Abstract
Background
The duration of rectal gonococcal and chlamydial infection remains unknown. This basic epidemiologic parameter is needed to understand transmission dynamics.
Methods
We conducted a prospective, longitudinal, observational cohort study of 140 men who have sex with men (MSM) at risk of gonorrhea and chlamydia acquisition. For 48 weeks, enrolled men collected rectal swabs (Aptima multi-test kit) at home and responded to an electronic survey about sexual behavior and health conditions weekly. Swabs remained untested until participants completed the study. We used Kaplan-Meier estimates to determine the median duration of infection, censoring infections for treatment, loss to follow-up, and end-of-study. We used log-rank test to compare duration of infection by human immunodeficiency virus (HIV) status, history of infection with gonorrhea or chlamydia, and coinfection with the other pathogen.
Results
140 enrolled MSM contributed 70.5 person-years of follow-up. Eighteen men had 20 incident rectal gonococcal infections, which persisted for 2–23 weeks; 30% were censored for treatment. The estimated median duration of rectal gonorrhea was 9 weeks (95% confidence interval [CI]: 3–12 weeks). Twenty-four men experienced 32 rectal chlamydial infections, persisting between 2 and 42 weeks; 60% were censored. The estimated duration of rectal chlamydia was 13 weeks (95% CI: 6 weeks–undefined). There were no differences in the duration of rectal gonorrhea or chlamydia by HIV status, history of chlamydia/gonorrhea, or coinfection.
Conclusions
On average, rectal gonorrhea and chlamydial infections last 2–3 months, although some infections persisted for 6–11 months. Further understanding into predictors of persistence is needed.
Keywords: gonorrhea, chlamydia, rectal infections, epidemiology, natural history study
Using weekly at-home, self-collected rectal Nucleic Acid Amplification Tests over a 48-week study period, we estimated the duration of rectal gonorrhea and chlamydia to be 9 and 13 weeks, respectively, in a cohort of Men who have Sex with Men at risk of sexually transmitted infections.
Infection with Neisseria gonorrhoeae and Chlamydia trachomatis at the anorectum is common among men who have sex with men (MSM). In a sexual health clinic setting, 11.8% and 12.6% of MSM screen positive for gonorrhea and chlamydia, respectively, at the rectum [1]. Although these infections are primarily asymptomatic, in the absence of human immunodeficiency virus (HIV) pre-exposure prophylaxis they increase the risk of HIV acquisition over 2-fold [2]. Moreover, given their high asymptomatic prevalence, to the extent that these infections go undetected through screening they may serve as a reservoir for ongoing community transmission [3].
Little is known about the natural history of rectal gonorrhea and chlamydia. In particular, the duration of rectal gonococcal and chlamydial infection remains unknown. Chow and colleagues [4] estimated the duration of these infections by dividing the estimated prevalence by the estimated incidence, reporting that rectal gonorrhea and chlamydia persist for a mean of 346 days (nearly 1 year) and 579 days (over 18 months). To the best of our knowledge, there are no published, empirically derived estimates of duration for these infections in the absence of treatment. We aimed to determine the duration of rectal gonorrhea and chlamydia using a prospective design of weekly self-collected specimens in a cohort of MSM.
METHODS
Between March 2016 and December 2018 we recruited 140 MSM from the Public Health—Seattle & King County (PHSKC) Sexual Health Clinic (SHC; formerly STD Clinic) and the University of Washington’s Center for AIDS Research patient registry for the ExGen Study, a prospective cohort study to define the incidence and duration of extragenital gonorrhea and chlamydia that has been previously described [5]. This study was approved by the University of Washington’s Institutional Review Board (#50028).
Study Population
Eligible men were at least 18 years of age, spoke and read English, had internet access, and reported receptive anal intercourse in the last 12 months and performing oral sex in the past 2 months. Additionally, they needed to meet at least 1 of the following criteria in the past 12 months for eligibility: (1) diagnosis of gonorrhea, chlamydia or syphilis; (2) use of methamphetamine or amyl nitrate (“poppers”); or (3) more than 2 sexual partners in the past 2 months or more than 5 sexual partners within 12 months. All enrolled men completed a written informed consent.
