Abstract
Background:
Industrial chemicals are increasingly recognized as potential developmental neurotoxicants. Di(2-ethylhexyl) phthalate (DEHP), used to impart flexibility and temperature tolerance to polyvinylchloride, and bisphenol A (BPA), used to manufacture polycarbonate, are commonly present in medical devices. The magnitude of exposure during hospitalization for cardiac surgery in neonates is unknown.
Methods:
We quantified urinary concentrations of DEHP metabolites and BPA preoperatively and post-operatively in neonates undergoing cardiac surgery and their mothers. Urinary concentrations of these biomarkers reflect recent exposures (half-lives are approximately 6–24 hours). Biomarker concentrations in mothers and infant preoperative and post-operative samples were compared.
Results:
Eighteen infants, age 5 (Mean) ± 4 (SD) days, underwent surgery. The maternal sample was obtained on postpartum day 4 ± 4. The preoperative urine sample was obtained on day-of-life (DOL) 4 ± 2 and the post-operative sample on DOL 6 ± 4. Mean maternal concentrations for DEHP metabolites and BPA were at the 50th percentile for females in the U.S. general population. Infant preoperative concentrations of one DEHP metabolite and BPA were significantly higher than maternal concentrations. For all DEHP metabolites, post-operative concentrations were significantly greater than preoperative concentrations.
Conclusions:
There is considerable perioperative exposure to DEHP and BPA for neonates undergoing cardiac surgery. For both BPA and DEHP metabolites, infant concentrations were significantly higher than maternal concentrations, consistent with infant’s exposure to medical devices. Further study is needed to determine the potential role of these suspect neurotoxicants in the etiology of neurodevelopmental disability after cardiac surgery.
Congenital heart defects (CHD) are the most common developmental defects in humans, affecting 8 per 1000 live births with 1/3 of affected children requiring intervention as neonates or in early infancy. Improved survival following surgical repair of CHD has led to recognition that neurobehavioral disabilities (mild cognitive dysfunction, language difficulties, impaired attention and executive function) are the most common, and potentially most disabling, long-term adverse outcomes of infants with CHD. Despite the efforts of multiple investigators, there has been minimal improvement in early neurodevelopmental (ND) outcomes for these children over the past 20 years.1 Currently identified risk factors do not adequately explain the incidence, mechanisms, or considerable inter-individual variability in severity of neurobehavioral disability following cardiac surgery in infancy.2
There is growing evidence of adverse impacts on early childhood neurodevelopment of even low-level exposures to environmental contaminants (e.g. smoke, pesticides, industrial chemicals).3 Exposure of a fetus or newborn to low-levels of environmental contaminants in conjunction with the post-natal risk factors associated with CHD [e.g. abnormal brain development, hypoxia/ischemia, exposure to cardiopulmonary bypass (CPB)] may result in more severe brain injury than either factor alone.4 Industrial chemicals are increasingly recognized as potential endocrine disruptors and developmental neurotoxicants. Di(2-ethylhexyl) phthalate (DEHP), used to impart flexibility and temperature tolerance to polyvinylchloride, and bisphenol A (BPA), used to manufacture polycarbonate, are commonly present in medical devices.5–7 DEHP and BPA are commonly used in components of the CPB circuit, endotracheal tubes, and blood storage bags. The magnitude of exposure during hospitalization for cardiac surgery in neonates is unknown. In this study, we assessed urinary levels of DEHP metabolites and BPA in infants before and during cardiac surgery.
