Abstract
Context
Seasonal variation in thyroid function, especially serum free triiodothyronine (FT3) and free thyroxine (FT4) levels, in healthy subjects remains unclear.
Methods
We examined thyroid function, including serum FT3 and FT4 levels, in healthy Japanese subjects using data of more than 7,000 health check-up participants and applied the analysis of means with transformed ranks (ANOMTR) to compare each month. In addition, we reviewed reports published in the last 2 decades.
Results
The median serum thyrotropin (TSH) level was the highest in January (1.61 mIU/L), and the lowest in May (1.16 mIU/L). ANOMTR revealed that serum TSH levels are high in winter and low in summer. Conversely, the median serum FT3 level was higher in July than in other months, and the ANOMTR plot demonstrated serum FT3 levels to be significantly higher in summer and lower in winter. In contrast, serum FT4 levels were more consistent throughout the year, but statistically, those in February and March, October, and November were higher than those in other months. ANOMTR revealed variations in serum FT4 levels to be small through the year but biphasic.
Conclusions
Taken together with previous reports, our study demonstrated seasonal changes in the serum TSH levels to be high in winter in the northern hemisphere; however, the serum FT3 differed among countries, and those of Japanese, an iodine-sufficient country, were high in summer. In contrast, FT4 levels were more consistent. These changes should be taken into account to precisely evaluate thyroid function.
Keywords: serum TSH, thyroid hormone levels, healthy Japanese, analysis of means with transformed ranks, ANOMTR
Serum thyroid hormone levels are regulated mainly by the hypothalamic-pituitary-thyroid (HPT) axis [1, 2]. The serum TSH level is sensitive to slight changes in the serum thyroid hormones levels even within the reference range and is used as the most sensitive biomarker for assessing thyroid function. We can therefore diagnose subclinical both hypothyroidism and hyperthyroidism using serum TSH levels in subjects with serum thyroid hormone levels in the reference range [3].
In healthy individuals, serum thyroid hormone levels exhibit substantial interindividual variability, resulting in wide reference ranges. However, the intraindividual variability lies within a much narrower range. This suggests that every individual may have a distinct set point of the HPT axis [4], although this remains controversial. Patients therefore can still be in relatively hypothyroid or hyperthyroid states when serum TSH and thyroid hormones levels are within the reference ranges due to the individual set point of the HPT axis.
These set points may be affected factors related to seasons. Studies in several countries revealed different characteristic seasonal changes [5-16]. Therefore, there are no consistent seasonal changes in thyroid function, which may affect the definition of thyroid dysfunction such as subclinical hypo- and hyperthyroidism.
Seasonal variations in serum TSH levels were recently reported using a large database of Japanese patients with several thyroid disorders [10]. However, it remains unclear whether the variations in serum thyroid function, including serum FT4 and FT3 levels, are observed in healthy subjects.
We therefore investigated variations in thyroid function in healthy subjects, including serum thyroid hormone levels, in Gunma, Japan, which has 4 distinct seasons and is an iodine-sufficient region. Furthermore, we performed the analysis of means (ANOM) with transformed ranks (ANOMTR) to compare each month, which is suitable for graphical representation of testing for simple comparative experiments. This ANOM was introduced for testing the equality of population means by Ott in 1967 [17], and it became popular during the early 1980s when it was applied to experimental data for manufacturing and quality control. Furthermore, compared with ANOM, ANOMTR provides reliable results regardless of the shape of the distribution and number of replications, as long as the variances are homogeneous [18].
Materials and Methods
Subjects and Methods
This was a cross-sectional study including 7,256 Japanese subjects (4,066 men and 3,190 women) who underwent annual health checkups at Takasaki Hidaka Hospital in Gunma prefecture between April 2020 and March 2021. Subjects were asked to complete a self-questionnaire by the same nurses, which included questions on medical and medication histories and smoking habits. Exclusion criteria were overt hyperthyroidism or hypothyroidism; history of thyroid disease; liver cirrhosis; renal failure; subjects currently on medications, including levothyroxine, antithyroid drugs, insulin, and steroid hormones; and subjects with missing data.
In total, 6,343 subjects (3,667 men and 2,676 women) were enrolled in this study. The average age of the groups was 53.8 ± 11 years (54.8 ± 11 years for men and 52.5 ± 10 years for women).
Regarding the number of participants each month, the largest number of participants was 735 in December, and the smallest number was 248 in May (Table 1). These numbers were sufficient for statistical evaluation of hormone levels each month.
Table 1.
