Abstract
Eighty-seven out of 575 gonococci isolated in Greece from 1991 to 1998 belonged to serovar Bropyst and exhibited resistance to penicillin, tetracycline, erythromycin, and chloramphenicol. Conventional and molecular typing showed three clusters, A, B, and C, that were associated with networks of high- frequency transmitters (cluster A with homosexuals and clusters B and C with refugees from Eastern Europe). Study of one isolate revealed mutations in the penA, mtrR, and porB genes that may explain the multidrug-resistant phenotype.
The decrease in the incidence of gonorrhea in Greece since 1986 was accompanied by changes in the epidemiology of the disease and an increase in the relative frequency of multidrug-resistant (Multi-R) Neisseria gonorrhoeae isolates (10, 16). Similar changes have occurred in other European countries and the United States (4, 17). Plasmid-determined resistance to penicillin and tetracycline and resistance to one or more antibiotic classes due to various chromosomal mutations have been described in gonococci (4, 6). In this study, we examined a group of Multi-R N. gonorrhoeae strains isolated in Greece from 1991 to 1998.
A total of 575 gonococcal isolates were collected in the National Reference Center for Neisseria gonorrhoeae during the 8-year study period. They were from cases of male gonococcal urethritis seen in the two major Greek sexually transmitted disease hospitals located in Athens and Thessaloniki (483 and 92 patients, respectively). All isolates were accompanied by a patient questionnaire with epidemiologic information about nationality, residence, sexual orientation, previous antibiotic use, recent travel abroad, and sexual partner. Antimicrobial susceptibilities of the isolates were determined by the Etest (AB Biodisk) on GC agar supplemented with Vitox (Oxoid) at 35°C and 5% CO2. Susceptibility to Triton X-100 was assessed by an agar dilution technique. Penicillinase production was detected with a nitrocefin assay (Oxoid). Serological classification with the Phadebact GC serovar test (Boule Diagnostics), auxotyping, and plasmid DNA analysis were performed as described previously (16). Phenotypically identical isolates were further typed by pulsed-field gel electrophoresis (PFGE) after digestion of genomic DNA with SpeI endonuclease (New England BioLabs) as described previously (11). For PCR amplification, total DNA prepared by the method of Murray and Thomson (12) was used as template DNA. A region including the transpeptidase domain of the penA gene (nucleotides 933 to 1794; GenBank accession no. X07468) was amplified by using oligonucleotide primers PA1 (5′-CGATATGATCGAACCTGG-3′) and PA2 (5′-ACAATCTCGTTGATACTCG-3′). To amplify the promoter and coding regions of the multiple transferable resistance regulator (mtrR) gene (nucleotides 860 to 1775; GenBank accession no. Z25796), primers MTR1 (5′-AACAGGCATTCTTATTTCAG-3′) and MTR2 (5′-TTAGAAGAATGCTTTGTGTC-3′) were used. For the amplification of a part of the porin (por) gene (nucleotides 160 to 917; EMBL accession no. AJ004943), primers PorB1 (5′-AAAGGCCAAGAAGACCTCGGC-3′) and PorB2 (5′-GAGAAGTCGTATTCCGCACCG-3′) were used. PCR products were prepared with a PCR Product Pre-Sequencing kit (United States Biochemicals), and the nucleotide sequences were determined with a Sequenase 2.0 kit (United States Biochemicals).
Analysis of serotyping and antibiotic susceptibility data from all 575 gonococci revealed a strong association between a newly emerged serovar and a Multi-R phenotype. Eighty-nine isolates, including 16 (36.4%) out of 44 penicillinase-producing and 73 (13.7%) out of 531 non-penicillinase-producing strains, were allocated to serovar Bropyst, which was not encountered among gonococci isolated in Greece before 1991. For all but two of the Bropyst isolates, the MICs were above or close to the respective resistance breakpoints for erythromycin, tetracycline, and chloramphenicol, and also for penicillin G, even in the absence of penicillinase production (13). MICs of cefotaxime were increased compared with those for the rest of the gonococcal population. Similarly, MICs of norfloxacin and ciprofloxacin, although within the susceptible range (9, 13), were consistently higher than those for the other non-quinolone-resistant isolates (Table 1). The same phenotype was observed in only 16 non-Bropyst strains scattered among 11 IB serovars (chi-square test, P < 0.001). Triton X-100 did not inhibit the growth of the Multi-R Bropyst isolates at a concentration of 8,000 μg/ml, while its MICs ranged from 16 to 125 μg/ml for 40 randomly selected isolates with other susceptibility phenotypes that were tested in parallel. Resistance to Triton X-100 suggested the operation of a multidrug efflux mechanism in Bropyst isolates (14).