Patients and Methods
This is an analysis of a subgroup of infants enrolled in prospective study investigating the impact of in utero exposure to environmental chemicals on ND outcomes after cardiac surgery in newborns. Inclusion criteria were: 1) infants with CHD and expected surgery with CPB; 2) age ≤ 44 weeks postconception age; 3) availability of biological parents of the infant; and 4) informed consent. Exclusion criteria were: 1) presence of an identified genetic syndrome; 2) major extra-cardiac anomaly; or 3) language other than English spoken in the home. The parent study enrolled 140 infants. For the last 18 subjects, both preoperative and postoperative samples were obtained. These infants form the cohort for the current study. The Institutional Review Board at The Children’s Hospital of Philadelphia approved the study. Written informed consent was obtained from the parent or guardian. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
Intraoperative Management
Subjects were enrolled between 11/4/2014 and 8/13/2015. Operations were performed by one of four cardiac surgeons with a dedicated team of cardiac anesthesiologists. Deep hypothermic circulatory arrest (DHCA) was used at the surgeon’s discretion. Modified ultrafiltration was performed in all patients. According to the manufacturers, DEHP and BPA are present in multiple components of the bypass circuit (cannula, tubing, and cardioplegia sets).
Urine Collection
Spot infant urine samples were collected from a cotton ball placed in the diaper, an adhesive urine collection bag or a Foley catheter collection bag. The cotton balls were placed into a 3–5-cc polypropylene syringe (Becton-Dickinson, Franklin Lakes, NJ), and the urine transferred into a polypropylene cryovial. After surgery, samples were collected from the Foley catheter collection bag.
Maternal specimens were collected as a random clean-catch voided urine sample. Mothers were instructed to wash their hands and avoid touching the container. The collected specimen was sealed inside a zip bag and placed in a refrigerator until picked up by study personnel. Specific gravity (SG) was measured at room temperature using a handheld refractometer. Normal saline control samples were collected in the same fashion. Urine and control samples were frozen at –20°C and shipped overnight on dry ice to the CDC for analysis.
Assessment of Exposure to DEHP and BPA
We quantified five urinary biomarkers in 100 μL of maternal and infant spot samples using online solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry after enzymatic deconjugation to hydrolyze urinary conjugates and following standard quality assurance/quality control procedures as described before.8, 9 The target analytes were BPA and four DEHP metabolites (mono (2-ethyl-5-carboxypentyl) phthalate [MECPP], mono (2-ethyl-5-hydroxyhexyl) phthalate [MEHHP], mono (2-ethyl-5-oxohexyl) phthalate [MEOHP], and mono (2-ethylhexyl) phthalate [MEHP]). The limits of detection (LOD) were 0.2 µg/L (BPA, MECPP, MEHHP, MEOHP), and 0.5 μg/L (MEHP).
Statistical Analysis
Descriptive statistics were computed for all subjects. Prior to formal analysis, if measured concentrations for each analyte were between 0 and its LOD, we used the measured concentration; however, if a measured concentration was actually 0, then we imputed a value as LOD/2.10, 11 Urinary biomarker concentrations were adjusted for dilution using specific gravity. We computed SG-corrected biomarker concentrations using the following formula: Pc = P [(x – 1.000)/SG – 1.000], where Pc is the SG-corrected biomarker concentration (μg/L), P is the measured biomarker concentration (μg/L), x = specific gravity value for a given subject, and SG is the median specific gravity value for the specific study cohort (maternal, infant preoperative or infant postoperative).12 Biomarker concentrations were summarized using geometric means and 95% confidence intervals. Two sets of comparisons were conducted: (a) infant’s preoperative measurements with their mothers (H1), and, (b) infant’s preoperative and postoperative measurements (H2). The statistical test of choice in both cases was a nonparametric Wilcoxon Signed Rank Test. This test was deemed most appropriate given the repeated nature of the data as well as the small and markedly skewed nature of the distributions. The hypothesis-wise error rate was adjusted using Tukey, Ciminera, and Heyse’s correction for the five moderately correlated endpoints (αADJ = 0.01).13 National Health and Nutrition Examination Survey (NHANES) population-based (2011–2012) reference values and confidence limits for all females (6 years and older) are provided for context (see Table 2). All data were analyzed using SAS (v9.4, Cary, NC).
