Abstract
The proton concentration gradient (ΔpH) and membrane potential (Δψ) formed across the thylakoid membrane contribute to ATP synthesis in chloroplasts. Additionally, ΔpH downregulates photosynthetic electron transport via the acidification of the thylakoid lumen. K+ exchange antiporter 3 (KEA3) relaxes this downregulation by substituting ΔpH with Δψ in response to fluctuation of light intensity. In the Arabidopsis (Arabidopsis thaliana) line overexpressing KEA3 (KEA3ox), the rate of electron transport is elevated by accelerating the relaxation of ΔpH after a shift from high light (HL) to low light. However, the plant cannot control electron transport toward photosystem I (PSI), resulting in PSI photodamage. In this study, we crossed the KEA3ox line with the line (Flavodiiron [Flv]) expressing the Flv proteins of Physcomitrium patens. In the double transgenic line (Flv-KEA3ox), electrons overloading toward PSI were pumped out by Flv proteins. Consequently, photodamage of PSI was alleviated to the wild-type level. The rate of CO2 fixation was enhanced in Flv and Flv-KEA3ox lines during HL periods of fluctuating light, although CO2 fixation was unaffected in any transgenic lines in constant HL. Upregulation of CO2 fixation was accompanied by elevated stomatal conductance in fluctuating light. Consistent with the results of gas exchange experiments, the growth of Flv and Flv-KEA3ox plants was better than that of WT and KEA3ox plants under fluctuating light.
Flavodiiron proteins alleviated the photodamage of photosystem I and enhanced the rate of CO2 fixation under fluctuating light in the Arabidopsis plants overexpressing K+ exchange antiporter 3.
Introduction
In light reactions of photosynthesis, solar energy is converted into chemical energy as ATP and NADPH. The core machinery of oxygenic photosynthesis is conserved from cyanobacteria to angiosperms. However, the evolutional strategy may not have been straightforward because in natural light environments, especially under terrestrial conditions, the intensity of light drastically fluctuates in the time scale of seconds to a few minutes. Low light (LL) limits the efficiency of photosynthesis. On the other hand, high light (HL) may damage the photosynthetic apparatus. Plants have to optimize the efficiency of light energy utilization in response to rapid changes in light intensity. Despite the conservation of the core machinery of electron transport, the regulatory mechanism, including the light-harvesting system, is divergent among phototrophs to acclimate to the respective light environments.
Coupled with photosynthetic electron transport, protons are taken up by the thylakoid membrane. Under HL, excess energy is dissipated from photosystem II (PSII) antennae as heat. The extent of thermal dissipation is measured by an energy-dependent (qE) component of nonphotochemical quenching (NPQ) of chlorophyll fluorescence (Ruban, 2016; Ruban and Wilson, 2021). In the Arabidopsis (Arabidopsis thaliana) mutants defective in the qE induction, the efficiency of light energy utilization by PSII {Y(II)} was not upregulated (Niyogi et al., 1998), suggesting that the induction of qE does not limit the rate of electron transport under the constant HL. Under fluctuating light intensity, however, the qE induced at HL intensity may decrease the rate of electron transport through PSII by dissipating the limited light energy in the subsequent LL period. To avoid the resulting energy loss, the qE should be rapidly relaxed after the shift from HL to LL. Artificial modification of the kinetics of NPQ relaxation successfully improved the yield of photosynthesis in tobacco (Nicotiana tabacum) under natural light environments (Kromdijk et al., 2016), although contradicting results were reported in Arabidopsis (Garcia-Molina and Leister, 2020).
The acidification of the thylakoid lumen also downregulates the rate of electron transport through the cytochrome (Cyt) b6f complex, the process of which is called photosynthetic control (Rumberg and Siggel, 1969; Tikhonov et al., 1981; Nishio and Whitmarsh, 1993). Photosynthetic control is essential to optimize the rate of electron transport toward photosystem I (PSI). The Arabidopsis proton gradient regulation 5 (pgr5) mutant is defective in cyclic electron transport around PSI and cannot induce ΔpH-dependent downregulation of photosynthesis, qE, and photosynthetic control (Munekage et al., 2002; Yamamoto and Shikanai, 2019). Mainly because of the impaired photosynthetic control, the pgr5 mutants are sensitive to fluctuating light intensity due to the severe photodamage of PSI (Suorsa et al., 2012). The Arabidopsis pgr1 mutant has an amino acid alteration in the petC gene encoding the Rieske subunit of the Cyt b6f complex (Munekage et al., 2001). The pgr1 mutation makes the Cyt b6f complex hypersensitive to the lumenal acidification (Jahns et al., 2002), consequently strengthening the ΔpH-dependent brake at the Cyt b6f complex. The introduction of the pgr1 mutation into the pgr5 background restored the tolerance of PSI to the fluctuating light intensity (Yamamoto and Shikanai, 2019).
Transfer of protons from the stroma to the thylakoid lumen generates an electrochemical proton gradient (ΔµH+) consisting of the membrane potential (Δψ) formed across the thylakoid membrane and the proton concentration gradient (ΔpH; Bailleul et al., 2010; Davis et al., 2017). Both components of ΔµH+ contribute to ATP synthesis as proton motive force (pmf). Although ΔpH downregulates photosynthetic electron transport via lumenal acidification, this is not the case for Δψ. Regulation of partitioning of two pmf components optimizes the balance of an accelerator (ATP synthesis) and a brake (ΔpH-dependent downregulation) on electron transport (Shikanai and Yamamoto, 2017). K+ efflux antiporter 3 (KEA3) is localized in the thylakoid membrane and is considered to be an H+/K+ antiporter mainly on the basis of its structural similarity with Escherichia coli K+ efflux system protein C (Armbruster et al., 2014; Kunz et al., 2014), although only K+ transporting activity has been experimentally confirmed (Tsujii et al., 2019). The C-terminal K+ transport/nucleotide (KTN)-binding domain downregulates KEA3 activity probably by sensing NADPH or ATP (Armbruster et al., 2014, 2016; Correa Galvis et al., 2020). The truncation of the KTN domain constitutively activates KEA3, resulting in the low level of NPQ during the induction of photosynthesis (Armbruster et al., 2014). A similar phenotype was observed when the wild-type (WT) KEA3 was overexpressed (Armbruster et al., 2016; Wang and Shikanai, 2019). On the other hand, the knockout of the KEA3 gene resulted in the delay in the relaxation of NPQ after a shift from HL to LL (Armbruster et al., 2014; Wang et al., 2017). KEA3 collaborates with the chloroplast NADH dehydrogenase-like (NDH) complex for the efficient induction of photosynthesis after an overnight dark adaptation by optimizing the lumenal acidification (Basso et al., 2020). Overexpression of the WT KEA3 accelerates the relaxation of NPQ after the shift from HL (700 µmol photons m−2 s−1) to LL (70 µmol photons m−2 s−1), resulting in the higher yield of PSII in the LL period (Armbruster et al., 2016). However, the lines overexpressing KEA3 also exhibited the limitation of acceptors from PSI immediately after the shift from LL (47 µmol photons m−2 s−1) to HL (1,529 µmol photons m−2 s−1) because the photosynthetic control was not fully induced (Wang and Shikanai, 2019). The balance of ΔpH and Δψ may have already been optimized under the fluctuating light environments in wild plants.
