Abstract
Background
Standard testing fails to identify a pathogen in most patients with febrile neutropenia (FN). We evaluated the ability of the Karius microbial cell-free DNA sequencing test (KT) to identify infectious etiologies of FN and its impact on antimicrobial management.
Methods
This prospective study (ClinicalTrials.gov; NCT02912117) enrolled and analyzed 55 patients with FN. Up to 5 blood samples were collected per subject within 24 hours of fever onset (T1) and every 2 to 3 days. KT results were compared with blood culture (BC) and standard microbiological testing (SMT) results.
Results
Positive agreement was defined as KT identification of ≥1 isolate also detected by BC. At T1, positive and negative agreement were 90% (9/10) and 31% (14/45), respectively; 61% of KT detections were polymicrobial. Clinical adjudication by 3 independent infectious diseases specialists categorized Karius results as: unlikely to cause FN (N = 0); definite (N = 12): KT identified ≥1 organism also found by SMT within 7 days; probable (N = 19): KT result was compatible with a clinical diagnosis; possible (N = 10): KT result was consistent with infection but not considered a common cause of FN. Definite, probable, and possible cases were deemed true positives. Following adjudication, KT sensitivity and specificity were 85% (41/48) and 100% (14/14), respectively. Calculated time to diagnosis was generally shorter with KT (87%). Adjudicators determined real-time KT results could have allowed early optimization of antimicrobials in 47% of patients, by addition of antibacterials (20%) (mostly against anaerobes [12.7%]), antivirals (14.5%), and/or antifungals (3.6%); and antimicrobial narrowing in 27.3% of cases.
Clinical Trials Registration
Conclusion
KT shows promise in the diagnosis and treatment optimization of FN.
Keywords: Febrile neutropenia, infection, next-generation sequencing
Microbial cell-free DNA sequencing using the Karius test (KT) detects organisms not identified by conventional microbiological tests. KT can be useful in the etiologic diagnosis of infection of febrile neutropenia and may facilitate prompt and optimal antimicrobial therapy.
(See the Editorial Commentary by Vijayvargiya and Thoendel on pages 1669–70.)
Fever frequently complicates chemotherapy-induced neutropenia in patients with hematologic malignancies [1, 2]. Although its etiology is believed infectious in a majority of cases and potentially from a broad array of microorganisms, classical microbiology often fails to identify a pathogen. As a consequence, empiric antimicrobial therapy remains unfocused and may prove inappropriate [3]. Emerging nucleic acid amplification-based strategies can identify nonculturable organisms and are less affected by prior antimicrobial administration, but the use of selected primers limits the range of potential organisms detected [4, 5]. In contrast, next-generation sequencing (NGS) allows sensitive, broad, unbiased pathogen detection and has shown promise as a diagnostic tool for infections in immunocompetent [6] and immunocompromised hosts [7–10], including patients with febrile neutropenia (FN) in a pilot study [11]. The Karius plasma microbial cell-free DNA (mcfDNA) NGS test (KT) has proved useful in promptly diagnosing culturable and unculturable organisms when standard workup failed to identify an infectious culprit [12, 13], also enabling the noninvasive diagnosis of deep-seeded infections with atypical organisms that otherwise would require invasive diagnostic procedures [14–17].
We examined the performance of the KT in the etiologic diagnosis of FN in adults with acute leukemia and its clinical utility and potential impact on antimicrobial management.
METHODS
Study Design
We conducted a prospective, observational study at Stanford University Hospital comparing the KT with the final microbiologic diagnosis in patients with FN (ClinicalTrials.gov: NCT02912117). The study was approved by the Stanford institutional review board (#32817) and eligible patients enrolled after signing an informed consent.
Adults with acute leukemia and neutropenia (<500 neutrophils/mm3) enrolled at the time of their first FN episode. Samples were collected within 24 hours of fever onset (enrollment sample, T1) and every 2 to 3 days until resolution of neutropenia, for a maximum of 5 samples. mcfDNA was prepared and sequenced in a Clinical Laboratory Improvement Amendments/College of American Pathologists laboratory with human reads excluded; aligned to a curated pathogen database including bacteria, viruses, fungi, and parasites; and microbial concentrations compared with that of each respective taxon in negative control samples to determine whether the taxon was significantly above baseline, as previously described [18]. KT results were qualitative and not available to the care providers. All results were clinically adjudicated by an independent panel of 3 infectious disease (ID) specialists as described in the following section.
Primary Objective
Examine the diagnostic performance of KT at T1 compared with blood culture (BC) and with the final diagnosis determined by a composite reference standard of conventional microbiological diagnostics, radiological studies, and clinical adjudication.
Secondary Objectives
Examine the diagnostic performance of KT on subsequent testing.
Examine the time to diagnosis (TTD) for KT performed at T1 relative to standard microbiology testing (SMT).
Determine whether real-time availability of T1-KT results would have resulted in changes in antimicrobial management.
Definitions
Fever and neutropenia were defined according to guidelines [19] (Table 1).
Table 1.
Definitions, Case Classification in Clinical Adjudication
| Febrile neutropenia | Oral temperature >38.3°C or 2 consecutive readings of >38.0°C over 1 hour [6] and an absolute neutrophil count <500 neutrophils/mm3 or expected to fall within 48 hours below 500 neutrophils/mm3 for 7 days or longer. |
| BC and KT comparison | |
| True positive (positive agreement) | KT was concordant with initial BC for at least 1 pathogen. |
| False positive | KT was positive and BC was negative. |
| False negative | KT was negative and BC was positive (excluding contaminants), or both were positive for different microorganisms (discordant positives). |
| True negative (negative agreement) | Both KT and BC were negative. |
| Adjudication case classification | |
| Definite | Karius pathogen result is concordant with at least 1 pathogen identified on BC or other microbiologic tests performed within 7 days of Karius sample collection and is a likely cause of FN. |
| Probable | Karius test and BC results are discordant. Karius pathogen result is a likely cause of FN based on clinical, radiologic, or laboratory findings. |
| Possible | KT and BC results are discordant. Karius result is consistent with an infection but not a common cause, based on adjudicators clinical experience, and available literature, of FN. Diagnosis must be made using history, examination, and nonmicrobiologic testing and no other focal infection has been reported |
| Unlikely | KT is positive and discordant with MT and/or not a plausible cause of infection; or there is a more likely explanation for the febrile neutropenic event not meeting “possible” or “probable” classification criteria. |
| False negative | KT is negative but (1) microbiologic tests are positive and adjudicated as the cause of infection or (2) results from other microbiological tests are negative but there is a localized infection diagnosed using history, examination, and nonmicrobiologic data. |
| True negative | KT-negative result is concordant with other negative microbiologic tests and fever is attributed to a noninfectious etiology. |
| Indeterminate | The committee did not have enough information to adequately adjudicate and classify the case. |
Abbreviations: BC, blood culture; FN, febrile neutropenia; KT, Karius test; MT, microbiology testing.
