ABSTRACT
Objective
We previously showed that a training intervention comprising a combination of meditation, exposure to cold, and breathing exercises enables voluntary activation of the sympathetic nervous system, reflected by profoundly increased plasma epinephrine levels, and subsequent attenuation of the lipopolysaccharide (LPS)-induced inflammatory response. Several elements of the intervention may contribute to these effects, namely, two different breathing exercises (either with or without prolonged breath retention) and exposure to cold. We determined the contribution of these different elements to the observed effects.
Methods
Forty healthy male volunteers were randomized to either a short or an extensive training in both breathing exercises by either the creator of the training intervention or an independent trainer. The primary outcome was plasma epinephrine levels. In a subsequent study, 48 healthy male volunteers were randomized to cold exposure training, training in the established optimal breathing exercise, a combination of both, or no training. These 48 participants were subsequently intravenously challenged with 2 ng/kg LPS. The primary outcome was plasma cytokine levels.
Results
Both breathing exercises were associated with an increase in plasma epinephrine levels, which did not vary as a function of length of training or the trainer (F(4,152) = 0.53, p = .71, and F(4,152) = 0.92, p = .46, respectively). In the second study, the breathing exercise also resulted in increased plasma epinephrine levels. Cold exposure training alone did not relevantly modulate the LPS-induced inflammatory response (F(8,37) = 0.60, p = .77), whereas the breathing exercise led to significantly enhanced anti-inflammatory and attenuated proinflammatory cytokine levels (F(8,37) = 3.80, p = .002). Cold exposure training significantly enhanced the immunomodulatory effects of the breathing exercise (F(8,37) = 2.57, p = .02).
Conclusions
The combination of cold exposure training and a breathing exercise most potently attenuates the in vivo inflammatory response in healthy young males. Our study demonstrates that the immunomodulatory effects of the intervention can be reproduced in a standardized manner, thereby paving the way for clinical trials.
Trial Registration:ClinicalTrials.gov identifiers: NCT02417155 and NCT03240497.
Key words/Abbreviations: innate immunity, human endotoxemia, cold exposure, breathing exercise, AUC = area under the curve, BRT = breathing exercise without retention group, CBR = cold exposure and the breathing exercise without retention group, CEX = cold exposure group, CON = control group, IL = interleukin, IP = interferon gamma-induced protein, LPS = lipopolysaccharide, MCP = monocyte chemoattractant protein, MIP = macrophage inflammatory protein, TNF = tumor necrosis factor
INTRODUCTION
Previous work from our group revealed that healthy volunteers who followed a training program were able to voluntarily activate their sympathetic nervous system and attenuate their inflammatory response during experimental human endotoxemia, a standardized, controlled, and reproducible model of systemic inflammation elicited by intravenous administration of bacterial lipopolysaccharide (LPS) (1). The training program was devised by a Dutch individual, who holds several world records with regard to withstanding extreme cold, in whom initial indications for the previously described effects of the intervention were observed (2). The training consists of three elements, namely, meditation, exposure to cold, and breathing exercises. Trained participants, who practiced the breathing exercises during experimental endotoxemia, exhibited high plasma concentrations of epinephrine, which were related to a rapid and profound increase of the anti-inflammatory cytokine interleukin (IL) 10 and subsequent attenuation of the proinflammatory response (e.g., plasma levels of tumor necrosis factor [TNF] α, IL-6, and IL-8) (1).
The anti-inflammatory effects of this intervention could represent a novel treatment modality that may empower patients with inflammatory conditions, such as autoimmune diseases. However, there are several questions that need to be addressed first. Most importantly, it needs to be established which (combination of) element(s) is/are responsible for the effects observed, as feasibility may increase if potential users of the intervention would have to learn and practice less elements, but still attain the same efficacy. The meditation exercise is likely of limited relevance, as it was a very minor part of the training program and was not practiced during the endotoxemia experiments (1). The breathing exercises both involved cyclic hyperventilation (1). In one exercise, each cycle of hyperventilation was followed by breath retention for up to several minutes, resulting in profound decreases in oxygen saturation, whereas in the other exercise, participants only very shortly held their breath after each cycle of hyperventilation during which all body muscles were tightened, which was not associated with a decrease in oxygen saturation. Because both hyperventilation and hypoxia have been shown to result in epinephrine release (3–6), it is unknown which of these exercises is responsible for the observed effects. Furthermore, it is unclear whether it is necessary to be trained by the creator of the intervention (with regard to the so-called guru effect, in which the mere presence of an authoritarian figure influences symptomatology (7,8)) and whether or not a short instruction instead of an extensive training would be sufficient to increase plasma epinephrine levels (1).