Table 2:
U.S. General Population and Study Sample Biomarkers Urinary Concentrations (µg/L)
| NHANES | Infant | Infant | |||
|---|---|---|---|---|---|
| All Females (6 years and older) | Maternal | Pre-Operative | Post-Operative | ||
| Biomarkers | 50%ile | 95%ile | (n = 18) | (n = 16) | (n = 18) |
| Phthalate metabolites | |||||
| MECPP | 12.4 (11.2, 13.7) | 64.9 (54.4, 76.8) | 10.1 (7.2, 14.1) | 58.5 (29.4, 116.5) a | 1166.1 (576.7, 2357.9) b |
| MEHHP | 7.6 (6.9, 8.2) | 40.7 (35.1, 46.4) | 5.6 (3.9, 7.9) | 10.7 (5.7, 20.0) | 229.0 (119.6, 438.4) b |
| MEOHP | 5.0 (4.7, 5.6) | 25.4 (21.9, 29.6) | 4.6 (3.4, 6.3) | 7.2 (3.9, 13.2) | 148.6 (72.2, 305.6) b |
| MEHP | 1.2 (1.0, 1.4) | 8.1 (6.8, 9.2) | 0.9 (0.4, 1.9) | 1.7 (0.8, 3.9) | 27.2 (14.9, 49.5) c |
| Phenols | |||||
| BPA | 1.2 (1.1, 1.3) | 7.2 (5.6, 8.1) | 1.3 (0.8, 1.9) | 9.8 (7.3, 13.3) a | 13.9 (10.8, 18.0) |
Notes: National Health and Nutrition Examination Survey = NHANES. Population data for phthalate metabolites are unadjusted values for 2011–2012. Population data for BPA are unadjusted values for 2013–2014.Values presented here are geometric means defined as and 95% confidence intervals (in parenthesis).
Denotes a statistically significant difference between infant and maternal pre-operative analyte levels at the α = 0.0001 level.
Denotes a statistically significant difference between infant pre-operative and post-operative analyte levels at α = 0.0001 level
(denotes significance at the α = 0.0002 level).
Results
We quantified urinary concentrations of four DEHP metabolites and BPA preoperatively (n = 16) and post-operatively (n = 18) in neonates undergoing cardiac surgery and their mothers (n = 18). Patient characteristics are shown in Table 1. All infants underwent cardiac surgery, and CPB was utilized in 17 of the 18 infants. The mean age at surgery was 5.4 ± 3.8 days. Maternal samples were obtained on the infant’s day of life (DOL) 4 ± 4. The infant preoperative samples were obtained on DOL 4 ± 2 and the postoperative sample on DOL 6 ± 4. All postoperative samples were obtained on the day of surgery or postoperative day 1. In the control samples, concentrations of all four DEHP metabolites and BPA were < LOD.
Table 1:
Patient Characteristics
| Preoperative | |
|---|---|
| Gender | Males (9) Females (10) |
| Gestational Age (weeks) | 38.5 ± 1.5 (3 < 37 weeks) |
| Birth Weight (kg) | 3.05 ± 0.57 |
| Mechanical Ventilation (y/n) | 5 yes / 14 no |
| Operative | |
| Age at Surgery (days) | 5.4 ± 3.8 |
| Weight at Surgery (kg) | 3.0 ± 0.5 |
| Procedure (CPB, n=18) | |
| Arch/VSD repair | 1 |
| Aortic arch repair | 3 |
| Arterial switch procedure | 5 |
| Norwood (Stage 1) | 6 |
| TOF repair | 1 |
| Shunt | 2 |
| Procedure (No CPB, n=1) | |
| Coarctation repair, Left thoracotomy | 1 |
| CPB Time (minutes, n = 18) | 80.1 ± 18.6 |
A DEHP metabolite or BPA was detected in all subjects. MECPP, MEHHP, MEOHP, and BPA were present at or above LOD in 100% of the maternal samples. MEHP was detected in 9/18 (50%) maternal samples. MECPP, MEHHP, MEOHP, and BPA were present at or above LOD in 100% of the infant preoperative and postoperative samples. MEHP was detected in 11/16 (69%) preoperative samples and in 100% of the postoperative samples.
