Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA (vRNA) is detected in the bloodstream of some patients with coronavirus disease 2019 (COVID-19), but it is not clear whether this RNAemia reflects viremia (ie, virus particles) and how it relates to host immune responses and outcomes.
Methods
SARS-CoV-2 vRNA was quantified in plasma samples from observational cohorts of 51 COVID-19 patients including 9 outpatients, 19 hospitalized (non–intensive care unit [ICU]), and 23 ICU patients. vRNA levels were compared with cross-sectional indices of COVID-19 severity and prospective clinical outcomes. We used multiple imaging methods to visualize virions in plasma.
Results
SARS-CoV-2 vRNA was detected in plasma of 100%, 52.6%, and 11.1% of ICU, non-ICU, and outpatients, respectively. Virions were detected in plasma pellets using electron tomography and immunostaining. Plasma vRNA levels were significantly higher in ICU > non-ICU > outpatients (P < .0001); for inpatients, plasma vRNA levels were strongly associated with higher World Health Organization (WHO) score at admission (P = .01), maximum WHO score (P = .002), and discharge disposition (P = .004). A plasma vRNA level >6000 copies/mL was strongly associated with mortality (hazard ratio, 10.7). Levels of vRNA were significantly associated with several inflammatory biomarkers (P < .01) but not with plasma neutralizing antibody titers (P = .8).
Conclusions
Visualization of virus particles in plasma indicates that SARS-CoV-2 RNAemia is due, at least in part, to viremia. The levels of SARS-CoV-2 RNAemia correlate strongly with disease severity, patient outcome, and specific inflammatory biomarkers but not with neutralizing antibody titers.
Keywords: COVID, 19 outcome, SARS, CoV, 2 RNAemia, SARS, CoV, 2 viremia
Plasma viral RNA was detected in most hospitalized coronavirus disease 2019 patients including all critically ill patients, and the levels of RNA are strongly associated with disease outcome. SARS-CoV-2 virions were identified in plasma using multiple complementary approaches.
The coronavirus disease 2019 (COVID-19) pandemic is the largest public health emergency in modern history, resulting from global spread of the newly emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an approximately 30-kb single-stranded positive-sense RNA virus [1, 2]. The respiratory tract epithelial cells are the primary target of SARS-CoV-2, and the primary clinical syndrome of COVID-19 is one of upper and lower respiratory tract infection. The broad expression of the SARS-CoV-2 receptor angiotensin-converting enzyme 2 in extrapulmonary tissues could expand viral tropism. Indeed, a wide range of extrapulmonary symptoms including loss of smell and taste, vomiting and diarrhea, and neurologic impairment have been reported [3]. Detection of SARS-CoV-2 RNA in the gastrointestinal tract, endothelium, and central nervous system implicates extrapulmonary dissemination as an important contributor to disease manifestation severity [4–12]. Current data do not suggest that SARS-CoV-2 replicates in peripheral blood cells; however, systemic dissemination of virus through the bloodstream to extrapulmonary sites is possible. Aside from a central role in disease pathogenesis, viral RNA (vRNA) in blood could represent an important indicator of lung barrier breakdown, leading to release of intact virions, virion components (proteins and nucleic acids), or infected cell fragments into the bloodstream. Although some groups have found that the detection of SARS-CoV-2 RNA in plasma (SARS-CoV-2 RNAemia) is associated with severe disease [13–24], it is unclear whether vRNA detected using reverse-transcription polymerase chain reaction (RT-PCR) is in virions and thus reflects viremia.
Higher levels of serum antibodies to SARS-CoV-2 proteins have been reported in patients with more severe disease and in those who are immunocompetent [25, 26], suggesting that antibodies may not protect against severe disease. This raises the questions about the potential benefit of antibody-based therapies in severely ill people. Studies of monoclonal antibodies and convalescent plasma therapy have not shown clinical benefit in late stages of disease, which provides further evidence that antibodies may fail to clear infection in severely ill people [27–32]. Recently, however, combination monoclonal antibody therapy (casivirimab + imdevimab) was shown to modestly improve outcome in hospitalized patients who have not generated antibodies to SARS-CoV-2- [33]. In the absence of antibody therapy, inconsistent associations between SARS-CoV-2–specific neutralizing antibodies and SARS-CoV-2 RNAemia have been reported [16, 21, 22, 25, 34], suggesting that neutralizing antibody level and function vary between patients and may not prevent viral dissemination in some.
In the current study, we investigated whether RNAemia is an indicator of SARS-CoV-2 viremia (ie, virus particles in blood), how often RNAemia can be detected among outpatients and hospitalized patients, whether the level of RNAemia is associated with clinical outcome including mortality, and the relationship between RNAemia and host immune responses including neutralizing antibody and markers of inflammation.
METHODS
Study Cohorts
Inpatient.
From 4 April 2020 through 4 September 2020, we prospectively enrolled hospitalized patients with COVID-19 from 3 hospitals (UPMC Presbyterian, UPMC Shadyside, and UPMC East) in an observational cohort study (protocols STUDY19050099 and STUDY20040036). We included patients aged 18–90 years who were diagnosed with SARS-CoV-2 infection by a positive nasopharyngeal swab quantitative PCR (qPCR) test and had acute illness consistent with COVID-19 as the main reason for hospitalization. Patients were hospitalized either in an intensive care unit (ICU) or a dedicated hospital ward (non-ICU setting) for COVID-19.
Outpatient.
