Bezlotoxumab was significantly associated with reduced odds of recurrent Clostridioides difficile infection and all-cause readmission at 90 days on both unadjusted and adjusted analysis. Bezlotoxumab was well tolerated with low frequency of adverse events observed.
Keywords: bezlotoxumab, Clostridioides difficile, CDI, recurrence
Abstract
Background
Bezlotoxumab (BEZ) is a monoclonal antibody used to prevent recurrent Clostridioides difficile infection (rCDI). This study investigates BEZ effectiveness in relation to rCDI and patient-specific risk factors in a real-world setting.
Methods
A matched, retrospective cohort study was conducted from 2015 to 2019 to compare BEZ to historical standard of care (SoC) therapy with vancomycin or fidaxomicin. The primary outcome was incidence of 90-day rCDI. Secondary outcomes were incidence of all-cause hospital readmission and all-cause mortality at 90 days, infusion-related reactions, and incidence of heart failure exacerbation. Baseline confounding was addressed using inverse probability of treatment weighting (IPTW).
Results
Overall, 107 participants were included (54 BEZ and 53 SoC). Mean number of prior CDI episodes was 2, median number of risk factors for rCDI was 4, and 28% of participants had severe CDI. Incidence of 90-day rCDI was 11% BEZ vs 43% SoC (P = < .001) and 90-day all-cause readmission was 40% BEZ vs 64% SoC (P = .011). In IPTW-adjusted analyses, BEZ was associated with significantly reduced odds of rCDI (odds ratio [OR], 0.14 [95% confidence interval {CI}: .05–.41]) and all-cause readmission (OR, 0.36 [95% CI: .16–.81]). No safety signals were detected with BEZ use.
Conclusions
BEZ is effective for the prevention of rCDI and reduction in all-cause hospital readmission for patients at high risk for recurrence, supporting current guideline recommendations.
Clostridioides (formerly Clostridium) difficile infection (CDI) is a common cause of infectious colitis frequently associated with antimicrobial use [1]. Up to 30% of patients experience a recurrent CDI (rCDI) episode, which results in significantly increased morbidity including more hospitalizations, higher healthcare costs, and reduced quality of life [2, 3]. In addition, the risk of recurrence increases drastically from 20% following an initial episode to >60% after 2 or more recurrences [3]. As such, interventions to interrupt the cycle of recurrence early in the disease process are urgently needed.
Bezlotoxumab (BEZ), a novel monoclonal antibody designed to neutralize toxin B, is given in conjunction with standard of care (SoC) C. difficile therapy (oral vancomycin [VAN] or fidaxomicin [FDX]) to prevent rCDI [4]. From the phase 3 clinical trials, MODIFY I and II, BEZ demonstrated a 38% lower risk of rCDI compared to placebo [5]. Clinical response at end of CDI therapy was no different among BEZ recipients compared to placebo, suggesting that BEZ has limited impact on initial clinical cure, although additional trials are underway [6]. BEZ was well tolerated with low incidence of adverse events observed; however, an imbalance in heart failure exacerbation events was noted among BEZ recipients, leading to a Food and Drug Administration (FDA) precaution. Post hoc analyses of MODIFY I/II have additionally demonstrated that patients with multiple risk factors for recurrence are likely to derive greater benefit in rCDI prevention than those without risk factors [7].
Limited data exist regarding the effectiveness and safety of BEZ outside of clinical trials, particularly in those with multiple risk factors for recurrence [8, 9]. Previous real-world analyses are limited in size and lack a comparator group. In addition, several well-documented risk factors for rCDI, which may influence response to BEZ, were not assessed in the clinical trials. This study assessed the effectiveness of BEZ as compared with SoC treatments among patients with at least 1 risk factor for recurrence in a real-world setting.
PATIENTS AND METHODS
Study Design
This was a retrospective, matched cohort study conducted at the University of Colorado Hospital between 2015 and 2019. Inclusion criteria were age ≥18 years, SoC CDI treatment (VAN or FDX), ≥1 risk factor for rCDI, and documented follow-up 90 days after last dose of CDI therapy. Initial CDI episodes were identified on the basis of new onset of clinically significant diarrhea (≥3 stools of Bristol type 5, 6, or 7 in a 24-hour period) accompanied by a positive real-time polymerase chain reaction result for C. difficile toxin-producing genes and initiation of treatment with oral VAN or FDX as recommended by current practice guidelines [10–12]. Patients belonging to select vulnerable populations (physically or cognitively impaired, pregnant, or incarcerated individuals) were excluded in accordance with local institutional review board requirements. Patients treated with metronidazole monotherapy for CDI were not included, as recent updates to Infectious Diseases Society of America treatment guidelines no longer recommend metronidazole as first-line therapy [10].