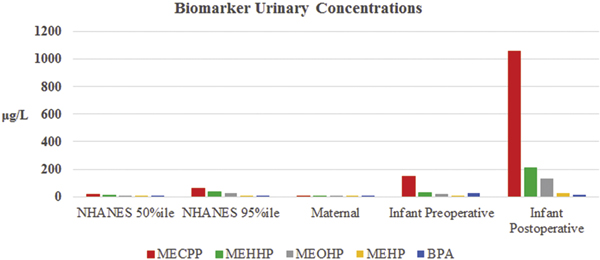
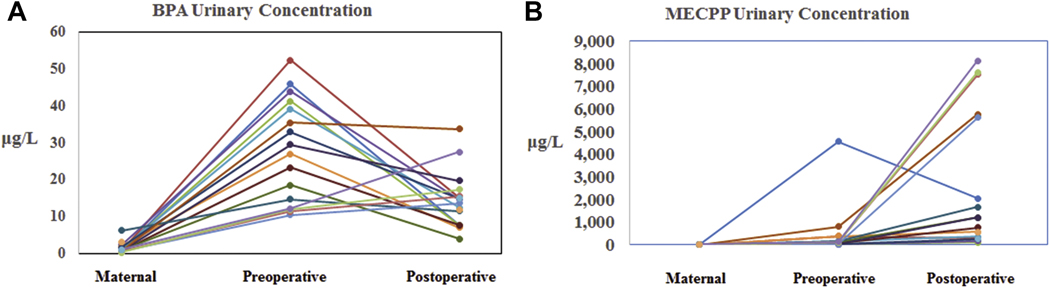
Geometric mean maternal urine concentrations for all DEHP metabolites and BPA in our population were at the 50th percentile for females in the U.S. general population (Table 2). Comparisions between the NHANES concentrations for females, the maternal concentrations, and the infant preoperative and postoperative concentrations are shown in Figure 1. There was variation between subjects in both pattern and magnitude of differences observed between the maternal and infant preoperative concentrations (Figure 2). Infant preoperative urinary concentrations were, on average, higher than maternal urinary concentrations for all metabolites, and statistically significantly higher for MECPP (p < 0.001) and BPA (p < 0.001). In addition, whereas infant post-operative concentrations were higher than pre-operative concentrations for most metabolites reported here, these differences were quite noticeably and statistically significantly higher for MECPP (p < 0.001), MEHHP (p < 0.001), MEOHP (p < 0.001), and MEHP (p = 0.002); BPA (p = 0.193) remained the lone exception.
Figure 1.
Comparison of Geometric Mean Maternal and Infant DEHP Metabolite and BPA Concentrations to U.S. General Population Concentrations for All Females.
Figure 2.
Individual Patient Urinary Concentrations for A) BPA and B) MECPP.
Comment
The magnitude of potential exposure to environmental toxicants is enormous. All children are exposed to multiple chemicals and environmental contaminants before and after birth. 3, 14, 15 We demonstrate that there is significant post-natal and perioperative exposure to DEHP and BPA in neonates undergoing surgery for CHD. Urinary concentrations of these biomarkers reflect recent exposures (half-lives ∼ 6–24 hours) to DEHP and BPA, most likely from exposure to medical devices. The infant preoperative concentrations tended to be higher than the maternal concentrations, consistent with post-natal exposure. DEHP metabolite concentrations (MECPP, MEHHP, MEOHP, and MEHP) measured in the postoperative period were significantly elevated compared to preoperative values, consistent with exposure in the operating room and early postoperative period. DEHP and BPA, increasingly recognized as potential endocrine disruptors and developmental neurotoxicants, are commonly present in medical devices. These exposures may have synergistic, additive, and/or antagonistic effects, with growing evidence of adverse effects at very low-level levels on development and function of multiple organ systems. 4, 16 There is no known “safe” threshold for exposure to these contaminants, with fetuses and infants having increased vulnerability. The fetal and infant brain is in a state of rapid growth, thus impairment of brain cognitive function may arise from a relatively modest toxic exposure. 3 The early post-natal period through the first two years of life is a particularly important time for brain development.
