Abstract
Objectives
This study evaluated the sustained kill and potential for resistance development of Acinetobacter baumannii exposed to human-simulated exposure of cefiderocol over 72 h in in vitro and in vivo infection models.
Methods
Seven A. baumannii isolates with cefiderocol MICs of 0.12–2 mg/L were tested. The sustained bactericidal activity compared with the initial inoculum and the resistance appearance over 72 h treatment were evaluated in both an in vitro chemostat and an in vivo murine thigh infection model under the human-simulated exposure of cefiderocol (2 g every 8 h as 3 h infusion).
Results
In the in vitro model, regrowth was observed against all seven tested isolates and resistance emergence (>2 dilution MIC increase) was observed in five test isolates. Conversely, sustained killing over 72 h and no resistance emergence were observed in six of seven tested isolates in vivo. The mechanism of one resistant isolate that appeared only in the in vitro chemostat studies was a mutation in the tonB-exbB-exbD region, which contributes to the energy transduction on the iron transporters. The resistance acquisition mechanisms of other isolates have not been identified.
Conclusions
The discrepancy in the sustained efficacy and resistance emergence between in vitro and in vivo models was observed for A. baumannii. Although the resistance mechanisms in vitro have not been fully identified, sustained efficacy without resistance emergence was observed in vivo for six of seven isolates. These studies reveal the in vivo bactericidal activity and the low potential for development of resistance among A. baumannii evaluated under human-simulated exposures.
Introduction
Carbapenem-resistant Acinetobacter baumannii is considered a critical priority for antibiotic research and development by WHO due to high mortality, limited treatments and healthcare burden, among others.1 Bacteraemia and hospital/ventilator-associated pneumonia are two of the most common infections associated with A. baumannii and have been associated with mortality ranging from 37%–55%.2–4 Prior antibiotic use and hospitalizations are associated with A. baumannii infections and ineffective antimicrobial therapy has been associated with mortality in A. baumannii bacteremia.3,5
Indeed, carbapenem-resistant A. baumannii leaves clinicians with limited treatments due to high-level resistance to numerous antibacterials (i.e. β-lactams, aminoglycosides, and fluoroquinolones, among others).6,7 Cefiderocol remains active against carbapenem-resistant A. baumannii due to the utilization of active iron transporters to penetrate through the outer membrane and high stability to both serine- and metallo-type β-lactamases.8 Cefiderocol has shown potent antibacterial activity in consecutive multinational surveillance studies (SIDERO-WT studies) against clinical isolates of A. baumannii in North America and Europe with MIC90 of 1 to 4 mg/L, and inhibited the growth of 91.0% to 96.9% of the isolates at ≤4 mg/L.9 The acquisition of β-lactamases such as blaPER has been reported to contribute to elevated cefiderocol MICs in clinical isolates of A. baumannii, although β-lactamase inhibitors such as avibactam have been shown to reduce cefiderocol MICs in such isolates.10
Cefiderocol exhibited efficacy against Gram-negative bacteria including A. baumannii in multiple clinical studies including APEKS-NP and CREDIBLE-CR.11,12 In the APEKS-NP study, cefiderocol was non-inferior to high-dose, extended-infusion meropenem in terms of all-cause mortality on Day 14 in patients with Gram-negative nosocomial pneumonia, with similar tolerability. In the CREDIBLE-CR study, cefiderocol had similar clinical and microbiological efficacy to best available therapy (BAT) in the heterogeneous patient population with infections caused by carbapenem-resistant Gram-negative bacteria, although there was numerically higher all-cause mortality among cefiderocol treated patients with infections caused by Acinetobacter sp. In these studies, the frequency of ≥4-fold MIC increase during the treatment in cefiderocol arm was similar to those of BAT and meropenem arms although the magnitude of increase to cefiderocol was smaller. Although actual cefiderocol MIC values remained ≤4 mg/L for many of the isolates which showed ≥4-fold MIC increase, careful monitoring for the appearance of cefiderocol resistance will be important.
We previously reported the discrepancy of resistance emergence of Stenotrophomonas maltophilia in in vitro chemostat models and in vivo murine thigh infection models where resistant mutants from the chemostat model were likely non-viable in the in vivo environment.13 Thus, the present study sought to compare the potential of cefiderocol resistance development of A. baumannii in an in vitro chemostat model and an in vivo murine thigh infection model, both utilizing human-simulated regimen (HSR) exposures over an extended treatment period of 72 h.
Materials and methods
Ethics
The present study was approved by the Institutional Animal Care and Use Committee of Hartford Hospital (Assurance #A3185-01). All animal experiments were conducted in concordance with the standards set by the National Research Council of the National Academy of Sciences.
Antimicrobial test agents
Cefiderocol 500 mg vials (Shionogi & Co. Ltd, Osaka, Japan, lot: 12M01) were used for all in vitro and in vivo experiments. Cefiderocol was reconstituted with normal saline (NS) prior to being further diluted to the desired final dosing concentration in NS to use in in vivo studies. All doses were delivered as 0.2 mL injections subcutaneously.