Beginning 11 March 2020, participants aged ≥2 years with confirmed or suspected SARS-CoV-2 infection were identified by provider-referral or self-referral in the outpatient clinic or community setting and enrolled into the Molecular and Epidemiological Study of Suspected Infection (MESSI, Pro00100241), as described previously [35]. The objective of the MESSI observational study is to enroll participants with suspected SARS-CoV-2 infection and to collect clinical and symptom information and bank biological samples for research use. All outpatient participants included in this study remained in the outpatient or at-home setting throughout the course of COVID-19 illness. SARS-CoV-2 virus testing was performed using qRT-PCR from nasopharyngeal swabs. COVID-19–positive participants included in these analyses demonstrated SARS-CoV-2 qPCR-positive results during acute infection.
Institutional review board approvals.
All research protocols were approved by the relevant institutional review boards and were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all research participants or their legally authorized representatives.
Clinical Data Extraction and Definitions
We classified patients based on the location of delivery of care at the time of patient enrollment and biospecimen acquisition into an ICU group (ie, critically ill patients requiring mechanical ventilation or high levels of oxygenation support), a non-ICU group (moderately ill inpatients), and an outpatient group of mildly symptomatic patients. For ICU patients, we considered the date of ICU admission the baseline timepoint; for non-ICU patients, the date of hospital admission was considered baseline. We recorded baseline demographics, COVID-19 severity by the World Health Organization (WHO) 10-point ordinal scale (baseline and peak during hospitalization), administered COVID-19–targeted therapies, duration of mechanical ventilation for intubated patients, length of hospital stay, and final outcome of hospitalization (death vs discharge to home care or inpatient facility). When available, we also recorded the cycle threshold (Ct) value of the nasopharyngeal swab qPCR that was used to establish the clinical diagnosis of COVID-19 as a surrogate of viral load in the respiratory tract. We used baseline clinical and laboratory variables for estimation of the Coronavirus Clinical Characterization Consortium (4C) scores for risks of mortality and inpatient deterioration. Clinical laboratory parameters of interest (white blood cell count, absolute lymphocyte count, platelets, D-dimer, ferritin, and lactate dehydrogenase) were provided when available as part of standard clinical care. We obtained laboratory values from the electronic medical record when corresponding clinical blood draws had occurred on the same date of research sample acquisition. When clinical laboratory parameters were not available on that same date, we used available clinical laboratory results for up to 2 days before or after the same date of research sample acquisition.
Experimental Analyses
For results of detailed experimental analyses, please see the Supplementary Methods.
RESULTS
Cohort Characteristics
We enrolled 51 patients with COVID-19, stratified by location of the clinical encounter in 3 groups: critically ill patients in the ICU (n = 23), moderately ill inpatients hospitalized in dedicated wards for COVID-19 (non-ICU inpatients; n = 19), and outpatients with mild illness (n = 9) [35, 36]. Comparisons of baseline characteristics, therapies, and outcomes are shown in Table 1. ICU patients had significantly higher 60-day mortality than non-ICU inpatients (n = 7, 30.4% vs 0%, P = .01). ICU patients had higher levels of interleukin (IL) 6, IL-8, IL-10, procalcitonin, suppression of tumorigenicity (ST)-2, and pentraxin-3 compared with non-ICU inpatients (all P =< .01; Supplementary Table 1). There was no significant difference in neutralizing antibody titer by plaque reduction assay between ICU and non-ICU inpatients (P = .22; Table 1). At the time of the study, there were no SARS-CoV-2 variants of concern identified at the enrolled patient locations.
Table 1.
Descriptive Characteristics of Outpatients and Inpatients With Coronavirus Disease 2019 Stratified by Moderately Ill (Hospitalized, Non–Intensive Care Unit [ICU]) vs Critically Ill (ICU)
| Characteristic | Outpatient | Inpatient, Non-ICU | Inpatient, ICU | P Value |
|---|---|---|---|---|
| N | 9 | 19 | 23 | |
| Demographics | ||||
| Age, median (IQR), years | 35.0 (26.9–52.4) | 60.0 (52.0–66.0) | 65.0 (57.5–75.5) | .11 |
| Males, n (%) | 2 (22.2) | 9 (47.4) | 16 (69.6) | .21 |
| White, n (%) | 4 (44.4) | 15 (78.9) | 12 (52.2) | .11 |
| Body mass index, median (IQR), kg/m2 | 31.1 (29.0–36.6) | 33.8 (27.0–39.4) | .52 | |
| Diabetes, n (%) | 4 (21.1) | 7 (31.8) | .50 | |
| Chronic obstructive pulmonary disease, n (%) | 2 (10.5) | 6 (26.1) | .26 | |
| Immunosuppressed host, n (%) | 2 (10.5) | 4 (17.4) | .67 | |
| Resident of nursing facility, n (%) | 0 (0) | 1 (5.3) | 4 (17.4) | .36 |
| Detectable plasma SARS-CoV-2 RNA, n (%) | 1 (11) | 10 (53) | 23 (100) | |
| SARS-COV-2 RNA copies, median (range) | 1 (<1–5) | 1 (<1–918) | 3349 (32–225, 320) | <.0001 |
| Plaque reduction neutralization titer, median (IQR) | 647.7 (23.4–2407.5) | 2487.0 (287.8–3695.8) | .22 | |
| Coronavirus disease 2019 treatment | ||||
| Remdesivir, n (%) | 0 (0) | 8 (42.1) | 15 (65.2) | .21 |
| Convalescent plasma, n (%) | 0 (0) | 2 (10.5) | 10 (43.5) | .04 |
| Glucocorticoids, n (%) | 0 (0) | 10 (52.6) | 15 (65.2) | .33 |
| Illness severity and outcome | ||||
| Baseline WHO scale, median (IQR) | 5.0 (4.0–5.0) | 7.0 (6.0–8.0) | <.001 | |
| Worst WHO scale during hospitalization, median (IQR) | 5.0 (5.0–5.0) | 9.0 (6.5–10.0) | <.001 | |
| 60-day mortality, n (%) | 0 (0) | 0 (0) | 7 (30.4) | .01 |
Nonparametric test comparisons were performed with Wilcoxon and Fisher exact tests for continuous and categorical variables, respectively.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Baseline Plasma Levels of SARS-CoV-2 RNA and Patient Outcomes
Plasma SARS-CoV-2 RNA was detectable at baseline in all 23 ICU patients but in only 10 of 19 (52.6%) non-ICU inpatients and in 1 (11.1%) outpatient (P < .0001). ICU patients had >3000-fold higher median number of vRNA copies per milliliter (3349; interquartile range [IQR], 756–8408) compared with non-ICU inpatients (median, 1; 1–72 copies/mL, P < .0001; Table 1, Figure 1A). There was only a moderate, borderline significant association between plasma vRNA copies and Ct values from RT-PCR performed on 20 paired nasal swab specimens (P = .052; Supplementary Figure 2A). We detected a weak nonsignificant inverse correlation between absolute lymphocyte count and plasma vRNA copies (r = –0.3, P = .06; Supplementary Table 2) but not with other available clinical laboratory parameters (Supplementary Table 2). The small overall sample size may have limited the detection of significant correlations.