Subjects who received BEZ, in addition to SoC, from 1 February 2017 to 30 June 2019 were compared to historical controls, receiving SoC alone, in the 2 years immediately prior to BEZ use. Controls were matched 1:1 to the BEZ arm according to incidence of concurrent antibiotic use and number of prior CDI episodes. BEZ dosing was 10 mg/kg based on actual body weight, administered as a single intravenous infusion over 60 minutes according to product labeling [4]. Doses were capped at 1000 mg (1 vial) for those weighing >100 kg according to an institutional policy to avoid waste, based on review of previously published pharmacokinetic and pharmacodynamic data [13]. This study was granted an exemption by the Colorado Multiple Institutional Review Board prior to initiation. A total of 66 patients were previously reported in an analysis specific to transplant receipt [14].
Data Collection and Definitions
Data collected included demographic and clinical characteristics such as comorbidities, CDI complications (eg, ileus, toxic megacolon), history of fecal microbiota transplant, and CDI treatment regimen. Risk factors for rCDI evaluated were age ≥65 years, immunocompromised status, prior episode of CDI, concomitant antibiotic use, proton pump inhibitor use, severe CDI (Zar score ≥2), and proteinuria (urine total protein ≥30 mg/dL). Extended-duration CDI treatment was defined as treatment with CDI antibiotics for >14 days, either as a tapered-pulsed regimen or initiation of a lower dose for prophylactic intent immediately after completion of therapy. Charlson Comorbidity Index and Zar scores were calculated for each patient to quantify comorbidity burden and CDI severity, respectively [15, 16].
Statistical Analysis and Outcomes
Baseline characteristics were evaluated using descriptive statistics. The χ 2 and Fisher exact tests were utilized to evaluate categorical variables, whereas continuous variables were analyzed via Wilcoxon rank-sum test. The primary outcome was the incidence of rCDI, defined as new onset of clinically significant diarrhea (≥3 stools of Bristol type 5, 6, or 7 in a 24-hour time period) accompanied by initiation of treatment with oral VAN or FDX, within 90 days following completion of last CDI antibiotic dose. Secondary outcomes included 90-day all-cause hospital readmission. Planned subgroup analysis included stratification of the primary outcome by the number and type of rCDI risk factors. Safety outcomes assessed were incidence of heart failure exacerbations, all-cause mortality at 90 days, and infusion-related reactions.
To control for biases associated with selection of BEZ treatment, inverse probability of treatment weighting (IPTW) was performed using the propensity score. The propensity score was generated using variables selected a priori, which are associated with selection of treatment that could influence outcome. Variables used in generation of the score were age, Zar score, number of prior CDI episodes, concomitant antibiotic use, CDI-related hospitalization within the prior 30 days, and FDX receipt. Variables used in the propensity score not achieving balance with a standardized mean difference <0.1 were planned for inclusion into a multivariable logistic regression. As a sensitivity analysis, a trimmed weight whereby the propensity scores were truncated below the 10th percentile and above the 90th percentile was evaluated. Analyses were conducted using R, version 4.0.2 software (R Core Team) [17]. The level of statistical significance was set at .05.
RESULTS
Baseline Characteristics
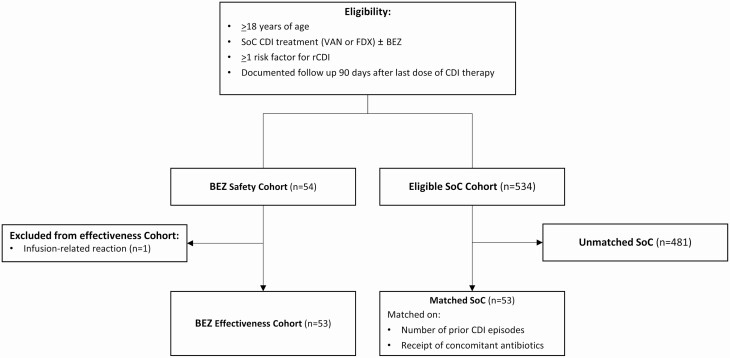
Overall, 106 patients (53 matched pairs) were included in the study. One additional patient who received BEZ was only included in the safety analysis given BEZ infusion was not completed following infusion reaction (Figure 1). Patients had a mean age of 56 (standard deviation [SD], 17) years and a mean Charlson Comorbidity Index of 4.1 (SD, 2.8), and the majority were white (76%). However, significantly more patients with black race were identified in the SoC group (23% vs 2%, P = .002). The mean number of prior CDI episodes was 2 (SD, 2), and 13% had previously received fecal microbiota transplantation. Regarding the index CDI episode, the median Zar score was 1 (interquartile range [IQR], 0–2), and the incidence of severe CDI was similar between the BEZ and SoC cohorts (23% vs 34%, respectively). Twelve percent of patients experienced a complication associated with CDI, primarily intensive care unit admission (8.5%) and shock (7.5%).
