Abstract
Background
Although efforts to treat hepatitis C virus (HCV) in people who inject drugs (PWID) yield high rates of sustained virologic response (SVR), the relationship between successful HCV treatment and health-related quality of life (HRQOL) among PWID is poorly understood. We examined HRQOL changes throughout HCV treatment and post-treatment for PWID achieving SVR.
Methods
Participants included 141 PWID who achieved SVR following HCV treatment onsite at 3 opioid agonist treatment (OAT) clinics in the Bronx, New York. EQ-5D-3L assesses 5 health dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), producing an index of HRQOL ranging from 0 to 1. EQ-5D-3L was measured at baseline; 4, 8, and 12 weeks during treatment; and 12 and 24 weeks post-treatment. Linear mixed effects regression models assessed changes in the mean EQ-5D-3L index over time.
Results
Mean EQ-5D-3L index baseline was 0.66 (standard error [SE] = 0.02). While over half the population reported no baseline problems with self-care (85.1%), usual activities (56.0%), and mobility (52.5%), at least two-thirds reported problems with pain/discomfort (78.0%) and anxiety/depression (66.0%). Twenty-four weeks post-treatment, proportions reporting pain/discomfort and anxiety/depression decreased by 25.7% and 24.0%, respectively. Mean EQ-5D-3L index significantly improved during treatment (P < .0001), and improvement was sustained following treatment completion, with mean EQ-5D-3L index of 0.77 (SE = 0.02) 12 weeks post-SVR.
Conclusions
HCV treatment led to sustained improvement in HRQOL for PWID on OAT who achieved SVR. Future research is necessary to determine whether improvements in HRQOL can be sustained beyond 12 weeks post-SVR.
Keywords: direct-acting antiviral, hepatitis C virus, health-related quality of life, opioid agonist treatment, people who inject drugs
People who inject drugs (PWID) successfully treated for hepatitis C virus (HCV) during opioid agonist treatment (OAT) had sustained increases in health-related quality of life (HRQOL). We describe HRQOL improvements during and post successful HCV treatment for PWID on OAT.
In 2017, nearly three-quarters (73.0%) of individuals with acute hepatitis C virus (HCV) infections in the United States reported injection drug use (IDU) [1]. Expanding coverage of HCV treatment to people who inject drugs (PWID) can improve patient-reported outcomes [2] and be an effective strategy to reduce prevalence of HCV among PWID [3, 4]. Concomitant HCV treatment with opioid agonist treatment (OAT) has shown promise as a model of care that promotes high adherence, treatment completion, and sustained virologic response (SVR), or HCV cure [5–8].
Studies show that health-related quality of life (HRQOL) of populations living with HCV is often low in comparison to the population norms within their respective countries [6, 9–13]. Many studies have reported an association between SVR and HRQOL [2, 8, 14–16], which appears to be driven by the type of HCV treatment [8]. Whereas interferon-based treatments appear to have little/no effect on HRQOL of individuals with HCV [10, 14, 17], those treated with direct-acting antivirals (DAAs) report increased HRQOL 3 to 12 months following treatment [17–19]. A study of patients with chronic HCV in Spain reported significant decreases in the proportion of patients reporting problems with mobility, pain/discomfort, and anxiety/depression 12 weeks post-DAA treatment [19], and individuals treated for HCV in Japan continued to report improvements in general health perception up to 3 years post-DAA administration [20]. While these findings are encouraging, many of these studies do not include PWID [10, 18], which limits the generalizability of findings to this population.
Assessing how HCV treatment might impact HRQOL for PWID is necessary to better understand the relationship between curing HCV and improving patient-centered health outcomes for a population whose stigmatization often creates barriers to accessing treatment [21–23]. Furthermore, understanding benefits of HCV cure for PWID beyond hepatic manifestations may motivate provider- and system-level changes that increase the provision of HCV treatment for PWID. The lack of research focusing on HRQOL of PWID with HCV is surprising, given that patients living with chronic HCV and a history of IDU report a lower quality of life compared with those with no history of IDU [24]. Overall, lower HRQOL among PWID may be attributed to the increased prevalence of severe mental health and physical comorbidities [25], as well as an increased likelihood of experiencing poverty [26], homelessness [27, 28], and incarceration [29]. These issues may persist following SVR, which could influence post-treatment HRQOL.
Despite encouraging findings from OAT treatment and the increase in studies using concomitant HCV treatment with OAT, only 1 study has investigated the relationship between DAA treatment and HRQOL among PWID. From 2016 to 2018, Schulte et al [6] assessed changes in HRQOL among German PWID treated with DAA, reporting small significant increases in mental health but no significant differences in physical health for individuals with and without SVR 24 weeks post-treatment. While encouraging, further research is necessary to assess generalizability of these results to PWID with SVR.