Medians and ranges of the serum thyrotropin, free triiodothyronine, and free thyroxine levels each month
| TSH (mIU/L) median (range) |
FT3 (pg/mL) median (range) |
FT4 (ng/dL) median (range) |
n | |
|---|---|---|---|---|
| January | 1.61 (0.51-5.22) | 2.97 (2.35-3.55) | 1.00 (0.83-1.20) | 628 |
| February | 1.52 (0.39-5.11) | 3.14 (2.48-3.64) | 1.04 (0.86-1.26) | 651 |
| March | 1.45 (0.49-4.82) | 3.10 (2.37-3.64) | 1.04 (0.83-1.29) | 554 |
| April | 1.28 (0.46-4.61) | 3.35 (2.46-3.70) | 1.00 (0.82-1.22) | 382 |
| May | 1.16 (0.37-4.02) | 3.32 (2.64-3.70) | 1.00 (0.81-1.21) | 248 |
| June | 1.17 (0.43-3.63) | 3.37 (2.75-3.68) | 1.02 (0.83-1.25) | 370 |
| July | 1.23 (0.41-4.30) | 3.39 (2.79-3.70) | 1.03 (0.83-1.30) | 452 |
| August | 1.24 (0.41-3.44) | 3.29 (2.43-3.69) | 1.02 (0.84-1.29) | 586 |
| September | 1.26 (0.45-3.69) | 3.00 (2.37-3.50) | 1.03 (0.82-1.28) | 551 |
| October | 1.30 (0.41-4.08) | 3.00 (2.33-3.56) | 1.04 (0.85-1.31) | 610 |
| November | 1.30 (0.38-3.86) | 3.05 (2.39-3.58) | 1.04 (0.84-1.32) | 576 |
| December | 1.34 (0.46-4.10) | 3.01 (2.36-3.62) | 0.99 (0.81-1.21) | 735 |
| All | 1.34 (0.43-4.27) | 3.13 (2.44-3.66) | 1.02 (0.83-1.27) | 6,343 |
Range represents 2.5th to 97.5th percentiles.
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyrotropin.
In the present study, we examined monthly variations in the serum FT4 and FT3 levels and serum TSH levels in healthy Japanese subjects, not patients with thyroid disorders. In addition, as the daily circadian rhythm of thyroid function, especially serum TSH values, increases from evening to midnight [19, 20], blood was sampled at 8:00 to 9:00 am to exclude the effects of diurnal variation. We are not using sunlight saving times in Japan.
All methods were performed in accordance with the relevant guidelines and regulations, including ethical guidelines for Medical and Health Research Involving Human Subjects presented by the Ministry of Health, Labour, and Welfare in Japan. This study was approved by the ethics committee on human research of Hidaka Hospital (approval number 3: Hidaka Hospital Human Genome Ethics Committee.). According to the ethical guidelines for Medical and Health Research Involving Human Subjects, with this study design, written informed consent is not necessarily required, but we widely disclosed the outline of our study and provided opportunities for unenrollment.
Blood Tests for Serum Thyroid Hormones and TSH Levels
Blood samples were collected from all subjects in the morning between 8:00 and 9:00 am after fasting for at least 11 hours. Serum thyroid hormone and TSH levels were measured using the following kits: TSH, Architect TSH CLIA (Abbott, Inc., Matsudo-shi, Chiba; the manufacturer’s reference range was 0.35~4.94 mIU/L), FT4, FT4•Abbott CLIA (Abbott, Inc., Matsudo-shi, Chiba; the manufacturer’s reference range was 0.70-1.48 ng/dL), and FT3, FT3•Abbott CLIA (Abbott, Inc., Matsudo-shi, Chiba; the manufacturer’s reference range was 1.71-3.71 pg/mL). All samples were measured with Architect i2000SR in the hospital laboratory.
Statistical Analyses
Medians, percentiles, P-values, and CIs were calculated using JMP 15.2.0 (SAS Institute Inc.). Multiple comparisons of the median of serum TSH, FT4, and FT3 levels in each month were performed using Dunn’s Multiple Comparison Test.
To compare serum TSH, FT4, and FT3 levels in each month with annual ranges, we used ANOMTR. ANOMTR compares the mean transformed rank of each group with the overall mean transformed rank. Transformed ranks suppose that there are n observations and were calculated as
The ANOM procedure was applied to the transformed Ri. We ranked all observations from smallest to largest, accounting for ties, and denoted the ranks by R1, R2, . . . , Rn. As the ranks have a uniform distribution, the transformed ranks have a folded normal distribution.
The estimated sample size between the groups was based on the difference in measured value and SD in serum TSH, FT4, and FT3 levels between groups, with a 2-sided type 1 error of less than 5% and power of 80%. The estimated sample size was sufficient for each month.
Results
Monthly Variation in the Median Serum TSH Levels
As shown in Table 1, the monthly median serum TSH levels were evaluated. The annual median TSH level was 1.34 mIU/L (0.43~4.27, the 2.5th~97.5th percentiles). The highest TSH level was 1.61 mIU/L in January (P < 0.01), which compared with other months. The lowest was 1.16 mIU/L in May (P < 0.01).
The 2.5th to 97.5th percentiles are shown in parentheses for each month in Table 1.
