Abstract
Background
Pregnancy increases the risk of tuberculosis and its complications. A 3-month regimen of weekly isoniazid and rifapentine (3HP) is safe and effective for tuberculosis prevention in adults and children, including those with HIV, but 3HP has not been evaluated in pregnancy.
Methods
IMPAACT 2001 was a phase I/II trial evaluating the pharmacokinetics and safety of 3HP among pregnant women with indications for tuberculosis preventative therapy in Haiti, Kenya, Malawi, Thailand, and Zimbabwe (NCT02651259). Isoniazid and rifapentine were provided at standard doses (900 mg/week). Pharmacokinetic sampling was performed with the first (second/third trimester) and twelfth (third trimester/postpartum) doses. Nonlinear mixed-effects models were used to estimate drug population pharmacokinetics.
Results
Of 50 participants, 20 had HIV and were taking efavirenz-based antiretroviral therapy. Among women without HIV, clearance of rifapentine was 28% lower during pregnancy than postpartum (1.20 vs 1.53 L/hour, P < .001), with area under the concentration-time curve (AUCSS) of 786 and 673 mg × hour/L, respectively. In pregnant women with HIV, clearance was 30% higher than women without HIV (P < .001), resulting in lower AUCss (522 mg × hour/L); clearance did not change significantly between pregnancy and postpartum. Pregnancy did not impact isoniazid pharmacokinetics. There were no drug-related serious adverse events, treatment discontinuations, or tuberculosis cases in women or infants.
Conclusions
3HP does not require dose adjustment in pregnancy. Rifapentine clearance is higher among women with HIV, but all women achieved exposures of rifapentine and isoniazid associated with successful tuberculosis prevention. The data support proceeding with larger safety-focused studies of 3HP in pregnancy.
Clinical Trials Registration
ClinicalTrials.gov, NCT02651259.
Keywords: maternal health, latent tuberculosis, rifapentine, pharmacokinetics, HIV
The regimen of weekly isoniazid and rifapentine for 3 months for tuberculosis prevention was well tolerated in pregnant women, and target concentrations of both drugs were achieved at standard doses among women with and without human immunodeficiency virus.
Active tuberculosis (TB) is twice as likely to develop postpartum than at any other time in a woman’s life [1]. Every year, an estimated 200 000 pregnant women develop active TB [2], which is a leading infectious cause of maternal mortality globally [2, 3]. Infants of mothers with TB have high rates of prematurity, low birth weight, and stillbirth [4, 5]. Maternal TB more than doubles the risk of mother-to-child transmission of human immunodeficiency virus (HIV) [6] and significantly increases mortality for all children in the household [4, 7–9].
The World Health Organization (WHO) currently recommends 6–9 months of daily isoniazid (INH) preventive therapy (IPT) to prevent TB in people living with HIV, but uptake during pregnancy varies widely by country [10–12]. TB Antepartum vs Postpartum Prevention with INH in HIV Seropositive mothers and their Exposed infants (TB APPRISE)—a randomized controlled IPT trial in pregnant women living with HIV (WLHIV)—showed an increased risk of adverse pregnancy outcomes from antepartum versus postpartum IPT [13]. But a meta-analysis of 9 other studies found inconsistent associations between IPT and adverse pregnancy outcomes [14]. Consequently, the optimal strategy for TB prevention in pregnant WLHIV remains unclear.
The WHO also endorses 3 months of weekly INH and rifapentine (3HP) for TB prevention [10]. This regimen has higher completion rates and decreased adverse events compared with IPT [15], including in people with HIV [16]. However, pregnant women were excluded from all 3HP trials. The intent of this study was to evaluate the effects of pregnancy on 3HP pharmacokinetics (PK) to generate initial safety and toxicity data in pregnancy, and to establish the appropriate rifapentine (RPT) dose in pregnant women with and without HIV.
METHODS
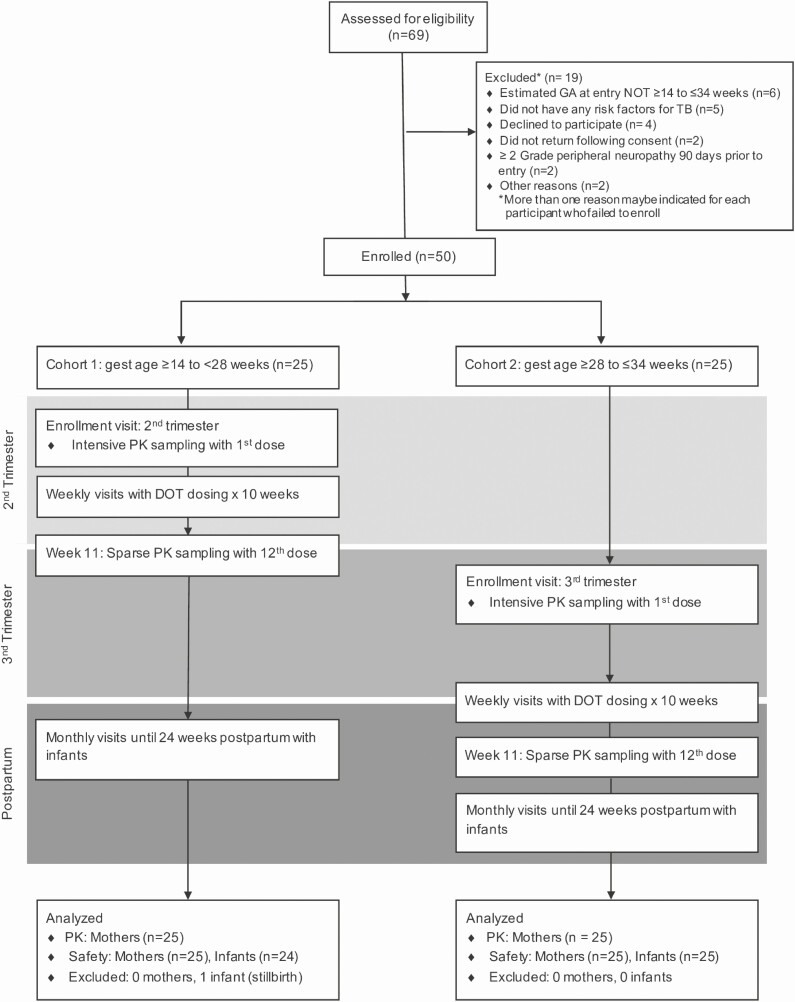
The International Maternal Pediatric Adolescent AIDS Clinical Trial Network (IMPAACT) 2001 study (NCT02651259) was a phase I/II, open-label, PK and safety study of 3HP among pregnant women living with and without HIV, with an indication for TB preventive therapy. Participants were enrolled in Haiti, Kenya, Malawi, Thailand, and Zimbabwe. Cohort 1 enrolled second-trimester women (≥14 to <28 weeks’ gestation, n = 25), and cohort 2 enrolled third-trimester women (≥28 to ≤34 weeks’ gestation, n = 25) (Figure 1). Participants received 12 directly observed, once-weekly doses of RPT (900 mg) and INH (900 mg), with pyridoxine. RPT was donated by Sanofi; INH was purchased from Macleod. Treatment completion was defined as receipt of at least 11 doses over 16 weeks [15].