Study Procedures
We asked enrolled men to attend 2 in-person visits at the PHSKC SHC (ie, the enrollment and exit visits) and to complete weekly self-collected swabs and an electronic survey at home for a period of 48 weeks. At the enrollment visit, after completing the eligibility screen and informed consent, study personnel collected basic demographic and clinical data and instructed the participant on study procedures. The study participant then completed the first sexual behavior survey and underwent complete sexually transmitted infection (STI) testing (ie, gonorrhea and chlamydia at the throat and rectum, syphilis, and HIV, if not known to be previously diagnosed with HIV) if they had not had verifiable clinical testing within 3 weeks of the enrollment visit. Enrolled participants diagnosed with an STI at the baseline visit were treated clinically and asked to defer starting weekly study procedures until 2 weeks (gonorrhea) [6] or 3 weeks (chlamydia) [7] after treatment.
We asked men to self-collect and return via US Postal Service a rectal swab in an Aptima multi-test collection system (Hologic, Inc, San Diego, CA) each week. (Mailed Aptima specimens for gonorrhea and chlamydia testing have been shown to be stable up to 84 days and in ambient temperatures between 2°C and 36°C [8].) Specimens were promptly stored in a −80°C freezer until the participant had completed the entirety of the study. Specimens were tested in the University of Washington Neisseria Reference Laboratory using the Aptima Combo 2 (AC2; Hologic, Inc) assay on the fully automated Panther machine (Hologic, Inc) according to the Clinical Laboratory Improvement Amendments (CLIA) regulatory requirements for quality assurance [9].
At enrollment, study personnel emphasized that study specimens would not be tested in real time and that participants should continue their usual STI screening practices. Insofar as participants continued to seek STI screening or treatment during the study, we asked them to receive these services at the PHSKC SHC so that we could capture clinical test positivity and treatment episodes. Each week, participants received an email or SMS text message with a link to a personalized, password-protected REDCap survey. The survey queried participants about intervening sexual behavior, symptoms, testing and treatment, and adjuvant health and hygiene practices since their last completed survey. All data were collected and managed through REDCap [10]. Study participants were compensated for their participation.
Statistical Analysis
Definitions
For the primary analysis, we defined a rectal infection as at least 2 consecutive weeks of positive specimens. Clearance of an infection was defined as 2 consecutive weeks of negative specimens. We imputed missing, negative, and/or equivocal specimens that occurred in-between positive specimens as follows: (1) a single negative, missing, or equivocal specimen between 2 single-positive specimens was considered positive; (2) 2 consecutive negative, missing, or equivocal specimens in-between 2 sets of 2 or more consecutive positive specimens (ie, infections) were imputed as positive. Because there were a high number of single-positive specimens that did not meet criteria for a true infection, we conducted a secondary analysis in which we considered a single-positive specimen to be a “true infection” and clearance to be 2 consecutively negative specimens. Imputation rules remained the same as in the primary analysis.
Censoring
We censored infections for receipt of pathogen-specific treatment, loss to follow-up, and for infections occurring on the last week of the study. Specifically, for gonococcal infections, we considered receipt of ceftriaxone alone, ceftriaxone plus either azithromycin and/or doxycycline, or azithromycin alone to be pathogen-specific treatment. (No one received gentamicin during the study period.) Given uncertainty about RNA clearance, we consider antibiotics given during the final, penultimate, or week following the final week of specimen positivity to have cleared an infection. For rectal chlamydia infections, we considered azithromycin or doxycycline received in the week of, the week prior to, or the week after the final positive week of infection to have cleared the infection. However, if a participant received antibiotic treatment (eg, azithromycin) and remained positive, that treatment was not considered effective.
Analyses
Although we requested that participants collect specimens and survey data every 7 days, we allowed a 3-day window beyond the intended collection date for reimbursement purposes and included all received data in analyses. We calculated time in days between collected specimens and converted days to weeks for reporting purposes. We calculated the incidence of infection as the number of new infections divided by the time at-risk. That is, we removed time when individuals had an infection (positive weeks) from the time at-risk. We excluded infections present on week 1 of collection if they were also present at the enrollment visit. For the duration analyses, we used Kaplan-Meier methods to estimate the median duration of infection. We assessed for differences in duration by HIV status, history of gonorrhea or chlamydial infection consistent with the analysis, and coinfection with the other pathogen using log-rank test. All analyses were conducted using Stata 15.0 (StataCorp, College Station, TX), and an ɑ of 0.05 was considered statistically significant.