Figure 1.
Patient flow diagram. Abbreviations: BEZ, bezlotoxumab; CDI, Clostridioides difficile infection; FDX, fidaxomicin; SoC, standard of care; VAN, vancomycin.
Patients were overall at high risk of recurrence, evidenced by a mean of 4 (SD, 1) rCDI risk factors. The cohorts were well matched with respect to specific rCDI risk factors including concomitant antibiotic use, number of prior CDI episodes, and proton pump inhibitor use (Table 1). Patients in the BEZ cohort, however, were more likely to have an immunocompromising condition (77% vs 49%, P = .003). Prior transplantation (solid organ or hematopoietic cell) was the most common immunocompromising condition among both cohorts (80% BEZ vs 73% SoC, P = .503).
Table 1.
Baseline Characteristics
| Variable | BEZ (n = 53) |
SoC (n = 53) |
P Value |
|---|---|---|---|
| Age, y, mean (SD) | 55 (16) | 57 (17) | .874 |
| Female sex | 29 (55) | 28 (53) | .846 |
| Race/ethnicity | |||
| White | 44 (83) | 37 (70) | .109 |
| Asian | 2 (3.8) | 1 (1.9) | .999 |
| Black or African American | 1 (1.9) | 12 (23) | .002 |
| American Indian/Alaska Native | 0 | 1 (1.9) | .999 |
| >1 race | 3 (5.7) | 1 (1.9) | .618 |
| Unknown/unreported | 3 (5.7) | 1 (1.9) | .618 |
| Weight, kg, median (IQR) | 72.6 (58.5–87.8) | 69.1 (59.4–83.0) | .645 |
| Obesity (BMI ≥30 kg/m2) | 12 (23) | 10 (19) | .480 |
| Charlson Comorbidity Index, mean (SD) | 4 (3) | 4 (3) | .753 |
| Heart failure | 7 (13) | 9 (17) | .587 |
| Chronic kidney disease | 5 (9.4) | 7 (13) | .540 |
| Diabetes mellitus | 15 (28) | 18 (34) | .529 |
| Inflammatory bowel disease | 10 (19) | 11 (21) | .807 |
| Cirrhosis | 8 (15) | 10 (19) | .605 |
| Clostridioides difficile complication | 5 (9.4) | 8 (15) | .374 |
| ICU admission | 4 (7.5) | 5 (9.4) | .999 |
| Ileus | 1 (1.9) | 1 (1.9) | .999 |
| GI surgical intervention | 0 | 1 (1.9) | .999 |
| Pseudomembranous colitis | 2 (3.8) | 2 (3.8) | .999 |
| Shock | 3 (5.7) | 5 (9.4) | .716 |
| Risk factors for recurrence, median (IQR) | 4 (3–4) | 4 (3–4) | .643 |
| Immunocompromised | 41 (77) | 26 (49) | .003 |
| Transplantation | 33 (80) | 19 (73) | .503 |
| Active cancer | 6 (15) | 7 (27) | .215 |
| IBD biologics | 2 (4.9) | … | .518 |
| Concomitant antibiotic receipt | 31 (59) | 37 (70) | .224 |
| Prior C. difficile episode | 40 (76) | 34 (64) | .204 |
| No. of prior CDI episodes, mean (SD) | 2 (2) | 2 (2) | .685 |
| Age ≥65 y | 20 (38) | 15 (28) | .302 |
| PPI receipt | 24 (45) | 28 (53) | .437 |
| Proteinuria | 25 (47) | 29 (55) | .437 |
| Zar score, median (IQR) | 1 (0–1) | 1 (0–2) | .352 |
| Severe CDIa | 12 (23) | 18 (34) | .196 |
| Prior FMT | 8 (15) | 6 (11) | .566 |
| CDI treatmentb | |||
| Vancomycin | 48 (91) | 45 (85) | .374 |
| Fidaxomicin | 17 (32) | 5 (9.4) | .004 |
| Combination/sequential therapy | 13 (25) | 14 (27) | .779 |
| Combination IV MTZ | 8 (15) | 20 (38) | .008 |
| Extended-duration CDI treatmentc | 41 (77) | 33 (62) | .091 |
| Tapered-pulse regimen | 30 (57) | 14 (26) | .002 |
| Prophylaxis | 12 (23) | 4 (7.5) | .030 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BEZ, bezlotoxumab; BMI, body mass index; CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation; GI, gastrointestinal; IBD, inflammatory bowel disease; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; MTZ, metronidazole; PPI, proton pump inhibitor; SD, standard deviation; SoC, standard of care.