Isolates
Nine clinical A. baumannii isolates were used. Seven of nine clinical isolates (AB230, AB231, AB232, AB233, AB235, AB236 and AB237) were used for both in vitro and in vivo studies. The two remaining clinical isolates (AB84 and AB87), were used in previously published in vivo experiments using the same model, and thus used for the in vivo studies as internal controls to validate the stability of the model and the consistency of the in vivo response to the cefiderocol HSR across studies.14,15
The cefiderocol MICs for isolates used for both in vitro and in vivo studies were determined to be 0.12–2 mg/L by the broth microdilution (BMD) method using iron-depleted CAMHB (ID-CAMHB), which was prepared based on CAMHB (Becton-Dickinson, Sparks, MD, USA), as recommended by CLSI (Table 1).16,17 In addition, the β-lactamase gene profile was also determined by WGS or PCR analysis (Table S1, available as Supplementary data at JAC-AMR Online) prior to inclusion in the study (Table 1).7,18 Isolates were randomly selected based on the cefiderocol MICs to have a larger population of the isolates with MIC of 1 or 2 mg/L, which is near the susceptibility breakpoint, to evaluate the risk of resistance emergence as well as having a relevant background of genotypically identified β-lactamases.7,12
Table 1.
Clinical isolates included the in vitro and in vivo models
| Isolate ID | MLST (PubMLST Oxford) | MIC (mg/L) | β-lactamase | |
|---|---|---|---|---|
| cefiderocol | meropenem | |||
| AB230a | 281 | 2 | 32 | ADC-33, OXA-82 |
| AB231a | 281 | 1 | 32 | ADC-33, OXA-23, OXA-82 |
| AB232a | 944 | 0.125 | >64 | ADC-152, CARB-16, CTX-M-115, OXA-72, OXA-90 |
| AB233a,b | N/Ac | 0.25 | >64 | ADC, OXA-24-like |
| AB235a | 281 | 2 | 64 | ADC-33, OXA-23, OXA-82 |
| AB236a | 1418 | 1 | 32 | ADC-52, CARB-16, ADC-199, OXA-23, OXA-91 |
| AB237a,b | N/Ac | 2 | 16 | ADC, OXA-58-like |
| AB87d | ND | 4 | ND | ND |
| AB84d | 1289 | 16 | 32 | ADC-25, OXA-23, OXA-66, PER-1 |
N/A, not applicable; ND, not determined.
Evaluated in vitro and in vivo.
β-lactamase profile was determined by PCR.
Not identified.
Internal control isolates previously evaluated in the in vivo model to assess stability of the model.15
In vitro chemostat studies
Seven clinical isolates were used for the in vitro chemostat models to evaluate the bacterial killing and the emergence of resistance under the human pharmacokinetic (PK) exposure of cefiderocol for 72 h, as described previously.19 Briefly, an exponentially growing bacterial suspension of 5.00 to 5.78 log10 cfu/mL was incubated at 37°C for 72 h in ID-CAMHB under conditions recreating the free cefiderocol concentration-time curves in plasma in healthy subjects (for AB230 and AB231) or patients, which was determined from the population PK studies in the patients from Phase II/III studies (for the remaining five isolates).20 The bacterial counts were determined at 4 h and every 8 h until 72 h by the incubation of 10-fold serially diluted bacterial suspension on drug-free tryptic soy agar (Becton-Dickinson). The cefiderocol MIC was determined by selecting five colonies that were randomly picked up from 72 h treatment cultures to make a bacterial suspension, which was then assessed with BMD methods using ID-CAMHB. Against the isolates with reduced susceptibility to cefiderocol, the cefiderocol MIC in the presence of avibactam (final concentration, 4 mg/L) was determined, since previous reports found avibactam augmented cefiderocol potency in vitro against some A. baumannii isolates with elevated cefiderocol MICs.10
Frequency of resistance
The bacterial suspensions of these strains prior to experimental antimicrobial exposure were used to evaluate the potential of resistance emergence observed in the chemostat studies, which showed ≥32-fold increase of MIC. A concentration of 107 or 108 cfu/mL was prepared by dilution of the culture grown overnight in ID-CAMHB and spread on Mueller–Hinton agar (MHA) containing 10-fold MIC of cefiderocol, respectively. After incubation at 35°C for 48 h, the colonies that appeared on the plates then underwent cefiderocol susceptibility testing.