A group of Flavodiiron (Flv) proteins reduces molecular oxygen to water by accepting electrons from NADPH (Vicente et al., 2002) or ferredoxin (Sétif et al., 2020) at the acceptor side of PSI (Allahverdiyeva et al., 2015). Because Flv proteins drain excess electrons that are harmful at high levels in PSI, they function as a safety valve for electrons from PSI in fluctuating light intensity in divergent phototrophs (Allahverdiyeva et al., 2013; Gerotto et al., 2016; Jokel et al., 2018). Angiosperms have relinquished this safety valve for electrons during their early evolution (Yamamoto et al., 2016; Ilik et al., 2017), even though they are frequently exposed to fluctuating light. The alternative mechanism to protect PSI from fluctuating light intensity is the operation of photosynthetic control, which is induced by the lumenal acidification mainly depending on PGR5-dependent cyclic electron transport around PSI (Suorsa et al., 2012; Yamamoto and Shikanai, 2019). To test the impact of the different evolutional scenarios, we cloned the FlvA and FlvB genes from Physcomitrium patens and introduced them into Arabidopsis WT and pgr5 mutant plants (Yamamoto et al., 2016). In the pgr5 mutant background, Flv mediates the electron transport from “water” oxidized by PSII to “water” generated by Flv-dependent O2 reduction. Instead of PGR5-dependent cyclic electron transport around PSI, the Flv-dependent pseudocyclic electron transport (water–water cycle) increased the size of pmf to the WT level. In the WT Arabidopsis background, Flv did function as a safety valve for electrons under fluctuating light to protect PSI from the photodamage (Yamamoto et al., 2016) but not during steady-state photosynthesis. A similar effect was also confirmed in rice (Oryza sativa; Wada et al., 2018).
Plants overexpressing KEA3 might utilize light energy more efficiently than the WT plants, but they also might be more prone to photodamage of PSI under fluctuating light intensity (Wang and Shikanai, 2019). The Flv-dependent safety valve for electrons may solve this problem. In this study, we tested the artificial regulatory system depending on KEA3 and Flv and found an unexpectedly positive effect on photosynthesis mainly supported by the Flv proteins.
Results
Introduction of Flv suppresses the P700 phenotypes of the KEA3ox line under constant light
To create the double transgenic line, we selected the Flv expressing line no. 13 (Flv; Yamamoto et al., 2016) and the KEA3 overexpressing line #32 (KEA3ox; Wang and Shikanai, 2019). The KEA3ox line accumulates approximately 24 times more KEA3 protein than WT plants. We crossed the two transgenic lines and selected T2 plants homozygous for both transgenes (Flv-KEA3ox). The T3 generation was used for genotyping and further study. The growth of plants was unaffected in any genotype (Flv, KEA3ox, or Flv-KEA3ox) under the growth chamber conditions (50 µmol photons m−2 s−1, 16-h light/8-h dark at 23°C).
We analyzed the light intensity dependence of PSI and PSII photochemistry by measuring the absorption changes of P700 and chlorophyll fluorescence, respectively, using a Dual-pulse amplitude modulation (PAM) system. Light intensity-dependence of PSII yield {Y(II)} and the induction of NPQ were unaffected in any genotype (Figure 1, A and B). As reported previously (Wang and Shikanai, 2019), the NPQ level was mildly decreased at moderate light intensities (198 to 331 µmol photons m−2 s−1) in the KEA3ox line, although the difference was not statistically significant in this study.
Figure 1.
Light-intensity dependence of chlorophyll fluorescence and P700 parameters in constant light intensity. NPQ (A), Y(II) (B), Y(I) (C), Y(ND) (D), and Y(NA) (E) were analyzed in detached leaves from WT and three transgenic lines. Data represent means ± sd (n = 3 for WT, 4 for Flv and KEA3ox and 8 for Flv-KEA3ox, biological replicates). Different letters indicate the statistical differences by the Tukey–Kramer test (P < 0.05).
Consistent with previous work (Wang and Shikanai, 2019), the level of Y(I) was slightly elevated at HL intensities higher than 544 µmol photons m−2 s−1, although the difference was significant only at 687 µmol photons m−2 s−1 from the WT (Figure 1C). Y(I) was restored to the WT level by introducing Flv into the KEA3ox background. Y(ND) is calculated as the ratio of oxidized P700 (P700+) per total P700 and represents the nonphotochemical energy dissipation from oxidized PSI. Y(ND) is used to estimate the operation of the ΔpH-dependent downregulation of the Cyt b6f complex (photosynthetic control). Consistent with the normal NPQ level, Y(ND) was not affected in the Flv line (Figure 1D), as reported previously (Yamamoto et al., 2016). However, it was decreased in the KEA3ox line at light intensities higher than 544 µmol photons m−2 s−1. In the Flv-KEA3ox line, Y(ND) was complemented to the levels of WT and Flv plants. The Y(NA) parameter represents the fraction of reduced P700 that cannot be oxidized by a saturating pulse (SP) due to the lack of acceptors from PSI. The Y(NA) level was higher at light intensities higher than 544-µmol photons m−2 s−1 in the KEA3ox line than in other genotypes (Figure 1E). As in the Y(ND) levels, the Y(NA) levels were restored to the levels of WT and Flv plants in the Flv-KEA3ox line.