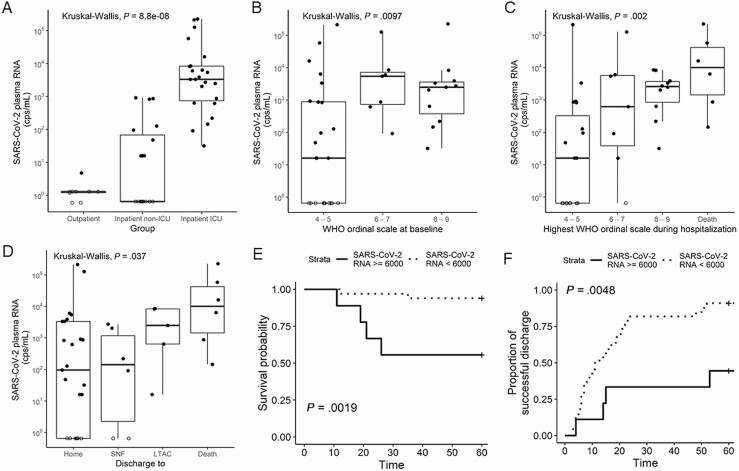
Figure 1.
SARS-CoV-2 RNA levels in plasma are associated with disease severity and outcome. Plasma SARS-CoV-2 RNA levels (copies per milliliter) by location of clinical care at baseline (A), severity of illness by WHO ordinal scale at baseline (B), and worst WHO scale during hospitalization (C) for outpatient (n = 9), inpatient non-ICU (n = 19), and inpatient ICU (n = 24). Kaplan-Meier curves for time to discharge from hospital admission (D) and 60-day survival (E) for inpatients (both ICU and non-ICU) stratified by high (>6000 copies/mL) vs low (≤6000 copies/mL) initial viral RNA level. Plasma SARS-CoV-2 RNA levels (copies per milliliter) by outcome of hospitalization among inpatients (F). Patients with undetected SARS-CoV-2 RNA in plasma are represented by open circles and graphed as one-half the Lower limit of detection (LLOD). Abbreviations: cps, copies; ICU, intensive care unit; LTAC, long-term acute care; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNF, skilled nursing facility; WHO, World Health Organization.
Higher plasma vRNA levels at hospital or ICU admission were significantly associated with higher baseline severity of illness (as quantified by a WHO ordinal scale of >6) compared with patients who had a baseline WHO scale <6 (ie, mild hypoxemia not requiring high-flow oxygen or mechanical ventilation; Figure 1B). Viral RNA levels at baseline were also significantly associated with the worst severity of illness (peak WHO score) during hospitalization (Figure 1C; P = .002), as well as hospitalization outcome (death vs discharge to an inpatient facility or home care; P = .037; Figure 1D).
We performed a receiver operating characteristic (ROC) curve analysis to define an optimal cutoff for vRNA levels as a predictor of mortality (Supplementary Figure 1). A cutoff of >6000 copies/mL among all inpatients (ICU and non-ICU) was significantly associated with greater 60-day mortality (log-rank P = .002) and longer hospital stay (log-rank P = .005) in unadjusted Kaplan-Meier curve analyses (Figure 1E, 1F). The sensitivity and specificity of the cutoff for mortality were 67% and 86%, respectively (Supplementary Figure 1). Similarly, analysis of the ROC curve with an optimal Youden index (0.56) indicated a threshold of 6352 copies/mL that offered 67% sensitivity and 89% specificity. In Cox proportional hazards models adjusting for the confounding effects of age, sex, and treatment with corticosteroids, high vRNA levels were associated with a higher hazard ratio (HR) for death (adjusted HR = 10.7, 95% confidence interval [CI] = 1.49–76.9) and longer time to hospital discharge among survivors (adjusted HR = 5.12, 95% CI = 1.65–15.88). We did not find a significant difference in plasma viremia in patients treated with remdesivir or glucocorticoids 2–3 days before sampling compared with patients with no treatment. Larger studies of the effects of remdesivir, glucocorticoids, and other COVID-19 therapies on viremia are needed to draw conclusions.