aSevere CDI defined as Zar score ≥2.
bMay have received >1 agent.
cExtended duration defined as treatment for >14 days.
With regard to CDI therapy, VAN was predominantly utilized (88%) and the majority of patients (70%) received extended courses (>14 days) of therapy, largely owing to a higher use of tapered-pulsed regimens and initiation of prophylaxis after standard treatment dosing. FDX use was more common in the BEZ cohort (32% vs 9%, P = .004), whereas receipt of combination therapy involving metronidazole was more common in the SoC cohort (15% vs 38%, P = .008). A single patient received concurrent rifaximin in the SoC cohort; however, the prescribed indication was for management of hepatic encephalopathy rather than as an adjunct for CDI management. BEZ was administered at a median of 19 (IQR, 12–35) days after SoC antibiotic initiation. BEZ was administered in the outpatient setting in 87% (n = 46) of cases, and the majority of patients (70%, n = 37) were receiving SoC antibiotics at time of BEZ administration. The median time to BEZ administration in patients receiving SoC was 14 (IQR, 8–27) days compared to 33 (IQR, 17–43) days among those already completing SoC at time of BEZ administration.
Outcomes
Unadjusted 90-day effectiveness and safety outcomes are displayed in Table 2. Recurrent CDI occurred in 6 (11%) patients treated with BEZ compared to 23 (43%) patients in the SoC cohort (absolute risk reduction [ARR], 32.1% [95% confidence interval [CI]: 16.2%–47.9%). Additionally, BEZ was associated with lower incidence of all-cause hospital readmission at 90 days (ARR, 24.5% [95% CI: 6.1%–43.0%]). BEZ was associated with fewer occurrences of rCDI for patients with ≤3 risk factors for recurrence (7.7% vs 51.9%, P < .001), with the highest disparity seen among those with 1–2 risk factors (Supplementary Appendix 1). Furthermore, BEZ recipients demonstrated numerically lower rates of rCDI among all risk factors evaluated (Supplementary Appendix 1). Among BEZ recipients, incident 90-day rCDI was lower among those with <2 prior episodes for rCDI compared to those with ≥2 prior episodes (7.7% vs 14.8%, P = .026). Moreover, all 6 recurrences in the BEZ group were in those receiving SoC at time of administration.
Table 2.
Unadjusted 90-Day Outcomes After Completing Clostridioides difficile Treatment
| Outcome | BEZ (n = 53) |
SoC (n = 53) |
P Value |
|---|---|---|---|
| rCDI | 6 (11) | 23 (43) | <.001 |
| All-cause hospital readmission | 21 (40) | 34 (64) | .011 |
| Heart failure exacerbationa | 1 (2.9) | 1 (7.1) | .503 |
| Infusion-related reactionb | 1 (1.9) | … | |
| All-cause mortality | 1 (1.9) | 0 | .999 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BEZ, bezlotoxumab; rCDI, recurrent Clostridioides difficile infection; SoC, standard of care.
aPercentages are of those with underlying heart failure.
bSafety analysis included all patients that received any BEZ administration (n = 54).