Monthly Variation in Median of Serum FT3 and FT4 Levels
The monthly median serum FT3 and FT4 levels are also shown in Table 1. The median FT3 level in the entire year was 3.13 pg/mL, the 2.5th percentile was 2.44 pg/mL, and the 97.5th percentile was 3.66 pg/mL. The serum FT3 level in July was 3.39 pg/mL, which was significantly higher than those in other months (P < 0.01). The lowest value was in January at 2.97 pg/mL.
The median FT4 in the entire year was 1.02 ng/dL, the 2.5th percentile was 0.83 ng/dL, and the 97.5th percentile was 1.27 ng/dL. The FT4 levels in February, March, October, and November were significantly higher than those in other months (P < 0.01). The lowest value was observed in December at 0.99 ng/dL.
ANOMTR of Thyroid Function in Each Month
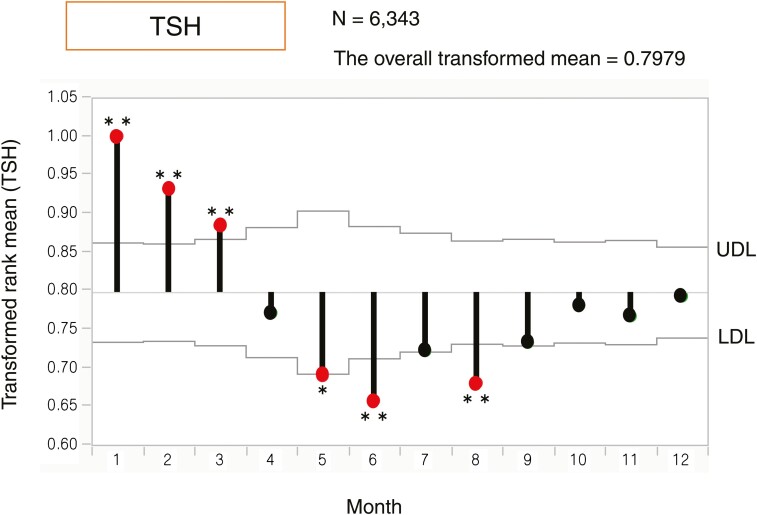
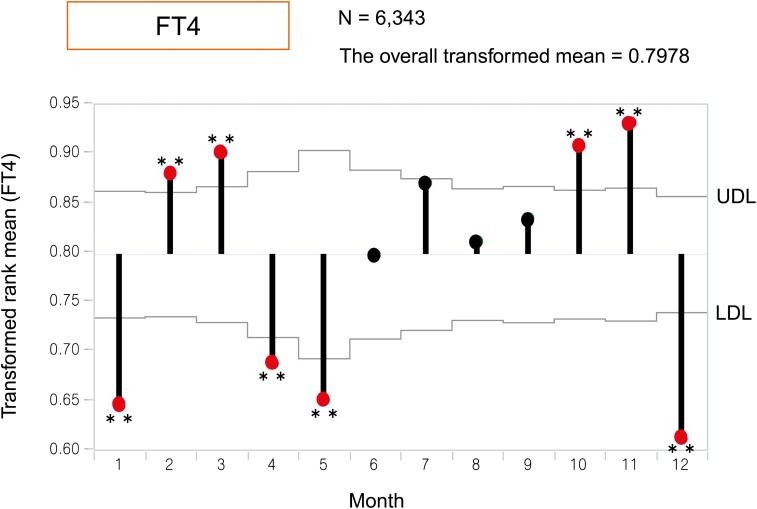
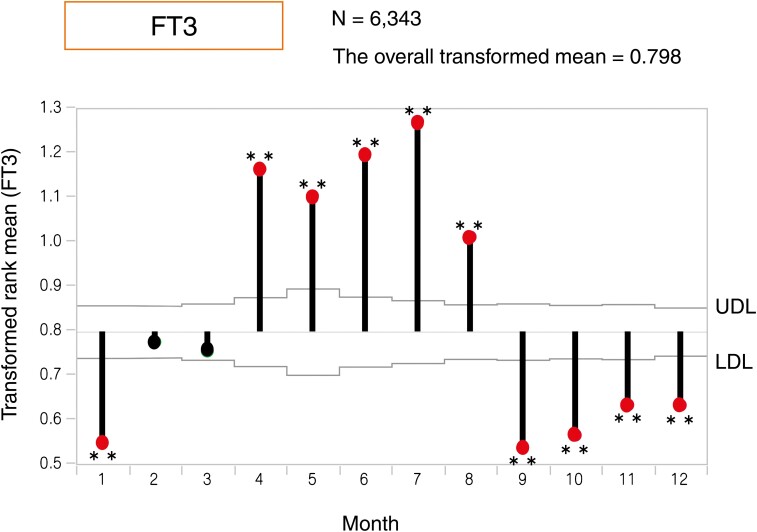
To assess the variation in the annual mean and create a visual image, we applied ANOMTR for thyroid function. The ANOM plot demonstrates a transformed rank mean in each month compared with the overall mean, and when the transformed rank mean is outside of the upper definition lines (UDL) and lower definition lines (LDL) considering the sample number in each month, the value is significant. The red dots in Figures 1 to 3 indicate significantly different months based on UDL or LDL.