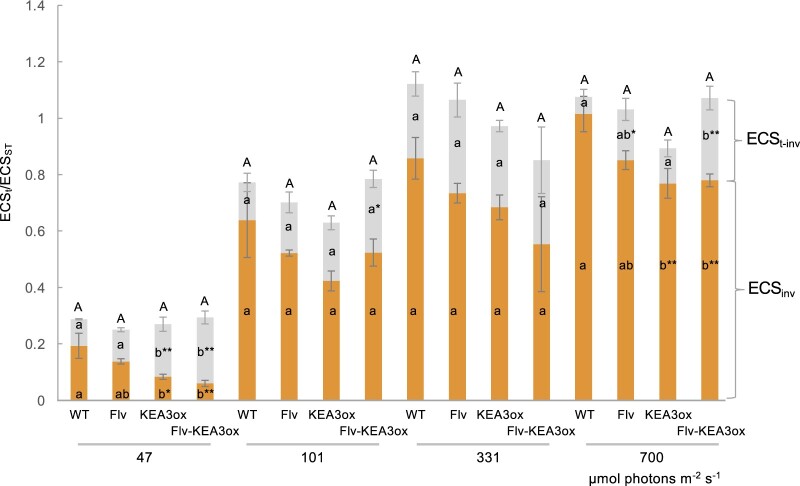
We analyzed the light intensity dependence of the size of pmf and the partitioning of pmf components (Figure 2). Because the partitioning analysis is influenced by NPQ-related signals (Johnson and Ruban, 2014), we used ECSint for the difference between the lowest ESCt and the steady-state signal in the dark and ESCt-int for the difference between ECSt and ECSint, as defined in Wilson et al. (2021). We used the same light intensities with our previous study (Wang and Shikanai, 2019) and confirmed the similar results in the WT and KEA3ox plants, although the size of ESCt-int was significantly larger in the KEA3ox line than that in the WT plants only at 47 µmol photons m−2 s−1. Because the effect of NPQ is minimum at LL intensity, the ratio of ESCt-int to ECSt represents the contribution of Δψ. Because the contribution of Δψ to ECSt is not negligible at 47 µmol photons m−2 s−1, the size of pmf is likely overestimated by evaluating ECSt/ECSST. The observation of higher contribution of Δψ to ECSt is consistent with the idea that KEA3 is active at LL intensity to rapidly relax ΔpH-dependent downregulation (Armbruster et al., 2014). The introduction of Flv into the KEA3ox background exaggerated the higher contribution of Δψ to ECSt. Although the minor contribution of ΔpH was also observed at 700 µmol photons m−2 s−1 in the KEA3ox background than in the WT plants, careful discussion is necessary because of contamination of NPQ-related signals (Johnson and Ruban, 2014; Wilson et al., 2021).
Figure 2.
Size and components of pmf. ECS signals were analyzed at different light intensities (µmol photons m−2 s−1). At LL intensity, ECSint and ECSt-int are proportional to the ratio of ΔpH and Δψ to ECSt, respectively. Error bars represent sd for ECSint and ECSt-int (biological replicates, n = 4). Different letters indicate the statistically significant differences by Tukey–Kramer test (P < 0.05). Uppercase and lowercase letters are for total pmf and each component, respectively.
In summary, the Flv line did not show any alteration in steady-state photosynthesis in the WT background, which is consistent with the previous work (Yamamoto et al., 2016). In contrast, the KEA3ox affected P700 parameters especially at HL intensities. The phenotypes were suppressed by the introduction of Flv.
Introduction of Flv complemented the sensitivity of PSI to the fluctuating light intensity in the KEA3ox background
The contribution of both transgenes should be more evident under fluctuating light intensity on the basis of previous studies (Armbruster et al., 2016; Yamamoto et al., 2016; Wang and Shikanai, 2019; Yamamoto and Shikanai, 2019). Photosynthetic performances of the four genotypes were analyzed under fluctuating light condition consisting of 5-min LL (47 µmol photons m−2 s−1) and 1-min HL (1,529 µmol photons m−2 s−1). After the five independent experiments, we still could not conclude on some minor phenotypes. We decided to show two different datasets representing all the analyses (Figure 3; Supplemental Figure S1). The level of NPQ was slightly higher in the KEA3ox line than in other genotypes in the second and third HL periods (Figure 3A). PSII photodamage may have contributed to the enhanced NPQ slightly, although PSII is not the main target of photodamage in the fluctuating light (Yamamoto and Shikanai, 2019). As observed previously (Armbruster et al., 2016), the relaxation of NPQ was faster in the KEA3ox line after the shift from HL to LL than in the WT. This phenotype is consistent with the idea that KEA3 functions in the rapid relaxation of NPQ by substituting ΔpH with Δψ. In an independent experiment, however, the NPQ level was similar between the KEA3ox and WT plants and the rapid relaxation of NPQ was also observed in the Flv background (Supplemental Figure S1A). The level of Y(II) was lower in the KEA3ox line in the third and fourth LL periods (Figure 3B; Supplemental Figure S1B). The decrease in Y(II) was accompanied by the reduction of the PQ pool monitored by the 1-qL parameter (Figure 3C; Supplemental Figure S1C). In contrast, the level of Y(II) was slightly higher in the Flv lines than in other genotypes, especially in the third and fourth LL periods. The phenotype was accompanied by the lower level of 1-qL parameter.
Figure 3.
Effects of fluctuating light intensity on photosynthetic parameters. Parameters were Y(II) (A), NPQ (B) and 1-qL (C) of PSII and Y(I) (D), Y(ND) (E), and Y(NA) (F) of PSI. Detached leaves from plants dark adapted for 30 min were exposed to three cycles of fluctuating light. Each cycle consisted of 5 min of LL (47 µmol photons m−2 s−1; yellow bars) followed by 1 min of HL (1,529 µmol photons m−2 s−1; red bars). Data represent means ± sd (n = 5, biological replicates). The results of an independent experiment are shown in Supplemental Figure S1.
The level of Y(I) was lower in the KEA3ox line than in other genotypes. This phenotype was enhanced by the repetition of the HL treatment (Figure 3D; Supplemental Figure S1D). On the contrary, the level of Y(I) was higher in the Flv line in LL after HL than in the WT and Flv-KEA3ox plants. Consistent with a previous report (Wang and Shikanai, 2019), induction of Y(ND) was decreased in the KEA3ox line and the decline was enhanced by the repetition of the HL treatment (Figure 3E; Supplemental Figure S1E). The Y(ND) parameter represents the operation of photosynthetic control, ΔpH-dependent downregulation of the Cyt b6f complex. The higher level of Y(ND) was induced more rapidly in the Flv lines. To reach the steady-state level of Y(ND) in the WT, >15–30 s was required, although the full induction of Y(ND) was attained within 15 s in the Flv lines (Figure 3E). In addition to the rapid induction of photosynthetic control, the Flv most likely oxidized P700 by accepting electrons from PSI as a safety valve (Yamamoto et al., 2016; Yamamoto and Shikanai, 2019).