RESULTS
Study Population
The 140 enrolled MSM had a mean age of 37 years (range: 20–75 years) and just over half (n = 71) had HIV (Table 1). Most (74.3%) had been diagnosed with gonorrhea, chlamydia, or syphilis in the past year, and one-quarter (n = 37) tested positive for a bacterial STI at their enrollment visit. Twenty-eight (20%) of the 140 enrolled men only completed the enrollment visit, leaving 112 men who completed a median follow-up time of 39 weeks (range: 1–48 weeks). The total follow-up time was 70.5 person-years (py).
Table 1.
Study Population Demographics, Sexual Behavior, and Sexually Transmitted Infection History and Diagnoses at Enrollment Overall and by Rectal Gonorrhea and/or Chlamydial Infection
| Characteristics | Study Participants (N = 140) | Study Participants with ≥1 Incident Rectal GC Infection (n = 18) | Study Participants with ≥1 Incident Rectal CT Infection (n = 24) |
|---|---|---|---|
| Age, mean (range), years | 37 (20–75) | 36 (20–75) | 36 (20–73) |
| Race/ethnicity, n (%) | |||
| Non-Hispanic White/Caucasian | 88 (63) | 13 (72) | 20 (83) |
| Non-Hispanic Black/African-American | 15 (11) | 1 (6) | 0 |
| Hispanic/Latino | 25 (18) | 3 (17) | 3 (12.5) |
| Asian/Pacific Islander | 8 (5.7) | 1 (6) | 1 (4.2) |
| Other | 3 (2.5) | 1 (6) | 0 |
| Income, n (%) | |||
| <$14 999 | 54 (39) | 6 (33) | 9 (38) |
| >$15 000–$29 999 | 26 (19) | 5 (28) | 4 (17) |
| >$30 000–$49 999 | 28 (20) | 3 (17) | 6 (25) |
| >$50 000–$99 999 | 25 (18) | 3 (17) | 5 (21) |
| >$100 000 | 7 (5.0) | 1 (6) | 0 |
| Educational attainment, n (%) | |||
| Grade school | 3 (2.1) | 0 | 0 |
| High school | 30 (21) | 5 (28) | 7 (29) |
| Some college/associate or technical degree | 50 (36) | 7 (39) | 6 (25) |
| Bachelor’s degree/some graduate school | 39 (28) | 5 (28) | 9 (37) |
| Graduate degree | 18 (13) | 1 (6) | 2 (8) |
| HIV, n (%) | |||
| Infected | 71 (51) | 14 (78) | 13 (54) |
| Uninfected on PrEP | 40 (58) | 2 (50) | 8 (73) |
| Self-reported lifetime STI history, n (%) | |||