WGS analysis and analyses of MLST and β-lactamase
The isolates that showed an increase in cefiderocol MIC in in vitro chemostat studies were analysed using WGS. Genomic DNA from each bacterial sample was extracted and WGS was performed using the Illumina MiSeq system (Illumina, San Diego, CA, USA) as described previously.10,13 The raw FASTQ reads were first trimmed to a quality score limit of 0.05 (Q13) with maximum two ambiguous nucleotides and assembled into contigs for each test sample using CLC Genomics Workbench version 11.0.1 (Qiagen, Hilden, Germany) as described previously.10,13 To investigate β-lactamase genes, all contig datasets of test samples were loaded to the Pipeline Pilot version 18.1.100.11 (PP) (Dassault Systèmes Biovia, San Diego, CA, USA), and subjected to blastn-based search against the in-house β-lactamase gene database, which consists of β-lactamase genes from the ResFinder database (https://cge.food.dtu.dk/services/ResFinder/) and NCBI gene (https://www.ncbi.nlm.nih.gov/gene/) with some manual curation. The subtype of β-lactamase was identified by the amino acid sequence of each gene. The A. baumannii MLST profiles were determined by comparison of seven allele sequences, which were determined by blastn search for generated contigs using the PP, from the public Oxford database (https://pubmlst.org/bigsdb? db=pubmlst_abaumannii_oxford_seqdef).
Long-read sequencing and analyses
For AB231 and its resistant mutant, WGS was performed using both Illumina MiSeq short-read sequencing and Oxford Nanopore MinION (Oxford, UK) long-read sequencing technologies. The long-read sequencing and de novo assembly were performed at GeneBay (Yokohama, Japan). MiSeq and Nanopore reads were assembled using Unicycler.21 The assembled contigs were error corrected using Nanopolish (available from https://github.com/jts/nanopolish). The protein-coding genes along with their functional annotation of the long contigs were predicted and specific mutations in AB231 derivative were detected using Genedata Selector 5.2.3 (Genedata, Switzerland). Detected mutations were confirmed by Sanger sequencing using BigDye Terminator v3.1.
mRNA expression analysis
The mRNA expressions were determined by real time RT-PCR. The RNA of A. baumannii in the exponential growth phase in ID-CAMHB was extracted with RNeasy mini kit (Qiagen). Real time RT-PCR was conducted with One Step PrimeScript RT-PCR Kit (Takara-bio, Shiga, Japan) and Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific) according to the manufacturer’s instruction. The mRNA expression of tonB, exbB and exbD genes were tested using strain AB231 and its derivatives, and those of blaADC, blaOXA-23 and blaOXA-82 were tested using strains AB230 and AB235 and their derivatives. Comparative Ct method was used to determine the relative expression level of target genes using recA as a reference gene.
Animals
Specific-pathogen-free, female, CD-1 mice (20–22 g) were obtained from Charles River Laboratories Inc. (Raleigh, NC, USA). All animals acclimatized for 48 h prior to study procedures housed in groups of six animals per HEPA-filtered cage (Innovive, San Diego, CA, USA) at controlled room temperature. Cages were used with nourishment and enrichment as previously described.13 Monitoring for morbidity was conducted as previously described and animals were euthanized if found moribund and tissues were harvested.13
Neutropenic murine thigh infection model
Prior to all in vivo efficacy studies, animals were prepared as follows: neutropenia was induced using intraperitoneal (IP) cyclophosphamide 150 mg/kg and 100 mg/kg administered on Days −4 and −1, respectively. On study Day −3, 5 mg/kg of uranyl nitrate was administered IP to aid in developing the cefiderocol HSR.22,23 An inoculum of ∼1 × 107 cfu/mL was needed as previously described to establish infection with this bacterium in the thigh model.14,15 The cefiderocol HSR was administered as previously described in the neutropenic murine thigh infection model to produce plasma exposure in the mice similar to that of cefiderocol 2 g IV every 8 h over 3 h in humans.14,15,22,23
In vivo efficacy studies
One group of three mice per isolate was sacrificed via CO2 asphyxiation and cervical dislocation at 0 h (2 h post-inoculation) and both thighs were harvested aseptically to enumerate baseline bacterial burden. Groups of three mice received either sham control (NS) or cefiderocol HSR subcutaneously for 24, 48 or 72 h. At each designated timepoint (24, 48 and 72 h), control and cefiderocol HSR groups were euthanized as described above and thighs were aseptically harvested. Bacterial enumeration was conducted on each thigh at each timepoint as previously described.13 Animals that were sacrificed due to morbidity or succumbed to infection were assessed at the closest following timepoint and thus some timepoints did not have any animals survive to be assessed. Efficacy was assessed using the change in log10 cfu/thigh for each treatment by subtracting the log10 cfu/thigh at each assessment point (24 h, 48 h or 72 h) from the 0 h control. Log10 change in cfu/thigh was reported as mean ± SD for each treatment per isolate. Achievement of 1 log10 bacterial kill at 24 h, 48 h and 72 h relative to the 0 h controls was assessed as a surrogate endpoint for prediction of clinical efficacy.24
Resistance determinant study in the in vivo model
To evaluate resistance development during cefiderocol treatment, post-exposure MICs were determined for isolates recovered during the in vivo efficacy studies from the infected murine thighs as previously described.13 Post-exposure MICs were determined by BMD per CLSI standards using made-to-order ID-CAMHB produced by Thermo Fisher (YT3464-5, Oakwood Village, OH, USA, lot: M20261).16,17 Quality control was conducted per CLSI guidance using Pseudomonas aeruginosa ATCC 27853 and MIC endpoints were determined as outlined in the CLSI guidelines where trailing was not interpreted as the endpoint.16 The post-exposure BMD MICs were considered to have resistance development if the cefiderocol-treated isolates had a greater than two dilution increase in the MIC compared with control (saline)-treated isolates. Post-exposure specimens with recoverable colonies were frozen at −80°C in skim milk.