SMT considered for comparison included cultures, single-analyte, and multiplex polymerase chain reaction (PCR) tests, fungal and bacterial ribosomal sequencing from body fluids and tissue specimens, as well as pathogen-specific antigens and serology assays. Results were blinded to the Karius analytic team.
Comparison of KT to BC
Definitions and result categorizations are summarized in Table 1.
Clinical Adjudication
Following a standardized algorithm (Supplement F1) involving review of laboratory data, radiology results, and discharge summaries, each of 3 board-certified ID specialists independently classified results according to the likelihood that a pathogen identified by NGS caused the FN episode. The committee chair had final discretion to adjudicate discrepancies. The definitions of definite, probable, possible, unlikely, indeterminate cases, and false and true negatives are summarized in Table 1. Definite, probable, and possible cases were considered true positives.
The panel also evaluated the antimicrobial management of these patients, categorizing the likelihood of its effectiveness against the organisms detected by KT based on clinical knowledge and published susceptibilities. Finally, it determined whether real-time availability of KT results could have changed management, specifying whether antibiotic, antiviral, antifungal, or antiparasitic agents would have been withdrawn or added.
Additional detail on the adjudication process is available in the Supplement materials.
Statistical Analysis
The full analysis data set included all subjects with data at any timepoint. The modified-intent-to-diagnose (mITD) data set included all subjects with the sample collected at enrollment passing quality control criteria, providing a valid KT result and a valid standard clinical diagnostic result for the same specimen collection time.
We calculated the positive percent agreement (PPA) and negative percent agreement (NPA) of the KT compared with BC results for samples collected at enrollment (T1) and later timepoints. Ninety percent confidence intervals were estimated by the exact binomial method. For the calculation of sensitivity and specificity, KT results were compared with final clinical diagnosis as determined by clinical adjudication and estimated along with the associated confidence intervals.
At the time of the study, turnaround time for KT was calculated to be 52 hours after phlebotomy for samples with a result provided with first sampling and 100 hours for those requiring a second aliquot; this includes time for shipment and analysis (28-76 hours) using metrics extrapolated from Clinical Laboratory Improvement Amendments laboratory performance. TTD was calculated for the KT performed at T1. For BC, TTD was defined as the time elapsed from the T1-BC draw to result reporting by the Stanford Microbiology Laboratory. For other SMT drawn between enrollment and throughout hospitalization, TTD was defined as the time from initial BC draw (surrogate of time of initial FN event) to the time of result. Because some subjects did not have a final etiological diagnosis using all SMT, we used Kaplan-Meier curves and log-rank tests to compare TTD for KT vs all SMT, including BC.
All statistical analyses were performed using SAS statistical software, version 9.3.
The KT
KT was performed following the methodology previously described in validation studies [18] and in the supplemental material.
RESULTS
Patient Characteristics
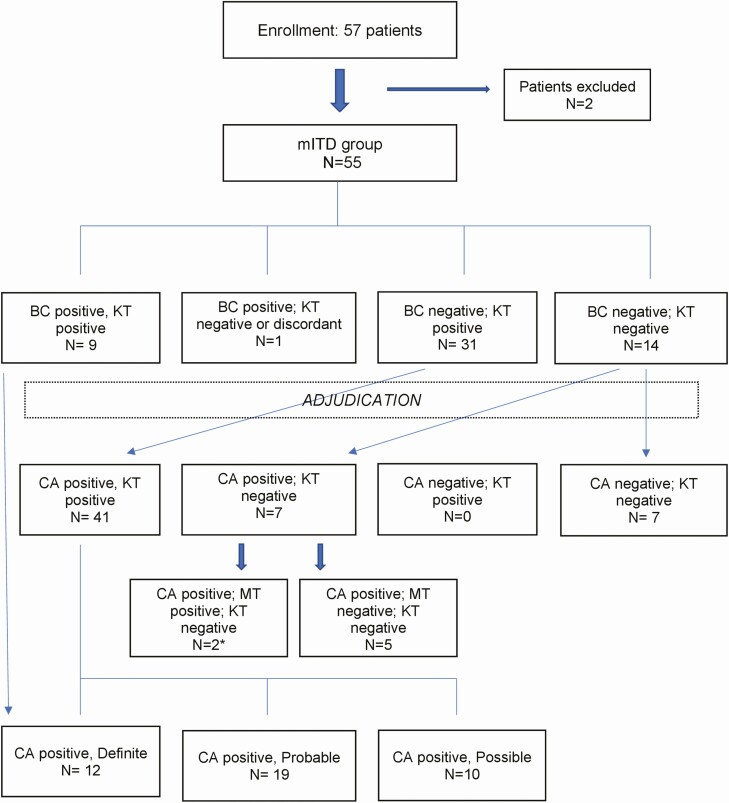
The mITD group included 55 of 57 enrolled subjects (Figure 1, Table 2). Table 3 summarizes patient characteristics. The median age was 60 years (range, 20-82), and 60% of patients were male. The most common underlying diagnosis was acute myeloid leukemia in 43 (78%) subjects. Three patients with acute myeloid leukemia had undergone stem-cell transplantation; 2 were excluded from the study because of poor sample quality. At enrollment, 71% of patients were receiving therapeutic or prophylactic antibiotics (35%), antivirals (20%), or antifungals (16%) (Supplement table ST1).
Figure 1.
Patient enrollment and overall results at T1. Exclusion reasons: enrollment blood sample for Karius test did not pass quality acceptance criteria (n = 1), blood culture at enrollment was contaminated (n = 1). *1 case with positive tooth abscess culture (Prevotella spp.); 1 case with positive bronchoalveolar lavage culture (A niger). Abbreviations: BC, blood culture; CA, clinical adjudication; KT, Karius test; mITD, modified intent to diagnose; MT, standard microbiological test.