| Syphilis | 66 (47) | 8 (44) | 12 (50) |
| Gonorrhea | 104 (74) | 16 (89) | 21 (88) |
| Chlamydia | 95 (68) | 17 (94) | 20 (83) |
| Herpes | 31 (22) | 4 (22) | 4 (17) |
| Criteria for study entry, n (%) | |||
| Bacterial STI diagnosis <12 months | 104 (74) | 13(72) | 16 (67) |
| Methamphetamine or popper use | 35 (25) | 3 (17) | 3 (13) |
| Number of sexual partners | 117 (84) | 16 (89) | 20 (83) |
| Early syphilis diagnosis at enrollment, n (%) | 5 (4) | 3 (17) | 3 (13) |
| HIV diagnosis at enrollment, n (%) | 1 (1) | 0 | 0 |
| Gonorrhea diagnoses at enrollment, n (%) | 19 (14) | 3 (17) | 3 (13) |
| Pharyngeal | 11 (8) | 1 (6) | 1 (4) |
| Rectal | 13 (9) | 2 (11) | 2 (8) |
| Urethral | 2 (1) | 0 | 0 |
| Chlamydia diagnoses at enrollment, n (%) | 17 (11) | 2 (11) | 8 (33) |
| Pharyngeal | 2 (1) | 0 | 1 (4) |
| Rectal | 14 (10) | 2 (11) | 7 (29) |
| Urethral | 2 (1) | 0 | 0 |
| Number of sexual partners <2 months, median (IQR) | 6 (4–12) | 11 (6–15) | 9 (5–15) |
| Number of receptive anal sex acts <2 months, median (IQR) | 11 (4–37) | 11.5 (8–30) | 12.5 (8–22) |
| Oral-anal sex received <2 months, n (%) | 88 (63) | 13 (72) | 13 (54) |
Abbreviations: CT, chlamydia; GC, gonorrhea; HIV, human immunodeficiency virus; IQR, interquartile range; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Rectal Gonorrhea
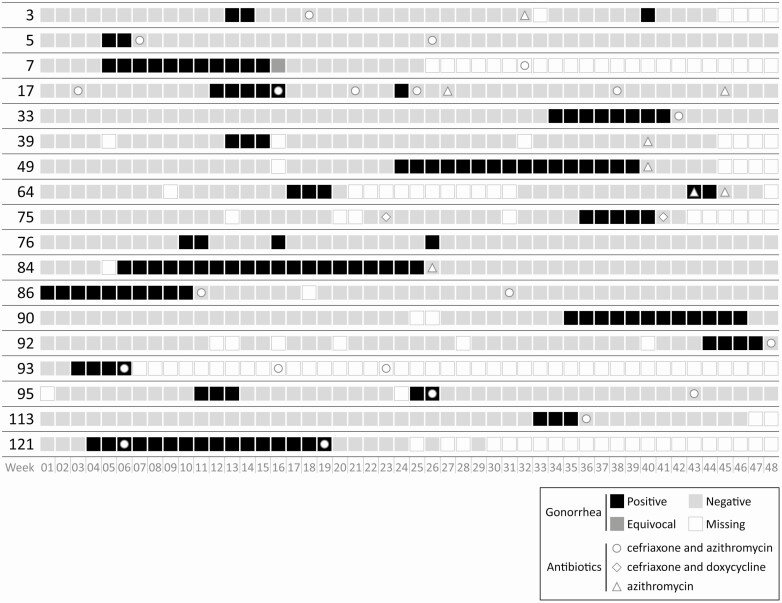
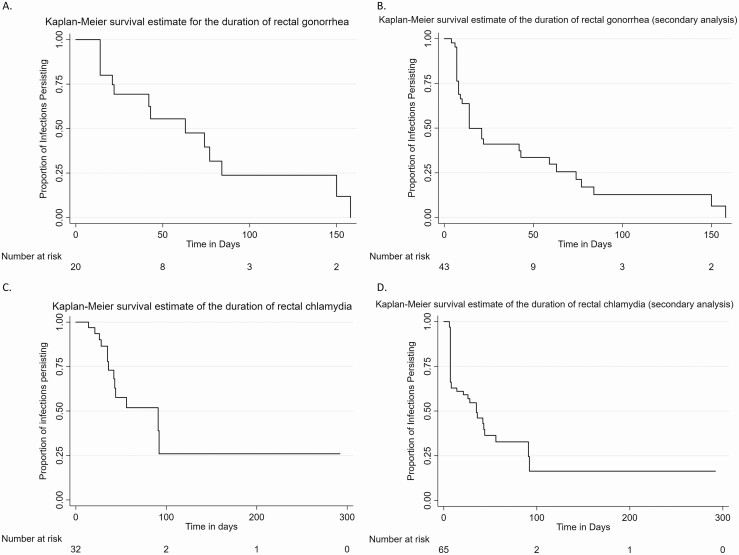
In the primary analysis, there were 20 incident infections among 18 men, or an incidence of 30.3 rectal gonorrhea infections per 100 py (95% confidence interval [CI]: 19.6–47.0 per 100 py) (Figure 1). The 20 rectal gonococcal infections were observed for a duration between 2 and 22.5 weeks. The majority (n = 14, 70%) were censored, all due to treatment. The estimated median duration of infection was 9 weeks (95% CI: 3–12 weeks) (Figure 3). There were no statistically significant differences between the duration by HIV status, lifetime history of gonorrhea, or coinfection with chlamydia.