Figure 1.
CONSORT diagram and study schema: CONSORT flow diagram showing participant flow through the IMPAACT 2001 trial. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; DOT, directly observed therapy; GA/gest age, gestational age; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trial Network; PK, pharmacokinetics; TB, tuberculosis.
Participants
Eligibility requirements included age of 18 years or older, singleton pregnancy by ultrasound with gestational age of 14– 34 weeks, high risk for TB (household contact with pulmonary TB, or WLHIV with latent TB), and the following laboratory values: hemoglobin of 7.5 g/dL or greater, white blood cell count of 1500 cells/mm3 or greater, absolute neutrophil count of 750 cells/mm3 or greater, platelet count of 100 000/mm3 or greater, alanine aminotransferase less than 2.5 times the upper limit of normal (ULN), and total bilirubin less than 1.6 times the ULN. Women living with HIV had to be taking an efavirenz (EFV)-based antiretroviral regimen (ART). Exclusion criteria included active TB within 2 years, treatment for latent TB, personal history or exposure to drug-resistant TB, fetal abnormality by ultrasound, liver cirrhosis, or peripheral neuropathy. Mother–infant pairs were followed until 24 weeks postpartum.
Study Procedures
At study entry, participants were screened for TB symptoms. At study visits, safety laboratory tests (complete blood count, liver function panel) were collected monthly and fetal movement and heart sounds assessed until delivery. Maternal prothrombin time (PT) was collected at baseline and at 34 or more weeks of gestation, if still taking the study drug. Intensive PK sampling was performed with the first dose (pre-dose, then 0.5, 1, 2, 4, 5, 8, 12, 24, 48, and 72 hours post-dose). Sparse PK sampling occurred with the 12th dose (1, 4, 24, and 48 post-dose) (Figure 1). Infants were assessed within 3 days of delivery. If the mother was taking the study drug at delivery, infant PT was checked. Follow-up visits assessed interim health changes and adverse events.
Oversight/Safety
All women provided written informed consent for themselves and their infants. The study was approved by local institutional review boards and/or ethics committees at participating sites. An independent Safety Monitoring Committee within the IMPAACT Network reviewed accrual, safety, PK data, and quality of study implementation. The Protocol Team reviewed PK and safety data regularly.
Primary and Secondary Outcomes
The primary outcome measures were as follows: (1) PK parameters of RPT and INH (eg, absorption, oral clearance, volume of distribution) 3 (2) maternal safety of 3HP (eg, serious adverse events [SAEs], grade 2+ drug-related adverse events [AEs], grade 3+ AEs, and permanent study drug discontinuation), and (3) infant drug-related SAEs. Attribution of AEs to the study drug was determined by the study team.
Secondary outcomes included drug tolerability, as assessed by discontinuation of the study drug regimen due to intolerance, and incidence of active TB in mother–infant pairs.
Pharmacokinetics
Drug Concentration Analysis
Plasma RPT and INH concentrations were analyzed with validated liquid chromatography–tandem mass spectrometry assays developed at the Division of Pharmacology, University of Cape Town [17] (Supplementary Methods). The lower limit of quantification (LLOQ) for rifapentine was 0.039 μg/mL.
Pharmacokinetics Analysis
Pharmacokinetics parameters for RPT were determined from plasma concentration-time profiles using a nonlinear mixed-effects model (version 7.4; ICON PLC, Dublin, Ireland). The PK model described the oral absorption of RPT and estimated antepartum RPT clearance by HIV status and the apparent volume of distribution relative to bioavailability (Vc/F). For the effect of trimester on clearance, cohort 1 intensive PK sampling was used for second-trimester analysis; sparse and intensive PK sampling from cohorts 1 and 2, respectively, were used for third-trimester analysis. For the effect of pregnancy on clearance, pooled second- and third-trimester data were compared with sparse postpartum PK sampling. Model-based secondary PK parameters included maximum concentration (Cmax) and steady-state area under the concentration-time curve (AUCSS), which describes drug exposure after weekly dosing. A similar model-based approach was used for INH.
An interim PK analysis was conducted after 12 participants enrolled into each cohort to determine if a dose adjustment was indicated. The median estimates were within 25% of historical controls, so no dose adjustments occurred [16].