Many flexible plastic medical devices used in the care of neonates with CHD are made of polyvinyl chloride (PVC); including blood collection and storage bags, IV tubing, enteral feeding tubes, umbilical catheters, extracorporeal membrane oxygenation (ECMO) circuit tubing, components of the CPB circuit, and endotracheal tubes.5, 17, 18 The plasticizer DEHP is used to confer pliability.6 DEHP does not bind covalently to PVC and migrates into surrounding fluids and tissues.6, 17 DEHP is metabolized via glucuronidation pathways to its monoester, MEHP, and further oxidized and hydroxylated into subsequent metabolites, including MEHHP, MECPP, and MEOHP.17, 19 Neonates have an impaired ability to clear phthalates from their bodies which may increase the potential for adverse effects.17 BPA is used primarily in manufacturing polycarbonate plastic and epoxy resins.20 BPA is present in a variety of common products including water bottles, sports equipment, and medical devices.7 BPA is added to plastics like polycarbonate used in medical devices such as catheters and ventilator tubing to improve strength and clarity.7
Phthalates and phenols are biologically active compounds with endocrine disrupting properties, including anti-androgenicity and impairment of thyroid function.21, 22 Exposure to these substances has been associated with risk of obesity, as well as altered genital and pubertal development.23, 24 There is some evidence that exposure to phthalates, both in utero and later in life, is associated with neurobehavioral disability. A systematic review of the literature by Ejaredar and associates found evidence that prenatal exposure to phthalates is associated with adverse cognitive and behavioral outcomes, including lower IQ, and problems with attention, hyperactivity, and poorer social communication. 25 Kim and coworkers evaluated the association between prenatal DEHP exposure and neurodevelopmental outcomes assessed using the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant Development II. They found a strong inverse association between prenatal exposure to MEHHP and MEOHP in the third trimester of pregnancy with the MDI and PDI scores of males.26 Tewar and colleagues found that higher urinary BPA concentrations collected at 8 to 15 years of age were associated with ADHD, and the association was stronger in boys than in girls.27 Braun and coworkers reported that increasing prenatal exposure to BPA was associated with more anxious and depressed behavior, as well as poorer emotional control and inhibition.28 The effect was greater in girls compared to boys. Won and coinvestigators found that exposure to DEHP in Korean children and adolescents was significantly associated with social, thought, and attention problem scores on the Child Behavior Checklist.29 The pattern of neurobehavioral disability described after DEHP and BPA exposure is very similar to the pattern seen after repair of CHD in neonates and infants.
Our findings support previous studies showing that critically ill infants and children are exposed to plasticizers via medical devices. Calafat and colleagues investigated exposure to BPA in a neonatal intensive care unit (NICU) and found that the BPA geometric mean urinary concentration among premature infants undergoing intensive therapeutic interventions was an order of magnitude higher than that among the general population.20 These same investigators also observed a strong relationship between the use of DEHP-containing medical devices in the NICU and urinary concentrations of DEHP metabolites. 30 Su and coworkers also evaluated exposures from PVC containing devices in the NICU and found that neonates who received an endotracheal tube and a feeding tube had approximately three-fold higher DEHP urinary metabolite concentrations than those who did not.19 Mallow and Fox showed that infants in the NICU receive substantial amounts of DEHP from direct and indirect contact with PVC containing devices. The total daily exposure often significantly exceeded known thresholds for toxicity.6 Blood transfusions, endotracheal tubes and feeding tubes were significant sources of exposure.