Table 2.
Patient Enrollment and Disposition
| Total (N = 57) | |
| All subjects enrolled, n | 57 |
| All subjects who discontinued from study, n (%) | 8 (14) |
| Reason for discontinuation, n (%) | |
| Stem cell transplant recipients | 2 (25) |
| Karius test collected >24 hours following onset of fever | 1 (13) |
| Discharge before complete sample collection | 2 (25) |
| Neutropenia not chemotherapy-induced | 1 (12.5) |
| Subject withdrew voluntarily, n (%) | 2 (25) |
| All subjects included in full analysis data Set, n (%) | 57 (100) |
| All subjects included in modified intent-to-diagnose analysis data set, n (%)a | 55 (96.5) |
aExclusion reasons: enrollment blood sample for Karius test did not pass quality acceptance criteria (n = 1); blood culture at enrollment was contaminated (n = 1).
Table 3.
Patient Demographics and Characteristics in the Modified Intent-to-Diagnose Group
| Total (N = 55) | ||
| Gender, n (%) | Male | 31 (56.4) |
| Female | 24 (43.6) | |
| Age, y | N | 55 |
| Mean ± SD | 55.1 ± 16 | |
| Median | 60 | |
| Range | 20, 82 | |
| Race, n (%) | American Indian/Alaska Native | 0 (0) |
| Asian | 8 (14.5) | |
| Black or African American | 0 (0) | |
| White | 32 (58.2) | |
| More than 1 race | 0 (0) | |
| Native Hawaiian or Other Pacific Islands | 2 (3.6) | |
| Unknown/not reported | 1 (1.8) | |
| Hispanic/Latino | 12 (21.8) | |
| Weight, kg | N | 55 |
| Mean ± SD | 77.4 ± 24 | |
| Median | 69 | |
| Range | 47.3-170.7 | |
| Is patient HIV positive, n (%) | No | 53 (96.4) |
| Missing | 2 (3.6) | |
| AML, n (%) | Yes | 39 (70.9) |
| No | 16 (29.1) | |
| Secondary AML from MDS, n (%) | Yes | 4 (7.3) |
| No | 51 (92.7) | |
| ALL, n (%) | Yes | 9 (16.4) |
| No | 46 (83.6) | |
| AUL, n (%) | Yes | 2 (3.6) |
| No | 53 (96.4) | |
| MDS, n (%) | Yes | 1 (1.8) |
| No | 54 (98.2) | |
| Total (N = 55) | ||
| Transplant history, n (%) | Yes | 3 (5.5) |
| No | 52 (94.5) | |
| Transplant type, n (%) | Allogeneic stem-cell transplant | 3 (5.5) |
| Indication for transplant, n (%) | Acute myelogenous leukemia | 3 (5.5) |
| Allogeneic stem cell source, n (%) | Peripheral blood | 3 (5.5) |
| Total (N = 55) | ||
| Donor type, n (%) | HLA-matched related donor | 1 (1.8) |
| HLA-matched unrelated donor | 1 (1.8) | |
| Haploidentical | 1 (1.8) | |
| Number of stem cell transplants, n (%) | First HSCT | 3 (5.5) |
| Chronic medical conditions, n (%) | Yes | 33 (60) |
| No | 22 (40) | |
| Chronic condition type, n (%) | Asthma | 3 (5.5) |
| COPD | 1 (1.8) | |
| Congestive heart disease | 1 (1.8) | |
| Diabetes | 6 (10.9) | |
| Hypertension | 8 (14.5) | |
| Other | 14 (25.5) |
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia, AUL: acute undifferentiated leukemia; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplant; MDS, myelodysplastic syndrome; SD, standard deviation.
KT and Microbiology
At time of fever onset (T1), 10 patients (18%) had positive BC and 41 (75%) had positive KT; 25 of 41 (61%) being polymicrobial. The most frequent pathogens detected by KT at T1 were Escherichia coli (10.1%), Herpes simplex virus 1 (HSV-1) (5.9%), Streptococcus mitis (5%), and Enterococcus spp. (4.2%), followed by Pseudomonas aeruginosa, Bacteroides fragilis, Staphylococcus aureus, and Rothia mucilaginosa (3.4% each) (ST2).
Overall, 85% of all KT samples were positive. Enterococcus spp. (6.8%), streptococci (6.3%), staphylococci (6.3%), and E coli (6%) were the predominant bacteria. HSV-1 (7.4%) and Candida albicans (2%) were the most frequent nonbacterial organisms. For 1 patient, Pneumocystis jirovecii was identified at all timepoints. Invasive molds were detected in 3 subjects (5.5%): Aspergillus spp. in 2 subjects at T1 and Rhizopus spp. at 2 consecutive timepoints for a single patient.
Positive and Negative Agreement
Concordant KT and BC results were observed in 9 of 10 patients at T1 (PPA = 90%) (Table 4), involving mostly gram-negative rods (GNR): E coli, Klebsiella pneumoniae, and P aeruginosa (n = 2 each); Morganella morganii, Proteus mirabilis, and Serratia marcescens (n = 1, each). S mitis and Enterococcus faecium were codetected in 1 case (ST3). One patient had discrepant results: Streptococcus parasanguinis in BC but HSV-1 and Prevotella oralis by KT. The estimated TTD of KT was faster in 4 of 9 BC-positive cases. At T1, KT was negative in 14 of 45 BC-negative cases, resulting in an NPA of 31% (Table 5). In 31 patients, KT yielded at least 1 organism not detected by BC. The composite PPA and NPA with BC at all timepoints were 71% and 28%, respectively (Table 4).
Table 4.
Comparison of Karius Test and Blood Culture Results
| Timepoint (Days Since Fever Onset) | PPA % (Frequency) | NPA % (Frequency) | 90% Confidence Interval |
|---|---|---|---|
| 1 | 90 (9/10) | 60.6–99.5 | |
| 31.1 (14/45) | 19.9–44.3 | ||
| 2 (days 3–4) | 0 (0/2) | 0–77.6 | |
| 16.7 (4/24) | 5.9– 34.2 | ||
| 3 (days 5–7) | 0 (0/0) | … | |
| 7.7 (1/13) | 0.4–31.6 | ||
| 4 (days 7–10) | 100 (1/1) | … | |
| 30.8 (4/13) | 11.3–57.3 | ||
| 5 (days 9–13) | 0 (0/1) | … | |
| 75 (6/8) | 40–95.4 | ||
| Composite PPA, NPA | 71.4 (10/14) | 28.2 (29/103) |
Abbreviations: NPA, negative percent agreement; PPA, positive percent agreement.