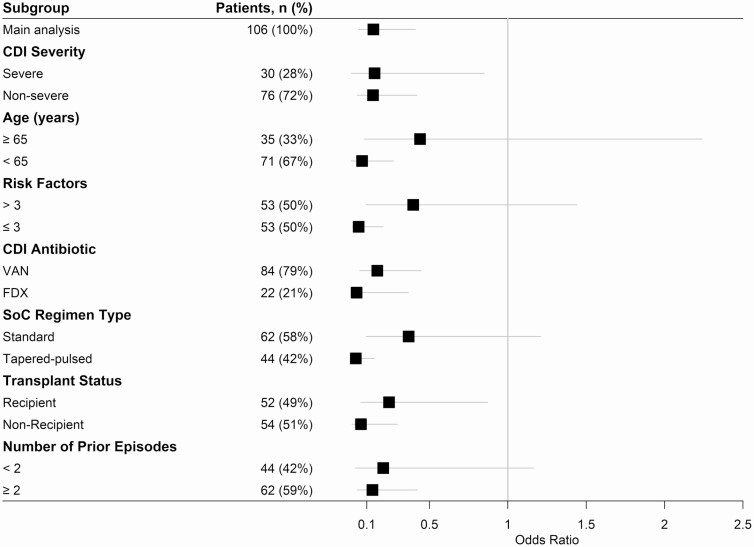
Propensity scores generated from the multivariable model ranged from 0.097 to 0.842, with an area of common support between cohorts of 88.7%. Weighting by the inverse of the propensity score resulted in standardized mean differences <0.1 for all variables included (Supplementary Appendix 2). In IPTW analysis, BEZ was associated with reduced odds of 90-day rCDI (odds ratio [OR], 0.14 [95% CI: .05–.41]), which was consistent in the trimmed analysis (OR, 0.16 [95% CI: .06–.46]) and across all clinically important subgroups including comparison of transplant and nontransplant recipient status (Figure 2). Furthermore, BEZ was associated with lower odds of 90-day all-cause hospital readmission in IPTW analysis (OR, 0.36 [95% CI: .16–.81]).
Figure 2.
Main inverse probability of treatment weighting analysis and subgroup analysis of 90-day recurrent Clostridioides difficile infection. Odds ratio <1 favors bezlotoxumab. Abbreviations: BEZ, bezlotoxumab; CDI, Clostridioides difficile infection; FDX, fidaxomicin; SoC, standard of care; VAN, vancomycin.
With respect to safety, BEZ was well tolerated. One of the 54 patients (1.9%) in the BEZ safety cohort was unable to receive the complete administration due to infusion-related nausea and vomiting. Among patients with underlying heart failure, exacerbation occurred in 14% (n = 1/7) of patients in the BEZ cohort and 11% (n = 1/9) in the SoC cohort. The episode of heart failure exacerbation following BEZ occurred 4 weeks after administration. All-cause mortality at 90 days was not different between groups (ARR, –1.9% [95% CI: –5.6% to 1.8%]).
Discussion
To our knowledge, this study represents the first real-world analysis comparing BEZ to a SoC cohort for prevention of rCDI. In unadjusted analysis, we observed a 32% absolute risk reduction in 90-day rCDI, with reductions consistently observed across all stratified risk factors. Based on these results, between 3 and 4 patients would need to receive BEZ to prevent 1 episode of rCDI. After successfully balancing prognostic factors by IPTW, BEZ remained significantly associated with lower odds of 90-day rCDI and all-cause hospital readmission. BEZ use was generally well tolerated with low incidence of serious adverse events or heart failure exacerbation. Overall, our findings support the utility of BEZ for reduction of rCDI among high-risk patient populations in a real-world setting.
Recently published guidelines for C. difficile management recommend FDX and BEZ earlier in the disease course, thereby placing greater emphasis on the importance of preventing rCDI [12, 18]. Provided FDX’s impact alone on preventing rCDI compared to VAN, it remains unclear the additive impact BEZ provides when partnered with FDX in further reducing rCDI over FDX alone. Interestingly, subgroup analysis from MODIFY I/II and previously published real-world evidence did not identify differences in rCDI by SoC antibiotic received [8, 19]. A potential shortcoming in FDX efficacy described by several studies is the lack of observed differences in recurrence compared to VAN among patients infected with BI/NAP1/polymerase chain reaction ribotype 027 strains [20]. In contrast, post hoc analysis of MODIFY I/II demonstrates that BEZ efficacy was unaffected by presence of the BI/NAP1/ribotype 027 strain and remained significantly associated with lower incidence of rCDI at 12 weeks compared to placebo [21]. Together, these data suggest value in identifying and prioritizing patient subsets infected with BI strain, and administration of BEZ earlier in the disease course could be of greater importance than initial FDX selection; however, limitations on the available sample size of this data warrant additional studies.