TABLE 1.
Antimicrobial susceptibilities of Multi-R Bropyst isolates compared to those of the rest of the gonococci isolated from 1991 to 1998
| Antimicrobial agent and parameter | MIC (μg/ml) for isolates exhibiting phenotype:
|
|
|---|---|---|
| Multi-R Bropyst (n = 87) | Other (n = 488) | |
| Penicillina | ||
| MIC range | 0.5–>256 (6) | ≤0.016–256 (4) |
| MIC50b | 1 | 0.19 (0.125) |
| MIC90c | 32 (2) | 1.5 (1) |
| Cefotaxime | ||
| MIC range | 0.012–0.5 | ≤0.002–0.25 |
| MIC50 | 0.047 | 0.012 |
| MIC90 | 0.125 | 0.064 |
| Tetracyclinea | ||
| MIC range | 0.5–4 | ≤0.016–128 (4) |
| MIC50 | 3 | 0.5 |
| MIC90 | 3 | 2 (1.5) |
| Erythromycin | ||
| MIC range | 0.5–8 | ≤0.016–6 |
| MIC50 | 1.5 | 0.38 |
| MIC90 | 3 | 1 |
| Chloramphenicol | ||
| MIC range | 2–12 | 0.125–12 |
| MIC50 | 4 | 0.5 |
| MIC90 | 8 | 3 |
| Ciprofloxacina | ||
| MIC range | 0.008–0.125 (0.023) | ≤0.002–16 (0.016) |
| MIC50 | 0.012 | 0.004 (0.003) |
| MIC90 | 0.023 | 0.012 (0.006) |
| Norfloxacina | ||
| MIC range | 0.023–0.38 (0.094) | ≤0.016–48 (0.064) |
| MIC50 | 0.047 | ≤0.016 |
| MIC90 | 0.064 | 0.047 (0.032) |
In parentheses are the MICs of the respective agents when isolates with plasmid-mediated resistance to penicillin (16 Bropyst, 28 other) or tetracycline (21 other) or quinolone-resistant isolates known to carry gyrA and parC mutations (1 Bropyst, 30 other) (11) are not included.
MIC50, MIC for 50% of strains tested.
MIC90, MIC for 90% of strains tested.
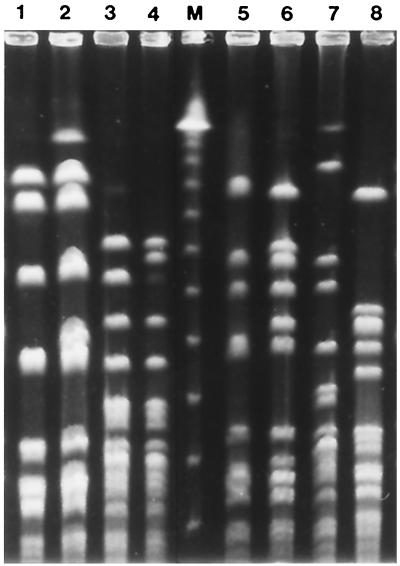
Typing results are summarized in Table 2. Auxotyping, combined with plasmid content analysis, classified the majority of the Bropyst isolates into three main groups. The largest consisted of 49 prototrophs carrying only the cryptic plasmid (Proto/cryptic) and was followed by 12 proline-requiring isolates with the cryptic plasmid (P−/cryptic) and 12 hypoxanthine-requiring isolates with the African-type penicillin resistance plasmid plus the conjugative and cryptic plasmids (H−/African+). Accordingly, PFGE classified Multi-R isolates with Proto/cryptic, P−/cryptic, and H−/African+ characters into three clusters, A, B, and C, respectively, each comprising isolates with patterns differing in less than four bands. Four proline-dependent isolates with Asian-type penicillin resistance and the cryptic plasmid (P−/Asian−) exhibited pulsotypes similar to those of the P−/cryptic isolates and were therefore classified in cluster B. With the exception of penicillin resistance status, the antibiotic susceptibility patterns observed in the three clusters were similar. The two antibiotic-sensitive Bropyst strains (Proto/cryptic and P−/cryptic) displayed unique pulsotypes (Fig. 1).