Table 5.
Diagnosis of Infection With Karius Test, Blood Culture, and Clinical Diagnosis at T1
| Total (N = 55) | Blood Culture Positive | Blood Culture Negative | Clinical Diagnosis Positivea | Clinical Diagnosis Negativeb |
|---|---|---|---|---|
| Karius test positive | 9 | 31 | 41 | 0 |
| Karius test negative | 1c | 14 | 7 | 7 |
Categories at T1 where adjudicated with 100% agreement among all 3 adjudicators.
aDefinite (n = 12), probable (n = 19), possible (n = 10), and false negatives (n = 7) adjudicated cases. False negatives included: 2 cases of cellulitis, 2 tooth infections, 2 cases of pulmonary nodules, and 1 case of persistent neutropenic fever with intensive care unit transfer.
bUnlikely (n = 0) and true negative (n = 7) adjudicated cases.
cDiscordant positive. Blood culture detected Streptococcus parasanguinis. Karius test detected human herpesvirus 1 and Prevotella oralis.
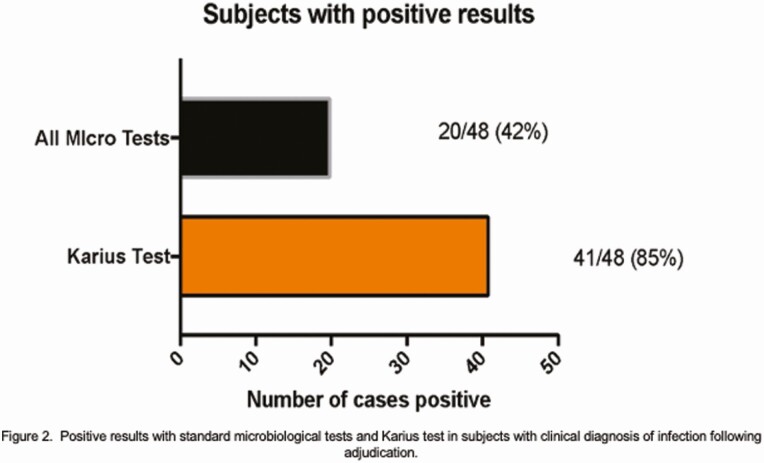
KT Performance and Clinical Adjudication
At T1, the adjudication panel agreed in all categorizations and concluded infection was the etiology of FN in 48 patients (87%). SMT was positive in 20 cases (42%) and KT in 41 (85%) (Figure 2). At T1, KT identified a plausible cause of FN in all 41 cases: 12 were definite, 19 probable, and 10 possible upon adjudication. In the probable and possible categories, the most common organisms by KT (37.8%) were common microbes of the oral and gastrointestinal (GI) flora (predominantly B fragilis, R mucilaginosa); followed by Enterobacteriaceae spp. (21.7%, predominantly E coli), gram-positive cocci (9.5%, predominantly S mitis), and herpesviruses (8%) (ST4).
Figure 2.
Positive results with standard microbiological tests and Karius test in subjects with clinical diagnosis of infection. Abbreviation: Micro tests, microbiological tests.
KT identified polymicrobial infections associated with intra-abdominal syndromes (eg, mucositis, typhlitis, enterocolitis, biliary infection) in 12 cases (5 definite, 7 probable) and detected HSV-1 or HSV-2 in 8 patients, 4 of which were confirmed by PCR from mucocutaneous lesions. A fumigatus was detected in 2 patients; 1 had imaging findings compatible with fungal pneumonia.
Seven of 14 negative KT at T1 were adjudicated as true negatives (Table 5) with fever attributed to relapsed or progressive disease (n = 3), unclear etiology (n = 2), or infection with an RNA virus (n = 2; Norovirus, Influenza B).
KT sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at T1 were 85%, 100%, 100%, and 50%, respectively (Table 6). PPV and NPV reached 92% and 100%, respectively, at timepoint 3. With clinical adjudication, the cumulative sensitivity, specificity, PPV and NPV were of 92%, 70%, 93%, and 63%, respectively.
Table 6.
Karius Test Performance Following Clinical Adjudication at All Timepoints
| Timepoint (Days Since Fever Onset) | Sensitivity % (Frequency) | Specificity % (Frequency) | 90% Confidence Interval | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|
| 1 | 85.4 (41/48) | 74.4–93 | 100 | ||
| 100 (7/7) | 65.2–100 | 50 | |||
| 2 (days 3–4) | 94.1 (32/34) | 82.6–98.9 | 97 | ||
| 80 (4/5) | 34.3–99 | 66.7 | |||
| 3 (days 5–7) | 100 (22/22) | 87.3–100 | 91.7 | ||
| 33.3 (1/3) | 1.7–86.5 | 100 | |||
| 4 (days 7–10) | 94.4 (17/18) | 76.2–99.7 | 89.5 | ||
| 33. (1/3) | 1.7–86.5 | 50 | |||
| 5 (days 9–13) | 88.9 (8/9) | 57.1–99.4 | 88.9 | ||
| 50 (1/2) | 2.5–97.5 | 50 | |||
| Cumulative | 91.6 (120/131) | 70 (14/20) | 93.4 | 63.3 |
KT False Negatives
At T1, infection was clinically suspected in 7 KT-negative cases (14.5%) summarized in Table 7, 5 of which also had negative SMT. Two patients diagnosed with odontogenic infections underwent tooth extraction; 1 grew Prevotella spp. from a tooth abscess. Two subjects were clinically diagnosed with cellulitis improved with antibiotics. Another patient developed shock, thought to be septic but had a negative microbiological workup. Two other patients were diagnosed with fungal pneumonia. The first was treated empirically with voriconazole based on computed tomography (CT) findings. The second had a consolidation with surrounding ground-glass opacities (GGO) on initial chest CT, and voriconazole was started. On day 10, 6 colonies of A niger grew from bronchoalveolar lavage (BAL). Repeat CT showed progressive cavitating pneumonia and antifungals were changed to amphotericin-B on day 12. Imaging showed a brain abscess on day 28. Fungal hyphae were identified on tissue from a brain needle biopsy, morphologically compatible with Mucorales, but fungal cultures and sequencing were negative. In this case, despite a negative result at T1, KT yielded Rhizomucor miehei at timepoints 2 and 3 (10 days before amphotericin initiation and 34 before the brain biopsy) and detected low levels of the organism at T1.