Plasma Contains SARS-CoV-2 Virions
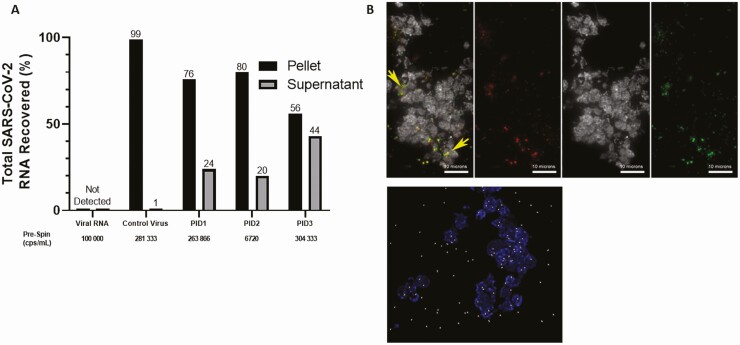
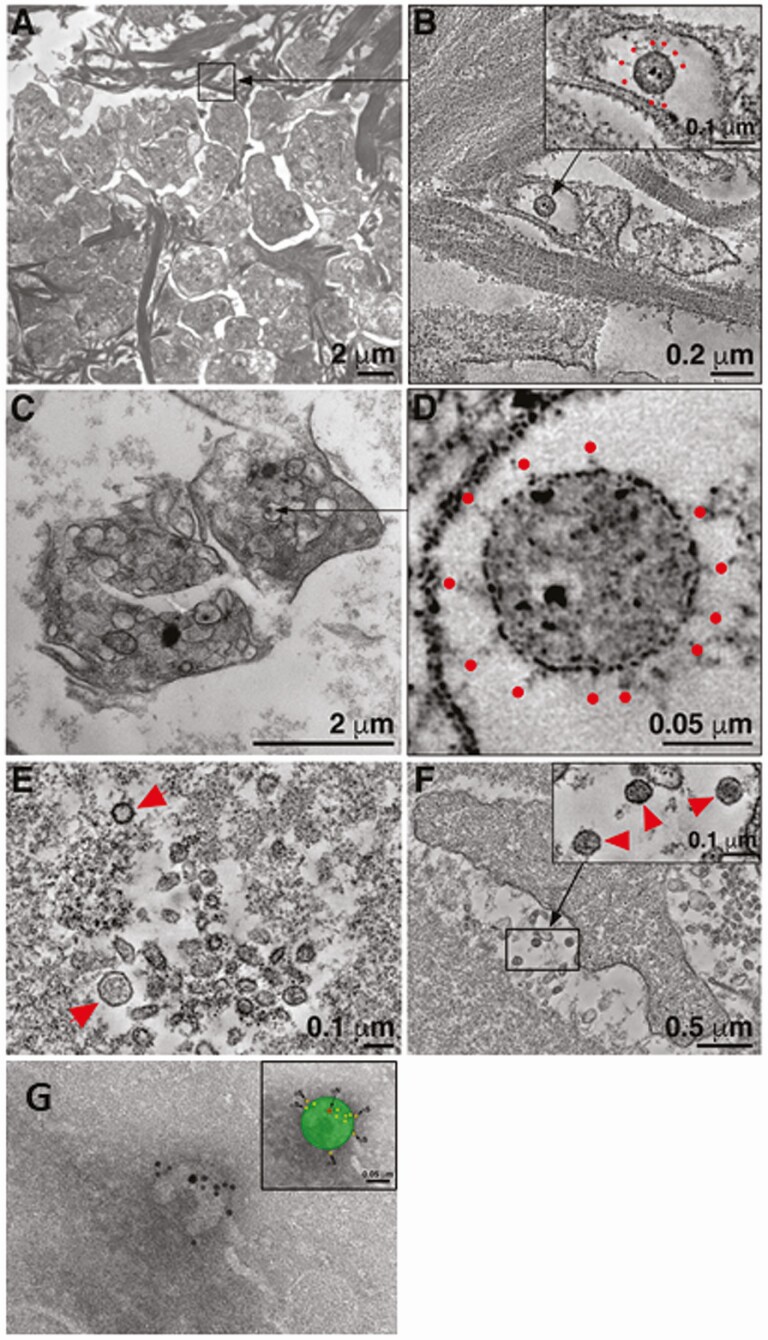
Next, we sought to characterize the vRNA present in plasma to determine whether it includes intact viral particles. After high-speed centrifugation of a subset of plasma samples chosen to reflect a wide range of vRNA levels (6720–304, 333 copies/mL) with sufficient available sample volume, a median of 76% (56%–80%) of total recovered vRNA was detected in the pellet fraction (Figure 2A), indicating the vRNA detected is contained within or associated with a pelletable structure. Notably, SARS-CoV-2 RNA from positive controls (SARS-CoV-2 virions spiked into healthy human donor plasma) was found almost exclusively in the pellet fraction (99%), confirming that cell-free SARS-CoV-2 virions are pelletable using the same centrifugation conditions. In addition, when free SARS-CoV-2 RNA was added to SARS-CoV-2–negative plasma prior to centrifugation, vRNA was not recovered in either fraction, indicating that free vRNA is not stable in plasma and likely does not account for the vRNA detected in the supernatant fraction. When a subset of plasma samples was prepared for cyto-spin analysis, immunofluorescence of cyto-spin slides stained with 2 independent SARS-CoV-2–specific antibodies (antinucleocapsid and antispike) and a platelet marker (anti-CD41) revealed punctate double-positive structures that were absent in negative controls prepared from SARS-CoV-2–negative plasma (Figure 2B). A mean of 41.5% of double-positive structures colocalized with CD41 (Figure 2B). Presumptive SARS-CoV-2 virions were identified in the pellet fraction of a subset of high-speed centrifuged plasma samples by electron microscopy (EM) and tomographic reconstruction (Figure 3A–3F). Virions observed ranged from 1 to 4 per field of view, and presumptive virions were found within or in close proximity to presumptive platelets, as well as in regions distant from identifiable cell fragments (Figure 3A–3F, Supplementary Movies 1–3). Presumptive SARS-CoV-2 virions were confirmed by immuno-EM using 2 independent antibodies (Figure 3G).