TABLE 2.
Phenotypic characteristics and PFGE patterns of 89 Bropyst isolates
| Auxotype and plasmid content | No. of strains | No. of strains with PFGE pattern
|
||||
|---|---|---|---|---|---|---|
| A | B | C | Ua | NDb | ||
| Proto | ||||||
| Cryptic | 49 | 48 | 1 | |||
| Conjugative + cryptic | 2 | 2 | ||||
| P− | ||||||
| Cryptic | 12 | 11 | 1 | |||
| Asian + cryptic | 4 | 4 | ||||
| H− | ||||||
| Conjugative + African + cryptic | 12 | 12 | ||||
| Cryptic | 2 | 2 | ||||
| Conjugative + cryptic | 1 | 1 | ||||
| Otherc | ||||||
| Cryptic | 5 | 5 | ||||
| Conjugative + cryptic | 2 | 2 | ||||
| Total | 89 | 48 | 15 | 12 | 2 | 12 |
U, unique patterns, represented by the two antibiotic sensitive strains.
ND, not determined.
Seven auxotypes, represented by single strains.
FIG. 1.
Representative PFGE patterns of gonococcal isolates of serovar Bropyst. Lanes: 1 and 2, type A (Proto/cryptic) isolates; 3 and 4, type B isolates with P−/cryptic and P−/Asian− phenotypes, respectively; 5 and 6 type C (H−/African+) isolates; 7 and 8, two antibiotic-sensitive Bropyst isolates displaying unique (U) patterns; M, λ DNA ladder (New England BioLabs).
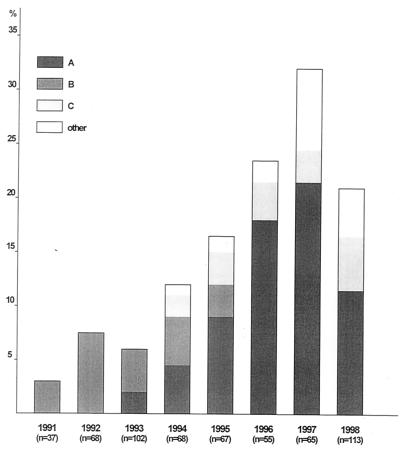
Isolates of all three types were obtained in both participating hospitals. By reviewing patient data, we could not trace any association of cases within each cluster. Nevertheless, type B infections that occurred between 1992 and 1995, as well as type C infections appearing since 1994, were clearly associated with acquisition of infection either in Eastern Europe (mainly in Russia and Romania) or in Greece from contacts involving immigrants from Eastern Europe (chi-square test, P < 0.001 for type B and P < 0.005 for type C). Type A emerged in 1993 and persisted through 1998, with a peak in frequency during 1997. Except for the two initial isolates, deriving from patients stating that they had been infected “abroad,” all of the other isolates in this cluster were from infections acquired in Greece. Up to 1995, type A gonococci were isolated only from heterosexuals. Since 1996, however, type A was associated with homosexual contacts (among 37 type A isolates in the period 1996 to 1998, 16 were from homosexuals, representing 37% of all homosexual patients seen during the same period; chi-square test, P < 0.002). Time and type distribution of the Bropyst isolates is presented in Fig. 2.
FIG. 2.
Time and type distribution of 89 Bropyst N. gonorrhoeae isolates (percentage of the total number of gonococci isolated yearly).