Materials used in CPB and extracorporeal membrane oxygenation (ECMO) circuits may also expose infants to plasticizers. Takahashi and associates demonstrated significant DEHP exposure from PVC containing tubing in adults undergoing cardiac surgery.18 This exposure could be reduced by using non-PVC containing tubing. Eckert and coworkers evaluated DEHP exposure using a mock CPB circuit. They demonstrated that a CPB circuit using standard PVC tubing material leads to considerable plasticizer migration into blood and thus significant exposures to patients. However, the type of plasticizer significantly impacted the migration rate of contaminants into solutions. For example, the leaching of DEHP was several times higher than that of its substitute tri-2-ethylhexyl trimellitate.5 No studies have evaluated exposure to DEHP during CPB in infants, but our data suggest the exposure may be considerable.
There is considerable evidence linking in utero and early life exposures to plasticizers to neurobehavioral problems. There is also considerable evidence demonstrating very concerning levels of exposure in hospitalized infants and children. However, there is little evidence directly evaluating in-hospital exposures and later neurodevelopmental sequelae. In an important study, Verstraete and colleagues investigated the relationship of circulating phthalate metabolites in a cohort of over 400 critically ill children (ages 0 to 16 years) to subsequent development of ADHD at compared to normal controls.31 The phthalate exposure explained half of the risk of attention deficit in the cohort. They concluded that “Iatrogenic exposure to DEHP metabolites during intensive care was independently and robustly associated with the important attention deficit observed in children 4 years after critical illness.”31 Importantly, the risk was due to chronic exposure above a threshold, rather than the magnitude of a single exposure. This finding may, in part, explain the well described association of longer length of hospital stay with worse neurodevelopmental outcomes. It has usually been explained by increased severity of illness, but may be partly related to toxicity from medical devices.
There are several limitations to the current study. The sample size is small and we only evaluated exposure over a few days early in the hospitalization. We did not characterize the specific type of medical devices used for each child, the levels of DEHP or BPA in the medical devices, or the duration of exposure. Finally, we did not evaluate neurodevelopmental outcomes in this preliminary study.
In conclusion, we demonstrated significant perioperative exposure to DEHP and BPA in neonates undergoing cardiac surgery. Concentrations in the infants were much higher than those in the mothers, consistent with exposure from medical devices. The magnitude and pattern of biomarker concentrations were similar to those reported for critically ill preterm newborns undergoing treatment in neonatal intensive care units. Review of the literature suggests that exposure to these neurotoxicants could be an important cause of neurobehavioral disability after cardiac surgery in infants. Future studies are needed to fully characterize the magnitude and pattern of exposure to DEHP and BPA over the entire hospitalization and to clarify potential long-term sequelae.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS, Goldberg CS, Hovels-Gurich H, Ichida F, Jacobs JP, Justo R, Latal B, Li JS, Mahle WT, McQuillen PS, Menon SC, Pemberton VL, Pike NA, Pizarro C, Shekerdemian LS, Synnes A, Williams I, Bellinger DC, Newburger JW and International Cardiac Collaborative on Neurodevelopment I. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, Nord AS, Clancy RR, Nicolson SC and Spray TL. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–53, 1353 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandjean P and Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 5.Eckert E, Münch F, Göen T, Purbojo A, Müller J and Cesnjevar R. Comparative study on the migration of di-2-ethylhexyl phthalate (DEHP) and tri-2-ethylhexyl trimellitate (TOTM) into blood from PVC tubing material of a heart-lung machine. Chemosphere. 2016;145:10–6. [DOI] [PubMed] [Google Scholar]
- 6.Mallow EB and Fox MA. Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. J Perinatol. 2014;34:892–7. [DOI] [PubMed] [Google Scholar]