The level of Y(I) was higher in LL in the Flv line than in the Flv-KEA3ox line (Figure 3D; Supplemental Figure S1D). Because the level of Y(ND) was similar between two lines in LL, the higher level of Y(I) in the LL periods was due to the lower level of Y(NA) in the Flv line than in the Flv-KEA3ox line (Figure 3F; Supplemental Figure S1F): Y(I) + Y(ND) + Y(NA) = 1 (Klughammer and Schreiber, 1994). The Y(NA) parameter represents the acceptor limitation from PSI. In the WT plants, the Y(NA) level was transiently high (0.2–0.4) immediately after the shift to HL (15 s), but was relaxed to the lower level (0.1–0.25) by the operation of photosynthetic control (Figure 3F; Supplemental Figure S1F). The level of transient Y(NA) was higher (0.35–0.7) and the level was also higher in the LL phases in the KEA3ox line than in other genotypes probably because the photosynthetic control was only partially induced. In the Flv background, however, the level of Y(NA) decreased by the shift to HL by the Flv-dependent safety valve, which pumps electrons away from PSI. In contrast to the KEA3ox line, the Y(NA) level was lower in the LL periods in the Flv line than the WT. In the Flv-KEA3ox line, the P700 parameters were between those of Flv and KEA3ox lines and similar to those of the WT plants (Figure 3, D–F). The minor inconsistency was observed in the P700 parameters in the independent experiments (Supplemental Figure S1), in which the level of Y(NA) sharply dropped to the level of the Flv line immediately after the shift to HL. Consequently, the P700 parameters in the Flv-KEA3ox line behaved like those in the Flv line rather than in the WT plants.
To evaluate the photodamage of PSI under fluctuating light, the maximum P700+ levels (Pm) were compared before and after the experiment (Figure 4). The Pm level was severely decreased (˂80%) in the KEA3ox plants. In the Flv-KEA3ox plants, the Pm level was restored between that of Flv and KEA3ox plants.
Figure 4.

Photodamage of PSI after exposed to the fluctuating light. The Pm (the maximum P700+) levels were recorded before and after expositing the fluctuating light in Figure 2 (1,529 µmol photons m−2 s−1) and Supplemental Figure S2 (500 µmol photons m−2 s−1). The remaining PSI activity after the experiment is represented by the ratio of Pm levels (n = 5) with sd. The Tukey–Kramer test was performed within the same light condition. Different letters indicate the statistically significant differences (P < 0.01). The impact of the different light condition in the same genotype was analyzed by t test (*P < 0.05, **P < 0.01).
Flv proteins enhance the plant growth under fluctuating light intensity
To evaluate the impact of transgenes on plant growth, the transgenic lines and the WT were cultured under fluctuating light intensity. The fluctuating light consists of 10-min HL (500 µmol photons m−2 s−1) and 10-min LL (50 µmol photons m−2 s−1) from 6:00 to 18:00 in a day. We selected the lower light intensity of HL than that used in the Dual-PAM analysis (1,529 µmol photons m−2 s−1) because the HL of 1,529 µmol photons m−2 s−1 might photodamage PSI in the WT plants. This would highlight the photoprotective contribution of Flv and mask the positive effect of KEA3ox. As a control, the plants were cultured under constant light for 5 weeks. To unify the total amounts of photons exposed to plants, the constant light also consists of the LL periods (6:00–9:00 and 15:00–18:00) and the HL period (9:00–15:00) (Figure 5A). Plants were precultured for 2 weeks under the constant light condition and then exposed to the fluctuating light for 3 weeks.
Figure 5.
Plant growth in the constant light or fluctuating light condition. A, A scheme of light conditions. In the fluctuating light condition, HL (500 µmol photons m−2 s−1) and LL (50 µmol photons m−2 s−1) were alternated for 12 h a day. All plants were precultured in the constant light conditions for 14 d after sowing and then moved to the fluctuating light condition or kept under the constant light condition for a further 21 d. B, A representative image of growth in 4 genotypes at 35 d after sowing. C, Dry weight of a seedling culture at the same timing with (B). Error bars represents ± sd (n = 7, biological replicates). Different letters indicate the significant difference by the Tukey–Kramer test (P < 0.05). No statistically significant difference was observed among genotypes under the constant light condition.
To evaluate the effect of the lower light intensity in the HL period, the experiment in Figure 3 was repeated with the HL of 500 µmol photons m−2 s−1 (Supplemental Figure S2). The results indicate a similar tendency with the experiment using 1,529 µmol photons m−2 s−1 (Figure 3), but the phenotypes of KEA3ox plants were milder with 500 µmol photons m−2 s−1 than with 1,529 µmol photons m−2 s−1. Although the Flv-KEA3ox and WT plants behaved similarly with 1,529 µmol photons m−2 s−1, the phenotypes of the Flv-KEA3ox plants were between those of the WT and KEA3ox plants with 500 µmol photons m−2 s−1. The photodamage of PSI was alleviated in the WT, KEA3ox, and Flv-KEA3ox plants by decreasing the HL intensity to 500 µmol photons m−2 s−1 but PSI was still more severely photodamaged in the KEA3ox plants compared to the other genotypes (Figure 4).
To evaluate the photodamage of both PSs after the longer exposure to the fluctuating light, the plants were exposed to the fluctuating light as in the growth test. The levels of Fv/Fm and Pm were measured before and after the exposure to the fluctuating light for 2 and 4 d (Supplemental Figure S3). The Fv/Fm level was not affected in any genotype. Under the constant light, the Pm level was elevated to the WT level in the KEA3ox plants after the exposure to the constant light for 4 d. To monitor the photodamage in the same leaf successively, we used the short-day condition (8-h light and 16-h dark) with relatively LL (100 to 150 µmol photons m−2 s−1) for the preculture. The increase in the Pm level observed in the KEA3ox plants probably reflected the acclimation to the constant light condition with HL of 500 µmol photons m−2 s−1. Although PSI was photodamaged in the KEA3ox plants after the short exposure to the fluctuating light (Figure 4), the Pm level was not affected in any genotype after the longer exposure to the fluctuating light (Supplemental Figure S3). The longer exposure to the fluctuating light induced the acclimation of plants to the condition rather than aggravating the photodamage.
Under fluctuating light conditions, the plant size was decreased in all four genotypes compared to plants grown under constant light (Figure 5, B and C). Interestingly, the Flv lines (Flv and Flv-KEA3ox) grew more than the other genotypes. The growth of the KEA3ox plants was slightly inferior to that of WT plants and Flv-KEA3ox plants grew slightly better than Flv plants, although the differences were not statistically significant in the dry weight (Figure 5C). In this growth test, we could not see any positive effect of the KEA3ox but Flv proteins enhanced the plant growth under the fluctuating light intensity.