Understanding how SVR may influence HRQOL in PWID is necessary in order to increase HRQOL in this population. Our objective in this study was to examine changes in HRQOL throughout HCV treatment and post-treatment for PWID receiving OAT who achieve SVR.
METHODS
Study Population and Design
This is a secondary analysis of data collected from participants enrolled in the PREVAIL study, a randomized clinical trial investigating intensive models of HCV care for individuals who inject drugs (NCT01857245) [30]. Patients living with HCV were recruited from OAT clinics in the Bronx, New York, from October 2013 to May 2016. The majority of the population (75.0%) reported previous IDU.
HCV treatment regimens were based on guidelines from the American Association for the Study of Liver Diseases at the time of treatment initiation. The majority of participants received second-generation DAAs, defined as combination regimens that were interferon-free, ribavirin-free, or interferon- and ribavirin-free, including simeprevir and sofosbuvir (SIM/SOF) and sofosbuvir and ledipasvir (SOF/LDV). Few received first-generation DAAs, defined as regimens that contain ribavirin, interferon, or ribavirin and interferon, including sofosbuvir and ribavirin (SOF/RBV); telaprevir, ribavirin, and pegylated interferon (TVR/RBV/PEG); and sofosbuvir, ribavirin, and pegylated interferon (SOF/RBV/PEG). As only 7 of 150 individuals did not achieve SVR (2 deceased prior to determining SVR status), there was an insufficient sample size to perform stratified analysis as a function of SVR. Additionally, a sensitivity analysis showed no significant difference for the main outcome when all 150 participants were included compared with a sample limited to those who achieved SVR (N = 141) (Supplementary Figure 1). Therefore, the primary analytic sample for the current study was limited to 141 individuals (94.0%) who achieved SVR.
Study Participants
Individuals were eligible for participation in the PREVAIL study if they were aged ≥18 years, were treatment-naive (or treatment experienced after 3 December 2014), and spoke English or Spanish. Individuals were excluded if they had decompensated cirrhosis, were unable to provide informed consent, were pregnant or breastfeeding, or had hypersensitivity to HCV medication. Eligibility for the current study was further limited to participants who achieved SVR, defined as anyone whose HCV RNA viral load was undetectable, lower than 43 mm/IU at an earlier stage of the trial, or lower than 15 mm/IU at a later stage of the trial (as per laboratory guidelines).
Patient demographics and clinical information were collected at the baseline assessment; during the 4-, 8-, and 12-week research visits during treatment; and 12 and 24 weeks following treatment. Additional information for the study population and study design is provided in detail elsewhere [30, 31].
Health-related Quality of Life
The EQ-5D-3L is a standardized measure of health status that provides a simple descriptive profile and single index value for health status [32]. Although applicable to a wide range of health conditions, EQ-5D-3L has frequently been used to measure HRQOL in populations living with HCV [9, 10, 14, 33]. EQ-5D-3L uses responses for 5 health domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) to measure a respondent’s health state. Health states are determined by the scores reported for each domain, where 1 = “no problems,” 2 = “some problems,” and 3 = “extreme problems” [32]. Final health states were converted into a summary index using US-specific valuation weights at each level of every domain with a standardized formula provided by the Agency for Healthcare Research and Quality [34, 35]. Final EQ-5D-3L scores range from 0 to 1, where 1 is full health and 0 indicates death [32]. Participants were assessed with the EQ-5D-3L at baseline, during treatment (4, 8, and 12 weeks), and post-treatment (12 and 24 weeks).
Statistical Analyses
χ 2 tests and T tests were used to examine differences in the baseline demographic and clinical characteristics by treatment type (second-generation DAAs vs first-generation DAAs). Percent differences were calculated to examine changes in the proportion of individuals reporting any problems (ie, some problems or extreme problems) for each EQ-5D-3L domain from baseline to 12 weeks post-SVR. Linear mixed effects models were used to determine changes in HRQOL over time. A first-order autoregressive correlation structure was incorporated to account for temporal correlations among repeated values. Sensitivity analyses were performed to compare HRQOL over time as a function of treatment type: second-generation DAAs (SOF/LDV, SIM/SOF) and first-generation DAAs (SOF/RBV/PEG, SOF/RBV, and TVR/RBV/PEG). All statistical analyses were conducted using SAS 9.4 (SAS Inc, Cary, NC).