Figure 1.
Analysis of means (ANOM) with transformed ranks of monthly changes in the serum thyrotropin (TSH) levels. UDL indicates upper definition lines, and LDL indicates lower definition lines. When the value was outside of the area representing P < 0.05 within UDL and LDL, it was significant and indicated by a red dot. The overall transformed mean was 0.7979. The ANOM plot demonstrated that the TSH levels were significantly higher from January to March and significantly lower in May, June, and August than the overall transformed mean. *P < 0.05, **P < 0.01.
Figure 3.
Analysis of means with transformed ranks (ANOMTR) of monthly changes in the serum free thyroxine (FT4) levels. ANOMTR of monthly changes in serum FT4 levels. The overall transformed mean was 0.7978. ANOMTR demonstrated that the FT4 levels were significantly higher in February, March, October, and November and significantly lower in January, April, May, and December than the overall transformed mean. *P < 0.05, **P < 0.01.
Regarding the serum TSH level each month, the values in January, February, and March were significantly higher than the overall transformed mean (P < 0.01) (Fig. 1).
The FT3 levels from April to August were higher (P < 0.01), and those from September to January were lower than the overall mean (P < 0.01) (Fig. 2).
Figure 2.
Analysis of means with transformed ranks (ANOMTR) of monthly changes in the serum free triiodothyronine (FT3) levels. ANOMTR of monthly changes in serum FT3 levels. The overall transformed mean was 0.798. The ANOM plot demonstrated that the FT3 levels were significantly higher from April to August and significantly lower in January and from September to December than the overall transformed mean. *P < 0.05, **P < 0.01.
Regarding FT4, although the values were more consistent through the year and the differences among months were small, those in February, March, October, and November were higher, and those in January, April, May, and December were lower (P < 0.01), demonstrating small biphasic changes throughout the year (Fig. 3).
Table 2 shows the number of subjects with normal thyroid function who were diagnosed as subclinical dysfunction using the manufacturer’s reference range. The distribution was significantly different (P < 0.05), suggesting the clinical relevance of seasonal changes particularly when subclinical dysfunction was diagnosed.
Table 2.
Number of subjects with normal thyroid function who were diagnosed as subclinical dysfunction using the manufacturer’s reference range in each month
| Subclinical hyperthyroidism, n (%) | Subclinical hypothyroidism, n (%) | |
|---|---|---|
| January | 4 (0.64) | 17 (2.71) |
| February | 10 (1.54) | 17 (2.61) |
| March | 3 (0.54) | 12 (2.17) |
| April | 4 (1.05) | 5 (1.31) |
| May | 5 (2.02) | 3 (1.21) |
| June | 3 (0.81) | 4 (1.08) |
| July | 5 (1.11) | 7 (1.55) |
| August | 8 (1.37) | 6 (1.02) |
| September | 8 (1.45) | 3 (0.54) |
| October | 9 (1.48) | 4 (0.66) |
| November | 8 (1.39) | 7 (1.22) |
| December | 5 (0.68) | 5 (0.68) |
| All | 72(1.14) | 90 (1.42) |
Discussion
In the present study, we investigated annual variation in thyroid function in over 7,000 healthy subjects in Japan, an iodine-sufficient country.
Several reports have been published regarding seasonal variations in thyroid function worldwide, and previous reports regarding seasonal variation in thyroid function in the last 2 decades using PubMed with the search terms “seasonal” and “thyroid function” or “TSH” and “thyroid hormones” are shown in Table 3 [5-16]. As shown in Table 3, 8 out of 12 reports demonstrated seasonal variation in serum TSH levels to be high in winter and low in summer [7, 9-12, 14-16], whereas 2 studies reported no changes in Iraq and Siberia, which has no clear seasonal changes and is a relatively cold area, respectively [8, 13]. A recent study from Ito Hospital in Japan investigating many patients with thyroid disorders reported similar results [10]. The studies listed in Table 3 are all from northern hemisphere countries; however, studies conducted in the Antarctic, which were excluded because of the small number of studies, reported that TSH was elevated in winter [21]. Therefore, taken together with the present study, at least in the northern hemisphere, the serum TSH levels in healthy subjects are high in winter.
Table 3.