In the first part of the current study, we addressed these issues by investigating the effects of the two different breathing exercises and different training modalities (i.e., training by the creator of the intervention versus an independent trainer, and a short instruction versus extensive training) on plasma epinephrine levels, as these are implicated to be the main determinant of the anti-inflammatory effects of the intervention (1). In the second part of this study, we investigated the effects of the optimal breathing exercise established in the first part and of cold exposure, both independently and combined, on the inflammatory response during experimental human endotoxemia. In this highly controlled and reproducible model, a systemic inflammatory response is elicited by intravenous administration of bacterial LPS to healthy volunteers (9). This model is used to investigate the inflammatory response and possible therapeutics in sepsis and other inflammatory conditions, but also offers possibilities to study mechanisms underlying cytokine-induced behavioral changes and to characterize potential targets of therapies against inflammation-associated depression (10). Cold exposure may influence the inflammatory response either through a direct, epinephrine-independent effect or by enhancing epinephrine levels elicited by the breathing exercise.
By identifying the efficacy of the different training modalities and elements, this study aims to shed light on the underlying mechanisms responsible for the previously observed anti-inflammatory effects and will aid future clinical development of the training intervention.
METHODS
Ethical Approval and Participants
All procedures were approved by the local ethics committee of the Radboud university medical center (Commissie Mensgebonden Onderzoek—Human Research Ethics Committee Arnhem-Nijmegen, reference numbers are provided in the corresponding sections hereinafter) and were conducted in accordance with the Declaration of Helsinki including current revisions and Good Clinical Practice guidelines. Data were collected between December 2014 and June 2016. Participants were community volunteers, mostly students, who were recruited through paper leaflets, posters, and online communities within the campus of the Radboud University in Nijmegen, the Netherlands. All participants provided written informed consent to participate in the study and were screened before the start of the experiment to confirm a normal physical examination, electrocardiography, and routine laboratory values. Exclusion criteria were prior experience with any of the elements of the intervention developed by the creator of the intervention or other breathing, meditation, or cold exposure exercises (including mindfulness, yoga, exposure to cold showers, and frequent visits to sauna facilities [more than once per month]). Additional exclusion criteria were use of any medication, smoking, previous spontaneous vagal collapse, use of recreational drugs within 21 days before the experiment day, surgery or trauma with significant blood loss or blood donation, hospital admission or surgery with general anesthesia, participation in another study within 3 months before the experimental day, or clinically significant acute illness (including infections) within 4 weeks before the experiment day.
Breathing Exercises Study
Study Design
After ethical approval (reference number: 2014-1374/NL51237.091.14), 40 males provided written informed consent to participate in this prospective randomized study registered at ClincialTrials.gov (NCT02417155). A schematic overview of the study is depicted in Figure 1. Participants were randomized to four different groups (n = 10 per group) by an independent research nurse using the sealed envelope method: extensive training by the creator of the intervention, extensive training by an independent trainer, short training by the creator of the intervention, and short training by an independent trainer. All participants were trained in both breathing exercises, with and without the prolonged breath retention (detailed in the Breathing Exercises section), in the week before the experiment day.
FIGURE 1.

Schematic overview of the procedures of the breathing exercises study. Dots indicate blood sampling from the arterial catheter at the corresponding time points.
Training Procedures
In the group who received the extensive training by the creator of the intervention, participants were trained every morning for 2 hours for 4 days, and after these initial 4 days of training, participants were instructed to practice the learned exercises at home, both analogous to our previous study (1). In the group who received the short training by the creator of the intervention, participants were trained for only 2 hours on the morning of the fourth day (Figure 1), and participants were instructed not to practice the learned exercises at home. The training procedures in the other two groups were exactly the same, with the exception that the creator was substituted by an independent trainer from our research group (R.v.G.), and that participants also received a detailed written instruction of both breathing exercises (Appendix, “Written instructions for breathing exercises,” Supplemental Digital Content 1, http://links.lww.com/PSYMED/A820).