Figure 2.
SARS-CoV-2 RNA in plasma includes a pelletable fraction that contains virus particles. A, Percent of total recovered SARS-CoV-2 RNA detected in the pellet or supernatant fractions of spiked-in control SARS-CoV-2 virus or plasma from 3 inpatients with coronavirus disease 2019 (COVID-19) centrifuged at 21 000 × g for 2 hours. B, Immunofluorescence of cytospin slides prepared from plasma of COVID-19 inpatients PID3 (upper) and PID2 (lower) including the platelet marker CD41 (upper, white; lower, blue), and SARS-CoV-2 S and N proteins (upper, red and green or yellow for colocalization; lower, white). Abbreviations: cps, copies; PID, patient identifier; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 3.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in plasma includes a pelletable fraction containing SARS-CoV-2 virus particles. Electron micrographs of pelleted fraction of plasma from 2 independent coronavirus disease 2019 inpatients, PID2 (A, B, and G) and PID1 (C–F). Montaged overview of a field of platelets surrounded by amorphous plasma material and large clusters of fibrils, possibly collagen (A, B). B, Tomographic reconstruction of the region indicated by the square in upper left showing a single presumptive SARS-CoV-2 virion within a membrane-bound compartment of a platelet. C, Tomogram detail of the virion with notable core puncta, clearly delineated membrane bilayer, and distinct surface spikes (B, red dots). Two-dimensional overview image of 3 platelets. D, High-magnification tomographic slice of a presumptive SARS-CoV-2 virion within an enclosed compartment of a platelet. Tomogram detail of a group of vesicles. Two spherical structures conform to presumptive SARS-CoV-2 virions (E, red arrowheads). F, Tomogram of a second platelet within the field, similarly surrounded by small vesicles. Tomogram detail of the area indicated by the rectangle in F showing 3 presumptive virions adjacent to the platelet (F, inset). Presumptive virions were identified as described (Methods section) and by comparisons with analogous electron microscopy (EM) of SARS-CoV-2–infected cultured cells. Immuno-EM image from pelleted fraction of plasma of PID2; presumptive SARS-CoV-2 virion labeled with anti-N (large 15 nm) gold particle and anti-S (smaller 10 nm) gold particles (G).
Baseline Plasma Levels of SARS-CoV-2 RNA and Host Responses
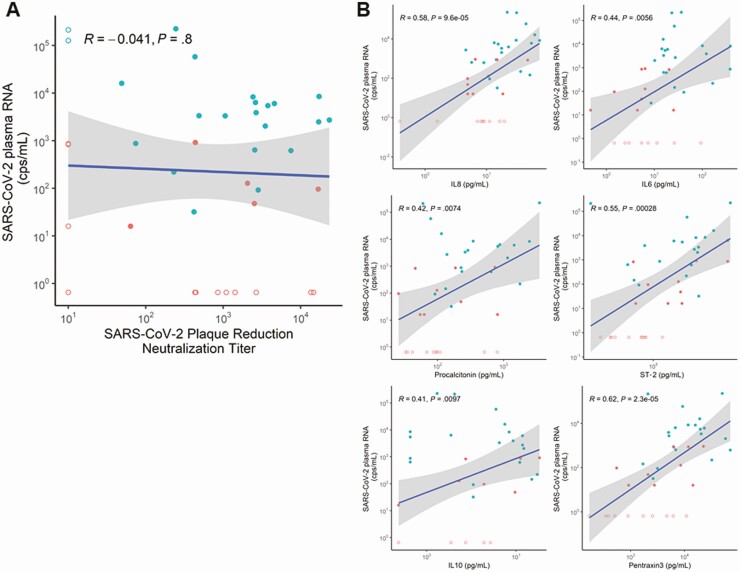
Viral RNA levels at baseline were not significantly associated (P = .8) with neutralizing antibody titers by plaque reduction neutralization test (Figure 4A). By contrast, vRNA levels were significantly correlated with some plasma biomarkers of innate immunity (IL-6, P = .006; IL-8, P = .000096; IL-10, P = .0097; ST-2, P = .00028) and inflammation (procalcitonin, P = .0074; pentraxin-3, P = .000023; Figure 4B, Supplementary Figure 2B). Notably, these biomarkers were significantly different in ICU patients compared with non-ICU inpatients (P ≤ .1 for all), mirroring the corresponding differences in vRNA levels (Supplementary Table 1). These findings suggest that dissemination of viral infection is associated with a systemic inflammatory host response.
Figure 4.
Plasma SARS-CoV-2 RNA levels are not significantly correlated with SARS-CoV-2–specific neutralizing antibody levels but are correlated with multiple host-response inflammatory biomarkers in hospitalized patients. Correlations between SARS-CoV-2 RNA levels and anti–SARS-CoV-2 neutralizing antibodies measured by plaque reduction neutralization titer assay (A) or the inflammatory biomarkers IL-8, IL-6, procalcitonin, ST-2, IL10, and pentraxin 3 measured in the plasma of coronavirus disease 2019 inpatients (B). Red dots represent non–intensive care unit (ICU) patients, and blue dots represent ICU patients. Undetected values are represented by open circles and graphed as one-half the lower limit of detection (LLOD). Abbreviations: cps, copies; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ST-2, suppression of tumorigenicity 2.