Specimens that resulted in elevated MICs post-exposure were subsequently subcultured from frozen stock to undergo disc diffusion susceptibility testing to confirm post-exposure changes in cefiderocol phenotype since trailing can complicate BMD endpoint assessment.16 Briefly, cefiderocol discs (30 µg) (Hardy Diagnostics, CA, USA, lot: 442073) were tested by placing the disc on an MHA plate (Becton Dickinson, NJ, USA) lawned with a bacterial suspension per the manufacturer’s instructions. Results were measured by two independent qualified personnel after 16–18 h of incubation.
Results
In vitro chemostat studies
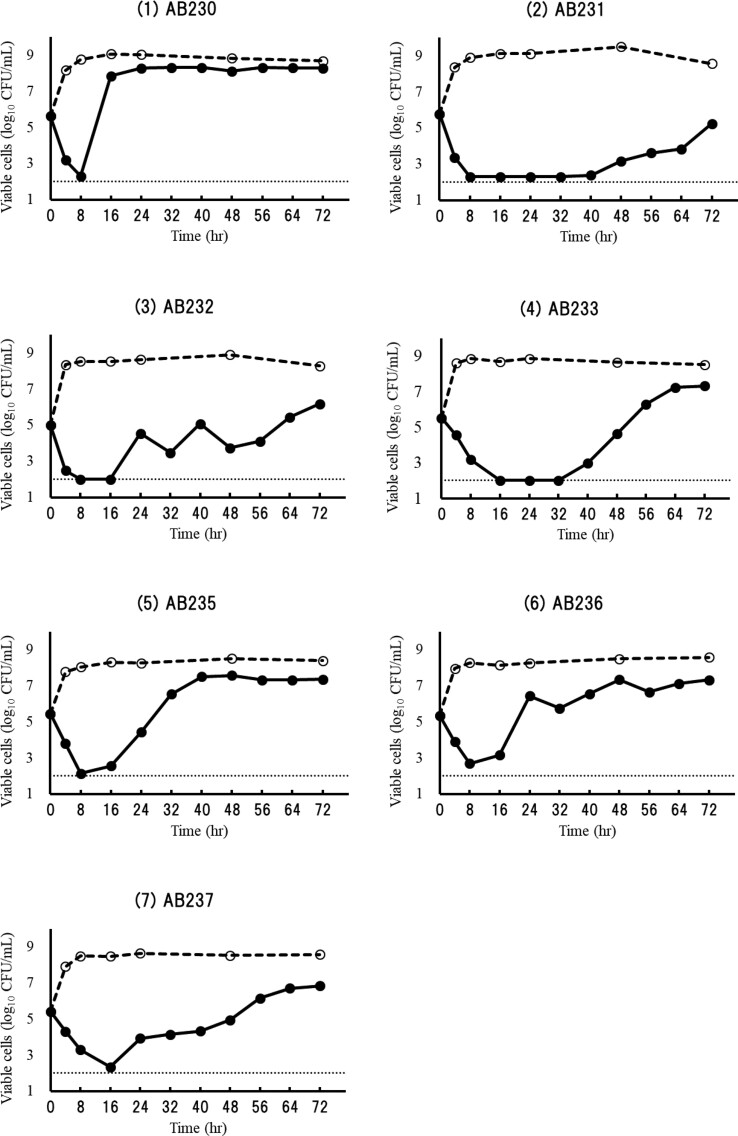
Cefiderocol showed greater than 2 log10 kill against all seven test strains with MICs of 0.12 to 2 mg/L within 8 h (>3 log10 reduction for four strains and 2 to 3 log10 reduction for three strains) under the human exposure in the chemostat models, but regrowth was observed for all cases during the 72 h treatment (Figure 1). Reduced susceptibility was observed in five of the seven isolates assessed. Resistant colonies were not obtained for isolates AB233 and AB237. For the remaining five isolates (AB230, AB231, AB232, AB235 and AB236), 5/5, 5/5, 2/5, 5/5 and 5/5 isolates showed ≥32-fold increase in cefiderocol MIC, and the MIC of these five isolates after 72 h treatment ranged from 8 to >128 mg/L. As for AB230 and AB235, which showed regrowth within 24 h treatment, the appearance of the resistant isolates was confirmed in the bacterial suspension at 24 h treatment, and the MIC of the resistant isolates from 24 h and 72 h treatment was the same.
Figure 1.
Bactericidal activity against A. baumannii isolates in the in vitro chemostat model. Solid lines and dashed lines indicate the growth curves under the human PK of cefiderocol and no treatment, respectively.