A post hoc analysis of MODIFY I/II previously demonstrated that patients with risk factors for rCDI derived greater benefit than those without risk factors [5]. The absolute risk reduction in our cohort was larger than that demonstrated in MODIFY I/II, likely due to inclusion of a patient population with a greater number of risk factors for recurrence [5]. Notably, a large number of immunocompromised hosts were included, and the majority of patients had experienced several prior CDI episodes. Our study also assessed proteinuria, proton pump inhibitor use, and receipt of concomitant antibiotics as risk factors for rCDI, which were not previously evaluated in MODIFY I/II. Stratification of rCDI by these risk factors was comparable to the overall analysis, suggesting that the effectiveness of BEZ is not diminished by these factors. Similar to another real-world study conducted by Hengel et al, BEZ administration in patients with <2 episodes resulted in lower rates of rCDI compared to those with ≥2 episodes, suggesting that the early use of BEZ may be more beneficial. Further investigation into the impact of broad-spectrum antibiotic coadministration or administration after receipt of BEZ is warranted [8].
We observed an association with BEZ and decreased 90-day all-cause hospital readmission on both adjusted and unadjusted analyses. These findings are consistent with a post hoc analysis of MODIFY I/II, which demonstrated a lower incidence of 30-day CDI-related admissions and a trend toward lower all-cause readmissions [22]. The direct drug costs of BEZ remain problematic for inpatient administration; however, such costs should be considered in light of the potential cost savings from reductions in hospital readmissions and rCDI. In addition, the direct drug costs of BEZ were mitigated at our center by deferring administration in many patients to the outpatient setting after hospital discharge, where reimbursement is generally more favorable for health systems. While several pharmacoeconomic analyses suggest that BEZ may be cost-effective for prevention of rCDI, further studies should investigate the financial impact of a deferred administration approach [23, 24].
BEZ was well tolerated in our patient population. Of 54 BEZ recipients, 1 patient experienced infusion-related nausea and vomiting that required cessation of the infusion prior to completion. In the MODIFY trials, 9% of patients reported infusion-related reactions, including nausea, headache, dizziness, and fatigue, yet cessation of medication administration occurred in only 0.1% of recipients [5]. Among those with congestive heart failure in MODIFY I/II, 13% of BEZ-treated patients experienced an exacerbation compared to only 5% in the placebo arm, leading to a labeled warning for BEZ. One case of heart failure exacerbation among those with baseline heart failure was observed in our BEZ cohort, requiring hospitalization 4 weeks after BEZ administration, which was deemed unrelated to BEZ in the opinion of clinical providers at our institution. This finding was no different than the SoC arm, where 1 incidence of heart failure exacerbation was reported. No cases were reported in 2 previously published real-world studies, demonstrating that the incidence of BEZ-induced heart failure exacerbation may be lower than seen in clinical trials [5, 8, 9].
Our findings are limited by several factors, including the retrospective nature of the study and inclusion of patients from a single academic health system, which could limit generalizability. In addition, the administration of BEZ in this study was later in the CDI disease course than in MODIFY I/II. However, this pragmatic approach reflects real-world practice, where BEZ often cannot be feasibly administered early to hospitalized patients, as there are numerous barriers including unfavorable inpatient reimbursement, insurance requirement for prior authorizations, and challenges with timely scheduling for outpatient infusions. This study was also limited by a 90-day follow-up period for the primary and secondary outcomes, which does not capture the late effects of BEZ on prevention of rCDI given the concentrations are expected to persist up to 6 months. [13] Although there have been concerns regarding the long-term effects of BEZ, a recently published 12-month follow-up analysis of MODIFY I/II demonstrated persistent efficacy up to 12 months, demonstrating prevention, rather than delayed onset, of rCDI [25]. Provided the incomplete reporting of C. difficile ribotype at our institution, the impact of BI strain on this study’s outcomes is unclear. As described above, presence of BI strain may impart higher rCDI frequency compared to those without this strain, but current evidence suggests that BEZ efficacy is not impacted by strain type [21]. Finally, institutional practices regarding dose capping may affect the overall impact of BEZ for rCDI reduction, and further study of overweight and obese populations is needed.