- 7.Huygh J, Clotman K, Malarvannan G, Covaci A, Schepens T, Verbrugghe W, Dirinck E, Van Gaal L and Jorens PG. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ Int. 2015;81:64–72. [DOI] [PubMed] [Google Scholar]
- 8.Silva MJ, Samandar E, Preau JL, Jr., Reidy JA, Needham LL and Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–12. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Kuklenyik Z, Needham LL and Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–13. [DOI] [PubMed] [Google Scholar]
- 10.Hornung JP, Fritschy JM and Tork I. Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J Comp Neurol. 1990;297:165–81. [DOI] [PubMed] [Google Scholar]
- 11.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L and Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeniger MF, Lowry LK and Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–27. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Quan H, Ng J and Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Control Clin Trials. 1997;18:204–21. [DOI] [PubMed] [Google Scholar]
- 14.Mendola P, Selevan SG, Gutter S and Rice D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment Retard Dev Disabil Res Rev. 2002;8:188–97. [DOI] [PubMed] [Google Scholar]
- 15.Bellinger DC. Assessing environmental neurotoxicant exposures and child neurobehavior: confounded by confounding?[comment]. Epidemiology. 2004;15:383–4. [DOI] [PubMed] [Google Scholar]
- 16.Bellinger D Very low lead exposures and children’s neurodevelopment. Current Opinions in Pediatrics. 2008;20:172–177. [DOI] [PubMed] [Google Scholar]
- 17.Calafat AM, Needham LL, Silva MJ and Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–34. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Shibata T, Sasaki Y, Fujii H, Bito Y and Suehiro S. Di(2-ethylhexyl) phthalate exposure during cardiopulmonary bypass. Asian Cardiovasc Thorac Ann. 2008;16:4–6. [DOI] [PubMed] [Google Scholar]
- 19.Su PH, Chang YZ, Chang HP, Wang SL, Haung HI, Huang PC and Chen JY. Exposure to di(2ethylhexyl) phthalate in premature neonates in a neonatal intensive care unit in Taiwan. Pediatr Crit Care Med. 2012;13:671–7. [DOI] [PubMed] [Google Scholar]
- 20.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K and Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM and Wolff MS. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environ Res. 2017;152:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgenstern R, Whyatt RM, Insel BJ, Calafat AM, Liu X, Rauh VA, Herbstman J, Bradwin G and Factor-Litvak P. Phthalates and thyroid function in preschool age children: Sex specific associations. Environ Int. 2017;106:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miodovnik A, Edwards A, Bellinger DC and Hauser R. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology. 2014;41:112–22. [DOI] [PubMed] [Google Scholar]
- 24.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejaredar M, Nyanza EC, Ten Eycke K and Dewey D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ Res. 2015;142:51–60. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, Hong YC, Chang N and Kim BN. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119:1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tewar S, Auinger P, Braun JM, Lanphear B, Yolton K, Epstein JN, Ehrlich S and Froehlich TE. Association of Bisphenol A exposure and Attention-Deficit/Hyperactivity Disorder in a national sample of U.S. children. Environ Res. 2016;150:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN and Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Won EK, Kim Y, Ha M, Burm E, Kim YS, Lim H, Jung DE, Lim S, Kim SY, Kim YM, Kim HC, Lee KJ, Cheong HK, Kang HT, Son M, Sakong J, Oh GJ, Lee CG, Kim SY, Ryu JM and Kim SJ. Association of current phthalate exposure with neurobehavioral development in a national sample. Int J Hyg Environ Health. 2016;219:364–71. [DOI] [PubMed] [Google Scholar]
- 30.Weuve J, Sanchez BN, Calafat AM, Schettler T, Green RA, Hu H and Hauser R. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect. 2006;114:1424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.. Verstraete S, Vanhorebeek I, Covaci A, Güiza F, Malarvannan G, Jorens PG and Van den Berghe G Circulating phthalates during critical illness in children are associated with long-term attention deficit: a study of a development and a validation cohort. Intensive Care Med. 2016;42:379–92. [DOI] [PubMed] [Google Scholar]