher AM, Kelly MEM, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br J Pharmacol. 2015;172:4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hill AJ, Williams CM, Whalley BJ, et al. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97. [DOI] [PubMed] [Google Scholar]
- 175. Silvestro S, Mammana S, Cavalli E, et al. Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules. 2019;24:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Iuvone T, Esposito G, De Filippis D, et al. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ignatowska-Jankowska B, Jankowski MM, Swiergiel AH. Cannabidiol decreases body weight gain in rats: involvement of CB2 receptors. Neurosci Lett. 2011;490:82–84. [DOI] [PubMed] [Google Scholar]
- 178. Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. [DOI] [PubMed] [Google Scholar]
- 179. Azouzi S, Santuz H, Morandat S, et al. Antioxidant and membrane binding properties of serotonin protect lipids from oxidation. Biophys J. 2017;112:1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Resstel LBM, Tavares RF, Lisboa SFS, et al. 5-HT 1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hegde VL, Singh UP, Nagarkatti PS, et al. Critical role of mast cells and peroxisome proliferator–activated receptor γ in the Induction of myeloid-derived suppressor cells by marijuana cannabidiol in vivo. J Immunol. 2015;194:5211–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Gervois P, Mansouri RM. PPARα as a therapeutic target in inflammation-associated diseases. Expert Opin Ther Targets. 2012;16:1113–1125. [DOI] [PubMed] [Google Scholar]
- 183. Ma X, Wang D, Zhao W, et al. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front Endocrinol (Lausanne). 2018;9:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Parray HA, Yun JW. Cannabidiol promotes browning in 3T3-L1 adipocytes. Mol Cell Biochem. 2016;416:131–139. [DOI] [PubMed] [Google Scholar]
- 185. Han KH, Lim S, Ryu J, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–386. [DOI] [PubMed] [Google Scholar]