Table 7.
Karius Test False Negatives at T1
| Patient | KT Result | Other SMT Result | Clinical Diagnosis | Antibiotics Received (all) | Case Details |
|---|---|---|---|---|---|
| 1 | Negative | Negative, no tooth cultures | Dental infection | PIP-TAZ; AMOX-CLAV; ERT | Underwent tooth extraction; periodontal disease and “lucencies” on imaging |
| 2 | Negative | Tooth abscess culture: Prevotella spp | Dental abscess | PIP-TAZ; VORI | Underwent tooth extraction |
| 3 | Negative | Negative | Facial cellulitis | PIP-TAZ; VANC | Jaw pain, swelling, tenderness of left mandibular region—swelling on CT, improved with antimicrobials |
| 4 | Negative | Negative | RLE cellulitis | CEF; VANC | Purpura and DVT on the same leg |
| 5 | Negative | Negative | Sepsis of unclear etiology | CEF; VANC; VORI; PIP-TAZ; ACV | Persistent fever on day 3 developed shock. Noted hemoglobin drop (7 to 4.8 g/dL); no identified source of bleeding; improvement within 24 hours following steroids, PRBC, and broad antimicrobials |
| 6 | Negative | Negative, no fungal cultures, Asp GM and 1,3 BDG negative |
Fungal pneumonia | PIP-TAZ; MERO; VORI | CT chest with 7-mm nodule and small areas of GGO |
| 7 | Rhizopus spp at T2, T3 | BAL: 6 colonies of Aspergillus niger Brain abscess: fungal cultures, fungal sequencing negative Brain histopathology: fungal forms consistent with Rhizopus spp. |
Necrotizing fungal pneumonia and brain abscess | CEF; PIP-TAZ; VORI; L-AMB | CT chest: right lower lobe consolidation with surrounding GGO, VORI initiated On day 10, growth of A niger CT chest day 12: progressive cavitating pneumonia, changed to L-AMB. - Day 28; MRI brain: abscess - Day 30: Fungal hyphae identified on tissue |
Abbreviations: 1,3 BDG, 1,3 beta-D-glucan; ACV, acyclovir; AMOX-CLAV, amoxicillin-clavulanic; Asp GM, aspergillus galactomannan; CEF, cefepime; CT, computed tomography; ERT, ertapenem; GGO, ground-glass opacities; KT, Karius test; L-AMB, liposomal amphotericin; MERO, meropenem; MRI, magnetic resonance imaging; PIP-TAZ, piperacillin-tazobactam; PRBC, packed red blood cell; RLE, right lower extremity; SMT, standard microbiological testing; VANC, vancomycin; Vori, voriconazole.
Time to Diagnosis
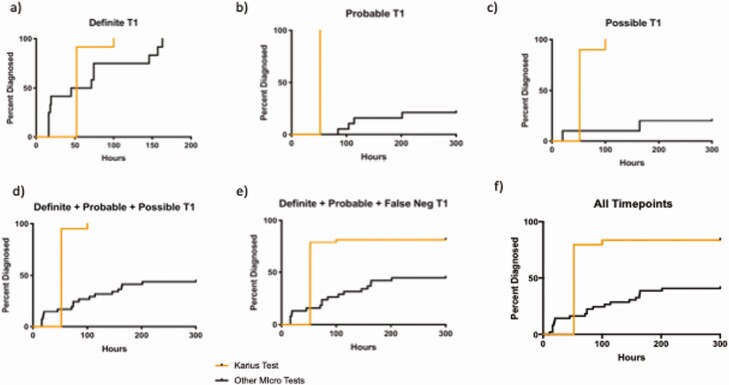
As described, KT TTD was 52 hours [18] for most samples, and 100 hours for those requiring repeat testing (<5%).
A comparison of KT TTD to that of all SMT is shown in Figure 3. Performed in real time, KT would have provided an earlier result in 87.3% overall and would have facilitated an earlier diagnosis in 50% of the definite cases with positive BC (n = 9) or SMT (n = 3).
Figure 3.
Time to diagnosis.
In 1 particular case, KT detected P jirovecii at all timepoints and 5 days before SMT. CT of the chest on day 3 showed bilateral GGO (Supplement F2). On day 5, the patient was transferred to the intensive care unit for worsening hypoxia, diagnosed with P jirovecii pneumonia on BAL, and started on appropriate treatment.
Antimicrobial Adequacy and Management
The committee evaluated the efficacy of the empirical antimicrobial regimens against the organisms KT detected (Table 8, Supplemental Table ST5a-b). At T1, empirical antimicrobials were at least likely effective in 38% of cases, and likely ineffective or not effective in 36.3%. At the time that KT would have been available (52-100 hours), the antimicrobials were at least likely to be effective in 42% of cases and likely ineffective or not effective in 32.7%. Real-time availability of KT results could have resulted in antimicrobial changes in 26 patients (47.3%). Addition of antibacterials—mainly of anaerobic coverage (12.7%, n = 7)—was indicated in 20%. Addition of antivirals (mostly anti-HSV-1 coverage) and antifungals followed in 14.5% (n = 8) and 3.6 % (n = 2), respectively. De-escalation or withdrawal of antibacterials would have been indicated in 25.5% (n = 14) of cases, including discontinuation of methicillin-resistant S aureus coverage in 9% (n = 5).
Table 8.