Flv proteins upregulate the CO2 assimilation rate independently of KEA3ox in fluctuating light intensity
To clarify the underlying mechanism for the increased growth phenotype in the Flv background, we analyzed the gas exchange in the four genotypes (Figure 6). Because of the requirement of larger leaves in the gas exchange analysis, seedlings were precultured at 100 to 150 µmol photons m−2 s−1 (8-h light/16-h dark) for 8–9 weeks. We used the same cycle of 10-min LL (50 µmol photons m−2 s−1) and 10-min HL (500 µmol photons m−2 s−1) with the growth test (Figure 5) for 2 h (Figure 6A). As a control (constant light), the 60-min HL period was sandwiched by two LL periods (30 min) so that the total amount of photons exposed to plants was the same between the two conditions. Under the constant light, the kinetics of induction and the maximum rate of CO2 assimilation were similar among the four genotypes (Figure 6B), consistent with the same growth rate (Figure 5). In contrast, the kinetics of induction and the maximum rate of CO2 assimilation depended on the genotypes under the fluctuating light (Figure 6B). Because 1 h was required to reach the maximum CO2 assimilation rate in the HL period in the WT, we divided the time course into two phases, the induction phase (<1 h) and the subsequent steady-state phase (>1 h) (Supplemental Figure S4A). In the steady-state phase, the maximum rate of CO2 assimilation in the HL period was higher in the Flv background than in other genotypes (Figure 6B), although the difference was significant only between WT and Flv plants, and WT and Flv-KEA3ox plants in the cumulative CO2 assimilation in this phase (Supplemental Figure S4B). This result suggests that Flv upregulates the rate of CO2 assimilation. Because we did not observe any impact of the KEA3ox, the positive effect of Flv observed in the steady-state phase was likely independent of the function of KEA3ox. On the contrary, induction of CO2 assimilation was faster in the order of Flv-KEA3ox > Flv > KEA3ox > WT in the HL periods of the induction phase, although the difference was significant only between the WT and Flv-KEA3ox plants in the cumulative CO2 assimilation (Supplemental Figure S4B). We did not observe any difference in the LL periods except for the enhanced drop of CO2 fixation in the Flv line in the second LL period.
Figure 6.
Rate of CO2 assimilation. A, Time course of the photosynthetic rate in an artificial fluctuating light. The CO2 assimilation rate (B) and the gs (C) at the CO2 concentration of 400 μmol mol−1 at the cuvette temperature of 25°C were measured under two light conditions, constant light condition and fluctuating light condition. Under the constant light condition, the photosynthetic rate was measured at the PFD of 50 μmol m−2 s−1 for 30 min and maintained at the PFD of 500 μmol m−2 s−1 for 60 min, and then again at the PFD of 50 μmol m−2 s−1 for 30 min. On the other hand, under the fluctuating light condition, the PFD was alternated between 50 μmol m−2 s−1 for 10 min and 500 μmol m−2 s−1 for 120 min. The leaves of the plants kept in the dark overnight were used. The photosynthetic rate was recorded every 5 s. Values are the mean (n = 3–4, biological replicates). Statistical analyses of Figure 4, B and C are summarized in Supplemental Figure S4 and Supplemental Table S1, respectively.
Relaxation of NPQ was faster in the KEA3ox plants (Figure 3A). However, the quick recovery from the photoprotection after the shift from HL to LL did not upregulate the rate of CO2 fixation (Figure 6B). Although the reason is unclear, stomatal conductance (gs) was higher in the Flv background than in WT and KEA3ox plants but this was not the case under the constant light condition (Figure 6C). The gs was higher in the Flv-KEA3ox plants than in other genotypes even in the dark before the exposure to the fluctuating light (Figure 6C) but the difference was not statistically significant (Supplemental Table S1). We did not observe the same difference in the dark before the exposure to the constant light (Figure 6C). In contrast, statistically significant differences of gs were observed during the induction phase between the WT and the Flv-KEA3ox line and during the steady state between the Flv and KEA3ox lines (Supplemental Table S1).
In summary, KEA3ox efficiently induced the CO2 fixation but this positive effect was observed only during the first 1 h of the 12-h light period (Figure 6B) and is unlikely to improve the plant growth significantly. Although PSI was slightly photodamaged even in the milder condition of fluctuating light consisting of 50 and 500 µmol photons m−2 s−1 (Figure 4), it unlikely limited the rate of CO2 fixation in the KEA3ox line (Figure 6B). In fact, the Pm levels were not affected by the longer exposure to the fluctuating light (Supplemental Figure S3). In contrast to the minor effects of the KEA3ox on the rate of CO2 assimilation, it was enhanced in the Flv background (Figure 6B). The phenotype was accompanied by elevated gs (Figure 6C). This is one of the reasons for the enhanced growth of the Flv background plants under the fluctuating light intensity (Figure 5).
Discussion
KEA3 is necessary for the rapid relaxation of the ΔpH-dependent downregulation of electron transport via substitution of ΔpH by Δψ during the induction of photosynthesis (Armbruster et al., 2014; Kunz et al., 2014; Wang et al., 2017). We also confirmed this function in the rapid relaxation of NPQ after the shift from HL to LL in the KEA3ox line (Figure 3A). The high level of NPQ induced in the HL period may limit the PSII photochemistry transiently in the subsequent LL period. Although the rate of CO2 fixation was transiently dropped immediately after the shift from HL to LL (Figure 6B), this is probably related to the high activity of photorespiration in HL (postillumination CO2 burst; Kaiser et al., 2014). We did not observe any positive effect of the transgenes in the recovery of the rate of CO2 assimilation to the LL level.
The fluctuating light condition consisted of 47 and 1,529 µmol photon m−2 s−1 in the Dual-PAM analysis (Figure 3), as used in the previous studies (Wang and Shikanai, 2019; Yamamoto and Shikanai, 2019). The high level of Y(NA) was induced in the KEA3ox line both in the HL and LL periods, resulting in the decline of Y(I) in the LL periods (Figure 3, D and F). The phenotype observed in the HL periods is likely due to the lower induction of the downregulation at the Cyt b6f complex (photosynthetic control), as observed in the low Y(ND) (Figure 3E). In contrast to the lower Y(ND) levels in the HL periods, NPQ was even higher in the KEA3ox line than in the WT plants (Figure 3A). The discrepancy is not explained by the requirement of the larger ΔpH in photosynthetic control than in NPQ (Takizawa et al., 2007). We do not eliminate the possibility that the levels of PsbS or xanthophyll cycle pigments were altered in the KEA3ox line. However, overexpression of KEA3 may disturb the induction of ΔpH-dependent regulation independently of the lumenal acidification at least partly.
The high Y(NA) level observed in the LL periods is partly due to the PSI photodamage (Figures 3 and 4). We may have to be more careful on this parameter because Y(NA) may be affected by the balance between electrons moved to and from P700 and may not simply reflect the limitation in electron acceptors from PSI at LL intensity (Theune et al., 2021). The introduction of Flv successfully reduced the Y(NA) level even lower than the WT level (Figure 3F). The photodamage of PSI was also significantly alleviated in the Flv-KEA3ox plants (Figure 4). We selected the milder fluctuating condition for the growth test because a longer exposure to the stressful light cycle likely affects even the growth of the WT plants. Furthermore, the protective function of the exogenous Flv systems has already been evaluated in Arabidopsis (Yamamoto et al., 2016, Yamamoto and Shikanai, 2019; Tula et al., 2020), rice (Wada et al., 2018), and barley (Hordeum vulgare; Shahinnia et al., 2021). In fact, the photodamage of PSI was alleviated in the genotypes except for KEA3ox by decreasing the intensity of HL to 500 µmol photons m−2 s−1 (Figure 4). Although KEA3ox potentially accelerates the rate of electron transport, it may induce undesirable photodamage of PSI. In the growth and gas exchange analyses, we focused on the impact of the positive effect of the KEA3ox by using the milder condition of fluctuating light and found that the positive effect was restricted in the induction phase.