The resistance phenotype observed was reversed by the addition of avibactam to the MIC testing of three isolates. In the case of AB230, AB235 and AB236, the cefiderocol MIC of the post-treatment isolates decreased to the parent level by the addition of avibactam. On the other hand, in the case of AB231 and AB232, the addition of avibactam did not decrease the cefiderocol MIC significantly (Table 2).
Table 2.
MIC and frequency of resistance for the test isolates derived from the in vitro model
| Isolate ID | Frequency of resistance | Cefiderocol MIC (mg/L) | |||
|---|---|---|---|---|---|
| pre-treatment | post-treatment | ||||
| alone | + avibactama | alone | + avibactama | ||
| AB230 | 4.0 × 10−7 | 2 | 1 | 128 | 0.5 |
| AB231 | <9.1 × 10−8 | 1 | 0.25 | 32 | 8 |
| AB232 | 5.5 × 10−7 | 0.125 | ≤0.06 | 8 | 1 |
| AB233 | 1.1 × 10−6 | 0.25 | NT | —b | —b |
| AB235 | 5.0 × 10−6 | 2 | 1 | >128 | 2 |
| AB236 | 3.0 × 10−6 | 1 | 0.12 | 64 | 0.5 |
| AB237 | 1.0 × 10−6 | 2 | NT | —b | —b |
NT, not tested.
Avibactam tested at 4 mg/L.
Not done due to no resistance emergence.
The frequency of the colonies that appeared on the agar medium containing 10-fold MIC of cefiderocol varied from 5.0 × 10−6 to <9.1 × 10−8 among these seven test strains. As for AB231, no colonies appeared on the agar medium in the frequency of resistance study, but resistant mutants appeared in the chemostat models. On the other hand, for AB233 and AB237, colonies appeared on the agar medium, but resistant mutants were not obtained in the chemostat models. When the mutants with decreased cefiderocol susceptibility were phenotypically assessed with and without the addition of avibactam, similar MICs were seen regardless of which study they were derived from (e.g. frequency of resistance versus chemostat study).
WGS analysis
Assessing the AB231 resistant mutants, no mutation was observed by the short-read analysis, but the insertion of IS4 family transposase ISAba1 (1189 bp with 99% identity with ISAba1) at the stop codon of the tonB gene was observed by long-read analysis in all five resistant mutants derived from this strain in the in vitro chemostat studies. As a result, this mutation caused the addition of eight amino acid residues after the C-terminus amino acid residue of the original TonB protein and 20-fold decrease of the mRNA expression of exbB and exbD genes, which are located downstream the tonB gene as tonB-exbB-exbD operon.
In addition, the expression level of multiple β-lactamase genes was observed using AB230, which had ADC-33 and OXA-82, and AB235, which had ADC-33, OXA-23 and OXA-82, and their corresponding cefiderocol-resistant mutants obtained in chemostat models. For both isolates, neither gene mutations nor different expression level were observed as determined by RT-PCR.
In vivo efficacy study
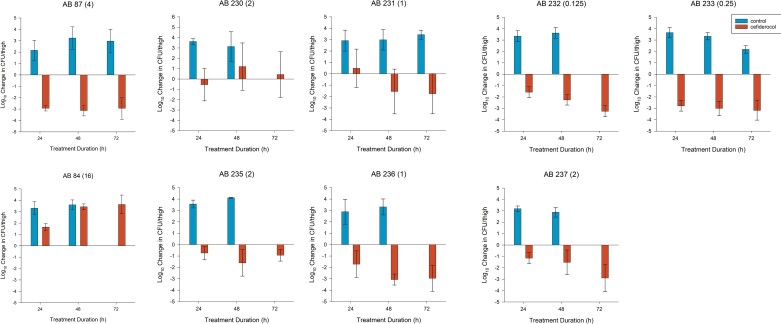
Two A. baumannii isolates previously evaluated in the model (AB87 and AB84) produced similar reduction and increase in bacterial burden to previously published data, respectively.15 Figure 2 displays the change in bacterial burden including averaged results for AB87 and AB84 internal controls.
Figure 2.
Change in bacterial density (mean ± SD) for untreated controls or mice receiving cefiderocol HSR against A. baumannii isolates including two internal controls (AB87 and AB84) and seven test isolates (AB230, AB231, AB232, AB233, AB235, AB236 and AB237). The absence of a result at a given timepoint represents no animals survived to the given timepoint in the group. Cefiderocol BMD MICs (mg/L) in parentheses were determined in iron-depleted media.