Antimicrobial Management Based on Clinical Adjudication, According to KT Results
| Characteristic | Effectivenessa | Frequency (%) |
|---|---|---|
| Was the initial antimicrobial treatment efficacious against the microorganisms detected by KT? | Very likely effective | 2/55 (3.6) |
| Definitely effective | 9/55 (16.4) | |
| Likely effective | 10/55 (18.2) | |
| Likely not effective | 8/55 (14.5) | |
| Not applicable | 14/55 (25.5) | |
| Not effective | 12/55 (21.8) | |
| Was antimicrobial treatment at 52 to 100 hours efficacious against the microorganisms detected by KT? | Very likely effective | 3/55 (5.5) |
| Definitely effective | 8/55 (14.5) | |
| Likely effective | 12/55 (21.8) | |
| Likely not effective | 7/55 (12.7) | |
| Not applicable | 14/55 (25.5) | |
| Not effective | 11/55 (20) | |
| Different antimicrobial treatment proposed had KT result been available in real time | No | 29/55 (52.7) |
| Yes | 26/55 (47.3) | |
| Antibiotic | Additionb | 11/55 (20) |
| No change | 33/55 (60) | |
| Withdrawalc | 14/55 (25.5) | |
| Antiviral | Addition | 8/55 (14.5) |
| No change | 47/55 (85.5) | |
| Antifungal | Addition | 2/55 (3.6) |
| No change | 51/55 (92.7) | |
| Withdrawal | 1/55 (1.8) | |
| Not applicable | 1/55 (1.8) | |
| MRSA coverage | Addition | 1/55 (1.8) |
| Withdrawal | 5/55 (9.1) | |
| Not applicable | 49/55 (89.1) | |
| Anaerobe coverage | Addition | 7/55 (12.7) |
| Not applicable | 48/55 (87.3) | |
| Narrowing of antibiotic spectrum | Yes | 8/55 (14.5) |
| Not applicable | 47/55 (85.5) | |
| Broadening of antibiotic spectrum | Yes | 1/55 (1.8) |
| Not applicable | 54/55 (98.2) | |
| Antiviral coverage CMV | Addition | 1/55 (1.8) |
| Not applicable | 54/55 (98.2) | |
| Antiviral coverage HSV | Addition | 7/55 (12.7) |
| Not applicable | 48/55 (87.3) | |
| Antiparasitic coverage | Addition | 1/55 (1.8) |
| Not applicable | 54/55 (98.2) | |
| Antimycobacterial coverage | Addition | 1/55 (1.8) |
| Not applicable | 54/55 (98.2) |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; KT, Karius test; MRSA, methicillin-resistant Staphylococcus aureus.
aEffectiveness was adjudicated based on available literature on antimicrobial susceptibility of the microorganism considered.
bIncludes 1 addition of trimethoprim-sulfamethoxazole for coverage of Pneumocystis jirovecii.
cWithdrawal of antibacterials including withdrawal of MRSA coverage (n = 5), narrowing of gram-negative rod coverage (n = 8), and 2 nonspecified cases. In 2 cases, addition and withdrawal of different antibacterials was recommended.
DISCUSSION
Compared with SMT, KT was twice as likely to provide a microbiological diagnosis, often without resorting to invasive diagnostic procedures.
BC was positive in 18% of patients, a rate concordant with the 10% to 25% previously reported in FN [20]. GNRs were the most frequent pathogens detected by BC and KT at FN onset. Worth noting, most patients were enrolled during their first FN episode; 34.5% (n = 19) were receiving antibiotics (4 on fluoroquinolone prophylaxis) at enrollment.
At T1, KT detected nearly all organisms isolated by BC (PPA 90%), failing to identify S parasanguinis yielded in 1 BC drawn before the patient became febrile (as defined by study criteria), >48 hours before KT draw. Possible explanations for this “miss” include BC contamination or bloodstream organism clearance from antibiotics received during this interval. KT NPA was only 31%, a predictably low proportion because of the relative lack of sensitivity of BC. The performance of KT in this regard is better apprehended when compared with a composite of clinical, radiological, and laboratory data, including all available microbiology tests. At T1, KT yielded a positive result twice as frequently as all other SMT (85% vs 42%, respectively), detecting a pathogen adjudicated as a plausible cause of FN in every case.
In addition, KT identified viral and fungal infections as well as clinically significant polymicrobial infections not detected by SMT, the latter mostly associated with GI syndromes. Previous evidence suggests GI microbiota in neutropenic patients can help predict bacterial translocation [21, 22]. In a recent study, KT performed 2 days before fever onset was able to predict and diagnose bacteremia in 7 of 9 patients with malignancy [23]. However, control KT samples preceding the onset of FN were not obtained in our study. This, with quantitative measurements of organism predominance and their correlation with the presence and severity of mucositis, could have provided a better understanding of the significance of polymicrobial detections and/or the degree of sterility of blood samples [24, 25]. In a validation study, KT detected mcfDNA in 22.8% of 167 asymptomatic patients, mostly low levels of human commensals [18], whereas high mcfDNA levels were associated with true infection.
The sensitivity of KT relative to adjudication was 85% (41 of 48) at T1, similar to results reported in adults with sepsis [6, 18] and immunocompromised children [23]. Among the 7 “misses” (15%), the likelihood of infection based on clinical findings alone is worth questioning in some. Of the 2 patients diagnosed with odontogenic infections, only 1 had evident abscess and positive tooth cultures. One of the 2 patients clinically diagnosed with leg cellulitis had ipsilateral deep vein thrombosis. One subject had possible fungal pneumonia per European Organization for Research and Treatment of Cancer/National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria [26], although there were minimal findings on chest CT. Finally, the patient with fungal pneumonia and brain abscess in whom the KT identified Rhizomucor miehei deserves special attention. A niger grew in small quantities on BAL. Cultures and fungal ribosomal sequencing of the brain abscess were negative but fungal organisms on histopathology were suspicious for Rhizopus spp. Although A niger may have caused necrotizing pneumonia, this organism is known to have low pathogenicity [27], and progression of the lung and brain lesions through voriconazole would be more consistent with a Rhizopus spp. infection. This case illustrates the complexity of clinical presentations in immunocompromised hosts, often co-infected with multiple organisms, and the challenges in obtaining an accurate diagnosis [4]. It also underscores the value of KT in detecting unusual, unexpected organisms when both conventional and advanced SMT have failed, and without resorting to invasive procedures. In a recent study, KT had a higher diagnostic yield compared with invasively obtained specimens (n = 39) (87% vs 67%, respectively); potentially avoiding 34 invasive procedures and noninvasively enabling the diagnosis of 17 fungal, 10 bacterial, and 7 viral infections [28]. Finally, KT detected R miehei at TP2, suggesting potential diagnostic benefit in serial KT in difficult cases.