Statistical Analysis
Sample Size
The sample size was based on precision for estimating PK parameters. The stochastic simulation-estimation (SSE) methodology for clinical trial simulation was used to determine sample sizes required to evaluate key PK parameters with adequate precision [18]. A sample size of 50 achieved a relative standard error less than 20% when comparing clearance between the second and third trimester. With this sample size we had 90% power to detect a safety event with a true occurrence of 5 per 100 women.
Safety
The safety dataset included women who had received at least 1 dose of the study drug and their infants. The proportion and exact 95% confidence interval were computed, overall and by cohort.
RESULTS
Trial Population
We enrolled 50 women from March 2017 to June 2018 (Table 1). Aside from gestational age, baseline characteristics of women in cohorts 1 and 2 were similar. The median age was 27 years (interquartile range [IQR], 20–32 years) and median weight was 61 kg (IQR, 56–67 kg), with 72% of participants of Black African, non-Hispanic race and ethnicity. Of the 20 (40%, 10 per cohort) WLHIV, median CD4 cell count was 510 cells/mm3 (IQR, 390–877 cells/mm3).
Table 1.
Baseline Maternal Characteristics
| Characteristic | Overall (N = 50) | Cohort 1 (Second Trimester, n = 25) | Cohort 2 (Third Trimester, n = 25) |
|---|---|---|---|
| Race/ethnicity, n (%) | |||
| Black African, non-Hispanic | 31 (62) | 13 (52) | 18 (72) |
| Black, Caribbean, non-Hispanic | 16 (32) | 11 (44) | 5 (20) |
| Asian, Pacific Islander | 3 (6) | 1 (4) | 2 (8) |
| Age at study entry, median (IQR), years | 27 (20–32) | 26 (22–33) | 27 (20–31) |
| HIV-positive, n (%) | 20 (40) | 10 (40) | 10 (40) |
| CD4, median (IQR), cells/mm3 | 510 (390–877) | 586 (415–846) | 489 (368–952) |
| Weight, median (IQR), kg | 61 (56–67) | 59 (55–66) | 61 (58–67) |
| Gestational age at entry, median (IQR), weeks | 26 (20–30) | 20 (16–24) | 30 (28–31) |
| Midupper arm circumference, median (IQR), cm | 27 (25–30) | 27 (25–31) | 27 (26–29) |
| Prothrombin time, median (IQR), seconds | 10 (10, 11) | 10 (10, 11) | 11 (10–12) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
Pharmacokinetics Analyses
Effect of Trimester
There was no significant difference in clearance of INH (data not shown) or RPT in samples collected in the second versus the third trimesters (Figure 2). This allowed us to pool second- and third-trimester PK data to assess the impact of HIV on antepartum clearance and to compare antepartum with postpartum PK.
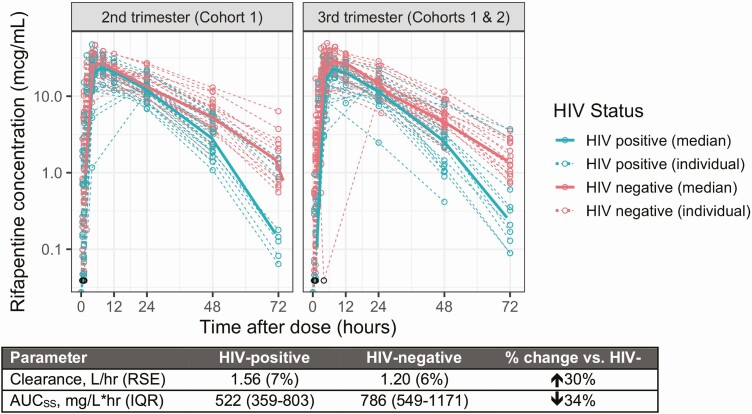
Figure 2.
Effect of HIV on clearance of RPT in second-trimester and third-trimester trimester. There were no differences in RPT concentration between second-trimester data (intensive PK from cohort 1; left panel) and third-trimester data (intensive PK from cohort 2, sparse PK from cohort 1; right panel). RPT clearance was 30% higher in pregnant WLHIV (blue) compared with women without HIV (pink) (P < .001). Data deemed to be below the LLOQ are displayed as open black circles as the LLOQ value (0.039 μg/mL). Abbreviations: AUCSS, steady-state area under the concentration-time curve; HIV, human immunodeficiency virus; IQR, interquartile range; LLOQ, lower limit of quantification; PK, pharmacokinetics; RPT, rifapentine; RSE, relative standard error; WLHIV, women living with HIV.
Effect of HIV/ART
During pregnancy, RPT clearance in WLHIV was 1.56 L/hour, 30% higher than in women without HIV (1.20 L/hour; P < .001). This resulted in a 34% lower AUCSS of 522 mg/L × hour (5th–95th percentile: 445–664 mg/L × hour) for pregnant WLHIV, compared to 786 mg/L × hour (5th–95th percentile: 639–904 mg/L × hour) in women without HIV (Figure 2). The INH PK profiles, stratified by HIV status, were comparable to one another. The AUCSS of INH was estimated to be 78.2 mg × hour/L (IQR, 21.9–78.2 mg × hour/L), with a maximum INH concentration of 7.74 mg/L (IQR, 5.68–10.6 mg/L) (Figure 3).
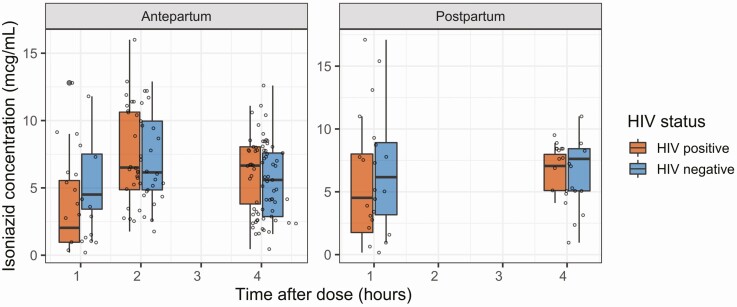
Figure 3.