We did not observe any positive effect of the KEA3ox line in the CO2 assimilation rate in the steady-state phase or any LL periods: the CO2 assimilation rate was the same between the WT and KEA3ox plants and between Flv and Flv-KEA3ox lines (Supplemental Figure S4). However, the CO2 assimilation was more rapidly induced during the induction phase in the KEA3ox background (Figure 6B). In contrast to the Flv background, the higher CO2 assimilation rate was not accompanied by the elevated gs in the KEA3ox line (Figure 6C). Most probably, the efficient induction in CO2 assimilation is due to the lesser limitation of electron transport at the Cyt b6f complex, accelerating both linear and cyclic electron transport. However, after the fifth HL period, the rate of CO2 assimilation became the same between the KEA3ox and WT plants (Figure 6B). Even in the HL periods, KEA3 may not be fully inactivated during the induction phase but the impact of the KEA3ox may be negligible in the steady-state phase. Consistent with this idea, the KEA3ox line is not sensitive to the constant HL (1,000 µmol photons m−2 s−1) for 30 min (Wang and Shikanai, 2019). The downregulation at the Cyt b6f complex is probably negligible in the LL periods, as observed in the immediate decline of Y(ND) after the shift from HL to LL (Figure 3E). To optimize the downregulation at the Cyt b6f complex, a more straightforward approach would be the alteration of the pH sensitivity (the strength of the brake) of the Cyt b6f complex.
In contrast to the impact of the KEA3ox, the positive effect of the Flv was observed in both the induction and steady-state phases under fluctuating light conditions (Figure 6B). Because the CO2 assimilation rate was not reduced in the KEA3ox line compared to that of the WT (Supplementary Figure S4), it is unlikely that the photodamage of PSI limited the CO2 assimilation. Furthermore, the PSI photodamage observed in the KEA3ox plants after the short exposure to the fluctuating light (Figure 4) was alleviated by the longer exposure to the condition probably via the acclimation process (Supplemental Figure S3). It is unlikely that the plant growth was enhanced under the fluctuating light by the Flv-dependent PSI photoprotection. Unexpectedly, the gs was elevated in the Flv background, the phenotype that was further enhanced in the Flv-KEA3ox line during the induction phase (Figure 6C; Supplemental Table S1). The stomatal opening is a factor limiting the rate of CO2 assimilation under the fluctuating light intensity (Kimura et al., 2020; Sakoda et al., 2020). In this study, the maximum level of the gs was lower in the fluctuating light condition (∼1.7 mol m−2 s−1) than in the constant light condition (∼2.3 mol m−2 s−1) in the WT and KEA3ox plants (Figure 6C). The elevated gs was also observed in the other genetically modified plants with the enhanced growth rate in the field, as in the above-mentioned tobacco plants with more rapid recovery from the photoprotection (Kromdijk et al., 2016) and also in the tobacco plants overexpressing the H-protein of the glycine cleavage system (Lopez-Calcagno et al., 2018). The positive association between the gs and the QA redox state (1-qL) was reported (Głowacka et al., 2018). However, the 1-qL level was lower in the Flv backgrounds than the WT and KEA3ox plants in the LL periods (Supplemental Figure S2C). Although the mechanism is unclear, upregulation of electron transport may indirectly affect gs. It is also possible that the exogenous Flv system may have disturbed the regulatory systems of the stomatal opening. Actually, the partitioning of pmf components (ΔpH and Δψ) was disturbed in the artificial Arabidopsis plants utilizing the Flv-dependent pseudocyclic electron transport instead of the PGR5-dependent cyclic electron transport around PSI (Yamamoto et al., 2016). At least, the elevated gs would be one of the reasons for the enhanced CO2 assimilation rate and the better growth in the Flv background. Notably, this unknown factor limits the CO2 assimilation and the plant growth specifically under the fluctuating light intensity but not under the constant light condition (Figures 5 and 6). Finally, it is also possible that the decreased rate of photorespiration may have enhanced the rate of CO2 assimilation because of O2 consumption by Flv in chloroplasts.
Materials and methods
Plant materials and growth conditions
Production of the Flv line (35Sp::P. patens FlvA-FlvB no. 13) and the KEA3ox line (35Sp::KEA3 #32) was described in the previous reports (Yamamoto et al., 2016; Wang and Shikanai, 2019). The locus of the transgene was single in both transgenic lines. For creating the double transgenic plants, both lines were crossed. From the resulting F2 generation, the plants homozygous for both transgenes were selected by analyzing the genotype in the F3 generation. As a control (WT), Arabidopsis (A. thaliana) accession Columbia gl1 was used. The F3 generation was used for the physiological analyses. For the Dual-PAM analysis, the plants were grown in soil in a growth chamber (50 to 60 μmol photons m−2 s−1, 16-h/8-h light/dark cycle, 23°C, 55% humidity) for 24–28 d.
In vivo measurements of chlorophyll fluorescence and P700 absorption changes
Chlorophyll fluorescence and P700 parameters were determined by a Dual-PAM 100 system (Walz, Effeltrich, Germany). Before the analysis, plants were adapted to the room light of 2 to 3 µmol photons m−2 s−1 at least for 30 min. Y(II) and NPQ were calculated as (Fm′ − Fs)/Fm′ and (Fm − Fm′)/Fm′, respectively. Fm and Fm′ are the maximum fluorescence levels emitted by the application of an SP (300 ms, 10,000 µmol photons m−2 s−1) in the dark and in the light, respectively. Fs is a steady-state florescence level monitored just before applying an SP.
The redox state of P700 was monitored by the absorbance changes of transmission light at 830 and 875 nm. The maximum P700+ (oxidized P700) level in the absence of AL (Pm) was recorded by the application of an SP in the background of far-red light (720 nm). The maximal P700+ level during AL illumination (Pm′) was also determined by an application of SP. The steady-state P700+ level (P) was recorded just before applying an SP. The acceptor side limitation of PSI {Y(NA)} was calculated as (Pm − Pm′)/Pm, whereas the donor side limitation of PSI {Y(ND)} was calculated as P/Pm (Yamamoto et al., 2016; Wang and Shikanai, 2019). Y(I) was calculated from the complementary PSI quantum yields of nonphotochemical energy dissipation, Y(ND) and Y(NA). Y(I) = 1 − Y(ND) − Y(NA) (Klughammer and Schreiber, 1994). To evaluate the photodamage of PSI, the Pm level was monitored again 3 min after turning off the fluctuating light. The light intensity dependence of fluorescence and P700 parameters was determined using a light-curve program of the Dual-PAM system.