Mean bacterial burden at 0 h for the other seven A. baumannii isolates tested in the model was 5.63 ± 0.31 log10 cfu/thigh. In all seven A. baumannii isolates tested, there was an increase in bacterial burden in control animals at each timepoint in the absence of mortality (Figure 2). The mean change in bacterial burden for cefiderocol treated animals ranged from −2.78 to +0.49 log10 cfu/thigh, −3.07 to +1.20 log10 cfu/thigh and −3.26 to +0.43 log10 cfu/thigh at 24, 48 and 72 h, respectively. Cefiderocol HSR produced net bacterial stasis in the initial 24 h for isolates AB230 and AB231; however, AB231 displayed bacterial reduction at 48 and 72 h compared with continued bacteriostasis with AB230. Mean bacterial reductions were noted at 24 h and sustained over 48 and 72 h in the remaining five A. baumannii isolates. Overall, 4/7, 6/7 and 5/7 isolates achieved 1 log10 kill at 24, 48 and 72 h, respectively (Figure 2). Additionally, 2 log10 kill was achieved in three of seven and four of seven isolates at 48 and 72 h, respectively.
Resistance determination studies of in vivo samples
For the seven A. baumannii isolates tested, 57 samples had sufficient growth post-exposure to conduct MIC testing. For isolates AB232 and AB237, each had a single specimen where the post exposure BMD MIC was >2-fold dilution higher than corresponding controls at 72 and 48 h, respectively. Disc diffusion testing for both AB232 and AB237 recovered from cefiderocol-treatment groups with elevated BMD MICs resulted in zone diameters similar to the bacteria recovered from the control mice as well as a fresh culture from the frozen stock without prior cefiderocol exposure. Thus, the disc diffusion confirmation studies on AB232 and AB237 are suggestive of variability in the interpretation of the BMD testing results not overt development of resistance.
AB230 had two, three and one sample at 24, 48 and 72 h with elevated BMD MICs, respectively. Disc diffusion from a single sample at 48 h and 72 h resulted in a decreased zone of inhibition consistent with the phenotypic change observed by BMD. All other post-exposure AB230 samples had disc diffusion zones similar to those of controls and the frozen bacterial stock without cefiderocol exposure.
Discussion
In this study, the sustained bactericidal efficacy and resistance acquisition under human-simulated exposure were evaluated using an in vitro chemostat and an in vivo murine thigh infection model. The results showed a discrepancy where in vivo cefiderocol HSR produced sustained efficacy and limited resistance development compared with regrowth and resistance as seen in vitro. These findings were similar to the observation with S. maltophilia.13
The present study reaffirmed the in vivo activity of cefiderocol HSR against six of the seven A. baumannii isolates tested. The greater than 1 log10 kill target associated with clinical outcomes in humans24 was achieved in four to six of the seven isolates tested depending on timepoint assessed, suggesting sustained microbiological activity over the 72 h model. This finding is consistent with pharmacokinetic/pharmacodynamic (PD) indices of fT>MIC predictive of success in murine infection models, including A. baumannii.25 Of note, the magnitude of the fT>MIC target for 1 log10 kill for A. baumannii was relatively higher than that of other tested species with 88% fT>MIC compared with 64%–82% for Enterobacterales and P. aeruginosa.22,25 The cefiderocol exposure in humans receiving 2 g IV every 8 h over 3 h, like the murine regimen used in this study, would be predictive of efficacy for MICs up to the CLSI breakpoint of 4 mg/L (Table 3) as supported by the microbiological success observed for AB235, AB237 and AB87, which had cefiderocol MICs of 2, 2 and 4 mg/L, respectively.14–16 Indeed, inter-strain differences in the fT>MIC magnitude have been described22 and may explain the limited activity of cefiderocol against AB230 (MIC = 2 mg/L). Alternatively, resistance emergence may explain the limited efficacy against AB230.
Table 3.
Comparative cefiderocol PK profiles in the in vitro chemostat and the in vivo murine model
| Drug | Model | % fT>MIC at MIC (mg/L) of: | |||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | 32 | 64 | ||
| Cefiderocol 2 g IV q8h, 3 h infusion | Human, infected patients Phase II/IIIa | 100 | 100 | 100 | 100 | 38 | 0 |
| In vitro chemostatb | 100 | 100 | 100 | 96 | 39 | 0 | |
| Human, healthy volunteers | 100 | 99 | 76 | 48 | 11 | 0 | |
| Mouse | 100 | 96 | 80 | 45 | 9 | 0 | |
The PK of the in vitro chemostat model mimicked the free-plasma profile of infected patients from the Phase III trials.18 Murine free-plasma PK mimicked the human free-plasma cefiderocol PK from healthy volunteers.14,15,22,24
Calculated from the steady-state PK for the pneumonia patient with creatinine clearance of 70 mL/min, body weight of 70 kg and albumin concentration of 3.0 g/dL.
Calculated from the non-steady-state PK for the pneumonia patient with creatinine clearance of 70 mL/min, body weight of 70 kg and albumin concentration of 3.0 g/dL.