RESULTS
The sample was predominantly male (63.8%) with a mean age of 51.3 years (range, 25–73). Half of the participants were Hispanic (56.0%), a quarter were Black (25.5%), 8.5% were White, and 9.9% were another race (Table 1). The majority received methadone (98.6%), and more than three-quarters were taking second-generation DAAs (77.3%). Sensitivity analyses showed no statistically significant differences in demographic or clinical characteristics by treatment type. Additional medical, mental health, and substance use comorbidities for this patient population are described elsewhere [36].
Table 1.
Baseline Demographic and Clinical Characteristics of People Who Inject Drugs on Opioid Agonist Treatment With Sustained Virologic Responsea
| Demographic Characteristic | N (%) |
|---|---|
| Ageb | 51.3 (10.6) |
| Sex | |
| Male | 90 (63.8) |
| Female | 50 (35.5) |
| Race | |
| Non-Hispanic White | 12 (8.5) |
| Non-Hispanic Black | 36 (25.5) |
| Hispanic | 79 (56.0) |
| Other | 14 (9.9) |
| Education | |
| Non-high school graduate | 59 (41.8) |
| High school or higher | 82 (58.3) |
| Marital status | |
| Married | 52 (36.9) |
| Not married | 89 (63.1) |
| Employment | |
| Employed | 27 (19.2) |
| Unemployed | 73 (51.8) |
| Disabled | 41 (29.1) |
| Clinical Characteristics | |
| Treatment typec | |
| Second-generation DAA | 109 (77.3) |
| First-generation DAA | 32 (22.7) |
| Type of opioid agonist treatment | |
| Methadone | 139 (98.6) |
| Buprenorphine | 2 (1.3) |
| Treatment group | |
| Treatment as usual | 46 (32.6) |
| Group treatment | 45 (31.9) |
| Directly observed treatment | 50 (35.5) |
| EQ-5D-3L indexd,e | 0.66 (0.02) |
| Liver disease severity | |
| Cirrhosis | 39 (27.7) |
| No cirrhosis | 102 (72.3) |
| Mobility | |
| No problems | 74 (52.5) |
| Some problems | 67 (47.5) |
| Extreme problems | 0 (0) |
| Self-care | |
| No problems | 120 (85.1) |
| Some problems | 20 (14.2) |
| Extreme problems | 1 (0.71) |
| Usual activities | |
| No problems | 79 (54.7) |
| Some problems | 55 (40.7) |
| Extreme problems | 7 (4.7) |
| Pain/Discomfort | |
| No problems | 31 (22.0) |
| Some problems | 79 (56.0) |
| Extreme problems | 31 (22.0) |
| Anxiety/Depression | |
| No problems | 48 (34.0) |
| Some problems | 63 (44.7) |
| Extreme problems | 30 (21.3) |
Abbreviation: DAA, direct-acting antiviral.
aSample Size = 141.
bMean (standard deviation).
cFirst-generation DAAs were defined as regimens that contain ribavirin and/or interferon, including sofosbuvir (SOF) and ribavirin; telaprevir, ribavirin, and pegylated interferon; and sofosbuvir, ribavirin, and pegylated interferon. Second-generation DAAs were defined as regimens that are interferon-free and/or ribavirin-free, including simeprevir/SOF and SOF/ledipasvir.
dMean (standard error).
The mean EQ-5D-3L index at baseline was 0.66 (standard error [SE] = 0.02). More than half reported no problems with self-care (85.1%), usual activities (56.0%), or mobility (52.5%). While nearly half reported problems with mobility and usual activities (47.3% and 45.3%, respectively), very few reported extreme problems; no patients reported extreme problems with mobility, and only 4.7% reported extreme problems with usual activities. In contrast, at least two-thirds reported problems with pain/discomfort (78.0%) or anxiety/depression (66.0%), with nearly one-quarter reporting extreme problems: 22.0% for pain/discomfort and 21.3% for anxiety/depression.