ECS measurements
The electrochromic shift (ECS) analysis was performed using a Dual-PAM 100 equipped with a P515/535 module (Walz). Plants were dark adapted for 30 min and then their detached leaves were exposed to AL for 3 min. After turning off AL, the ECS signal was monitored to record the level of ECSt and to chase the decay curve in the dark. ECSt represents the size of the light-induced pmf and was estimated from the total amplitude of the rapid decay of the ECS signal in the dark. ECSt levels were normalized against a 515-nm absorbance change induced by a single turnover flash, as measured in dark-adapted leaves before recording (Wang et al., 2015). The relative partitioning of the pmf into ECSint and ECSt-int was analyzed as previously described (Cruz et al., 2005; Wilson et al., 2021).
Plant growth in fluctuating light intensity
Plants were grown in plastic pots containing a mixture of equal volumes of vermiculite and nutrient soil (Metromix 350, Hyponex). They were grown in a growth chamber at 23°C at under the constant light condition for 2 weeks. Half of plants were then transferred to the fluctuating light condition and the remaining half was under the constant light condition for additional 3 weeks. Under both conditions, plants were exposed to HL at 500 μmol m−2 s−1 for 6 h and LL at 50 μmol m−2 s−1 for 6 h in a day. Under the constant light condition, the plants were exposed to LL for 3 h in the morning, and HL for 6 h in the middle of the day, and LL for 3 h in the evening (Figure 5A). Under the fluctuating light condition, HL for 10 min and LL for 10 min were alternated for 12 h a day. At 35 d after sowing, the aboveground parts of plants were sampled and dried at 80°C for several days and weighed.
To evaluate the photodamage of both PSs under the growth condition, plants were precultured, as described in the methods for the gas exchange analysis. After the short dark adaptation, the levels of Fv/Fm and Pm were monitored using a Dual-PAM system. The same leaf was successively used for the measurement before and after (2 and 4 d) exposed to the constant and fluctuating light conditions.
Gas exchange analysis
The gas exchange rate was measured in fully expanded young leaves with a portable gas exchange system (LI6400XT; LICOR, Lincoln, USA). Plants were preculture at 100- to 150-µmol photons m−2 s−1 under the short-day condition (8-h light/16-h dark) for 8–9 weeks. All photosynthetic parameters were analyzed at a cuvette temperature of 25°C and 65% relative humidity. Before the measurement, plants were kept in the dark overnight. We examined the photosynthetic responses to light intensity under two light conditions (Figure 6A). Under the constant light condition, the photosynthetic rate was initially measured at the photon flux density (PFD) of 50 μmol photons m−2 s−1 for 30 min and then at the PFD of 500 μmol photons m−2 s−1 for 60 min, and finally again at the PFD of 50-μmol photons m−2 s−1 for 30 min. Under the fluctuating light condition, the PFD was alternated between 50 μmol photons m−2 s−1 for 10 min and 500 μmol photons m−2 s−1 for 10 min and the cycle was repeated for 2 h.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers, NP_001190675.1 (KEA3, gene ID 825822), 168013124 (FlvA), and 168006765 (FlvB).
Supplemental data
The following supplemental materials are available in the online version of this article.
Supplemental Figure S1. Effects of fluctuating light intensity on photosynthetic parameters (1,529-µmol photons m−2 s−1).
Supplemental Figure S2. Effects of fluctuating light intensity on photosynthetic parameters (500-µmol photons m−2 s−1).
Supplemental Figure S3. Photodamage of both PSs under the culture conditions.
Supplemental Figure S4. Cumulative CO2 assimilation.
Supplemental Table S1. Statistical analysis of the gs.
Funding
This work was supported by the Japanese Society for the Promotion of Science KAKENHI (16H06555, 19H00992, and 21K19258) to T.S. and (16H06552, 20H05687, 21H02171) to W.Y.
Conflict of interest statement. None declared.
Supplementary Material
Contributor Information
Leonardo Basso, Department of Botany, Graduate School of Science, Kyoto University, Kyoto, 606-8502, Japan.
Kazuma Sakoda, Institute for Sustainable Agro-Ecosystem Services, Graduate School of Agriculture and Life Science, University of Tokyo, Tokyo, 188-0002, Japan; Japan Society for the Promotion of Science, Tokyo, Japan.
Ryouhei Kobayashi, Department of Botany, Graduate School of Science, Kyoto University, Kyoto, 606-8502, Japan.
Wataru Yamori, Institute for Sustainable Agro-Ecosystem Services, Graduate School of Agriculture and Life Science, University of Tokyo, Tokyo, 188-0002, Japan.
Toshiharu Shikanai, Department of Botany, Graduate School of Science, Kyoto University, Kyoto, 606-8502, Japan.
L.B. and T.S. designed research. L.B., K.S., R.K., and W.Y. performed experiments. All authors analyzed data and wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Toshiharu Shikanai (shikanai@pmg.bot.kyoto-u.ac.jp).