Among the isolates tested, one sample each from AB232 and AB237 recovered post-cefiderocol exposure had BMD MICs that were increased >2-fold dilutions compared with isolates recovered from control treated animals; however, the magnitude of these phenotypic changes was unconfirmed by the disc diffusion methodology, although testing was conducted after freezing so expression derived mechanisms could not be ruled out. Conversely, AB230 was the only isolate noted to have increased MICs relative to controls confirmed using both microbiological methods. Indeed, this isolate resulted in suppression of growth in vivo in the neutropenic model over the entire 72 h study. This in vivo efficacy profile contrasts with the development of adaptive-based resistance observed with previous siderophore antibiotic conjugate candidates, where significant bacterial regrowth comparable in magnitude to the control counterparts was observed.26,27 Similarly, adaptive-based resistance with previous siderophore antibiotic conjugate candidates was seen earlier in the experimental timeline at 24 h, which was not observed in the present study.26,27
The addition of avibactam reduced the cefiderocol MIC against the post-exposure resistant isolates from the chemostat model, including AB230, which was the only isolate that had sustained MIC increase after in vivo exposure. Indeed, the reductions in MICs varied by strain suggesting multiple mechanisms are contributing to elevated MICs. Although the increased production of any β-lactamases was not observed in the resistant mutants derived from at least two isolates (AB230 and AB235), the resistance mechanisms may be due to the induced production of β-lactamases in combination with decreased outer membrane permeability. The addition of avibactam is able to reduce the MIC by inhibition of β-lactamases that may free the cefiderocol to exert antimicrobial activity.10 Due to the multitude of mechanisms of resistance harboured by A. baumannii, combination therapy has been suggested.2 Despite this advocacy for multiple drug therapy; no consensus exists on optimal combination therapy or its impact on clinical outcomes and emergence of resistance.2,10 In vivo investigations using human-simulated exposures are needed to identify rational cefiderocol-based combination therapies to evaluate microbiological efficacy and prevention of resistance in A. baumannii.
Finally, WGS analysis of the resistant mutants derived from AB231 found a mutation in the tonB-exbB-exbD region with ISAba1 insertion at the C-terminus of tonB gene, causing the extension of eight amino acid residues at the C-terminus of TonB protein and the reduced expression of exbB and exbD genes. This mutation was not found in any of 168 clinical isolates from the multinational SIDERO-WT surveillance studies including cefiderocol-susceptible and -resistant isolates, and could cause the loss of the ability to acquire siderophore-iron complex due to the deficiency of energy transduction system to acquire iron via siderophore-iron receptor.28 The resistant mutants caused by the mutation in the tonB-exbB-exbD region did not manifest in the in vivo model, an observation that may be due to decreased fitness associated with reduced iron acquisition. The mutation in this region was also observed for S. maltophilia, and this could be related with the discrepancy of the resistance emergence between in vitro and in vivo studies.13,28 The mechanisms that conferred resistance to the other isolates were not identified by WGS. Similar discordant findings between in vitro and in vivo pre-clinical models have been due to inefficient clearance of β-lactamases in the in vitro system causing decreased microbiological killing of β-lactam antimicrobials.24,29 This was also seen in experiments comparing the in vitro and in vivo efficacy of ceftazidime/avibactam where regrowth was seen in vitro but not in vivo, suggesting the physiological clearance of β-lactamases in the in vivo model may better describe what is seen clinically compared with the in vitro systems.24,29 Considering the variation in cefiderocol MIC reductions in the presence of avibactam, multiple mechanisms are likely involved.
A. baumannii represents a challenging clinical pathogen as the evaluation of patient outcomes is confounded by many factors.30 Notably, patients infected with A. baumannii typically have multiple acute and chronic conditions that may be associated with morbidity and mortality making outcome determination challenging.30 Indeed, the CREDIBLE-CR study evaluated clinical outcomes of patients with carbapenem-resistant infections between cefiderocol-treated patients and best available therapy.12 There was a morality imbalance noted in patients with A. baumannii infections although clinical and microbiological outcomes were similar between groups. The present translational PK/PD murine model using clinically achievable cefiderocol exposures supports the microbiological efficacy of the agent against challenging A. baumannii clinical isolates. Translational data including combination therapy may better guide future clinical investigations for optimal therapy against difficult-to-treat A. baumannii infections.
In conclusion, a cefiderocol HSR mimicking the clinical dose of 2 g IV every 8 h over 3 h in humans displayed notable and sustained bacterial kill over 72 h in the neutropenic murine thigh infection model against A. baumannii isolates and the development of resistance was rare. The discrepancy of the efficacy and resistance emergence between in vitro and in vivo was observed, and the resistance acquisition observed in the in vitro chemostat model was partly due to the reduced ability to acquire iron by the mutation in tonB-exbB-exbD region, which developed in the highly enriched broth culture media. These data further support the in vivo activity of cefiderocol against A. baumannii.
Supplementary Material
Acknowledgements
We would all like to thank the staff from the Center for Anti-Infective Research and Development for their vital assistance in the conduct of this study.
Contributor Information
Christian M. Gill, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA
Kamilia Abdelraouf, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA.
Merime Oota, Shionogi TechnoAdvance Research & Co. Ltd, Osaka, Japan.
Rio Nakamura, Shionogi TechnoAdvance Research & Co. Ltd, Osaka, Japan.