Health-related Quality of Life
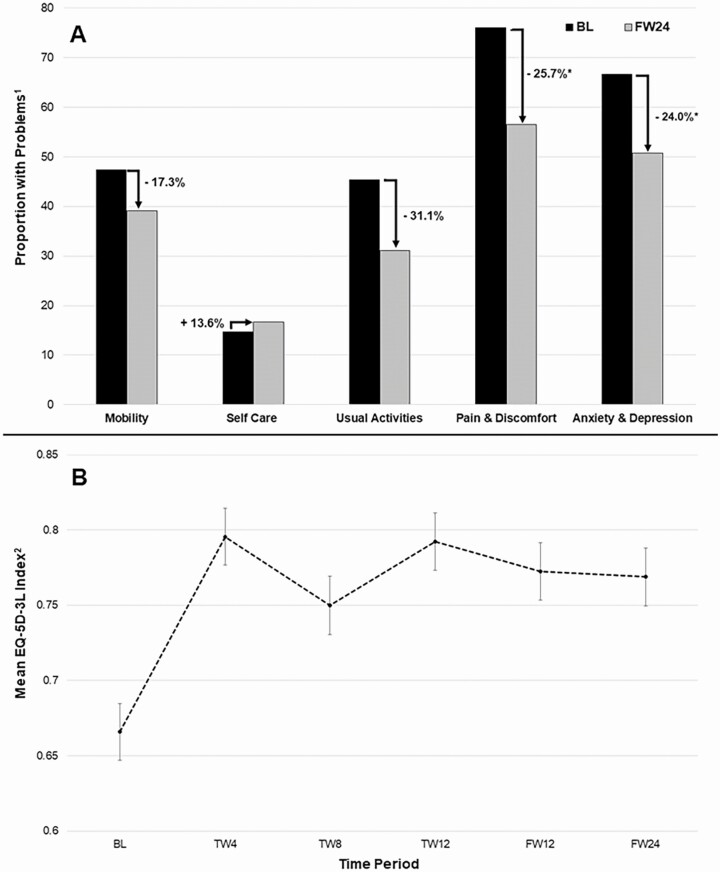
The proportion of individuals reporting any problems decreased from baseline to 24 weeks of follow-up for every domain but self-care, for which there was no evidence of change (14.7% vs 16.7%, P = .967; Figure 1A, Supplementary Table 1). The proportion reporting problems with mobility and/or usual activities decreased by 17.3% and 31.1%, respectively; however, neither difference was statistically significant. In contrast, for pain/discomfort, the proportion reporting problems at 24 weeks of follow-up was significantly lower compared with the baseline proportion(s) (76.0% vs 56.5%, P ≤ .0001) and/or anxiety/depression (66.7% vs 50.7%, P = .0028), yielding a 25.7% and 24.0% decrease in the proportion reporting problems for pain/discomfort and anxiety/depression, respectively.
Figure 1.
A, Five health domains of the EQ-5D-3L showing differences in the proportion of people who inject drugs (PWID) reporting problems [1] at baseline and at 12 weeks post-sustained virologic response (SVR). B, Mean EQ-5D-3L index [2] for PWID with SVR over time. The asterisk (*) indicates statistical significance at P ≤ .001. 1Individual reports either “some problems” or “extreme problems.” 2The EQ-5D-3L index is a summary score calculated using individual ratings of 5 health domains and valuation weights for the United States [34, 35]. Abbreviations: BL, baseline; FW, follow-up week; TW, treatment week.
Improvements in the mean EQ-5D-3L index from baseline peaked at 4 weeks of treatment (0.66 vs 0.80, P < .0001) and 12 weeks of treatment (0.66 vs 0.79, P < .0001; Figure 1B). Significant increases in the mean EQ-5D-3L index were also shown at 12 weeks of follow-up (0.66 vs 0.77, P < .0001) and 24 weeks of follow-up (0.66 vs 0.77, P < .0001), indicating the increase in overall HRQOL for PWID with SVR was sustained over time.
Sensitivity Analyses
Sensitivity analyses showed that changes in the mean EQ-5D-3L index were not significantly associated with HCV treatment type (P = .0617) and that increases in the mean EQ-5D-3L index between treatment types over time were not statistically significant (P = .1728; Supplementary Figure 2A). There were significant improvements in HRQOL for the second-generation DAA group over time (P = <.0001). Those on second-generation DAAs showed a significant increase in the mean EQ-5D-3L index that was sustained at 12 weeks (0.65 vs 0.75, P = .0003) and 24 weeks (0.65 vs 0.75, P = .0008) post-treatment. In contrast, although those on first-generation DAAs reported significant increases in the mean EQ-5D-3L index at 12 weeks (0.71 vs 0.86, P = .0086) and 24 weeks (0.71 vs 0.85, P = .0176) post-treatment, overall changes in the mean EQ-5D-3L over time were not significant for first-generation DAAs (P = .1425).
Similar trends were observed when specific HCV medications were examined (Supplementary Figure 2B). Only individuals taking second-generation DAAs regimens of SOF/LDV reported significant increases in the mean EQ-5D-3L index that were sustained at 12 weeks (0.66 vs 0.75, P = .0009) and 24 weeks (0.65 vs 0.75, P = .0024) post-treatment. No other treatment regimen showed a significant change in the mean EQ-5D-3L index over time.