Figure 1.
Weekly self-obtained rectal gonorrhea NAAT results and antibiotic receipt over 48 weeks of study participation among 18 MSM with incident rectal gonorrhea. Participant 86 was rectal gonorrhea negative at baseline. Abbreviations: MSM, men who have sex with men; NAAT, nucleic acid amplification test.
Figure 3.
Kaplan-Meier survival estimates for the duration of rectal infections. A, Rectal gonorrhea—primary analysis infection definition. B, Rectal gonorrhea—secondary analysis infection definition (ie, including single positive tests as “true infections”). C, Rectal chlamydia—primary analysis infection definition. D, Rectal chlamydia—secondary analysis infection definition (ie, including single-positive tests as “true infections”). Note different time scales between gonorrhea and chlamydia panels.
In the secondary analysis, there were 43 infections among 35 men, for an estimated incidence of 64.6 rectal gonococcal infections per 100 py (95% CI: 47.4–86.7 per 100 py). The range of the observed duration of these infections was from 4 days to 22.5 weeks. Eleven (26%) of these infections were censored, either for treatment (n = 7; 64%) or for loss to follow-up (n = 4; 36%). The estimated median duration of infection including single-positive specimens was 2 weeks (95% CI: 1–6 weeks), and there were no differences by HIV status, history of gonorrhea, or coinfection with chlamydia.
Rectal Chlamydia
In the primary analysis, there were 32 rectal chlamydial infections among 24 men (Figure 2). The calculated incidence was 46.8 infections per 100 py (95% CI: 32.8–66.3 per 100 py). Infections were observed between 2 and 42 weeks, and nearly 60% (n = 19) were censored. Censoring occurred most commonly due to treatment (n = 12; 37.5%), followed by end of the study (n = 5; 16%) and loss to follow-up (n = 2; 6%). The duration of rectal chlamydial infection was 13 weeks (95% CI: 6 weeks–undefined) (Figure 3). There were no differences in infection duration by HIV status, history of chlamydia, or coinfection with gonorrhea.
Figure 2.
Weekly self-obtained rectal chlamydia NAAT results and antibiotic receipt over 48 weeks of study participation among 24 MSM with incident rectal chlamydia. Participant 3 was rectal chlamydia negative at baseline. Abbreviations: MSM, men who have sex with men; NAAT, nucleic acid amplification test.
In the secondary analysis, there were 65 infections among 38 men for a calculated incidence of 90.0 rectal chlamydial infections per 100 py (95% CI: 69.8–115.9 per 100 py). Infections were observed for between 6 days and 42 weeks, and less than half were censored (n = 28; 43%). The reasons for censoring were primarily for treatment (n = 14; 22%), followed by loss to follow-up (n = 8; 12.3%) and, last, by end of study (n = 6; 9%). Including these single-positive specimens as true infections decreased the duration of infection to 5 weeks (95% CI: 1–8 weeks). There were no differences in infection duration by HIV status, history of chlamydia, or coinfection with gonorrhea.
Discussion
To the best of our knowledge, this is the first study to empirically measure the duration of rectal gonococcal and chlamydial infections from acquisition to clearance. Harnessing modern technologies such as nucleic acid amplification test (NAAT), self-collection of specimens, electronic surveys, and electronic medical records allowed us to test men frequently, and thus observe the entirety of an infection and ultimately provide valid estimates of their duration: rectal gonorrhea’s median duration is 9 weeks and rectal chlamydia’s duration is 13 weeks. However, these estimates are based on an infection definition that presumed that single-positive specimens, sometimes called “blips,” are false-positive tests. When we considered “blips” to be “true infections” in the secondary analysis, the estimated median duration of infection declined for both gonorrhea and chlamydia to 2 and 5 weeks, respectively. There remains a great deal of uncertainty about the importance of single-positive specimens and their validity. Importantly, most gonococcal infections and nearly half of the chlamydia infections were observed until they cleared spontaneously—meaning they were not detected through routine screening or symptomatic testing—and thus this has important implications for the current policies for gonorrhea and chlamydia control efforts among MSM.