Breathing Exercises
In the exercise with the prolonged retention of breath (henceforth designated as “with [+] retention”), participants hyperventilated for an average of 30 breaths using deep and powerful breaths. Subsequently, the participants exhaled and held their breath for approximately 2 minutes (“retention phase”). The duration of breath retention was entirely at the discretion of the participant. Breath retention was followed by a deep inhalation breath, which was held for 10 seconds. Subsequently a new cycle of hyper/hypoventilation began. In the exercise without retention of breath (henceforth designated as “without [−] retention”), participants also hyperventilated for an average of 30 times using deep and powerful breaths. Subsequently, participants held their breath for only 10 seconds, during which all body muscles were tightened, and then a new cycle of hyperventilation was initiated.
Procedures on the Experiment Day
The experiments were conducted at the research unit of the intensive care department of the Radboud university medical center, and an overview of the procedures is depicted in Figure 1. To allow for comparison with our previous study (1), participants refrained from caffeine and alcohol 24 hours before the experiment, and refrained from any intake of food and drinks 10 hours before the experiment. Fasting was maintained throughout the two breathing exercise sessions. A cannula was placed in the antecubital vein of the nondominant arm for hydration, and the radial artery of the same arm was cannulated under local anesthesia (lidocaine HCl 20 mg/mL) using a 20-gauge arterial catheter for continuous arterial blood pressure monitoring and blood withdrawal. After a 1-hour rest period, participants were randomized to start with one of the breathing exercises at 9:00 am (morning session): half of the participants started with the exercise with retentions, whereas the other half started with the exercise without retentions. They performed the exercise for 1.5 hours, after which they rested for 1.5 hours, and the second breathing exercise was started at noon (afternoon session), which also lasted 1.5 hours. Adherence was assured by a member of the research team who was present in the room during the entire experiment. Serial blood samples were obtained throughout the experiment (Figure 1).
Experimental Human Endotoxemia Study
Study Design
After ethical approval (reference number 2016-2312/NL56686.091.16), 48 males provided written informed consent to participate in this prospective randomized controlled study registered at ClincialTrials.gov (NCT03240497). A schematic overview of the study is depicted in Figure 2. We used a 2 × 2 design, in which 48 participants were randomized using the sealed envelope method to 4 different groups (n = 12 per group): cold exposure (CEX), breathing exercise without retention (BRT), cold exposure and the breathing exercise without retention (CBR), and a control group (CON). Participants of all groups except the control group were trained in the week leading up to the endotoxemia experiment day.
FIGURE 2.
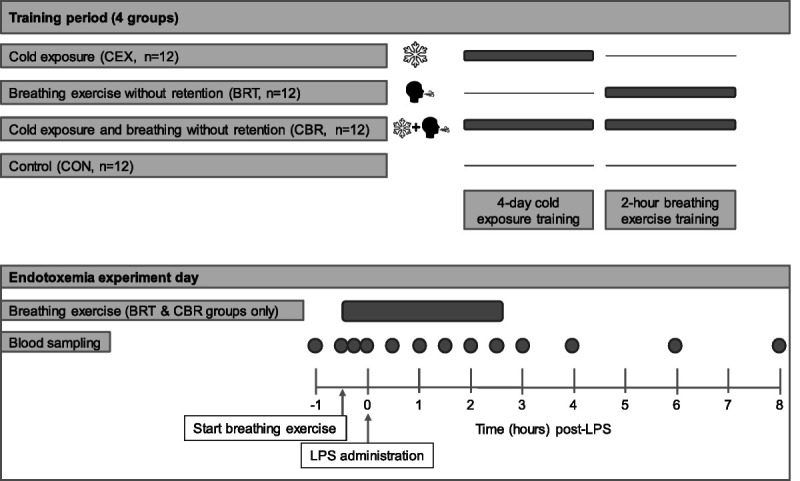
Schematic overview of the procedures of the human endotoxemia study. Dots indicate blood sampling from the arterial catheter at the corresponding time points. LPS = lipopolysaccharide.
Training Procedures
An impression of the training procedures is provided (Video, Supplemental Digital Content 2, http://links.lww.com/PSYMED/A821). All training procedures were provided by the same independent trainer (R.v.G.). The creator of the intervention was not involved in the training course. The study team, including an MD, was present during all training procedures. Participants in the CEX group followed an intensive 4-day cold exposure training program similar to that of our previous study (1), consisting of standing in snow with bare feet for up to 30 minutes, lying in snow in shorts for up to 20 minutes, and sitting and swimming in ice-cold water for up to 3 minutes (Video, Supplemental Digital Content 2, http://links.lww.com/PSYMED/A821). Participants were instructed to end their daily shower with a period of 60 seconds of cold water. Participants in the BRT group were trained in the breathing exercise without retentions of breath as described previously in the Breathing Exercises section. Similar to the short training by an independent trainer group in the breathing exercises study (see the previous section, Training Procedures), the independent trainer provided an instruction course of 2 hours. Participants were instructed not to practice the learned exercises at home. Participants randomized to the CBR group participated in both training procedures, and participants in the control group did not receive any training.