Discussion
In this study, we are the first to examine changes in HRQOL following SVR among PWID engaged in OAT in the United States. Over the course of treatment, HRQOL significantly increased among PWID achieving SVR, and those increases were sustained 24 weeks following HCV treatment. Increases in HRQOL could be attributed to the significant decreases in the proportion of individuals reporting problems with pain/discomfort and the decrease in the proportion of PWID reporting problems with anxiety/depression.
These findings show PWID on OAT report significant increases in HRQOL that were sustained 12 weeks post-SVR. Whereas this finding is consistent with the results of other studies that assessed the impact of DAA regimens on HRQOL in HCV-infected populations [6, 14, 17–19], including those on OAT [8], this study adds to the current body of knowledge by examining this relationship among PWID on OAT within the United States. Demonstrating the benefits to HRQOL that result from successful DAA treatment during OAT is of particular importance as PWID continue to face personal and provider- and system-level barriers that may prevent them from accessing HCV treatment [37]. PWID may not seek HCV treatment if actively injecting drugs out of fear that clinicians will reject them or reinfection may prevent them from accessing future treatment [22]. Additionally, many PWID continue to face challenges finding clinicians to provide HCV treatment; not all OAT programs are ready/willing to provide HCV treatment concurrently with OAT [22]. These barriers may explain the low baseline EQ-5D-3L index of our population (0.66; SE = 0.02). Although the mean baseline EQ-5D-3L index of the current study is similar to that of a population of PWID with chronic HCV in Scotland (0.69) [12] and a sample of HCV-infected individuals receiving OAT in Germany (0.71) [38], it remains low in comparison to baseline indices reported by HCV-infected non-PWID populations, which range from 0.823 to 0.92 [14, 39, 40]. Although the mean EQ-5D-3L index in the current study increased to 0.77 (SE = 0.02), this is still below those for the US population, which fall between 0.83 and 0.94 [39, 41, 42]. Future research might build on the momentum of successful HCV treatment by providing additional services that may sustain or increase HRQOL for PWID. Following SVR, clinicians may shift focus to addressing co-occurring substance use and tobacco cessation or encourage and provide resources for harm reduction services to prevent reinfection. Perhaps these services could be incorporated into a standardized checklist implemented during reinfection follow-up to increase the overall health and well-being of PWID following SVR.
Significant decreases were noted in the proportion of PWID reporting problems with pain/discomfort. Consistent with previous studies, the baseline proportion of problems with pain/discomfort in the current population of PWID was high. In 1 study, only 25.7% of the non-PWID population in HCV treatment reported any baseline problems with pain/discomfort [14], whereas in another study where half of the population reported a history of IDU, 60.0% reported baseline problems with pain/discomfort [19]. This difference is not surprising as IDU is often associated with severe infections or poor vein health that likely contributes to the increased prevalence of pain/discomfort among PWID [22, 43, 44]. Perhaps the decrease in pain/discomfort following SVR results from the elimination of painful neuropathic and musculoskeletal conditions associated with HCV [45, 46]. However, SVR may not be solely responsible for decreasing pain/discomfort in PWID. One study reported HCV exposure was not significantly associated with pain/discomfort after accounting for other confounding factors [33], suggesting that other factors may have an impact on pain/discomfort during HCV treatment among PWID. Increased attention from medical personnel might provide opportunities for PWID to be treated for infections and have access to additional medical or psychological care, and increased trust in clinicians may influence the willingness of PWID to seek additional care. Many PWID report discrimination from healthcare workers [47], and discrimination is often associated with decreased mental health and physical functioning [48]. It is likely that both elimination of painful conditions associated with HCV and the relationship formed with clinicians that increased the comfort of PWID to address underlying medical issues were responsible for decreasing pain/discomfort following SVR.
We also found significant decreases in the proportion of PWID reporting problems with anxiety/depression from baseline to 12 weeks post-SVR. These findings are complementary to those of Schulte et al, who reported significant increases in overall mental health for PWID treated with DAAs during OAT up to 12 weeks following treatment completion [6]. Similar to pain/discomfort, anxiety/depression appears to be much more prevalent among HCV-infected PWID compared with HCV-infected non-PWID in HCV treatment, as only 25.6%–57.0% of HCV-infected non-PWID reported baseline problems with anxiety/depression [14, 19]. These decreases in anxiety/depression might be attributed to increased engagement with clinicians or the supportive environment among other PWID in treatment for OAT [36]. Further, decreases in the proportion reporting problems with anxiety/depression likely contributed to decreases in the proportion reporting pain/discomfort, as psychological distress has been linked to pain and poor health [33].
