When plants first colonized land about 450 million years ago, they had to maintain diffusion of carbon dioxide CO2 to chloroplasts while avoiding substantial water loss to dry air. The development of openings in the leaf epidermis, that is stomata, controlled gas exchange between the plant and the fluctuating conditions of the atmosphere (Harris et al., 2020). By modulating their shape, guard cells limit the opening of stomata and control the rate of gas exchange (including CO2 and water vapor; Kirkham, 2014). Coordination of stomatal opening and closing with environmental conditions is therefore important for the plant to maximize the amount of carbon assimilated for a given amount of water transpired. This characteristic is reflected by water-use efficiency (WUE), which is the ratio of carbon assimilation to water transpiration.
Carbon atoms are integrated into organic molecules through the process of photosynthesis. Light energy, captured by the photosystems, is transformed into chemical energy in the form of organic molecules. Photosynthesis is often defined by the number of carbon atoms in the first fixation product, whether the C3 product phosphoglycerate or the C4 product oxaloacetate. Both C3 and C4 photosynthesis types are observed in Poaceae species, which represents more than 60% of the world’s food production. C3 plants generally grow in relatively mild conditions, with relative abundance of water and mild temperature, such as in temperate zone (Pearcy and Ehleringer, 1984). Under elevated temperature, the oxygenase activity of Rubisco (Ribulose-1,5-bisphosphate carboxylase oxygenase) is higher than the carboxylase activity, resulting in an imbalance between photorespiration and carbon fixation. In contrast, C4 plants transfer C4 products from the mesophyll cells to the vascular bundle sheath cells, where CO2 is released to maintain a high CO2 pressure and thus enhance carboxylase activity. The CO2 concentration mechanism allows C4 plants to fix carbon even under warm temperatures, such as in tropical areas, or when stomata are closed due to water stress. Thus, C4 species often have a higher WUE than C3 species, but is this solely because of the CO2 concentration mechanism? In the present issue of Plant Physiology, Ozeki et al. (2022) tested whether stomatal kinetics could also contribute to higher WUE in C4 species.
Of the factors that control stomatal conductivity and photosynthetic efficiency, light is probably the most important. Light-induced stomatal opening maintains a high intercellular CO2 concentration when photosystems are stimulated, while closing stomata in the dark avoids inefficient water loss. Characterizing the kinetics of stomata as light changes is therefore an important factor in WUE. Rapid opening upon illumination allows diffusion and assimilation of CO2 (Figure 1). Rapid closure upon darkness blocks water loss when assimilation stops. Higher WUE is thus expected if the rate of stomatal closing and/or opening is rapid. An important factor in controlling maximum assimilation rate is the nitrogen content of the leaf, as it can be considered as a proxy for Rubisco content (Wright et al., 2004). Estimating whole-plant traits, such as transpiration € and stomatal conductance (gs), via high-throughput phenotyping is an interesting way to distinguish the influence of environmental and physiological parameters (Charrier et al., 2018).
Figure 1.
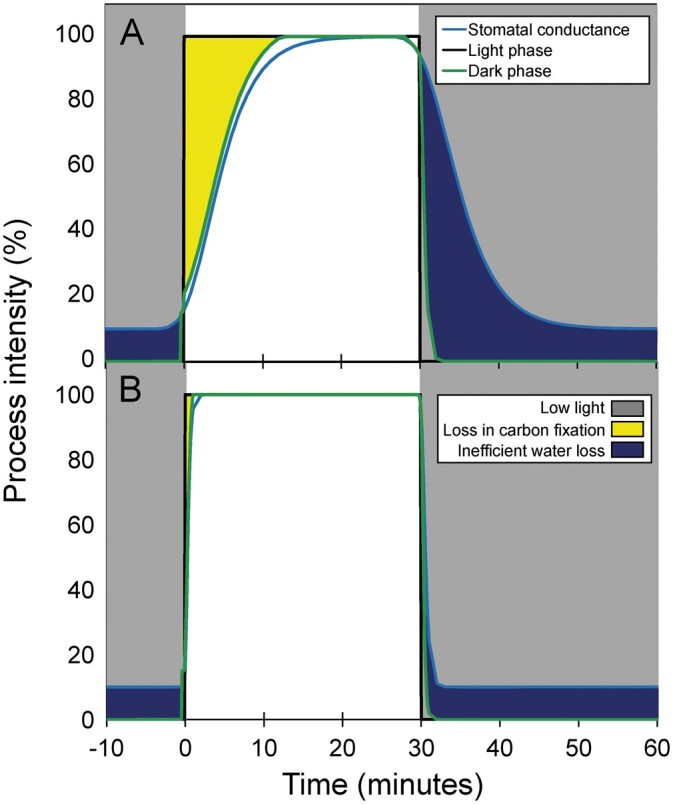
Stomatal kinetics during changes in light intensity. Stomatal conductance (blue line), light phase (black line) and dark phase of photosynthesis (green line) during changes in light regimes in C3 (A) and C4 species (B). The difference between light and dark phase corresponds to potential loss in carbon fixation when light turns on (yellow area). The difference between dark phase and stomatal conductance corresponds to inefficient water loss when light turns off (dark blue area). It should be noted that intercellular CO2 is limited by the diffusion through stomata although CO2 generated by maintenance respiration and photorespiration could modulate these patterns. Gray area corresponds to low light period.