The mechanisms contributing to the Multi-R phenotype were investigated in an isolate representative of the A cluster. The penA gene contained a GAC insertion between codons 345 and 346 of the wild-type sequence. Thus, PBP-2 contained an additional aspartic acid (Asp-345a). Five point mutations resulting in the following substitutions were also observed: Tyr383→His, Phe504→Leu, Ala510→Val, Ala516→Gly, and Pro551→Leu. The amplified mtr segments had a single base pair (A/T) deletion in the 13-bp inverted repeat between the mtrR and mtrC genes. Also, a C-to-T transition in the mtrR coding region resulted in substitution of Tyr for His-105. The deduced amino acid sequence of the por PCR product (codons 68 to 124) differed from wild-type Por (5) at two residues; positions 101 and 102 were occupied by Asn-Asp instead of Gly-Ala.
These mutations may partly explain the resistance phenotype of the Bropyst isolates. The extra Asp-345a in PBP-2 and the substitutions at positions 504, 510, 516, and 551 reduce affinity for β-lactam antibiotics (1, 3). The change at position 383 has not been reported previously. Resistance to hydrophobic antimicrobials could be attributed to the detected mutations in the mtr locus. The A/T deletion and the substitution of Tyr for His-105 in MtrR result in more efficient pump production and, consequently, increased efflux of the hydrophobic antibiotics erythromycin and chloramphenicol and the detergent Triton X-100 (14, 19). The amplified por segment included putative loop 3 (residues 100 to 128) of the Por protein. The presence of Asp-101 and Asp-102 has been associated with decreased permeability to penicillin and tetracycline (5). In the strain examined, aspartic acid was found only at position 102. The simultaneous presence of other chromosomal mutations does not allow an assessment of the role of the latter alteration.
The increasing incidence of isolates exhibiting a distinct Multi-R phenotype and belonging to a previously rare serovar suggested the spread of epidemiologically related strains. Detailed typing, however, showed a certain degree of diversity. The three main clusters found among Bropyst isolates were associated with particular transmission networks. Types B and C seemed to have originated in Eastern Europe and, once introduced, persisted in the Greek community for at least 5 years, sustained mostly by economic refugees. The origin of type A gonococci was not determined. Initially appearing among Greek heterosexuals, isolates of this type reached high frequencies after their introduction into the network of men having sex with men (MSM), where they are still present (unpublished observations).
The present study showed that a number of N. gonorrhoeae strains currently circulating in the Greek community possess chromosomal mutations that confer resistance to multiple antibiotic classes. Furthermore, epidemiological and typing data indicated that these strains persist in core population groups. A similar finding, i.e., an MSM-associated outbreak of Proto/IB-1 gonococci possessing mtr mutations, has been recently described in Seattle-King County, Wash. (2, 18). Outbreaks within groups of high-frequency transmitters due to resistant strains of N. gonorrhoeae have been repeatedly reported during the last decade (2, 7, 8, 11, 15, 18). In none of these studies was a clear association with antibiotic usage established, nor could such an association be shown in the present study. Only a few of the patients reported occasional use of antibiotics in the 3-month period preceding infection. As suggested previously, the persistence of resistant gonococcal strains in a community may be favored by core group-associated factors, such as higher transmissibility or longer duration of infectiousness, of which antibiotic resistance may be simply a marker (8). Nevertheless, the unregulated use of various antibiotics, including penicillins, macrolides, and fluorinated quinolones that are available over the counter in Greece may have contributed to the establishment of the Multi-R N. gonorrhoeae strains. Also, the persistence of such strains within the group of MSM may be facilitated by the mtr and penB mutations, which could be advantageous for survival on mucosal surfaces (14).
Irrespective of the selection process, it seems that gonococcal strains with decreased susceptibility to cephalosporins and fluoroquinolones have been established in the Greek community. In that respect, the selective use of spectinomycin, to which all of the isolates tested were susceptible (data not shown), should be considered.