Sensitivity analysis by treatment type showed sustained increases in HRQOL up to 12 weeks post-SVR for both second-generation DAAs and first-generation DAAs. However, in comparison to individuals taking second-generation DAAs, those on first-generation DAAs did not show a statistically significant change in HRQOL over time. This appears to be consistent with previous findings that show that first-generation DAAs containing interferon and/or ribavirin often negatively impact HRQOL during treatment [8, 17] or show no significant differences in HRQOL following treatment [14, 38]. This is often attributed to the negative side effects that occur with first-generation DAAs, including depressive symptoms, psychological syndromes, and fatigue [38]. One potential explanation for our disparate finding might be the receipt of OAT by all participants receiving HCV treatment, as OAT attendance is associated with improved HRQOL [49]. However, in a 2008 study of HCV-infected patients attending OAT in Germany, Schäfer et al reported no significant changes in HRQOL following HCV treatment with first-generation DAAs during OAT [38]. Nevertheless, this finding may further support the theory that individuals in the current study were able to access additional services and resources. Whereas those treated with first-generation DAAs may still experience adverse side effects, it is possible that better access to clinicians afforded them more opportunities to address those concerns.
Taken together, these findings highlight the importance of HCV treatment not only to cure HCV but also as a potential avenue for PWID to access additional services and resources instrumental to increasing HRQOL. These findings emphasize the overall health benefits of providing HCV treatment for PWID and should serve to support further efforts to engage and treat all PWID for HCV.
This study has several limitations. The PREVAIL study was composed of a small, predominantly male sample of PWID receiving OAT who were recruited from an urban environment in New York and may not be generalizable to non-PWID, women, individuals not receiving OAT, or rural populations. This sample size was too small to perform a stratified analysis as a function of SVR, highlighting the need for additional research in a large sample that can assess HRQOL in PWID on OAT with and without SVR. HRQOL was assessed using the EQ-5D-3L, a generic questionnaire. Although this questionnaire has been used to assess HRQOL in many populations with HCV [9, 10, 14, 33], future studies might consider combining these responses with those of an HCV-specific questionnaire. Additionally, it is possible that informing patients of their HCV RNA status once achieving SVR may have influenced their HRQOL ratings in successive visits. We did not examine the potential influence of treatment intervention group on HRQOL. Finally, future research may be warranted to determine long-term HRQOL following SVR in this high-priority population.
Conclusions
HCV treatment with first- and second-generation DAAs during OAT led to a sustained improvement in HRQOL for PWID achieving SVR, and significant decreases were seen in the proportion of individuals reporting problems with pain/discomfort and anxiety/depression. However, HRQOL reported by PWID continues to remain low in comparison to HRQOL in the general US population. Future efforts should consider the implementation of a standardized checklist for reinfection follow-up that would provide continued access to care for PWID and maintain increases in HRQOL following SVR. Future research is necessary to determine whether the increases in HRQOL can be sustained beyond 12 weeks post-SVR.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful for the collaborative support from the Addiction Research Center at the Prisma Health in Greenville, South Carolina.
Disclaimer. The parent PREVAIL trial is registered at ClinicalTrial.gov (NCT01857245). In addition to grant support, Gilead provided study medications. The contents of the work are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the US government.
Financial support. This work was supported in part by grants from the National Institute on Drug Abuse (R01DA034086) and Gilead Sciences (IN-337-1779).
Potential conflicts of interest. A. H. L. has served on advisory boards for Merck Pharmaceuticals, AbbVie, and Gilead Sciences and has received research grants from Merck Pharmaceuticals and Gilead Sciences. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Viral Hepatitis Surveillance United States. Centers for Disease Control and Prevention. 2017. Updated November 14, 2019. https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm. Accessed 1 July 2021.