Procedures on the Endotoxemia Experiment Day
Endotoxemia experiments were conducted at the research unit of the intensive care department of the Radboud university medical center according to our standard protocol (11) also used in our previous study into this intervention (1), and an overview of the procedures is depicted in Figure 2. Participants refrained from caffeine, alcohol, and intake of food and drinks in the same way as the participants of the breathing exercises study did. A cannula was placed in the antecubital vein of the nondominant arm for hydration, and the radial artery of the same arm was cannulated under local anesthesia (lidocaine HCl 20 mg/mL; Fresenius Kabi, Zeist, the Netherlands) using a 20-gauge arterial catheter for continuous arterial blood pressure monitoring and blood withdrawal. Participants received 1.5 L of 2.5% glucose/0.45% saline solution for 1 hour (prehydration) before LPS administration, followed by 150 mL/h until the end of the experiment (8 hours after LPS administration). Participants of the BRT and CBR groups practiced the learned breathing exercise from 30 minutes before administration of LPS to 2.5 hours afterward, identical to our previous study (1). Adherence was assured by a member of the research team that was present in the room during the entire experiment. Purified LPS (derived from Escherichia coli O:113, Clinical Center Reference Endotoxin) obtained from the Pharmaceutical Development Section of the National Institutes of Health (Bethesda, Maryland) and supplied as a lyophilized powder was reconstituted in 5 mL saline 0.9% for injection and vortex-mixed for 20 minutes before being administered as an intravenous bolus at a dose of 2 ng/kg body weight for 1 minute at T = 0 hours at 9:30 am. Blood samples were serially obtained throughout the experiment (Figure 2).
Epinephrine and Blood Gas Analysis
For circulating epinephrine measurements, blood was collected into lithium heparin tubes and was immediately placed on ice and centrifuged at 2000g for 10 minutes at 4°C, after which plasma was stored at −80°C until analysis. Plasma epinephrine concentrations were subsequently measured using high-performance liquid chromatography with fluorometric detection (12). Blood gas parameters were analyzed in lithium heparin anticoagulated arterial blood using an i-STAT Blood Gas Analyzer (Abbot, Hoofddorp, the Netherlands) and CG4+ cartridges.
Plasma Cytokines
EDTA-anticoagulated blood was centrifuged immediately at 2000g for 10 minutes at 4°C, after which plasma was stored at −80°C until analysis. Concentrations of TNF-α, IL-6, IL-8, IL-10, interferon gamma–induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein (MIP) 1α, and MIP-1β were measured in one batch using one assay with a simultaneous Luminex assay according to the manufacturer’s instructions (Milliplex; Merck-Millipore, Billerica, Massachusetts) on a Magpix instrument (Luminex Corporation, Austin, Texas). Intra-assay % coefficient of variation of the measured cytokines as provided by the manufacturer range from 1.5 to 2.6, whereas interassay % coefficients of variation ranged from 3.5 to 18.3. The detection range was 3.2 to 10,000 pg/mL for cytokines TNF-α, IL-10, IP-10, MCP1, MIP1a, and MIP1b and 1.4 to 10,000 pg/mL for IL-6 and IL-8. Samples below the detection limit were imputed by 3.2 and 1.4 pg/mL, respectively; no samples were above the upper detection limit.
Hemodynamic Parameters, Symptom Score, and Temperature
Heart rate (three-lead electrocardiogram), blood pressure (intra-arterial cannula), respiratory rate, and oxygen saturation (pulse oximetry) data were recorded from a Philips MP50 patient monitor (Eindhoven, the Netherlands) every 30 seconds by a custom in-house–developed data recording system. LPS-induced flu-like symptoms (headache, nausea, shivering, muscle, and back pain) were scored every 30 minutes on a 6-point Likert scale (0 = no symptoms, 5 = worst ever experienced, in case of vomiting 3 points were added), forming an arbitrary total symptom score with a maximum of 28 points. Body temperature was determined every 30 minutes using an infrared tympanic thermometer (First-Temp Genius; Sherwood Medical, Norfolk, Nebraska).