Reports on seasonal variation in thyroid function in the last two decades
| Country | Latitude | Number examined | Ethnicity | TSH levels | FT3 levels | FT4 levels | Diseases and blood withdrawal time | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Israel | 31°N | 46 million persons | Broad ethnic representation | Major peak in August and minor peak in winter | High in winter | High in late autumn | Tendler et al. (2021) [5] | |
| 2 | Italy (Modena) |
44°N | 1,506,495 data points | n/a | High in summer and winter | No change | No change | Santi et al. (2019) [6] | |
| 3 | China (Peking) |
39°N | 6,524 subjects | n/a | High in winter, low in summer | No change | No change | All subjects were >65 years old. | Wang et al. (2019) [7] |
| 4 | Iraq (Sulaymaniyah) |
35°N | 152 healthy volunteers, 25 SCH subjects | n/a | No change | High in winter | No change | Samples were taken between 9:00 and 11:00 am after an overnight (12 hour) fast. | Mahwi et al. (2019) [8] |
| 5 | China (Peking) |
39°N | 79,570 data points | n/a | High in winter, low in summer | n/a | n/a | Wang et al. (2018) [9] | |
| 6 | Japan (Tokyo) |
36°N | 135,417 patients | n/a | High in winter, low in summer | High in winter, low in summer | High in summer, low in autumn | All subjects had thyroid disorder. Samples were taken between 8:00 am and 5:00 pm. | Yoshihara et al. (2018) [10] |
| 7 | UK, Japan, Belgium |
51°N, 36°N, 50°N | The daily medians are calculated from at least 500 patients. | n/a | High in winter, low in summer | n/a | n/a | De Grande et al. (2017) [11] | |
| 8 | United States (Salt Lake City) |
40°N | 324,750 outpatients | Caucasian 78.4%, Native American 0.4%, Pacific Islander 0.7%, African American 0.8%, Asian 1.4%, Other | Low in August, high in December | n/a | No change | No diagnosis of thyroid disease. | Ehrenkranz et al. (2015) [12] |
| 9 | Russia (Siberia) | 62°N | 35 men, 59 women | Yakut (Sakha) adults | No change | Low in winter | Low in winter | Samples were taken in July/August and January in the morning after overnight fasting. | Leonard et al. (2014) [13] |
| 10 | Russia(Siberia) | 62°N | 51 men, 83 women | Yakut (Sakha) adults | High in winter | Low in winter | Low in winter | Samples were taken in July/August and January in the morning after overnight fasting. | Levy et al. (2013) [14] |
| 11 | Korea (Seoul) |
37°N | 28,096 subjects | n/a | High in winter and spring, low in summer and autumn | n/a | n/a | Samples were taken after 12-hour overnight fasting. | Kim et al. (2013) [15] |
| 12 | Finland (Northern Finland) |
67-68°N | 20 men | Caucasian | High in winter | High in summer | No change | Samples were taken between 9:00 and 11:00 am. | Hassi et al. (2001) [16] |
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; n/a, not available; SCH, subclinical hypothyroidism; TSH, thyrotropin.
In contrast, as shown in Table 3, there are no consistent data regarding seasonal variation in serum FT3 and FT4 levels. Regarding FT3, 3 out of 8 studies reported high values in winter [5, 8, 10], and 3 reported high values in summer [13, 14, 16]. As shown in Table 1, the present study revealed the difference in median serum FT3 levels between the highest and lowest month to be approximately 0.5 pg/mL, and we applied ANOMTR to these results. The ANOM plot demonstrated changes in serum FT3 levels to be high from April to August and low from September to January (P < 0.01) (Fig. 2). What causes these difference in serum FT3 levels among countries? Several possibilities were raised, including different conditions of iodine intake, lifestyle of the country, blood sampling, kits using for assays, and racial differences. In the present study, blood samples were taken at 8:00 to 9:00 am after fasting to exclude the effects of feeding and diurnal variation in thyroid hormone, and over 95% of subjects examined were Japanese.
Similar to FT3 levels, 5 out of 9 studies reported no changes in FT4 levels through the year [6-8, 12, 16], whereas 2 reported low levels in winter [13, 14] and 2 reported low levels in summer or autumn [5, 10] (Table 3). As shown in Table 1, the present study demonstrated the variation in the median serum FT4 levels throughout the year to be small. However, the ANOM plot revealed a smaller difference than that in the serum FT3 level but significant biphasic changes (Fig. 3). These discrepancies among countries may be similar to those of serum FT3 levels, as discussed earlier. In addition, each country or region has different temperatures, temperature differences throughout the year, sunshine hours, and humidity, in addition to a different prevalence of thyroid disease.