- 2. Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis 2015; 15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hickman M, De Angelis D, Vickerman P, Hutchinson S, Martin NK. Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence. Curr Opin Infect Dis 2015; 28:576– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zelenev A, Li J, Mazhnaya A, Basu S, Altice FL. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis 2018; 18:215– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pericot-Valverde I, Rennert L, Heo M, et al. Rates of perfect self-reported adherence to direct-acting antiviral therapy and its correlates among people who inject drugs on medications for opioid use disorder: the PREVAIL study. J Viral Hepat 2021; 28:548– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulte B, Schmidt CS, Manthey J, et al. Clinical and patient-reported outcomes of direct-acting antivirals for the treatment of chronic hepatitis C among patients on opioid agonist treatment: a real-world prospective cohort study. Open Forum Infect Dis 2020; 7:ofaa317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graf C, Mücke MM, Dultz G, et al. Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis 2020; 70:2355– 65. [DOI] [PubMed] [Google Scholar]
- 8. Stepanova M, Thompson A, Doyle J, Younossi I, de Avila L, Younossi ZM. Hepatitis C virus-infected patients receiving opioid substitution therapy experience improvement in patient-reported outcomes following treatment with interferon-free regimens. J Infect Dis 2018; 217:1033– 43. [DOI] [PubMed] [Google Scholar]
- 9. Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003; 98:630– 8. [DOI] [PubMed] [Google Scholar]
- 10. Jang ES, Kim YS, Kim KA, et al. Factors associated with health-related quality of life in Korean patients with chronic hepatitis C infection using the SF-36 and EQ-5D. Gut Liver 2018; 12:440– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Juanbeltz R, Castilla J, Martínez-Baz I, O’Leary A, Sarobe M, San Miguel R. Health-related quality of life in hepatitis C patients who achieve sustained virological response to direct-acting antivirals: a comparison with the general population. Qual Life Res 2019; 28:1477– 84. [DOI] [PubMed] [Google Scholar]
- 12. McDonald SA, Hutchinson SJ, Palmateer NE, et al. Decrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in Scotland. J Hepatol 2013; 58:460– 6. [DOI] [PubMed] [Google Scholar]
- 13. Dalgard O, Egeland A, Skaug K, Vilimas K, Steen T. Health-related quality of life in active injecting drug users with and without chronic hepatitis C virus infection. Hepatology 2004; 39:74– 80. [DOI] [PubMed] [Google Scholar]
- 14. Vera-Llonch M, Martin M, Aggarwal J, et al. Health-related quality of life in genotype 1 treatment-naïve chronic hepatitis C patients receiving telaprevir combination treatment in the ADVANCE study. Aliment Pharmacol Ther 2013; 38:124– 33. [DOI] [PubMed] [Google Scholar]
- 15. Loo N, Lawitz E, Alkhouri N, et al. Ombitasvir/paritaprevir/ritonavir + dasabuvir +/– ribavirin in real world hepatitis C patients. World J Gastroenterol 2019; 25:2229– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saeed S, Moodie EEM, Strumpf E, et al. ; Canadian Co-Infection Cohort Study Investigators. . Real-world impact of direct acting antiviral therapy on health-related quality of life in HIV/hepatitis C co-infected individuals. J Viral Hepat 2018; 25:1507– 14. [DOI] [PubMed] [Google Scholar]
- 17. Younossi ZM, Stepanova M, Nader F, Lam B, Hunt S. The patient’s journey with chronic hepatitis C from interferon plus ribavirin to interferon- and ribavirin-free regimens: a study of health-related quality of life. Aliment Pharmacol Ther 2015; 42:286– 95. [DOI] [PubMed] [Google Scholar]
- 18. Serper M, Evon DM, Amador J, et al. Patient-reported outcomes 12 months after hepatitis C treatment with direct-acting antivirals: results from the PROP UP study. Liver Int 2021; 41:692– 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juanbeltz R, Martínez-Baz I, San Miguel R, Goñi-Esarte S, Cabasés JM, Castilla J. Impact of successful treatment with direct-acting antiviral agents on health-related quality of life in chronic hepatitis C patients. PLoS One 2018; 13:1– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ichikawa T, Miyaaki H, Miuma S, et al. Direct-acting antivirals improved the quality of life, ameliorated disease-related symptoms, and augmented muscle volume three years later in patients with hepatitis C virus. Intern Med 2020; 59:2653– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodyear T, Brown H, Browne AJ, Hoong P, Ti L, Knight R. “Stigma is where the harm comes from”: exploring expectations and lived experiences of hepatitis C virus post-treatment trajectories among people who inject drugs. Int J Drug Policy 2021. doi:10.1016/j.drugpo.2021.103238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madden A, Hopwood M, Neale J, Treloar C. Beyond interferon side effects: what residual barriers exist to DAA hepatitis C treatment for people who inject drugs? PLoS One 2018; 13:e0207226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang K, Neil J, Wright J, Dell CA, Berenbaum S, El-Aneed A. Qualitative investigation of barriers to accessing care by people who inject drugs in Saskatoon, Canada: perspectives of service providers. Subst Abuse Treat Prev Policy 2013; 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998; 27:209– 12. [DOI] [PubMed] [Google Scholar]