Calculations and Statistical Analysis
Data are expressed as median and interquartile range or mean and 95% confidence interval, based on their distribution calculated by Shapiro-Wilk tests. For the sample size of the endotoxemia study, we wished to remain in line with our previous published endotoxemia study on this intervention (1), in which 12 participants per group were included. We calculated the achieved power using previous endotoxemia data of our group on the archetypal proinflammatory cytokine TNF-α. The mean of the TNF-α response (area under the time-concentration curve [AUC]) was 970 arbitrary units with a standard deviation of 300 arbitrary units (31% of the mean). Using these values, a detectable contrast (effect size) of 40% (388 arbitrary units), a two-sided α of .05, and 12 participants per group in an unpaired t test design, a power of 86% is achieved. There were no outliers that needed to be removed from any analysis. In the breathing exercises study, of the total of 280 sample moments, there were three missing values in the blood gas parameters because of technical issues. In the endotoxemia study, there was one missing value in the blood gas parameters (of a total of 312 sample moments) and five missing values in the epinephrine data (of a total of 432 sample moments), all because of technical issues. Serial data were analyzed using linear mixed-models analysis (p values of “time by column factors” are depicted in the figures, whereas the results of post hoc Sidak multiple comparison tests [only performed in case time by column, the p value was <.05] to evaluate differences at individual time points are provided in the supplemental tables, (Supplemental Digital Content 1, http://links.lww.com/PSYMED/A820). AUCs were calculated on a per-participant basis using the “Area under Curve” function in GraphPad Prism 8.0 (GraphPad Software, San Diego, California) to provide an integral measure of the cytokine responses. Multivariate multiple linear regression, entering AUC cytokine responses as dependent variables and the different groups as independent variables, was performed to assess the effects of cold exposure training and the breathing exercises on plasma levels of all measured cytokines. Demographic characteristics were analyzed using Kruskal-Wallis tests. A p value <.05 was considered statistically significant. Calculations and statistical analysis were performed using GraphPad Prism version 8.3.0 and SPSS v25.0.0.1 (IBM Corp, Armonk, New York).
RESULTS
Breathing Exercises Study
Demographic Characteristics
Demographic characteristics of the participants are listed in Table 1 and were not different between the study groups.
TABLE 1.
Demographic Characteristics
| Breathing Exercises Study | All Participants (n = 40) | Short Training by Independent Trainer (n = 10) | Extensive Training by Independent Trainer (n = 10) | Short Training by Creator (n = 10) |
Extensive Training by Creator (n = 10) | p |
|---|---|---|---|---|---|---|
| Age, y | 21 [19–24] |
20 [19–22] |
22 [19–26] |
21 [20–23] |
23 [19–26] |
.46 |
| BMI, kg/m2 | 22.9 [21.4–24.2] |
22.5 [21.5–24.8] |
23.9 [21.1–25.0] |
23.8 [22.2–24.6] |
22.3 [20.1–23.6] |
.60 |
| Systolic blood pressure, mm Hg | 140 [128–145] |
135 [123–148] |
143 [128–147] |
146 [137–150] |
135 [131–140] |
.26 |
| Diastolic blood pressure, mm Hg | 71 [64–79] |
71 [61–77] |
77 [70–85] |
69 [62–81] |
69 [62–81] |
.22 |
| Heart rate, bpm | 77 [60–88] |
77 [69–84] |
86 [59–103] |
77 [53–89] |
63 [54–82] |
.27 |
| Endotoxemia Study | All Participants (n = 48) | CON (n = 12) |
Cold Exposure (CEX) (n = 12) |
Breathing Exercise (BRT) (n = 12) |
Cold Exposure and Breathing Exercise (CBR) (n = 12) |
p |
|---|---|---|---|---|---|---|
| Age, y | 22 [20–24] |
22 [20–22] |
23 [20–26] |
22 [20–24] |
23 [20–25] |
.86 |
| BMI, kg/m2 | 23.3 [22.2–24.6] |
23.1 [22.2–23.9] |
22.8 [21.0–24.2] |
23.5 [22.7–24.7] |
24.5 [22.5–25.6] |
.37 |
| Systolic blood pressure, mm Hg | 140 [136–152] |
137 [122–156] |
142 [137–155] |
140 [136–152] |
144 [136–152] |
.88 |
| Diastolic blood pressure, mm Hg | 72 [64–80] |
73 [66–82] |
72 [64–81] |
70 [62–75] |
77 [68–82] |
.35 |
| Heart rate, bpm | 64 [56–71] |
66 [59–75] |
65 [56–73] |
62 [56–66] |
62 [51–67] |
.33 |
Data were obtained using the screening visit and are presented as median [interquartile range].
p Values were calculated using Kruskal-Wallis tests.