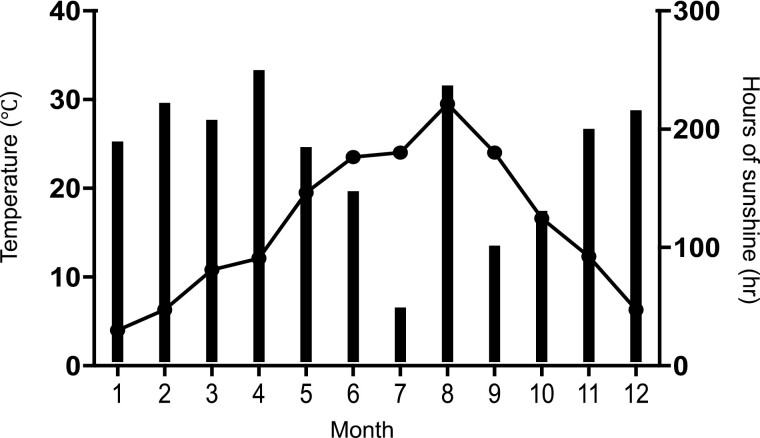
The present study was performed in Gunma prefecture, Japan, which has 4 distinct seasons. The temperature and sunshine time in each month examined in the present study are shown in Figure 4. The maximum temperature was observed in August, corresponding to the peak serum FT3 level, and the temperature was negatively correlated with the serum TSH throughout the year except for July. In Japan, during the transition from spring to summer, there is a seasonal phenomenon known as the rainy season. In this season, it rains more, and there is less sunlight than in the preceding and following seasons. In 2020, the rainy season began on June 11 and ended on August 1, which is thought to have resulted in shorter sunshine hours and relatively cool weather in July.
Figure 4.
The mean temperature and hours of sunshine in Maebashi, Japan. The data for the period when this study was performed in Maebashi City. The temperature each month is indicated by the line and hours of sunshine each month by the black bar. The data for April to December were from 2020 and the data for January to March were from 2021. The temperature was the highest in August, and the lowest temperature was in January. The hours of sunshine were highest in April and lowest in July. In Japan, there is a seasonal phenomenon called the “rainy season” in which the transition from spring to summer is marked by more rain and less sunshine than in the period before and after it. In 2020, the rainy season started on June 11 and ended on August 1, which is thought to have resulted in shorter sunshine hours in July. In addition, from the latter half of August to October, there is a phenomenon called “autumn rain” with shorter sunshine hours in September and October. These data were obtained from the Japan Meteorological Agency and Ministry of Land, Infrastructure, Transport, and Tourism. Hours of sunshine are calculated using observations from ground-based meteorological observation, and weather distribution are estimated from meteorological satellites. Historical Weather Data Search. The Japan Meteorological Agency and Ministry of Land, Infrastructure, Transport, and Tourism. https://www.jma.go.jp/jma/index.html. Accessed March 1, 2022.
Mechanisms of Variations in Thyroid Function
Based on the present study, serum FT3 levels, a strong factor in the downregulation of pituitary TSH production and secretion, seems to affect the serum TSH level, suggesting that seasonal changes in thyroid function are peripheral (thyroid hormone production-dependent). However, Hefco et al reported that experimental acute cold exposure increased serum TSH and thyroid hormone levels in rats and suggested both thyroid hormone and nonthyroid hormone-dependent mechanisms of changes in serum TSH [22]. On the other hand, as a study of cold exposure in humans, Leppäluoto et al reported changes in serum TSH and FT3 levels in healthy 20 men in Finland, a subarctic country [23], which were similar with the levels observed in our study. They suggested that changes in serum TSH levels are due to the photoperiod, and changes in serum FT3 may be polar T3 syndrome. Similarly, Reed et al evaluated the changes in thyroid functions in Antarctica and noted high serum TSH levels and low serum FT3 levels [21].
Furthermore, studies other than those cited here conducted between 1980s and 2000s reported possible mechanisms underlying seasonal variation of thyroid functions including the effect of sleeping metabolic rate, body composition, and serum leptin level [24]. Furthermore, other studies reported a greater response of TSH in the thyrotropin-releasing hormone test and high serum T4 and T3 values in winter [25]. On the other hand, another study reported high total T4, T3, and FT4 in summer and a relationship between relative weight and total T4 and FT4 levels [26]. In addition, a recent meta-analysis also reported that TSH levels were high in winter and FT4 levels were higher in autumn than in winter; however, FT3 levels were lower in summer. These results suggest that seasonal dynamics of TSH are influenced by the extent of the annual dynamics of the partial density of oxygen in the air as well as the magnitude of the annual dynamic of meteorological factors such as atmospheric pressure and relative humidity [27].
Several studies of circannual rhythms of HPT axis on pediatric cohorts have also been reported. Onsesveren et al reported that large differences in the reported reference ranges for TSH and FT4 during childhood showed significant elevation between day 1 and day 7 and gradually decreased until the age of 10 years. Furthermore, season at venipuncture was associated with serum FT4, with the highest being during autumn; however, there was no seasonal variation of serum TSH [28]. Nicolau et al reported that children around 11 years old showed seasonal variations in FT4 as well as FT3, with the highest values in the fall and the highest TSH values in the summer [29]. Bellastella et al reported that in prepuberty TSH was high in December while T4 and T3 did not show a circannual rhythm [30]. Although in pediatric subjects, differences in the time of blood withdrawal and fasting time may make the data inconsistent, thyroid function in the pediatric period may be affected by age and changes of life-style.