Effect of pregnancy and HIV on INH PK: The clearance of isoniazid was comparable between pregnant and postpartum women with HIV (orange) and without HIV (blue). The AUCSS for the entire population was 78.2 mg × hour/L (IQR, 21.9–78.2) and maximum isoniazid concentration was 7.74 mg/L (IQR, 5.68–10.6). Abbreviations: AUCSS, steady-state area under the concentration-time curve; HIV, human immunodeficiency virus; IQR, interquartile range.
Effect of Pregnancy
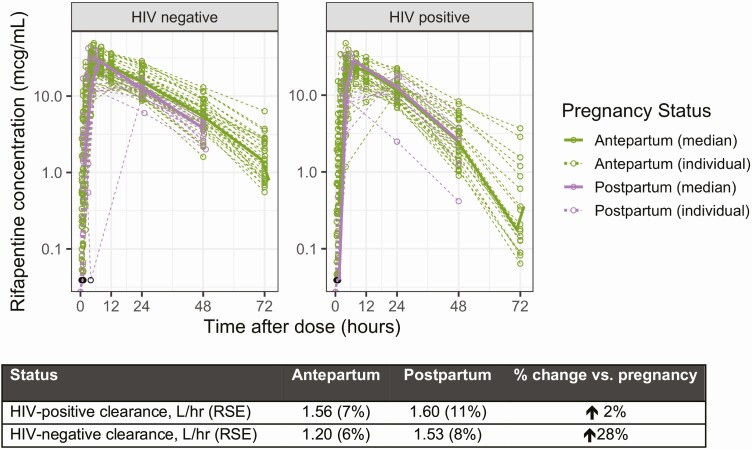
In WLHIV, the clearance of RPT antepartum and postpartum was comparable (1.60 vs 1.56 L/hour). However, in women without HIV, clearance was 28% higher in postpartum than in antepartum samples (1.53 vs 1.20 L/hour, P < .001) (Figure 4). Accordingly, the AUCSS for women without HIV was 786 mg/L × hour during pregnancy and 673 mg/L × hour postpartum, 21–50% higher than WLHIV at both time points (554 and 522 mg/L × hour) (Table 2). The INH PK profiles were unaffected by pregnancy status (Figure 3).
Figure 4.
Effect of pregnancy on clearance of RPT, by HIV status: In women without HIV (left panel), the postpartum clearance (purple) was 28% higher than antepartum clearance (green). In WLHIV, the clearance of RPT antepartum and postpartum was comparable to each other, and to postpartum clearance in women without HIV. Abbreviations: HIV, human immunodeficiency virus; RPT, rifapentine; RSE, relative standard error; WLHIV, women living with HIV.
Table 2.
Rifapentine Pharmacokinetic Results in IMPAACT 2001 and Other Studies in Nonpregnant Populations
| Rifapentine Parameter | IMPAACT 2001 (n = 50) | Weiner, JPIDS 2014 [16] (n = 80) |
|---|---|---|
| CL/F, L/hour (RSE) | ||
| Antepartuma | ||
| HIV-positive | 1.56 (7%) | … |
| HIV-negative | 1.20 (6%) | … |
| Postpartum | ||
| HIV- positive | 1.60 (11%) | … |
| HIV-negative | 1.53 (8%) | … |
| Nonpregnant (HIV-negative) | … | 2.32 (11%) |
| CLmet/F (RSE) | 2.75 (7%) | 2.05 (10%) |
| Median AUCSS (5th–95th percentile), mg × hour/L | ||
| Antepartuma | ||
| HIV-positive | 522 (359–803) | … |
| HIV-negative | 786 (549–1171) | … |
| Postpartum | ||
| HIV-positive | 554 (434–751) | … |
| HIV-negative | 673 (471–847) | … |
| Nonpregnant (HIV-negative) | … | 553 (326–931) |
| Median (IQR) Cmin, mg/L | 1.05 (0.455–2.01) | … |
| Median (IQR) Cmax, mg/L | 27.4 (24.7–34.6) | … |
Abbreviations: AUCSS, steady-state area under the concentration-time curve; CL/F, clearance; CLmet/F, clearance of metabolite; Cmax, observed maximum concentration; Cmin, observed minimum concentration; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trial Network; IQR, interquartile range; JPIDS, Journal of the Pediatric Infectious Diseases Society; RSE, relative standard error.
aIncludes combined second- and third-trimester data.
Comparison to Historic Nonpregnant Data
The clearance estimates of RPT in pregnant and postpartum women were within 25% of that reported in nonpregnant populations. Pregnant and postpartum women also achieved drug exposures of INH and RPT comparable to exposures associated with successful TB prevention (Table 2) [19].
Maternal Safety
All enrolled women completed the study regimen. None developed active TB. There were 5 (10%; 95% confidence interval [CI]: 3–22%) women who experienced SAEs; 2 (8%) in cohort 1 and 3e (12%) in cohort 2 (Table 3). None of the SAEs were deemed to be related to the study treatment. Ten weeks after completing the study regimen, 1 woman had a grade 5 placental abruption following physical trauma and, despite emergent C-section, suffered intrauterine fetal demise and died. Another woman had a grade 4 placental abruption related to pre-eclampsia. One woman had grade 3 pregnancy-induced hypertension, one had grade 3 postpartum hemorrhage requiring surgery, and one had grade 1 pregnancy-induced hypertension.
Table 3.