Ozeki et al. (2022) investigated the dynamics of stomatal opening and closing under intermittent light in C3 (wheat [Triticum aestivum], oat [Avena sativa], barley [Hordeum vulgare], and Ryegrass [Lolium multiflorum]) and C4 crop species (finger millet [Eleusine coracana], sorghum [Sorghum bicolor], millet [Panicum miliaceum], and maize [Zea mays], and its wild relative Zea nicaraguensis) under high and low nitrogen conditions. Alternating between 100 and 1,000 µmol m−2 s−1 photosynthetic photon flux density, they estimated gs by a continuous measurement of weight change. Fitting a sigmoid function to the stomatal response to the change in light regime provided time constant parameters, kopen and kclose, for opening and closure, respectively, which allowed comparison of species and fertilization treatments.
Both C3 and C4 species showed rapid changes in net assimilation (Anet) when irradiance was varied. However, the change in gs was slower in C3 compared with C4 species, leading to a lower WUE during the transition phase. Overall, C4 crops exhibited faster responses (smaller kopen and kclose), which contributed to a higher WUE in these species. All species showed consistent responses to change in light levels with a significant correlation between kopen and kclose. Under high nitrogen treatment, stomatal response correlated with morphological traits (leaf area, stomatal density, and length of the guard cells).
To assess the contribution of stomatal kinetic on WUE, Ozeki et al. (2022) performed a sensitivity analysis on a dynamic photosynthesis model. They simulated the effect of independent and simultaneous changes in kopen and kclose on total assimilation (Atotal), total transpiration (Etotal), and WUE. Rapid stomatal opening increased Atotal, providing better coordination between the light and dark phases, at the cost of higher Etotal (Figure 1). The increase in Atotal compared with Etotal was lower in C3 than in C4, resulting in a lower WUE in C3, while it was similar in C4. Rapid stomatal closure also allowed for better coordination between the light and dark phases with similar Atotal, reducing inefficient water loss and therefore increasing WUE. Finally, the concomitant rapid opening and closing of stomata induced a higher Atotal and similar Etotal, therefore increasing WUE in both C3 and C4.
The results from Ozeki et al. (2022) showed that higher WUE in C4 species can be attributed not only to higher Anet and lower E but also to more rapid stomatal opening and closing. Rapid stomatal kinetics are linked to the shape of the guard cells, which are dumbbell-shaped in Poaceae crops (McAusland et al., 2016). However, why do C4 Poaceae species exhibit a more rapid stomatal response compared with C3 species? Ozeki et al. (2022) suggested that the smaller guard cells would be more flexible. Osmotic control of guard cell volume would therefore involve mobilizing smaller amounts of solutes and/or water (Drake et al., 2013). In addition, it should be noted that the relationship between morphological traits and stomatal response was only observed under high nitrogen treatment. As Anet positively correlated with leaf nitrogen content (Wright et al., 2004), nitrogen-depleted plants exhibited lower maximum Anet whereas maximum gs was not systematically affected by the nitrogen treatment.
To improve WUE in the field and therefore decrease water consumption by food production of Poaceae crops, rapid stomatal closure seems to be a relevant avenue. Natural light fluctuations can induce substantial difference in WUE at different scales: at shorter timescales with the dynamic movement of sun/shade flecks in understory plants and at longer timescales along latitudinal gradients (Lawson and Blatt, 2014). During the early evolution of Poaceae in understory environment, a rapid stomatal response would have increased WUE in the early morning and under intermittent light (Hetherington and Woodward, 2003). In tropical areas, marked daily changes in light regimes may have generated important selective pressure in C4 crops. In temperate zones, the daily light changes are milder, resulting in less selective pressure for C3 crops.
Other related questions remain unanswered, such as does the carbon memory mechanism, that is a saturation of stomatal opening after successive open/close cycles, exist in Poaceae and differ across C3 and C4 crop species (Jezek et al., 2021)? And do stomatal response kinetics differ under drought stress, when guard cells are dehydrated and more reactive to vapor pressure deficit (Charrier et al., 2018)? A better understanding of the mechanisms of stomatal kinetics would help to understand whether kclose could be a relevant trait for the selection of Poaceae crops.
Funding
This work has been carried out with financial support from the Pack Ambition Recherche program “Doux-Glace” from the Auvergne-Rhône Alpes region. This work was also supported by the program ANR Acoufollow (ANR-20-CE91-0008) funded by the French National Agency for Research and the Austrian Science Fund.
Conflict of interest statement. None declared.
References
- Charrier G, Delzon S, Domec J-C, Zhang L, Delmas CEL, Merlin I, Corso D, King A, Ojeda H, Ollat N, et al. (2018) Drought will not leave your glass empty: Low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Sci Adv 4: eaao6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ (2013) Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 64: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BJ, Harrison CJ, Hetherington AM, Williams TA (2020) Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Curr Biol 30: 2001–2012 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Jezek M, Silva-Alvim FA, Hills A, Donald N, Ishka MR, Shadbolt J, He B, Lawson T, Harper JF, Wang Y, et al. (2021) Guard cell endomembrane Ca2+-ATPases underpin a ‘carbon memory’ of photosynthetic assimilation that impacts on water-use efficiency. Nat Plants 7: 1301–1313 [DOI] [PubMed] [Google Scholar]
- Kirkham MB (2014) Chapter 24—stomatal anatomy and stomatal resistance. In MB Kirkham, ed, Principles of Soil and Plant Water Relations. Academic Press, Amsterdam, New York, Elsevier, pp 431–451 [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki K, Miyazawa Y, Sugiura D (2022) Rapid stomatal closure contributes to higher water use efficiency in C4 compared to C3 major Poaceae crops. Plant Physiol 189: 188–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet‐Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light‐induced stomatal responses on photosynthesis and water‐use efficiency. New Phytol 211: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW, Ehleringer J (1984) Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ 7: 1–13 [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, et al. (2004) The worldwide leaf economics spectrum. Nature 428: 821–827 [DOI] [PubMed] [Google Scholar]