In addition, a relationship between the sexual cycle of migratory birds and TSH secreted from the pars tuberalis has been reported [31], suggesting that serum TSH does not originate from the anterior pituitary. In another animal experiment, there were reports showing that T4 had biphasic variations in deer, with high levels in early winter and spring, which is similar to the findings of the present study [32]. On the other hand, T4 in ewes was high in winter and low in summer [33]. It is interesting to note that laboratory rats were reported to show higher levels of T4 in summer [34], suggesting these hormonal changes may not be due only to climate factors.
Further studies are required to clarify the molecular mechanisms of seasonal variation in thyroid hormones in human including whether these thyroid changes may be due to the changing set points of the HPT axis.
Limitations of the Study
Several limitations of this study need to be considered when interpreting the results. First, compared with a population-based cohort study, this study only included health checkup participants; therefore, there was bias regarding the economic status of the subjects. Second, ethnicity may influence thyroid hormone levels, as the local population was evaluated using their own reference range. However, in the present study, more than 95% of the subjects were Japanese; thus, the effects of ethnicity and place of residence on thyroid hormones was minimal.
Conclusions
The present study demonstrated seasonal changes in the serum TSH levels, with high levels in winter, whereas serum FT3 levels of Japanese were high in summer. Seasonal variation in thyroid function should be taken into account to precisely evaluate thyroid function in clinical practice.
Acknowledgments
We would like to thank Prof. Haruyasu Fujita for statistical analysis. This study was supported in part by a Health Labour Science Research Grant (802) and Gunma University Initiative for Advanced Research (GIAR) (to M.Y.).
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Melmed S. The Pituitary. 3rd ed. Elsevier; 2010; 25-31. [Google Scholar]
- 2. Lewis E., Braverman DSC. Werner & Ingbar’s The Thyroid a Fundamental Clinical Text . [Google Scholar]
- 3. Razvi S, Bhana S, Mrabeti S. Challenges in interpreting thyroid stimulating hormone results in the diagnosis of thyroid dysfunction. J Thyroid Res. 2019;2019:4106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87(3):1068-1072. [DOI] [PubMed] [Google Scholar]
- 5. Tendler A, Bar A, Mendelsohn-Cohen N, et al. Hormone seasonality in medical records suggests circannual endocrine circuits. Proc Natl Acad Sci USA. 2021;118(7):e2003926118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santi D, Spaggiari G, Brigante G, et al. Semi-annual seasonal pattern of serum thyrotropin in adults. Sci Rep. 2019;9(1): 10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Yu S, Ma C, et al. Reference intervals for thyroid-stimulating hormone, free thyroxine, and free triiodothyronine in elderly Chinese persons. Clin Chem Lab Med. 2019;57(7):1044-1052. [DOI] [PubMed] [Google Scholar]
- 8. Mahwi TO, Abdulateef DS. Relation of different components of climate with human pituitary-thyroid axis and FT3/FT4 ratio: a study on euthyroid and SCH subjects in two different seasons. Int J Endocrinol 2019;2019:2762978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Li D, Guo X, et al. Effects of sex, age, sampling time, and season on thyroid-stimulating hormone concentrations: a retrospective study. Biochem Biophys Res Commun. 2018;506(3):450-454. [DOI] [PubMed] [Google Scholar]
- 10. Yoshihara A, Noh JY, Watanabe N, et al. Seasonal changes in serum thyrotropin concentrations observed from big data obtained during six consecutive years from 2010 to 2015 at a single hospital in Japan. Thyroid 2018;28(4):429-436. [DOI] [PubMed] [Google Scholar]
- 11. De Grande LA, Goossens K, Van Uytfanghe K, et al. Using “big data” to describe the effect of seasonal variation in thyroid-stimulating hormone. Clin Chem Lab Med. 2017;55(2):e34-e36. [DOI] [PubMed] [Google Scholar]
- 12. Ehrenkranz J, Bach PR, Snow GL, et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid. 2015;25(8):954-961. [DOI] [PubMed] [Google Scholar]
- 13. Leonard WR, Levy SB, Tarskaia LA, et al. Seasonal variation in basal metabolic rates among the Yakut (Sakha) of Northeastern Siberia. Am J Hum Biol. 2014;26(4):437-445. [DOI] [PubMed] [Google Scholar]