Miho Kuroiwa, Laboratory for Innovative Therapy Research, Shionogi & Co. Ltd, Osaka, Japan.
Yoshino Ishioka, Laboratory for Innovative Therapy Research, Shionogi & Co. Ltd, Osaka, Japan.
Miki Takemura, Laboratory for Drug Discovery and Disease Research, Shionogi & Co. Ltd, Osaka, Japan.
Yoshinori Yamano, Research Planning Department, Shionogi & Co. Ltd, Osaka, Japan.
David P. Nicolau, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA Division of Infectious Diseases, Hartford Hospital, Hartford, CT, USA.
Funding
This project was funded by Shionogi Co. Ltd, Japan. The funder provided financial support and did not exercise control over the conduct of or reporting of the research.
Transparency declarations
D.P.N. is a consultant, speaker bureau member and has received other research grants from Shionogi, the sponsor for the study. C.M.G. and K.A.: none to declare. M.O., R.N., M.K., M.T., Y.I. and Y.Y. are employees of Shionogi & Co. Ltd.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Tacconelli E, Carrara E, Savoldi A et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27. [DOI] [PubMed] [Google Scholar]
- 2. Wong D, Nielsen TB, Bonomo RA et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30: 409–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Son HJ, Cho EB, Bae M et al. Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. Open Forum Infect Dis 2020; 7: ofaa378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohd Sazlly Lim S, Zainal Abidin A, Liew SM et al. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J Infect 2019; 79: 593–600. [DOI] [PubMed] [Google Scholar]
- 5. Chopra T, Marchaim D, Johnson PC et al. Risk factors and outcomes for patients with bloodstream infection due to Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother 2014; 58: 4630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isler B, Doi Y, Bonomo RA et al. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2018; 63: e01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kazmierczak KM, Tsuji M, Wise MG et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53: 177–84. [DOI] [PubMed] [Google Scholar]
- 8. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019; 69(Suppl 7): S538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important Gram-negative bacteria. Clin Infect Dis 2019; 69 Suppl 7: S544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kohira N, Hackel MA, Ishioka Y et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist 2020; 22: 738–41. [DOI] [PubMed] [Google Scholar]
- 11. Wunderink RG, Matsunaga Y, Ariyasu M et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21: 213–25. [DOI] [PubMed] [Google Scholar]
- 12. Bassetti M, Echols R, Matsunaga Y et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. [DOI] [PubMed] [Google Scholar]
- 13. Gill CM, Abdelraouf K, Oota M et al. Discrepancy in sustained efficacy and resistance emergence under human- simulated exposure of cefiderocol against Stenotrophomonas maltophilia between in vitro chemostat and in vivo murine infection models. J Antimicrob Chemother 2021; 76: 2615–21. [DOI] [PubMed] [Google Scholar]
- 14. Monogue ML, Tsuji M, Yamano Y et al. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61: e01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stainton SM, Monogue ML, Tsuji M et al. Efficacy of humanized cefiderocol exposures over 72 hours against a diverse group of Gram-negative isolates in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 2019; 63: e01040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-First Edition: M100. 2021. [Google Scholar]
- 17. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018. [Google Scholar]
- 18. Yamano Y, Ishibashi N, Kuroiwa M et al. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J Glob Antimicrob Resist 2022; 28: 120–4. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto S, Kanazawa S, Sato T et al. Activities of cefiderocol with simulated human plasma concentrations against carbapenem-resistant Gram-negative bacilli in an in vitro chemostat model. Antimicrob Agents Chemother 2020; 64: e01128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawaguchi N, Katsube T, Echols R et al. Population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia, bloodstream infection/sepsis, or complicated urinary tract infection. Antimicrob Agents Chemother 2021; 65: e01437-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wick RR, Judd LM, Gorrie CL et al. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 2017; 13: e1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghazi IM, Monogue ML, Tsuji M et al. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents 2018; 51: 206–12. [DOI] [PubMed] [Google Scholar]
- 23. Chen IH, Kidd JM, Abdelraouf K et al. Comparative in vivo antibacterial activity of human-simulated exposures of cefiderocol and ceftazidime against Stenotrophomonas maltophilia in the murine thigh model. Antimicrob Agents Chemother 2019; 63: e01558-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bulitta JB, Hope WW, Eakin AE et al. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 2019; 63: e02307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura R, Ito-Horiyama T, Takemura M et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 2019; 63: e02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim A, Kutschke A, Ehmann DE et al. Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa: Assessing the risk for resistance and attenuated efficacy. Antimicrob Agents Chemother 2015; 59: 7743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomaras AP, Crandon JL, McPherson CJ et al. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57: 4197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun V. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev 1995; 16: 295–307. [DOI] [PubMed] [Google Scholar]
- 29. Crandon JL, Schuck VJ, Banevicius MA et al. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime/avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2012; 56: 6137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howard-Anderson J, van Duin D. Case commentary: uncertainty in evaluating treatment outcomes in carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2021; 65: e0142421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.