Frequency [n (%)] of Maternal Adverse Events
| Event | Total (N = 50) | Cohort 1 (Second Trimester, n = 25) | Cohort 2 (Third Trimester, n = 25) |
|---|---|---|---|
| Serious adverse eventsa | 5 (10%); 95% CI: 3–22% | 2 (8%); 95% CI: 1–26% | 3 (12%); 95% CI: 3–31% |
| Abruptio placentae | 2 | 2 | 0 |
| Hypertensive disorders | 3 | 1 | 2 |
| Postpartum hemorrhage | 1 | 0 | 1 |
| Death | 1 | 1 | 0 |
| Grade 3–4 adverse events | 9 (18%); 95% CI: 9–31% | 5 (20%); 95% CI: 7–41% | 4 (16%); 95% CI: 5–36% |
| Hematologic | 5 | 3 | 2 |
| Anemia | 4 | 2 | 2 |
| Elevated PT | 1 | 1 | 0 |
| Stillbirth | 1 | 1 | 0 |
| Premature delivery | 1 | 1 | 0 |
| Bacterial pneumonia | 1 | 1 | 0 |
| Drug-related grade 2 adverse events (muscle cramps) | 1 (2%); 95% CI: .05–11% | 1 (4%); 95% CI: .1–20% | 0 (0%); 95% CI: 0–14% |
| Permanent study drug discontinuation due to toxicity | 0 (0%); 95% CI: 0–7% | 0 (0%); 95% CI: 0–14% | 0 (0%); 95% CI: 0–14% |
Abbreviations: CI, confidence interval; PT, prothrombin time.
aParticipants could have experienced >1 of the listed events.
Overall, there were 9 (18%; 95% CI: 9–31%) women who had grade 3+ AEs: 5 (20%) in cohort 1 and 4 (16%) in cohort 2. None of these AEs were assessed as being related to the study treatment (Table 3). In cohort 1, the grade 3+ hemoglobin levels all occurred postpartum; the woman with the grade 5 placental abruption accounted for 2 of these events. The woman with the grade 4 placental abruption (described above) also had premature delivery and grade 3 pre-eclampsia. In cohort 2, the grade 3 hemoglobin levels also occurred after delivery, including the woman with postpartum hemorrhage described above. The other events are described in Table 3.
Only 1 (2%) participant had an AE assessed to be possibly related to the study regimen. She was in cohort 1 and had grade 2 muscle cramps of legs, feet, arms, hands, and face at week 7 of the study. She continued the study drug and her symptoms resolved within 24 hours.
Infant Safety
Of the 49 liveborn infants, none developed TB or study treatment–related SAEs (95% CI: 0–7%). There were 6 (12%) infants with SAEs; 4 (17%) born to women in cohort 1 and 2 (8%) from cohort 2 (Table 4). At 8 weeks of life, 1 infant had grade 3 anemia and grade 4 total bilirubin; both resolved by 12 weeks. There were 8 (16%) infants who had at least 1 adverse birth outcome: 2 (8%) in cohort 1 and 6 (24%) in cohort 2. In cohort 1, one had premature birth (<37 weeks) and low birth weight (<2500 g). In cohort 2, one had premature and low birth weight; one had premature birth, intrauterine growth retardation and low birth weight; and one had low birth weight and was small for gestational age. The remaining events are described in Table 4.
Table 4.
Frequency [n (%)] of Infant Adverse Events
| Event | Total (N = 49) | Cohort 1 (Second Trimester, n = 24) | Cohort 2 (Third Trimester, n = 25) |
|---|---|---|---|
| Serious adverse eventsa | 6 (12%); 95% CI: 5–25% | 4 (16.7%); 95% CI: 5–37% | 2 (8%); 95% CI: .1–26% |
| Neonatal sepsis | 4 | 3 | 1 |
| Hyperbilirubin | 2 | 1 | 1 |
| Neonatal respiratory distress | 1 | 1 | 0 |
| Premature birth (<32 weeks) | 1 | 1 | 0 |
| Anemia of prematurity | 1 | 1 | 0 |
| Small for gestational ageb | 1 | 0 | 1 |
| Subgaleal hematomaa | 1 | 0 | 1 |
| Adverse birth outcomesc | 8 (16%); 95% CI: 7–30% | 2 (8%); 95% CI: 1–27% | 6 (24%); 95% CI: 9–45% |
| Premature birth (<37 weeks) | 5 (10%); 95% CI: 3–22% | 1 (4%); 95% CI: .01–21% | 4 (16%); 95% CI: 5–36% |
| Low birth weight (<2500 g) | 4 (8%); 95% CI: 2–20% | 1 (4%); 95% CI: .01–22% | 3 (12%); 95% CI: 3–31% |
| Small for gestational ageb | 3 (7%); 95% CI: 4–12% | 1 (5%); 95% CI: .1–24% | 2 (9%); 95% CI: 1–29% |
| Intrauterine growth retardation | 1 (2%); 95% CI: .7–6% | 0 (0%); 95% CI: 0–16% | 1 (5%); 95% CI: .1–23% |
Abbreviation: CI, confidence interval; ICH, International Conference on Harmonization.
aInfants could have experienced >1 of the listed events.
bThere are 6 missing classification of newborn intrauterine growth and gestational age and only 1 of the 3 was a serious adverse event (had met ICH criteria of hospitalization).
c The adverse birth outcomes reported in this table are on the live born infants only, stillbirth is reported as a maternal adverse event in Table 3.
There were 2 (4%) infants who had grade 2 or higher AEs that were assessed to be possibly related to the study drug (not shown in Table 4). One infant had a grade 4 PT value at week 0 and another had a grade 3 PT value at week 4. Both mothers were taking the study drug at delivery. Neither infant had clinical signs of bleeding and repeat PT values were normal. Overall, the proportion of infants with AEs possibly related to the study drug was 4% (95% CI: .5–14%): 0% (95% CI: 0–14%) in cohort 1 and 8% (95% CI: 1–26%) in cohort 2.