Longitudinal Evolution of Plasma Levels of SARS-CoV-2 RNA
We examined longitudinal changes of vRNA levels among inpatients with detectable plasma RNA at baseline (day 1, n = 19) and available follow-up samples (post-enrollment day 5, n = 19 and/or day 10, n = 13; Supplementary Figure 3). Overall, we found a significant decrease in vRNA levels between day 1 and day 5 (P = .041, Wilcoxon test with Benjamini-Hochberg post hoc adjustment), between day 5 and day 10 (P = .041), and between day 1 and day 10 (P = .001). We further stratified the changes in vRNA according to 30-day mortality. Among survivors (n = 13 available samples for day 1, n = 13 for day 5, and n = 8 for day 10), we found a significant decrease in vRNA levels between day 1 and day 10 (P = .018). In nonsurvivors (n = 6 for day 1, n = 5 for day 5, and n = 5 for day 10), we did not detect significant change between any time point (day 1 vs day 5, P = .49; day 1 vs day 10, P = .11; day 5 vs day 10, P = .12). Although the analyses are limited by small sample size, results indicate a modest decrease in vRNA over 10 days in COVID-19 survivors and no measurable decline in nonsurvivors. Although these data suggest a trend, larger studies are needed to draw conclusions.
Additionally, viremia provided further prognostic information beyond established clinical risk stratification tools (4C scores that use baseline variables for predicting mortality and inpatient deterioration) [37]. In a bivariate regression model predicting the worst WHO ordinal scale as a surrogate of inpatient deterioration, both viremia (P = .002) and 4C deterioration scores (P = .007) were significant predictors. In a bivariate Cox proportional hazard model for 60-day survival, viremia was a significant predictor (P = .032) but not the 4C mortality score (P = .14).
Discussion
Here, we show that SARS-CoV-2 RNA is detected in a substantial fraction of hospitalized patients, including 100% of patients in the ICU, and that the levels of SARS-CoV-2 RNA associate with maximal in-hospital disease severity and 60-day mortality, independent of established clinical risk stratification tools. We have also visualized SARS-CoV-2 virions in centrifuged plasma pellets using complementary imaging-based approaches. In agreement with other recent studies [21, 23], SARS-CoV-2 RNA in plasma associates with some established markers of innate immunity and inflammation commonly elevated in patients with acute respiratory distress syndrome (IL-6, IL-8, IL-10, procalcitonin, and pentraxin-3) but not with SARS-CoV-2–specific neutralizing antibody measured by plaque reduction assay. Overall, these findings provide new insights into the pathogenesis and outcome of COVID-19. The strengths of our study include strong evidence that RNAemia is associated with viral particles in plasma (ie, plasma viremia) and absolute quantitation of vRNA using a SARS-CoV-2 RNA standard rather than estimation of viral levels by Ct value or DNA standards.
Although many studies have shown that the detection of SARS-CoV-2 RNA in plasma (SARS-CoV-2 RNAemia) is associated with severe disease and/or unfavorable outcome [13–24], our study adds unique measures of disease severity to extend these earlier findings, such as associations with maximal inpatient WHO disease severity score, discharge location (long-term acute care, skilled nursing facility [SNF], or home), and mortality, as well as additional prognostic discrimination beyond established, multivariable clinical predictors of disease severity International Severe Acute Respiratory Infection Consortium Clinical Characterisation (the ISARIC) Coronavirus Clinical Characterisation Consortium (4C). In addition, there has been inconsistency in the reported proportion of different patient groups with detectable plasma vRNA, as well as the levels of plasma vRNA. Specifically, SARS-CoV-2 RNA has been detected in plasma from 35% of patients hospitalized with COVID-19 [13] to 88% of critically ill patients [17], with clear trends in each study toward severe disease in those with RNAemia. This variability may result from differences in sample types tested (plasma or serum) or the type (digital droplet vs qPCR) and sensitivity of the RT-PCR methods used. Here, we report detection of vRNA in 79% of all hospitalized patients, in 100% of critically ill patients (hospitalized, ICU), and 52.6% of moderately ill patients (hospitalized, non-ICU). The higher proportion of patients with detectable vRNA in the current study is likely due to the ultrasensitive method that was used, which was based on a qRT-PCR assay of plasma human immunodeficiency virus type 1 (HIV-1) RNA that has a 95% limit of detection of 1 copy/mL [38]. Our finding that some viral particles associate with platelets suggests that testing of plasma samples that retain platelets may be a better sample type than sera for assessing SARS-CoV-2 presence in blood because of the removal of platelets from serum with clot formation. Despite the small sample size of our cohort, we observed that survivors of COVID-19 had statistically significant decreases in plasma vRNA in longitudinal samples, whereas those who succumbed did not have significant decreases, although the preliminary nature of this finding requires confirmation in additional studies. Similarly, the lack of an observed effect of therapeutic interventions such as remdesivir on plasma vRNA in the current study should be confirmed in larger studies. More extensive longitudinal sampling from patients across the spectrum of disease severity, including those with progressive disease during the observation period, as well as varying interventions, will provide additional insight into the usefulness of SARS-CoV-2 plasma RNA as a prognostic marker and a means to guide antiviral therapy.