To further assess the potential clinical utility of KT, we compared the TTD of KT with that of SMT and evaluated the impact KT results would have had in antimicrobial management. Performed in real time, KT could have provided earlier results in 87.3% of all cases, and would have allowed earlier targeted treatment in 1 patient with P jirovecii pneumonia. However, this was true only in one-half of the patients who had positive BC (3 identified by matrix-assisted laser desorption/ionization) or SMT.
Adjudicators would have changed antimicrobial management in nearly one-half of the patients on the basis of KT results, de-escalating or discontinuing antimicrobials in 27.3%, mostly narrowing GNR coverage and ceasing methicillin-resistant S aureus-targeted therapy. This suggests that KT could have an impact on antibiotic stewardship and the minimization of unnecessary antimicrobial exposure in these patients.
Our study had modest sample size and was performed at a single center. Additional limitations include the absence of control samples, organism quantification, or assessment of mucositis in patients with FN. Another caveat was the lack of adjudication of each organism identified in polymicrobial detections. For instance, if KT detected S mitis, HSV-1, and A fumigatus and BC isolated S mitis, the case was classified as definite. As a result, the likelihood of the other organisms of causing infection would not be strictly categorized. Finally, although a comprehensive patient chart review was used to determine whether infection was the etiology of FN, the results of KT were not blinded to the adjudicators, adding a potential for bias.
Other limitations are intrinsic to KT performance and NGS, like the potential for sample contamination or detection of background noise [29, 30]. KT detects >1000 DNA-based pathogens including bacteria, viruses, fungi, and parasites. During validation, its analytical specificity was of 99.99% after assessment of environmental DNA contamination, quantitative interference in coinfection, pathogen cross-reactivity, and diversity robustness [18]. Clinically, KT performance was assessed in 348 adults with suspected sepsis. Compared with BC, the PPA and NPA were 84.8% and 40%, respectively. After clinical adjudication, KT sensitivity was 92.9% with a specificity of 62.7%. Indeed, the enhanced sensitivity of KT and breadth of microorganisms detected compromises the ability to achieve high diagnostic specificity. In our study, the classification of the adjudicated possible cases as true positives could have overestimated both the sensitivity and specificity of KT, an inherent problem of analyzing tests with potentially greater sensitivity than the “gold standard.” And, although KT could diagnose opportunistic pathogens otherwise missed by SMT, it also yielded polymicrobial detections and organisms of uncertain clinical significance and actionability (eg, Mycobacterium abscessus, Enterocytozoon bieneusi, HHV7) [30]. In these instances, interpreting mcfDNA results can be particularly challenging. In a retrospective evaluation of the clinical impact of KT results, Hogan et al found 86.6% of 82 detections, half polymicrobial, had no clinical relevance [31]. Therefore, KT results should be examined with caution, by physicians with ID expertise and familiarity with the technology and result interpretation. On the other hand, the false-negative rate of KT was still 14.5% despite clinical adjudication, granted that 5 of 7 false-negative cases did not have an etiology determined by SMT and the adjudication results could be incorrect. Finally, KT current inability to provide susceptibility/resistance profiling or detect RNA virus, together with its cost, limit its routine use in clinical practice as a substitute of standard testing or a tool to target antimicrobial therapy.
In conclusion, KT can enhance the ability to diagnose infections in febrile neutropenic patients, especially when standard testing fails to detect an infectious culprit; it may also enable early optimization of antimicrobial therapy. Nevertheless, complete understanding of its utility in clinical practice and integration in diagnostic algorithms requires further investigation.
Supplementary Material
Acknowledgments
Author contributions. E. B.: study design, data collection, data analysis, manuscript writing and revision. K. G.: Study design, data collection, data analysis, manuscript writing and revision. J. N. A.: Data collection, data analysis, manuscript writing and revision. T. L.: Data collection, data analysis, manuscript writing and revision. C. A. G.: Data collection, data analysis, manuscript writing and revision. H. S.: Data collection, data analysis, manuscript writing and revision. R. A.: Data collection, data analysis, manuscript writing and revision. D. H.: Data collection, data analysis, manuscript writing and revision. D. K. H.: Study design, data collection, data analysis, manuscript writing and revision. T. B.: Data collection, data analysis, manuscript writing and revision. M. K.: Data collection, data analysis, manuscript writing and revision. L. B.: Data collection, data analysis, manuscript writing and revision. P. L. B.: Data collection, data analysis, manuscript writing and revision. B. C. M.: Data collection, data analysis, manuscript writing and revision. S. C.: Data collection, data analysis, manuscript writing and revision. S. Z.: Study design, data collection, data analysis, manuscript writing and revision. J. G. M.: Study design, data collection, data analysis, manuscript writing and revision. S. D.: Study design, data collection, data analysis, manuscript writing and revision.
The authors thank Joanna Schaenman, MD; Marisa Holubar, MD; and LauraLe Dyner, MD; for their contribution to the study and Sumedha Sinha for her contribution to the preparation of this manuscript.
Financial support. The study was funded by Karius Inc.
Potential conflicts of interest. E. B.: The work associated with this study was funded by Karius Inc. J. N. A.: The work associated with this study was funded by Karius Inc (grants and nonfinancial support). T. L.: The work associated with this study was funded by Karius Inc (grants and nonfinancial support). H. S. and R. A.: Employed at Karius Inc. D. H.: Employed at Karius Inc during the conduct of the study. D. H.: Employed at Karius Inc, during the conduct of the study (former employee); has a patent (Detection and Prediction of Infectious Disease) pending. T. B.: Employed at Karius Inc; has patent US20150133391A1 licensed to Karius, patent US9976181B2 issued, patent US20170016048A1 pending, and patent EP3497218A4 pending. M. K.: Employed at Karius Inc; has patent US20150133391A1 licensed to Karius, patent US9976181B2 issued, and patent US20170016048A1 pending. L. B.: Received personal fees while employed at Karius Inc. during the conduct of the study and outside the submitted work. S. Z.: Employed at Karius, Inc. until December 2018 (employed by Karius at the time of the manuscript writing) and consulting fees (February-August 2019), during the conduct of the study; has patent Pub. No. WO 2018/045359 (Detection and Treatment of Infection during Pregnancy) pending. J. G. M.: The work associated with this study was funded by Karius Inc (grants and nonfinancial support). S. D.: The work associated with this study was funded by Karius Inc.