DISCUSSION
IMPAACT 2001 was the first study to evaluate 3HP in pregnancy. We found that pregnancy did not significantly impact the clearance of RPT or INH. However, clearance of RPT in pregnant WLHIV was significantly higher than that in pregnant women without HIV. This amount of change in clearance does not warrant a change in RPT dosage for pregnant WLHIV. To understand whether this is an effect of HIV or of EFV-based ART, assessment of RPT PK given together with newer antiretrovirals, such as dolutegravir (DTG), will be helpful. Our study also did not find major safety issues in mothers or infants, which provides a strong rationale for a larger safety study of this regimen.
The most important first step to extend the use of a drug to pregnant women is establishing appropriate dosage. Pregnancy increases activity of progesterone-dependent cytochromes in the liver, for example, which may necessitate modifications to dose or schedule [20, 21]. In IMPAACT 2001, RPT concentrations were higher during pregnancy compared with postpartum. These increases, however, were modest, limited to women without HIV, and resulted in exposures that were within the range seen in nonpregnant adults taking 3HP [18]. Pregnancy did not alter INH disposition, which is in agreement with some previous studies, but in contrast to TB APPRISE [13, 22, 23]. To more fully characterize INH PK in this population, acetylation status and PK data collected in the absorption and elimination phase are needed.
The second step is to evaluate safety and tolerability during pregnancy. Given that 3HP is a 12-week regimen, it can be fully completed during pregnancy if started by the early third trimester. In this study, all 50 women completed the regimen, providing initial evidence of good tolerability during pregnancy. Furthermore, there were no major drug-related toxicities. These findings provide a strong rationale to proceed with a study adequately powered to evaluate maternal safety.
In our study, HIV was associated with a 30% higher clearance and 34% lower AUCss of RPT during pregnancy compared with women without HIV. This is consistent with the effects of HIV on RPT pharmacokinetics observed in nonpregnant adults, which has been attributed to malabsorption or, possibly, ART effects [25]. However, the AUCss for pregnant WLHIV in IMPAACT 2001 was similar to mean AUC values reported in nonpregnant adults without HIV [16]. Standard dosing in pregnant WLHIV, therefore, achieves drug exposures within the range of exposures associated with successful prevention of TB in registrational trials [15].
Interestingly, women without HIV experienced a 28% decrease in clearance while pregnant. A similar effect has been seen with rifampicin [25]. The increased clearance over time may be related to physiologic changes of pregnancy but may also be partly related to auto-induction of RPT-metabolizing enzymes [26–28]. A recent meta-analysis reported that RPT clearance may increase by 20% due to auto-induction with once-weekly dosing, but effects on AUC are modest [24].
In pregnant WLHIV, however, there was no significant change in clearance between antepartum and postpartum periods. A possible explanation is that EFV had already induced the enzymes responsible for RPT metabolism by the time 3HP was started, leaving less room for RPT’s induction of its own metabolism. A recent study of nonpregnant people with HIV reported decreased RPT clearance in slow N-acetyltransferase 2 acetylators taking EFV-based ART, likely because of increased INH exposure [29]. IMPAACT 2001 did not collect pharmacogenetic data to assess whether this association is present during pregnancy. As DTG replaces EFV as first-line treatment for WLHIV globally, additional PK studies during pregnancy may be useful to confirm that DTG does not significantly affect RPT PK, as has been shown in nonpregnant adults [30].
This study was designed to establish the RPT dose in pregnancy that achieves concentrations similar to those in nonpregnant adults. It was not powered to fully characterize the effect of 3HP on birth outcomes. The initial safety and tolerability data from our study, however, are encouraging, with complication rates comparable to global estimates [31–34]. The single maternal death was related to placental abruption from trauma weeks after completing the study drug. Although there were more adverse birth outcomes in those enrolled in the third trimester (cohort 2), the number of events was too small to determine their significance. One would expect similar or more adverse birth outcomes in cohort 1 because they were enrolled in the second trimester and had longer exposure to the study drug earlier in pregnancy, when most fetal development occurs. The only study drug–related AEs were grade 2 maternal muscle cramps, which resolved, and 2 grade 3/4 elevated PT in infants, which were not clinically significant.
A limitation of the study was the homogeneity of the population, both by race/ethnicity and ART used. For this initial PK study with a limited sample size, homogeneity increases statistical confidence in results. However, future safety studies should include more heterogeneous populations to increase generalizability. Rifapentine significantly decreases the plasma levels of protease inhibitors [35] (PIs); therefore, women on PIs were excluded. Dolutegravir was not approved for pregnancy at the time this study was initiated, but pregnant women on DTG will be included in future studies of 3HP. Because this is a single-arm study, we could not assess whether the shorter duration and small number of doses in 3HP is safer compared with other TB prevention regimens containing prolonged daily dosing in this population. An earlier study of 54 pregnant women who were inadvertently exposed to 3HP early in pregnancy also found no increased risk of fetal loss or congenital anomalies compared with women on nine months of daily INH or the general population [36], but it was similarly not powered for definitive comparison. Larger, postmarketing studies are needed to thoroughly evaluate the safety before definitively recommending 3HP in pregnant and postpartum women.