Our empirically derived estimates conflict with the only other published estimates of rectal infection duration, which were calculated from incidence and prevalence estimates. Chow and colleagues [4] estimated that rectal infections last for 1 year or more. However, the primary data source for these estimates was the Health In Men (HIM) Study, which was conducted between 2001 and 2005 in Australia and relied on a combination of annual rectal testing plus participant self-report to estimate incidence of disease, with enrollment test results considered the cohort’s prevalence [11]. Additionally, part of the HIM study was conducted prior to 2003 when culture was the primary testing technology, and after 2003, the study team employed a strand displacement assay (SDA) by BD Probe Tech, both of which are less sensitive than the Aptima [12]. In the HIM study, incidence of rectal gonorrhea was only 0.96 per 100 py and rectal chlamydia incidence was 2.75 infections per 100 py, both of which are less than 10% of the incidences that we observed using a more frequent testing interval. The discrepancies between these 2 studies serve to highlight the importance of frequent testing to detect and treat infections—with the ultimate goal of decreasing community prevalence. Annual testing will miss between two-thirds and three-quarters of rectal infections.
At the same time, the upper bound of the 95% CI for the rectal chlamydial duration estimate in our study was undefined. We suspect that this was due to a relatively high degree of censoring (60%) and that longer duration infections tended to be censored more often. It is possible that some rectal chlamydial infections can last 1.5 years as Chow and colleagues suggest, as we observed 1 infection that lasted for 42 weeks, which was censored for its presence at the end of the study. Understanding which infections persist and which do not could have major implications for clinical management. However, we did not observe any differences in persistence by HIV status, history of chlamydia (a surrogate marker for possible immunity), or coinfection with gonorrhea. Undoubtedly, there are other factors that we did not measure in our study, which might help predict persistence and/or clearance. Further research into this area is warranted.
One interesting observation in our study was the finding of many single-positive specimens for both gonorrhea and chlamydia. The importance of these single-positive NAAT is uncertain. While they could represent false positives derived from a recent sexual encounter, or from a contaminated environment [13], it is also possible that they represent short-duration infections that rapidly cleared. Further investigation into these single-positive specimens is needed. In the real world, these single-positive specimens could lead to unnecessary treatment, and subsequently antimicrobial resistance. Moreover, this is not a phenomenon unique to our study. In a recent randomized controlled trial of rectal chlamydial treatment, approximately 20% of MSM with a clinical diagnosis of rectal infection were NAAT negative at the baseline enrollment visit approximately 2 weeks later [14]. Although we cannot be certain that these were single-positive specimens that happened to be captured during screening, the high proportion of clearance seen prior to study enrollment for the rectal chlamydia treatment study, and the possibility of capturing even a portion of the single-positive NAATs seen in ExGen during a clinical visit, has the potential to result in an overuse of antibiotics, which could be contributing to the rising rates of azithromycin-resistant gonorrhea [15].
This study is subject to a number of limitations. Most important, we used an RNA-based testing technology to detect and define infection. RNA-based NAATs are highly sensitive for gonorrhea and chlamydia diagnosis, especially compared with culture [12]. However, since they only detect nucleic acids, there can be false-positive results resulting from detection of nucleic acid in the absence of viable infection, leading to an overestimate of the true duration of infection. However, this study could not have been conducted using culture, which requires in-clinic specimen collection. Moreover, given poor sensitivity of both gonorrhea and chlamydial culture, use of culture would likely have led to an underestimate of both incidence and duration. Second, in an effort to ensure a robust incidence of infection in the cohort, we enhanced our study population with MSM who would be considered at high risk of infection due to their history of sexual behavior and STIs. Thus, the incidences found in this study may not be generalizable to all MSM populations. However, we do not believe that the behavioral and STI history requirements for study entry affected the duration estimates. In fact, history of chlamydia and/or gonorrhea was not associated with the duration of infection in either primary or secondary analyses for either infection. Moreover, enrolling a lower-risk population would have required a much larger sample size than our funding mechanism could have supported.
In conclusion, rectal infection with gonorrhea or chlamydia is common among MSM and, on average, persists for 2–3 months in the absence of treatment, although it can last upwards of 6 to 11 months. Encouraging every-3-month screening among at-risk MSM likely will have a larger impact on decreasing community prevalence than 6- to 12-month screening. However, understanding clinical and behavioral differences that predict persistence and determining the significance of the high proportion of single-positive specimens are warranted to avert overtreatment.