References
- 1. Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis 2004; 39(Suppl 1):S32–7. [DOI] [PubMed] [Google Scholar]
- 2. Alp S, Akova M. Management of febrile neutropenia in the era of bacterial resistance. Ther Adv Infect Dis 2013; 1:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F; ESMO Guidelines Working Group . Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol 2010; 21(Suppl 5):v252–6. [DOI] [PubMed] [Google Scholar]
- 4. Montoya JG, Multani A. Diagnosis of infection in immunocompromised patients: from microscopy to next generation sequencing and host gene signatures. Curr. Opin. Infect. Dis. 2019; 32:295–9. [DOI] [PubMed] [Google Scholar]
- 5. Lamoth F, Jaton K, Prod’hom G, et al. . Multiplex blood PCR in combination with blood cultures for improvement of microbiological documentation of infection in febrile neutropenia. J Clin Microbiol 2010; 48:3510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thair S, Seng H, Hollemon D, et al. . The SEP-SEQ Trial: Clinical Validation of the Karius Plasma Next-Generation Sequencing Test for Pathogen Detection in Sepsis. Open Forum Infect Dis. 2017; 4(suppl_1):S735–S735. [Google Scholar]
- 7. Gyarmati P, Kjellander C, Aust C, Kalin M, Öhrmalm L, Giske CG. Bacterial landscape of bloodstream infections in neutropenic patients via high throughput sequencing. PLoS One 2015; 10:e0135756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Vlaminck I, Martin L, Kertesz M, et al. . Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci U S A 2015; 112:13336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gyarmati P, Kjellander C, Aust C, Song Y, Öhrmalm L, Giske CG. Metagenomic analysis of bloodstream infections in patients with acute leukemia and therapy-induced neutropenia. Sci Rep 2016; 6:23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fung M, Seng H, Hollemon D, et al. . The DISCOVER Trial: application of the Karius Plasma Next-Generation Sequencing Test for Pathogen Detection in Stem-Cell Transplant Patients. Open Forum Infect Dis. 2017; 4(suppl_1):S616–S616. [Google Scholar]
- 11. Benamu E, Gajurel K, Anderson JN, et al. . Performance of the Karius Plasma Next Generation Sequencing Test in Determining the Etiologic Diagnosis of Febrile Neutropenia: results from a pilot study. Open Forum InfectiDiseases. 2017; 4:S613 [Google Scholar]
- 12. Abril MK, Barnett AS, Wegermann K, et al. . Diagnosis of Capnocytophaga canimorsus sepsis by whole-genome next-generation sequencing. Open Forum Infect Dis 2016; 3:ofw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kondo M, Dalai SC, Venkatasubrahmanyam S, et al. . Diagnosis and genotyping of Coxiella burnetii endocarditis in a patient with prosthetic pulmonary valve replacement using next-generation sequencing of plasma microbial cell-free DNA. Open Forum Infect Dis 2019; 6:ofz242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong DK, Blauwkamp TA, Kertesz M, Bercovici S, Truong C, Banaei N. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis 2018; 92:210–3. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong AE, Rossoff J, Hollemon D, Hong DK, Muller WJ, Chaudhury S. Cell-free DNA next-generation sequencing successfully detects infectious pathogens in pediatric oncology and hematopoietic stem cell transplant patients at risk for invasive fungal disease. Pediatr Blood Cancer 2019; 66:e27734. [DOI] [PubMed] [Google Scholar]
- 16. Nomura J, Rieg G, Bluestone G, et al. . Rapid detection of invasive Mycobacterium chimaera disease via a novel plasma-based next-generation sequencing test. BMC Infect Dis 2019; 19:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nayakwadi-Singer M, Johnston S, Kulhanjian J, et al. . Detection of Nocardia cyriacigeorgica from a deep pulmonary infection using a novel plasma-based next generation sequencing assay. ASM Microbe 2017.
- 18. Blauwkamp TA, Thair S, Rosen MJ, et al. . Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019; 4:663–74. [DOI] [PubMed] [Google Scholar]
- 19. Baden LR, Swaminathan S, Angarone M, et al. . Prevention and treatment of cancer-related infections, version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016; 14:882–913. [DOI] [PubMed] [Google Scholar]
- 20. Gustinetti G, Mikulska M. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence 2016; 7:280–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taur Y, Pamer EG. The intestinal microbiota and susceptibility to infection in immunocompromised patients. Curr Opin Infect Dis 2013; 26:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taur Y, Xavier JB, Lipuma L, et al. . Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goggin K, Inaba Y, Gonzalez-Pena V, et al. . 1756. Prediction of Bloodstream Infection Prior to Onset of Symptoms by Plasma Metagenomic Sequencing in Pediatric Patients With Relapsed or Refractory Malignancy (PREDSEQ). Open Forum Infect Dis 2018; 5:S60. [Google Scholar]
- 24. Païssé S, Valle C, Servant F, et al. . Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016; 56:1138–47. [DOI] [PubMed] [Google Scholar]
- 25. Gosiewski T, Flis A, Sroka A, et al. . Comparison of nested, multiplex, qPCR; FISH; SeptiFast and blood culture methods in detection and identification of bacteria and fungi in blood of patients with sepsis. BMC Microbiol 2014; 14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gautam AK, Sharma S, Avasthi S, Bhadauria R. Diversity, pathogenicity and toxicology of A. niger: an important spoilage fungi. Res. J. Microbiol. 2011; 6:270–280. [Google Scholar]
- 28. Rossoff J, Chaudhury S, Soneji M, et al. . Noninvasive diagnosis of infection using plasma next-generation sequencing: a single-center experience. Open Forum Infect Dis. 2019; 6: ofz327. doi:10.1093/ofid/ofz327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol 2019; 14:319–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greninger AL. The challenge of diagnostic metagenomics. Expert Rev Mol Diagn 2018; 18:605–15. [DOI] [PubMed] [Google Scholar]
- 31. Hogan CA, Yang S, Garner OB, et al. . C linical Impact of Metagenomic Next-Generation Sequencing of Plasma Cell-Free DNA for the Diagnosis of Infectious Diseases: A Multicenter Retrospective Cohort Study. Clin Infect Dis 2021; 72:239–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.