Conclusions
The prevention of TB in pregnant women is an important priority for maternal-child health. Pregnant and postpartum women are at high risk of TB, which carries a serious risk of adverse outcomes for a woman and her child [5, 7, 8]. IMPAACT 2001 was the first dedicated study to evaluate the PK of 3HP in pregnancy, and it demonstrated that 3HP can be given without dose adjustment to both pregnant and postpartum women. In WLHIV taking EFV-based ART, RPT clearance was higher than in women without HIV but similar to nonpregnant populations. As 3HP was well tolerated, there is a strong rationale to proceed with a larger safety-powered study to ensure that pregnant and postpartum women have an equal opportunity to benefit from the revolutionary research occurring in TB prevention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the contributions of the site investigators and staff who conducted the IMPAACT 2001 study—Zimbabwe: University of Zimbabwe College of Health Sciences–Clinical Trials Research Centre: Petronella Matibe, MSc; Ruvimbo Mukonowenzou, SCM; Rufaro Katsande, BSC; Tichaona Vhembo, MPH; Haiti: Les Centres Gheskio Clinical Research Site: Dominique Lespinasse, MD; Marie Flore Pierre, MD; Maria Linda Aristhomene, RN; Elsie Jean, MD; Kenya: Kenya Medical Research Institute– Walter Reed Project Clinical Research Center: Lorner Soy, RN; Winnie Keter, Diploma Clinical Medicine; Priscillah Bii, Higher Diploma Clinical Medicine; Benard Rono, Diploma Clinical Medicine; Thailand: Siriraj Hospital, Mahidol University: Kulkanya Chokephaibulkit, MD; Winai Ratanasuwan, MD, MPH; Nirun Vanprapar, MD, MSc; Supattra Rungmaitree, MD, MSc; Malawi: University of North Carolina Project–Malawi: Lameck Chinula, MMED; Esnath Msowoya, MSc; Manly Kamija, BTech; Phaleda Kumwenda, Diploma Nursing; IMPAACT Operations Center: Jennifer Libous, MS, CCRP; Charlotte Perlowski, MSPH; IMPAACT Laboratory Center: William Murtaugh, MPH; Carolyn Yanavich, PhD, CCRP; IMPAACT Data Management Center: Bonnie Zimmer, BS; Rebecca LeBlanc, BS; Lauren Harriff, BS; Bobbie Graham, BS; and the women and families who participated in the trial.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding support and study product was provided by Sanofi.
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under award numbers UM1AI068632 (IMPAACT LOC; payments made to S. B.’s institution, FHI 360, through a subcontract from Johns Hopkins University), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT Lab center) and NICHD contract number HHSN275201800001I. Additionally, the University of Cape Town Clinical PK Laboratory is supported through the AIDS Clinical Trials Group (NIAID) under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. J. S. M. was supported by NIH K23AI129854; A. G. was supported by NIH UM1AI069465; and K. E. D. is supported by NIH K24AI150349.
Role of the Funding Source.
Pharmaceutical support was provided by Sanofi, including donation of standards for RPT assays. Neither the funder nor Sanofi had a role in writing the manuscript, study design, data collection, analysis or interpretation. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Potential conflicts of interest. A. G. reports funding to their institution from the NIH, during the conduct of the study; and reports funding from NIH, Unitaid, and the Centers for Disease Control and Prevention, outside the submitted work. J. S. M. reports travel support from NIH (NIH K23 award), outside the submitted work. G. M. reports support from NIH/NIAID (2 UM1 AI0686–15), payments made to her institution (Harvard T. H. Chan School of Public Health), during the conduct of the study; reports receiving NIH/NIAID (5 R01 AI142669–02) (subcontract from the University of Miami School of Medicine) payments made to her institution (Harvard T. H. Chan School of Public Health); and reports serving as an Advisory Board member for the OptiRif Kids trial (sponsored by TB Alliance), outside the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: A national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. Available at: http://www.atsjournals.org/doi/abs/10.1164/rccm.201106-1083OC. Accessed 22 July 2020. [DOI] [PubMed] [Google Scholar]
- 2. Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: An estimate of the global burden of disease. Lancet Glob Heal 2014; 2:e710–6. Available at: https://pubmed.ncbi.nlm.nih.gov/25433626/. Accessed 28 June 2021. [DOI] [PubMed] [Google Scholar]
- 3. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Glob Heal 2014; 2:e323–33. [DOI] [PubMed] [Google Scholar]
- 4. Salazar-Austin N, Hoffmann J, Cohn S, et al. Poor obstetric and infant outcomes in human immunodeficiency virus-infected pregnant women with tuberculosis in South Africa: The Tshepiso study. Clin Infect Dis 2018; 66:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: Epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: A risk factor for mother-to child transmission of human immunodeficiency virus. J Infect Dis 2011; 203:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis 2007; 45:241–9. [DOI] [PubMed] [Google Scholar]
- 8. Cantwell MF, Shehab ZM, Costello AM, et al. Congenital tuberculosis. N Engl J Med 1994; 330:1051–4. Available at: https://pubmed.ncbi.nlm.nih.gov/8127333/. Accessed 22 July 2020. [DOI] [PubMed] [Google Scholar]
- 9. Gomes VF, Andersen A, Wejse C, et al. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax 2011; 66:163–7. Available at: http://thorax.bmj.com/. Accessed 3 August 2020. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Guidelines on the management of latent tuberculosis infection. 2015. Available at: http://AppsWhoInt/Iris/Bitstream/10665/136471/1/9789241548908_EngPdf?Ua=1&Ua=1. Accessed 10 June 2021.