Conclusions
In our real-world cohort of patients at high risk for CDI recurrence, BEZ recipients experienced fewer rCDI episodes and all-cause hospital readmissions at 90 days compared to those treated with SoC antibiotics alone. These findings were consistent on IPTW-adjusted analyses. Overall, these results support the updated clinical practice guidelines, that BEZ effectively and safely prevents rCDI and should be routinely considered among patients with rCDI risk factors.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The contents of this work are the authors’ sole responsibility and do not necessarily represent official views of the National Institutes of Health (NIH).
Financial support. This project was supported by the NIH/National Center for Research Resources Colorado Clinical and Translational Sciences Institute (grant number UL1 RR025780).
Potential conflicts of interest. A related work was supported by an investigator-initiated research grant from Merck & Co, Inc, to M. A. M., K. C. M., and V. B. (paid to the University of Colorado, for time support only to complete the study). All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. . Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–40. [DOI] [PubMed] [Google Scholar]
- 4. Merck & Co, Inc. Bezlotoxumab [package insert]. Whitehouse Station, NJ: Merck & Co, Inc, 2016. [Google Scholar]
- 5. Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators. . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 6. Wenzler ER. Real-world evaluation of bezlotoxumab for the management of Clostridioides difficile infection.2021. Available at: https://clinicaltrials.gov/ct2/show/study/NCT04317963. Accessed 5 May 2021.
- 7. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018; 67:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hengel RL, Ritter TE, Nathan RV, et al. Real-world experience of bezlotoxumab for prevention of Clostridioides difficile infection: a retrospective multicenter cohort study. Open Forum Infect Dis 2020; 7:ofaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oksi J, Aalto A, Säilä P, Partanen T, Anttila VJ, Mattila E. Real-world efficacy of bezlotoxumab for prevention of recurrent Clostridium difficile infection: a retrospective study of 46 patients in five university hospitals in Finland. Eur J Clin Microbiol Infect Dis 2019; 38:1947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98. [DOI] [PubMed] [Google Scholar]
- 12. Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021; 116:1124–47. [DOI] [PubMed] [Google Scholar]
- 13. Yee KL, Kleijn HJ, Kerbusch T, et al. Population pharmacokinetics and pharmacodynamics of bezlotoxumab in adults with primary and recurrent Clostridium difficile infection. Antimicrob Agents Chemother 2019; 63:e01971-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson TM, Howard AH, Miller MA, et al. Effectiveness of bezlotoxumab for prevention of recurrent Clostridioides difficile infection among transplant recipients. Open Forum Infect Dis 2021; 8:ofab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 16. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 17. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing,2016. [Google Scholar]
- 18. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:e1029–44. [DOI] [PubMed] [Google Scholar]
- 19. Dubberke ER, Gerding DN, Kelly CP, et al. Efficacy of bezlotoxumab in participants receiving metronidazole, vancomycin, or fidaxomicin for treatment of Clostridioides (Clostridium) difficile infection. Open Forum Infect Dis 2020; 7:ofaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crook DW, Walker AS, Kean Y, et al. ; Study 003/004 Teams. . Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis 2012; 55(Suppl 2):S93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson S, Citron DM, Gerding DN, et al. Efficacy of bezlotoxumab in trial participants infected with Clostridioides difficile strain BI associated with poor outcomes [manuscript published online ahead of print 31 July 2020]. Clin Infect Dis 2021; 73:e2616–24. [DOI] [PubMed] [Google Scholar]
- 22. Prabhu VS, Cornely OA, Golan Y, et al. Thirty-day readmissions in hospitalized patients who received bezlotoxumab with antibacterial drug treatment for Clostridium difficile infection. Clin Infect Dis 2017; 65:1218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prabhu VS, Dubberke ER, Dorr MB, et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis 2018; 66:355–62. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Gong CL, Hitchcock MM, Holubar M, Deresinski S, Hay JW. Cost-effectiveness of bezlotoxumab and fidaxomicin for initial Clostridioides difficile infection [manuscript published online ahead of print 17 April 2021]. Clin Microbiol Infect 2021.. doi:10.1016/j.cmi.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldstein EJC, Citron DM, Gerding DN, et al. Bezlotoxumab for the prevention of recurrent Clostridioides difficile infection: 12-month observational data from the randomized phase III trial, MODIFY II. Clin Infect Dis 2020; 71:1102–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.