Notes
Previous presentation. These data were presented, in part, at the International Society for STD Research World Congress in Vancouver, British Columbia, Canada, 15 July 2019.
Acknowledgments. The authors thank their study participants and the Public Health—Seattle & King County Sexual Health Clinic for donating study space. They thank Rushlenne Pascual and Daphne Hamilton from the University of Washington Neisseria Reference Laboratory for laboratory support and Sean Proll of the Fred Hutchinson Cancer Research Institute for data visualization and graphics support.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant number K23 AI113185 to L. A. B.). Specimen collection kits and test reagents were provided in-kind by Hologic, Inc (San Diego, CA). REDCap (data collection and management system) at the University of Washington’s Institute for Translation Health Sciences (ITHS) is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1 TR002319. The University of Washington/Fred Hutchinson Center for AIDS Research (CFAR), is an NIH-funded program under award number AI027757, which is supported by the following NIH Institutes and Centers: NIAID, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Heart, Lung and Blood Institute, National Institute on Aging, National Institute of General Medical Sciences, and National Institute of Diabetes and Digestive and Kidney Diseases.
Potential conflicts of interest. L. A. B. reports that Hologic provided test kits in-kind during the conduct of the study, a grant and personal fees from Nabriva outside of the submitted work, and a grant from SpeeDx outside of the submitted work. C. M. K. and L. A. B. have received donations of specimen collection kits and reagents from Hologic, Inc, for studies unrelated to the submitted work. M. R. G. reports a grant from the NIH and a grant from Hologic that went to their associated institution outside of the submitted work. J. P. H. reports a grant from the NIH outside of the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Abara WE, Llata EL, Schumacher C, et al. Extragenital gonorrhea and chlamydia positivity and the potential for missed extragenital gonorrhea with concurrent urethral chlamydia among men who have sex with men attending sexually transmitted disease clinics—sexually Transmitted Disease Surveillance Network, 2015–2019. Sex Transm Dis 2020; 47(6):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang L, Regan DG, Chow EPF, et al. Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash. Sex Transm Dis 2017; 44:586–92. [DOI] [PubMed] [Google Scholar]
- 4. Chow EP, Camilleri S, Ward C, et al. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex Health 2016; 13:199–204. [DOI] [PubMed] [Google Scholar]
- 5. Barbee LA, Soge OO, Khosropour CM, et al. The duration of pharyngeal gonorrhea: a natural history study. Clin Infect Dis 2022; 74:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wind CM, Schim van der Loeff MF, Unemo M, Schuurman R, van Dam AP, de Vries HJC. Test of cure for anogenital gonorrhoea using modern RNA-based and DNA-based nucleic acid amplification tests: a prospective cohort study. Clin Infect Dis 2016; 62:1348–55. [DOI] [PubMed] [Google Scholar]
- 7. Wind CM, Schim van der Loeff MF, Unemo M, Schuurman R, van Dam AP, de Vries HJ. Time to clearance of Chlamydia trachomatis RNA and DNA after treatment in patients coinfected with Neisseria gonorrhoeae—a prospective cohort study. BMC Infect Dis 2016; 16:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moncada J, Clark CB, Holden J, Hook EW 3rd, Gaydos CA, Schachter J. Stability studies on dry swabs and wet mailed swabs for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Aptima assays. J Clin Microbiol 2017; 55:971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol 2011; 49:3610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin F, Prestage GP, Mao L, et al. Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health In Men Study. Sex Transm Infect 2007; 83:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima combo 2 assay and culture. Sex Transm Infect 2009; 85:182–6. [DOI] [PubMed] [Google Scholar]
- 13. Lewis N, Dube G, Carter C, et al. Chlamydia and gonorrhoea contamination of clinic surfaces. Sex Transm Infect 2012; 88:418–21. [DOI] [PubMed] [Google Scholar]
- 14. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis 2021; 73:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hook EW 3rd, Kirkcaldy RD. A brief history of evolving diagnostics and therapy for gonorrhea: lessons learned. Clin Infect Dis 2018; 67:1294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]