- 11. Kalk E, Heekes A, Mehta U, et al. Safety and effectiveness of isoniazid preventive therapy in pregnant women living with human immunodeficiency virus on antiretroviral therapy: an observational study using linked population data. Clin Infect Dis 2020; 71:E351–8. Available at: https://pubmed.ncbi.nlm.nih.gov/31900473/. Accessed 7 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaCourse S, Wagner A, Cranmer L, et al. Brief report: high programmatic isoniazid preventive therapy (IPT) use in pregnancy among HIV-infected women. J Acquir Immune Defic Syndr 2019; 82:41–5. Available at: https://pubmed.ncbi.nlm.nih.gov/31408031/. Accessed 7 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, Montepiedra G, Aaron L, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med –; 381:1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamada Y, Figueroa C, Martin-Sanchez M, Falzon D, Kanchar A. The safety of isoniazid tuberculosis preventive treatment in pregnant and postpartum women: Systematic review and meta-analysis. Eur Respir J 2020; 55. Available at: https://pubmed.ncbi.nlm.nih.gov/32217619/. Accessed 3 August 2020. [DOI] [PubMed] [Google Scholar]
- 15. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 16. Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: A randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and Isoniazid. JAMA Pediatr 2015; 169:247–55. Available at: https://pubmed.ncbi.nlm.nih.gov/25580725/. Accessed 22 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Medicines Agency. Guideline on bioanalytical method validation. London, UK: 2011. Available at: www.ema.europa.eu/contact. Accessed 22 July 2020. [DOI] [PubMed] [Google Scholar]
- 18. Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiner M, Savic RM, Mac Kenzie WR, et al. Rifapentine pharmacokinetics and tolerability in children and adults treated once weekly with rifapentine and isoniazid for latent tuberculosis infection. Orig Artic J Pediatr Infect Dis Soc 2014; 3:132–77. Available at: https://academic.oup.com/jpids/article-abstract/3/2/132/941088. Accessed 22 July 2020. [DOI] [PubMed] [Google Scholar]
- 20. Anderson GD. Pregnancy-induced changes in pharmacokinetics: A mechanistic-based approach. Clin. Pharmacokinet 2005; 44:989–008. [DOI] [PubMed] [Google Scholar]
- 21. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol 2015; 39:512–9. Available at: https://pubmed.ncbi.nlm.nih.gov/26452316/. Accessed 3 August 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdelwahab MT, Leisegang R, Dooley KE, et al. Population pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in pregnant South African women with tuberculosis and HIV. Antimicrob Agents Chemother 2020; 64. Available at: https://pubmed.ncbi.nlm.nih.gov/31844002/. Accessed 3 August 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gausi K, Wiesner L, Norman J, et al. IMPAACT P1078 (TB APPRISE) Study Group Team . Pharmacokinetics and drug-drug interactions of isoniazid and efavirenz in pregnant women living with HIV in high TB incidence settings: importance of genotyping. Clin Pharmacol Ther 2021; 109:1034–44. Available at: https://pubmed.ncbi.nlm.nih.gov/32909316/. Accessed 28 June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hibma JE, Radtke KK, Dorman SE, et al. Rifapentine population pharmacokinetics and dosing recommendations for latent tuberculosis infection. Am J Respir Crit Care Med 2020. Available at: https://pubmed.ncbi.nlm.nih.gov/32412342/. Accessed 22 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Denti P, Martinson N, Cohn S, et al. Population pharmacokinetics of rifampin in pregnant women with tuberculosis and HIV coinfection in Soweto, South Africa. Antimicrob Agents Chemother 2016; 60:1234–41. Available at: https://pubmed.ncbi.nlm.nih.gov/26643345/. Accessed 31 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savic RM, Lu Y, Bliven-Sizemore E, et al. Population pharmacokinetics of rifapentine and desacetyl rifapentine in healthy volunteers: Nonlinearities in clearance and bioavailability. Antimicrob Agents Chemother 2014; 58:3035–42. Available at: https://pubmed.ncbi.nlm.nih.gov/24614383/. Accessed 22 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dooley K, Flexner C, Hackman J, et al. Repeated administration of high-dose intermittent rifapentine reduces rifapentine and moxifloxacin plasma concentrations. Antimicrob Agents Chemother 2008; 52:4037–42. Available at: http://ctep.cancer.gov/forms. Accessed 22 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keung A, Eller MG, McKenzie KA, Weir SJ. Single and multiple dose pharmacokinetics of rifapentine in man: Part II. Int J Tuberc Lung Dis 1999; 3:437–44. [PubMed] [Google Scholar]
- 29. Haas DW, Podany AT, Bao Y, et al. Pharmacogenetic interactions of rifapentine plus isoniazid with efavirenz or nevirapine. Pharmacogenet Genomics 2020; 31:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brooks KM, George JM, Pau AK, et al. Cytokine-mediated systemic adverse drug reactions in a drug-drug interaction study of dolutegravir with once-weekly isoniazid and rifapentine. Clin Infect Dis 2018; 67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. PMNCH | Born Too Soon: The Global Action Report on Preterm Birth. WHO; 2016; Available at: http://www.who.int/pmnch/knowledge/publications/preterm_birth_report/en/. Accessed 28 June 2021. [Google Scholar]
- 32. Dolea C, Abouzahr C. Global burden of hypertensive disorders of pregnancy in the year 2000. Glob Burd Dis 2000 2003. [Google Scholar]
- 33. Black RE. Global prevalence of small for gestational age births. In: Nestle Nutrition Institute Workshop Series. Basel: S. Karger AG, 2015: 1–7. Available at: https://pubmed.ncbi.nlm.nih.gov/26111558/. Accessed 28 June 2021. [DOI] [PubMed] [Google Scholar]
- 34. Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Heal 2019; 7:e849–60. Available at: https://pubmed.ncbi.nlm.nih.gov/31103470/. Accessed 28 June 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Justesen US, Andersen AB, Klitgaard NA, Brosen K, Gerstoft J, Pedersen C. Pharmacokinetic Interaction between Rifampin and the Combination of Indinavir and Low-Dose Ritonavir in HIV-Infected Patients. Clin Infect Dis 2004; 38:426–9. Available at: https://academic.oup.com/cid/article/38/3/426/291867. Accessed 28 June 2021. [DOI] [PubMed] [Google Scholar]
- 36. Moro RN, Scott NA, Vernon A, et al. Exposure to latent tuberculosis treatment during pregnancy the PREVENT TB and the Iadhere trials. Ann Am Thorac Soc. 2018; 15:570–80. Available at: https://pubmed.ncbi.nlm.nih.gov/29393655/. Accessed 31 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.