- 14. Levy SB, Leonard WR, Tarskaia LA, et al. Seasonal and socioeconomic influences on thyroid function among the Yakut (Sakha) of Eastern Siberia. Am J Hum Biol. 2013;25(6):814-820. [DOI] [PubMed] [Google Scholar]
- 15. Kim TH, Kim KW, Ahn HY, et al. Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J Clin Endocrinol Metab. 2013;98(8):3420-3429. [DOI] [PubMed] [Google Scholar]
- 16. Hassi J, Sikkila K, Ruokonen A, Leppaluoto J. The pituitary-thyroid axis in healthy men living under subarctic climatological conditions. J Endocrinol. 2001;169(1):195-203. [DOI] [PubMed] [Google Scholar]
- 17. Ott ER. Analysis of means—a graphical procedure. Industrial Quality Control. 1967;24:101-109. [Google Scholar]
- 18. Yigit S. Comparison of ANOM and ANOMTR tests with regard to performances. Turk J Argic Res. 2019;6:193-198. [Google Scholar]
- 19. Roelfsema F, Pereira AM, Veldhuis JD, et al. Thyrotropin secretion profiles are not different in men and women. J Clin Endocrinol Metab. 2009;94(10):3964-3967. [DOI] [PubMed] [Google Scholar]
- 20. Brabant G, Prank K, Ranft U, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J Clin Endocrinol Metab. 1990;70(2):403-409. [DOI] [PubMed] [Google Scholar]
- 21. Reed HL, Reedy KR, Palinkas LA, et al. Impairment in cognitive and exercise performance during prolonged antarctic residence: effect of thyroxine supplementation in the polar triiodothyronine syndrome. J Clin Endocrinol Metab. 2001;86(1):110-116. [DOI] [PubMed] [Google Scholar]
- 22. Hefco E, Krulich L, Illner P, Larsen PR. Effect of acute exposure to cold on the activity of the hypothalamic-pituitary-thyroid system. Endocrinology. 1975;97(5):1185-1195. [DOI] [PubMed] [Google Scholar]
- 23. Leppaluoto J, Sikkila K, Hassi J. Seasonal variation of serum TSH and thyroid hormones in males living in subarctic environmental conditions. Int J Circumpolar Health. 1998;57(suppl 1):383-385. [PubMed] [Google Scholar]
- 24. Plasqui G, Kester AD, Westerterp KR. Seasonal variation in sleeping metabolic rate, thyroid activity, and leptin. Am J Physiol Endocrinol Metab. 2003;285(2):E338-E343. [DOI] [PubMed] [Google Scholar]
- 25. Harrop JS, Ashwell K, Hopton MR. Circannual and within-individual variation of thyroid function tests in normal subjects. Ann Clin Biochem. 1985;22(Pt 4):371-375. [DOI] [PubMed] [Google Scholar]
- 26. Pasquali R, Baraldi G, Casimirri F, et al. Seasonal variations of total and free thyroid hormones in healthy men: a chronobiological study. Acta Endocrinol. 1984;107(1):42-48. [DOI] [PubMed] [Google Scholar]
- 27. Kuzmenko NV, Tsyrlin VA, Pliss MG, Galagudza MM. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: a meta-analysis. Chronobiol Int. 2021;38(3):301-317. [DOI] [PubMed] [Google Scholar]
- 28. Onsesveren I, Barjaktarovic M, Chaker L, et al. Childhood thyroid function reference ranges and determinants: a literature overview and a prospective cohort study. Thyroid. 2017;27(11):1360-1369. [DOI] [PubMed] [Google Scholar]
- 29. Nicolau GY, Dumitriu L, Plinga L, et al. Circadian and circannual variations of thyroid function in children 11 ± 1.5 years of age with and without endemic goiter. Prog Clin Biol Res. 1987;227B:229-247. [PubMed] [Google Scholar]
- 30. Bellastella A, Criscuolo T, Mango A, Perrone L, Sinisi AA, Faggiano M. Circannual rhythms of plasma growth hormone, thyrotropin and thyroid hormones in prepuberty. Clin Endocrinol. 1984;20(5):531-537. [DOI] [PubMed] [Google Scholar]
- 31. Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Front Neuroendocrinol. 2013;34(3):157-166. [DOI] [PubMed] [Google Scholar]
- 32. Watkins BE, Ullrey DE, Nachreiner RF, Schmitt SM. Effects of supplemental iodine and season on thyroid-activity of white-tailed deer. J Wildlife Manage. 1983;47(1):45-58. [Google Scholar]
- 33. Webster JR, Moenter SM, Woodfill CJ, Karsch F, J. Role of the thyroid gland in seasonal reproduction. II. Thyroxine allows a season-specific suppression of gonadotropin secretion in sheep. Endocrinology. 1991;129(1):176-183. [DOI] [PubMed] [Google Scholar]
- 34. Wong CC, Dohler KD, Atkinson MJ, Geerlings H, Hesch RD, von zur Muhlen A. Influence of age, strain and season on diurnal periodicity of thyroid stimulating hormone, thyroxine, triiodothyronine and parathyroid hormone in the serum of male laboratory rats. Acta Endocrinol (Copenh). 1983;102(3):377-385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.