We are the first to show that SARS-CoV-2 vRNA in plasma is associated, at least in part, with intact SARS-CoV-2 virions in plasma. We found that vRNA is more abundant in the pelleted fraction of plasma compared with supernatant after high-speed centrifugation. While most vRNA was detected in the pellet fraction and therefore is likely associated with pelletable structures (eg, a virus particle or an infected cell), a fraction was contained in the supernatant. Our experimental results suggest that this nonpelletable RNA is unlikely to be free vRNA because free RNA was quickly degraded after spiking into plasma. It could represent free vRNA protected by RNA-binding proteins or viral or cellular fragments, or virus particles associated with lipids or lipid-rich structures that alter its density; and its composition may be heterogenous across individuals. Complementary methods including immunofluorescence, electron tomography, and immuno-EM confirmed the presence of SARS-CoV-2 virions in plasma from the subset of patient samples analyzed. Importantly, we were unable to determine if the SARS-CoV-2 virions were infectious because of limited sample availability. In a recent study, Andersson et al were unable to culture SARS-CoV-2 from vRNA-positive sera in Vero E6 cells [20], although optimal methods for culturing SARS-CoV-2 from blood have not been defined. To illustrate this point, we show that some SARS-CoV-2 virions associate with platelets in blood, thus it is possible that sensitivity of virus culture could be improved by using platelet-rich plasma. Future studies should optimize virus culture methods from blood and determine whether virions in blood are infectious and whether infected cells (if present) in blood are producing infectious virus. It is likely that if SARS-CoV-2 virions in blood are infectious, they will lead to dissemination of infection to multiple organs [4–12, 39]. Whether SARS-CoV-2 directly infects endothelial cells remains controversial [40]; thus, the mechanisms by which SARS-CoV-2 gains access to the bloodstream and infects extrapulmonary organs are unknown.
Neutralizing antibodies counter viral infections by preventing attachment and entry into cells. Clearance of infection that has disseminated to the blood in severely ill people would therefore depend, at least partially, on neutralizing antibodies to prevent seeding of extrapulmonary organs. The results from our study are in agreement with results from others that neutralizing antibody titer does not correlate with vRNA levels in blood [16, 21], suggesting that most viremic patients have already mounted a neutralizing antibody response, which underscores concern about the value of antibody-based therapies in severely ill patients. This is still an unsettled question that warrants further investigation because a few studies have found an association between RNAemia and neutralizing antibody response [22]. A failure of antibody to control viremia may be attributable to suboptimal antibody potency, specificity, or function in severely ill patients [41, 42]. We did identify a small group of patients with detectable viremia and lower neutralizing antibody titers (<100). It is possible that this patient subgroup could benefit from antibody-based therapies. Indeed, recently reported results from the RECOVERY trial showed a moderate improvement in outcome from treatment with monoclonal antibodies (casivirimab + imdevimab) in patients who do not have endogenously produced antibodies to SARS-CoV-2 in blood [33]. Reanalysis of existing datasets from clinical trials following quantification of viremia and pretreatment levels of endogenous neutralizing antibodies may help identify patient subgroups with favorable responses to antibody therapy.
In summary, our findings suggest that SARS-CoV-2 viremia is a strong and independent marker of COVID-19 disease severity and outcome. Future investigation should focus on whether therapies that prevent or reduce viremia improve clinical outcomes. Clinical studies with viremia-targeted outcomes may accelerate development of effective therapies for COVID-19, similar to what occurred with antiviral therapy of other viral infections including hepatitis B [43], hepatitis C [44], and HIV-1 [45].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the patients and patient families who have enrolled in our research studies at the University of Pittsburgh. We also thank the physicians, nurses, respiratory therapists, and other staff at the University of Pittsburgh Medical Center Presbyterian and Shadyside Hospital units for assistance with coordination of patient enrollment and collection of patient samples. We thank Heather Michael, Michelle Busch, Caitlin Schaefer, and Cathy Kessinger for assistance with patient enrollment and processing research samples. We also thank Lorraine Pollini for her careful review of the manuscript.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services; the National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute (NHLBI); or the National Institutes of Health (NIH) nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Funding. This work was supported in part by pilot coronavirus disease 2019 (COVID-19) awards received from the University of Pittsburgh Clinical and Translational Science Institute, the National Center for Advancing Translational Sciences, and the NIH (UL1TR001436 to G. D. K. and E. T. A. M.); a George Mason Fast grant (to P. J. B.); NIH/ NHLBI (P01HL114453 to B. J. M. and J. S. L.); NIH/National Institute of Allergy and Infectious Diseases (NIAID; U01AI066569 and UM1AI104681 to C. W. W., T. W. B., and E. P.); the US Defense Advanced Projects Agency (N66001-09-C-2082 and HR0011-17-2-0069 to C. W. W., T. W. B., and E. P.); the Virology Quality Assurance (75N93019C00015 to T. D.); in whole or in part by federal funds from the National Cancer Institute, NIH (under contract 75N91019D00024, task order 75N91020F00003 to J. W. M.), and intramural funds from the National Cancer Institute (to M. F. K.); NIH/NHLBI (agreement 1OT2HL156812-01 to W. B.); the US Department of Veterans Affairs Biomedical Laboratory R&D Career Development Award (IK2 BX004886 to WB.); and the University of Pittsburgh Vascular Medicine Institute, the Hemophilia Center of Western Pennsylvania, and the Institute for Transfusion Medicine (to W. B).