References
- Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro E-M (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110: 4111–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y, Isojärvi J, Zhang P, Aro E-M (2015) Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life 5: 716–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster U, Carrillo LR, Venema K, Pavlovic L, Schmidtmann E, Kornfeld A, Jahns P, Berry JA, Kramer DM, Jonikas MC (2014) Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun 5: 5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster U, Leonelli L, Correa Galvis V, Strand D, Quinn EH, Jonikas MC, Niyogi KK (2016) Regulation and levels of the thylakoid K+/H+ antiporter KEA3 shape the dynamic response of photosynthesis in fluctuating light. Plant Cell Physiol 57: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso L, Yamori W, Szabo I, Shikanai T (2020) Collaboration between NDH and KEA3 allows maximally efficient photosynthesis after a long dark adaptation. Plant Physiol 184: 2078–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B, Cardol P, Breyton C, Breyton C, Finazzi G (2010) Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res 106: 179–189 [DOI] [PubMed] [Google Scholar]
- Correa Galvis V, Strand DD, Messer M, Thiele W, Bethmann S, Hübner D, Uflewski M, Kaiser E, Siemiatkowska B, Morris BA, et al. (2020) H+ transport by K+ EXCHANGE ANTIPORTER3 promotes photosynthesis and growth in chloroplast ATP synthase mutants. Plant Physiol 182: 2126–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JA, Kanazawa A, Treff N, Kramer DM (2005) Storage of light-driven transthylakoid proton motive force as an electric field (Dc) under steady-state conditions in intact cells of Chlamydomonas reinhardtii. Photosynth Res 85: 221–233 [DOI] [PubMed] [Google Scholar]
- Davis GA, Rutherford AW, Kramer DM (2017) Hacking the thylakoid proton motive force for improved photosynthesis: modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Philos Trans R Soc Lond B Biol Sci 372: 20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Molina A, Leister D (2020) Accelerated relaxation of photoprotection impairs biomass accumulation in Arabidopsis. Nat Plants 6: 9–12 [DOI] [PubMed] [Google Scholar]
- Gerotto C, Alboresi A, Meneghesso A, Jokel M, Suorsa M, Aro E-M, Morosinotto T (2016) Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc Natl Acad Sci USA 113: 12322–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głowacka K, Kromdijk J, Kucera K, Xie J, Cavanagh AP, Leonelli L, Leakey ADB, Ort DR, Niyogi KK, Long SP (2018) Photosystem II subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat Commun 9: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilik P, Pavlovič A, Kouril R, Alboresi A, Morosinotto T, Allahverdieyeva Y, Aro E-M, Yamamoto H, Shikanai T (2017) Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol 214: 967–972 [DOI] [PubMed] [Google Scholar]
- Jahns P, Graf M, Munekage Y, Shikanai T (2002) Single point mutation in the Rieske iron-sulfur subunit of cytochrome b6/f leads to an altered pH dependence of plastoquinol oxidation in Arabidopsis. FEBS Lett 519: 99–102 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Ruban AV (2014) Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth Res 119: 233–242 [DOI] [PubMed] [Google Scholar]
- Jokel M, Johnson X, Peltier G, Aro E-M, Allahverdiyeva Y (2018) Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant J 94: 822–835 [DOI] [PubMed] [Google Scholar]
- Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM (2014) Dynamic photosynthesis in different environmental conditions. J Exp Bot 66: 2451–2462 [DOI] [PubMed] [Google Scholar]
- Kimura H, Hashimoto-Sugimoto M, Iba K, Terashima I, Yamori W (2020) Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J Exp Bot 71: 2339–2350 [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P7001-absorbance changes at 830 nm. Planta 192: 261–268 [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354: 857–861 [DOI] [PubMed] [Google Scholar]
- Kunz HH, Gierth M, Herdean A, Satoh-Cruz M, Kramer DM, Spetea C, Schroeder JI (2014) Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc Natl Acad Sci USA 111: 7480–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Calcagno PE, Fisk S, Brown KL, Bull SE, South PF, Raines CA (2018) Overexpressing the H-protein of the glycine cleavage system increases biomass yield in glasshouse and field-grown transgenic tobacco plants. Plant Biotech J 17: 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Takeda S, Endo T, Jahns P, Hashimoto T, Shikanai T (2001) Cytochrome b6f mutation specifically affects thermal dissipation of absorbed light energy in Arabidopsis. Plant J 28: 351–359 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Nishio JN, Whitmarsh J (1993) Dissipation of the proton electrochemical potential in intact chloroplasts (II. The pH gradient monitored by cytochrome f reduction kinetics). Plant Physiol 101: 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV (2016) Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol 170: 1903–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Wilson S (2021) The mechanism of non-photochemical quenching in plants: localisation and driving forces. Plant Cell Physiol 62: 1063–1072 [DOI] [PubMed] [Google Scholar]
- Rumberg B, Siggel U (1969) pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften 56: 130–132 [DOI] [PubMed] [Google Scholar]
- Sakoda K, Yamori W, Shimada T, Sugano SS, Hara-Nishimura I, Tanaka Y (2020) Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front Plant Sci 11: 589603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sétif P, Shimakawa G, Krieger-Liszkay A, Miyake C (2020) Identification of the electron donor to flavodiiron proteins in Synechocystis sp. PCC 6803 by in vivo spectroscopy. Biochim Biophys Acta – Bioenergetics 1861: 148526. [DOI] [PubMed] [Google Scholar]
- Shahinnia F, Tula S, Hensel G, Reiahisamani N, Nasr N, Kumlehn J, Gómez R, Lodeyro AF, Carrillo N, Hajirezaei MR (2021) Plastid-targeted cyanobacterial flavodiiron proteins maintain carbohydrate turnover and enhance drought stress tolerance in barley. Front Plant Sci 11: 613731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Yamamoto H (2017) Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol Plant 10: 20–29 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767: 1233–1244 [DOI] [PubMed] [Google Scholar]
- Theune ML, Hildebrandt S, Steffen-Heins A, Bilger W, Gutekunst K, Appel J (2021) In-vivo quantification of electron flow through photosystem I – Cyclic electron transport makes up about 35% in a cyanobacterium. Biochim Biophys Acta – Bioenerget 1862: 148353. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kera K, Hamamoto S, Kuromori T, Shikanai T, Uozumi N (2019) Evidence for potassium transport activity of Arabidopsis KEA1-KEA6. Sci Rep 9: 10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov AN, Khomutov GB, Ruuge EK, Blumenfeld LA (1981) Electron transport control in chloroplasts: effects of photosynthetic control monitored by the intrathylakoid pH. Biochim Biophys Acta 637: 321–333 [Google Scholar]
- Tula S, Shahinnia F, Melzer M, Rutten T, Gómez R, Lodeyro AF, von Wirén N, Carrillo N, Hajirezaei MR (2020) Providing an additional electron sink by the introduction of cyanobacterial flavodiirons enhances growth of A. thaliana under various light intensities. Front Plant Sci 11: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T (2019) PGR5-dependent cyclic electron flow protects PSI under fluctuating light at donor and acceptor sides. Plant Physiol 179: 588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Takahashi S, Badger MR, Shikanai T (2016) Artificial remodeling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat Plants 2: 16012. [DOI] [PubMed] [Google Scholar]
- Vicente JB, Gomes CM, Wasserfallen A, Teixeira M (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun 294: 82–87 [DOI] [PubMed] [Google Scholar]
- Wada S, Yamamoto H, Suzuki Y, Yamori Y, Shikanai T, Makino A (2018) Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol 176: 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shikanai T (2019) Modification of activity of the thylakoid H+/K+ antiporter KEA3 disturbs ΔpH-dependent regulation of photosynthesis. Plant Physiol 181: 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yamamoto H, Shikanai T (2015) Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochim Biophys Acta 1847: 931–938 [DOI] [PubMed] [Google Scholar]
- Wang C, Yamamoto H, Narumiya F, Munekage YN, Finazzi G, Szabo I, Shikanai T (2017) Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J 89: 540–553 [DOI] [PubMed] [Google Scholar]
- Wilson S, Johnson MP, Ruban AV (2021) Proton motive force in plant photosynthesis dominated by ΔpH in both low and high light. Plant Physiol 187: 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.