REFERENCES
- 1.Brannigan J A, Tirodimos I A, Zhang Q-Y, Dowson C G, Spratt B J. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1990;4:913–919. doi: 10.1111/j.1365-2958.1990.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Gonorrhea among men who have sex with men—selected sexually transmitted diseases clinics, 1993–1996. Morb Mortal Wkly Rep. 1997;46:889–892. [PubMed] [Google Scholar]
- 3.Dowson C G, Jephcott A E, Gough K R, Spratt B J. Penicillin-binding protein 2 genes of non-β-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1989;3:35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 4.Easmon C S F. The changing pattern of antibiotic resistance of Neisseria gonorrhoeae. Genitourin Med. 1990;66:55–56. doi: 10.1136/sti.66.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill M J, Simjee S, Al-Hattawi K, Robertson B D, Easmon C S F, Ison C A. Gonococcal resistance to β-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother. 1998;42:2799–2803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ison C A. Antimicrobial agents and gonorrhoea: therapeutic choice, resistance and susceptibility testing. Genitourin Med. 1996;72:253–257. doi: 10.1136/sti.72.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ison C A, Pepin J, Roope N S, Secka O, Easmon C S F. The dominance of a multiresistant strain of Neisseria gonorrhoeae among prostitutes and STD patients in the Gambia. Genitourin Med. 1992;68:356–360. doi: 10.1136/sti.68.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilmarx P H, Knapp J S, Xia M, St. Louis M E, Neal S W, Sayers D, et al. Intercity spread of gonococci with decreased susceptibility to fluoroquinolones: a unique focus in the United States. J Infect Dis. 1998;177:677–682. doi: 10.1086/514234. [DOI] [PubMed] [Google Scholar]
- 9.Knapp J S, Hale J A, Neal S W, Wintersheid K, Rice R J, Whittington W L. Proposed criteria for interpretation of susceptibilities of strains of Neisseria gonorrhoeae to ciprofloxacin, ofloxacin, enoxacin, lomefloxacin, and norfloxacin. Antimicrob Agents Chemother. 1995;39:2442–2445. doi: 10.1128/aac.39.11.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyriakis K P, Tzelepi E, Flemetakis A, Avgerinou H, Tzouvelekis L S, Frangouli E. Epidemiologic aspects of male gonococcal infection in Greece. Sex Transm Dis. 1999;26:43–48. doi: 10.1097/00007435-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Mavroidi A, Tzouvelekis L S, Tassios P T, Flemetakis A, Daniilidou M, Tzelepi E. Characterization of Neisseria gonorrhoeae strains with decreased susceptibility to fluoroquinolones isolated in Greece from 1996 to 1999. J Clin Microbiol. 2000;38:3489–3491. doi: 10.1128/jcm.38.9.3489-3491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray M G, Thomson W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing—sixth edition. Approved standard. NCCLS document M2–A6, supplement M100–S7. Vol. 17. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Shafer W M, Balthazar J T, Hagman K E, Morse S A. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology. 1995;141:907–911. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- 15.Tapsall J W, Limnios E A, Scultz T R. Continuing evolution of the pattern of quinolone resistance in Neisseria gonorrhoeae isolated in Sydney, Australia. Sex Transm Dis. 1998;25:415–417. doi: 10.1097/00007435-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Tzelepi E, Avgerinou H, Kyriakis K P, Tzouvelekis L S, Flemetakis A, Kalogeropoulou A, Frangouli E. Antimicrobial susceptibility and types of Neisseria gonorrhoeae in Greece. Data for the period 1990–1993. Sex Transm Dis. 1997;24:378–385. doi: 10.1097/00007435-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 17.van Duynhoven Y T H P. The epidemiology of Neisseria gonorrhoeae in Europe. Microbes Infect. 1999;1:455–464. doi: 10.1016/s1286-4579(99)80049-5. [DOI] [PubMed] [Google Scholar]
- 18.Xia M, Whittington W L H, Shafer W M, Holmes K K. Gonorrhea among men who have sex with men: outbreak caused by a single genotype of erythromycin-resistant Neisseria gonorrhoeae with a single-base pair deletion in the mtrR promoter region. J Infect Dis. 2000;181:2080–2082. doi: 10.1086/315510. [DOI] [PubMed] [Google Scholar]
- 19.Zarantonelli L, Borthagaray G, Eun-Hee L, Shafer W M. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob Agents Chemother. 1999;43:2468–2472. doi: 10.1128/aac.43.10.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]