- 25. Robaeys G, Grebely J, Mauss S, et al. ; International Network on Hepatitis in Substance Users. . Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis 2013; 57:S129– 37. [DOI] [PubMed] [Google Scholar]
- 26. Hall CS, Charlebois ED, Hahn JA, Moss AR, Bangsberg DR. Hepatitis C virus infection in San Francisco’s HIV-infected urban poor. J Gen Intern Med 2004; 19:357– 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gelberg L, Robertson MJ, Arangua L, et al. Prevalence, distribution, and correlates of hepatitis C virus infection among homeless adults in Los Angeles. Public Health Rep 2012; 127:407– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hotton A, Mackesy-Amiti ME, Boodram B. Trends in homelessness and injection practices among young urban and suburban people who inject drugs: 1997-2017. Drug Alcohol Depend 2021; 225:108797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Treloar C, Schroeder S, Lafferty L, et al. Structural competency in the post-prison period for people who inject drugs: a qualitative case study. Int J Drug Policy 2021; 95:103261. [DOI] [PubMed] [Google Scholar]
- 30. Akiyama MJ, Agyemang L, Arnsten JH, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis 2018; 18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170:594– 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. EQ-5D-3L user guide basic information on how to use the EQ-5D-3L instrument. Version 6.0. EuroQol Research Foundation. Updated December 2018. https://archive.ahrq.gov/professionals/clinicians-providers/resources/rice/EQ5Dscore.html. Accessed 1 February 2021. [Google Scholar]
- 33. Ng M, Hayashi K, Voon P, et al. Characterising the association between positive hepatitis C virus antibody and pain among people who inject drugs. Drug Alcohol Rev 2019; 38:639– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calculating the U.S. population-based EQ-5D index score: research initiative in clinical economics. 2005. Rockville, MD: Agency for Healthcare Research and Quality. https://archive.ahrq.gov/professionals/clinicians-providers/resources/rice/EQ5Dscore.html. Accessed 1 February 2021. [DOI] [PubMed] [Google Scholar]
- 35. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005; 43:203– 20. [DOI] [PubMed] [Google Scholar]
- 36. Pericot-Valverde I, Heo M, Niu J, et al. Declines in depressive symptoms among people who inject drugs treated with direct-acting antivirals while on opioid agonist therapy. Open Forum Infect Dis 2020; 7:ofaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heard E, Smirnov A, Massi L, Selvey LA. Personal, provider and system level barriers and enablers for hepatitis C treatment in the era of direct-acting antivirals: experiences of patients who inject drugs accessing treatment in general practice settings in Australia. J Subst Abuse Treat 2021; 127:108460. [DOI] [PubMed] [Google Scholar]
- 38. Schäfer A, Wittchen HU, Backmund M, et al. Psychopathological changes and quality of life in hepatitis C virus-infected, opioid-dependent patients during maintenance therapy. Addiction 2009; 104:630– 40. [DOI] [PubMed] [Google Scholar]
- 39. Vahidnia F, Stramer SL, Kessler D, et al. Recent viral infection in US blood donors and health-related quality of life (HRQOL). Qual Life Res 2017; 26:349– 57. [DOI] [PubMed] [Google Scholar]
- 40. Scalone L, Ciampichini R, Fagiuoli S, et al. Comparing the performance of the standard EQ-5D 3L with the new version EQ-5D 5L in patients with chronic hepatic diseases. Qual Life Res 2013; 22:1707– 16. [DOI] [PubMed] [Google Scholar]
- 41. Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ 2019; 20:205– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the national health measurement study. Med Care 2007; 45:1162– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open 2018; 1:e185220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gray ME, Rogawski McQuade ET, Scheld WM, Dillingham RA. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis 2018; 18:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005;72:1005– 19. [DOI] [PubMed] [Google Scholar]
- 46. Kumthekar A, Shull S, Lovejoy TI, Morasco BJ, Chang M, Barton J. Impact of hepatitis C treatment on pain intensity, prescription opioid use and arthritis. Int J Rheum Dis 2019; 22:592– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Couto e Cruz C, Salom CL, Dietze P, Burns L, Alati R. The association between experiencing discrimination and physical and mental health among PWID. Int J Drug Policy 2019; 65:24– 30. [DOI] [PubMed] [Google Scholar]
- 48. Couto e Cruz C, Salom C, Parsell C, Dietze P, Burns L, Alati R. Social domains of discrimination against people who inject drugs: links with health and wellbeing. Int J Drug Policy 2020; 77:188– 94. [DOI] [PubMed] [Google Scholar]
- 49. Aas CF, Vold JH, Skurtveit S, et al. Health-related quality of life of long-term patients receiving opioid agonist therapy: a nested prospective cohort study in Norway. Subst Abus Treat Prev Policy 2020; 15:1– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.