Potential conflicts of interest. H. Y. reports a scholarship to pursue study in the United States from the China Scholarship Council outside the submitted work. G. H. reports grants from NIH and Karius and personal fees for reviewing a legal case from Meyers, Rodbell & Rosenbaum, PA, outside the submitted work. T. W. B. reports grants from NIH/NIAID and Defense Advanced Research Projects Agency (DARPA) during the conduct of the study and equity options for consulting work from Predigen, Inc, outside the submitted work. J. S. L. discloses a paid consultantship with Janssen R&D unrelated to this work and discloses clinical adjudication of severity outcomes in the ENSEMBLE study of COVID-19 vaccine. C. W. W. reports personal fees from Duke University and Durham VA Health Care System for employment; grants from DARPA, NIH/Antibacterial Resistance Leadership Group (ARLG), equity/founder from Predigen, Inc, Sanofi, and NIH/Vaccine and Treatment Evaluation Unit (VTEU); personal fees from bioMerieux for consulting; personal fees from Roche Molecular Sciences for advisory board participation; personal fees for consulting from Biofire, Giner, Biomeme, FHI Clinical, and Arena; personal fees from Janssen for Data and Safety Monitoring Board (DSMB); and personal fees from Regeneron for advisory board participation outside the submitted work. B. J. M. reports grants from NIH/NHLBI, the Translational Breast Cancer Research Consortium, and the UPMC Learning While Doing Program during the conduct of the study; grants from Bayer Pharmaceuticals, Inc; and personal consultation fees from Boehringer Ingelheim, Inc, outside the submitted work. G. K. reports research funding from Karius, Inc. J. W. M. reports grants to the University of Pittsburgh from NIH, USAID, Gilead Sciences, Inc, and Janssen Pharmaceuticals; serves or has served as a consultant for Gilead Sciences, Inc; has served as a scientific advisory board member for Merck, Accelevir Diagnostics, and Xi’an Yufan Biotechnologies; owns share options in Co-Crystal Pharmaceuticals, Inc, and Infectious Diseases Connect; is a part-time employee and shareholder of Abound Bio, Inc; and is employed by the University of Pittsburgh. His holdings and roles in Co-Crystal Pharmaceuticals, Infectious Diseases Connect, and Abound Bio are unrelated to the current work. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782-93. [DOI] [PubMed] [Google Scholar]
- 4. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020; 369:50-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383:590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020; 383:989-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 2021; 218:e20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020; 20:389-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395:1417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831-3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181:1016-35.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hagman K, Hedenstierna M, Gille-Johnson P, et al. SARS-CoV-2 RNA in serum as predictor of severe outcome in COVID-19: a retrospective cohort study. Clin Infect Dis 2021; 73:e2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hogan CA, Stevens BA, Sahoo MK, et al. High frequency of SARS-CoV-2 RNAemia and association with severe disease. Clin Infect Dis 2020; 72:e291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs JL, Mellors JW. Detection of SARS-CoV-2 RNA in blood of patients with COVID-19: what does it mean? Clin Infect Dis 2021; 73:e2898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prebensen C, Hre PLM, Jonassen C, et al. SARS-CoV-2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID-19. Clin Infect Dis 2020; 73:e799-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veyer D, Kerneis S, Poulet G, et al. Highly sensitive quantification of plasma SARS-CoV-2 RNA sheds light on its potential clinical value. Clin Infect Dis 2021; 73:e2890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu D, Zhou F, Sun W, et al. Relationship between serum SARS-CoV-2 nucleic acid (RNAemia) and organ damage in COVID-19 patients: a cohort study. Clin Infect Dis 2020; 73:68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fajnzylber J, Regan J, Coxen K, et al. ; Massachusetts Consortium for Pathogen Readiness. . SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersson MI, Arancibia-Carcamo CV, Auckland K, et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res 2020; 5:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Schneider AM, Mehta A, et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest 2021; 131:e148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gutmann C, Takov K, Burnap SA, et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat Commun 2021; 12:3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bermejo-Martin JF, González-Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care 2020; 24:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 2020; 71:1937-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol 2020; 5: eabe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Guo X, Xin Q, et al. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin Infect Dis 2020; 71:2688-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators. . Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020; 371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; 324:460-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol 2020; 190:1680-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2020; 384:619-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horby PW, Estcourt L, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021. doi:10.1101/2021.03.09.21252736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundgren JD, Grund B, Barkauskas CE, et al. ; ACTIV-3/TICO LY-CoV555 Study Group. . A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med 2021; 384:905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021. Doi: 10.1101/2021.06.15.21258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin-Vicente M, Almansa R, Martínez I, et al. Absent or insufficient anti-SARS-CoV-2 S antibodies at ICU admission are associated to higher viral loads in plasma, antigenemia and mortality in COVID-19 patients. medRxiv 2021. Doi: 10.1101/2021.03.08.21253121. [Google Scholar]
- 35. McClain MT, Constantine FJ, Henao R, et al. Dysregulated transcriptional responses to SARS-CoV-2 in the periphery support novel diagnostic approaches. Nat Commun 2021; 12: 1079. Available at: https://doi.org/10.1038/s41467-021-21289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bain W, Yang H, Shah FA, et al. COVID-19 versus non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann Am Thorac Soc 2021; 18:1202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Consortium IC. 4C mortality and 4C deterioration calculator. https://isaric4c.net/risk/. Accessed 17 February 2021.
- 38. Tosiano MA, Jacobs JL, Shutt KA, Cyktor JC, Mellors JW. A simpler and more sensitive single-copy HIV-1 RNA assay for quantification of persistent HIV-1 viremia in individuals on suppressive antiretroviral therapy. J Clin Microbiol 2019; 57:e01714-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98:219-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses 2020; 13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atyeo C, Fischinger S, Zohar T, et al. Distinct early serological signatures track with SARS-CoV-2 survival. Immunity 2020; 53:524-32.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zohar T, Loos C, Fischinger S, et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell 2020; 183:1508-19.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ganem D, Prince AM. Hepatitis B virus infection— natural history and clinical consequences. N Engl J Med 2004; 350:1118-29. [DOI] [PubMed] [Google Scholar]
- 44. Li G, De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antiviral Res 2017; 142:83-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Katzenstein DA, Hammer SM, Hughes MD, et al. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med 1996; 335:1091-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.