BMI = body mass index; bpm = beats per minute; CON = control group; CEX = cold exposure group; BRT = breathing exercise group; CBR: cold exposure and breathing exercise group.
Plasma Epinephrine Levels and Blood Gas Parameters
Changes in blood gas parameters were identical during both the morning and afternoon sessions across all groups (saturation: F(6,462) = 0.74, p = .62; oxygen partial pressure [po2]: F(6,463) = 0.98, p = .44; pH: F(6,463) = 0.56, p = .76; and carbon dioxide partial pressure [pco2]: F(6,463) = 1.26, p = .28; Figures S1A–D and Table S5, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A820). During the morning session, plasma epinephrine levels sharply increased upon initiation of the breathing exercises across all groups (from 0.51 [0.33–0.72] nmol/L at T = 0 to 1.01 [0.64–1.48] nmol/L at T = 0.5, p < .0001; Figure S1E and Table S5, http://links.lww.com/PSYMED/A820). We previously hypothesized that this initial increase in epinephrine levels is the main driving factor inducing the anti-inflammatory phenotype (see Figure 5 in Ref. (1)). Epinephrine levels remained elevated for as long as the participants practiced the exercises in the morning (0.87 [0.51–1.24] nmol/L and 0.99 [0.56–1.68] nmol/L at T = 1, and 1.5 hours, respectively; Figure S1E and Table S5, http://links.lww.com/PSYMED/A820). In contrast, during the afternoon session plasma epinephrine levels failed to rapidly increase after commencing the breathing exercises, although concentrations were slightly elevated at later time points (T = 0: 0.48 [0.33–0.65] nmol/L, T = 0.5: 0.44 [0.30–0.73] nmol/L, T = 1: 0.54 [0.38–1.12] nmol/L and T = 1.5: 0.75 [0.54–1.26] nmol/L; Figure S1E and Table S5, http://links.lww.com/PSYMED/A820). Statistical comparison of plasma epinephrine levels over time between the morning and afternoon session yielded a highly significant difference (F(4,312) = 6.42, p < .001; Figure S1E and Table S5, http://links.lww.com/PSYMED/A820). Because of these findings, we restricted all further analyses to data obtained during the morning session.
A comparison of the breathing exercises with and without retention revealed that only the exercise with retention resulted in profound decreases in oxygen saturation levels at the end of each retention phase (from 98% ± 0.2% at T = 0 to 67% ± 5%, 58 ± 3% and 73 ± 4%, at T = 0.5, 1, and 1.5 hours, respectively; F(6,224) = 31.50, p < .001; Figure 3A and Table S1, http://links.lww.com/PSYMED/A820). Accordingly, sharp decreases in po2 were observed in this group (F(6,225) = 18.43, p < .001; Figure 3B and Table S1, http://links.lww.com/PSYMED/A820). pH and pco2 were largely comparable between the two breathing exercises, with only a slight but statistically significant difference at the last measured time point (pH: F(6,225) = 7.96, p < .001; pco2: F(6,225) = 4.67, p < .001; Figures 3C, D, and Table S1, http://links.lww.com/PSYMED/A820). The initial increase in plasma epinephrine levels was comparable between both breathing exercises (from 0.51 [0.38–0.75] nmol/L at T = 0 to 0.98 [0.67–1.78] nmol/L at T = 0.5 in the participants performing the breathing exercise with breath retention, and from 0.51 [0.32–0.68] nmol/L at T = 0 to 1.01 [0.63–1.46] nmol/L at T = 0.5 in the participants performing the breathing exercise without retention, p > .99; Figure 3E and Table S1, http://links.lww.com/PSYMED/A820). However, the increase in plasma epinephrine concentrations was slightly more sustained in the participants practicing the breathing exercise with retention, resulting in significantly higher levels at T = 1.5 compared with participants practicing the exercise without retention (F(4,152) = 4.19, p = .003; Figure 3E and Table S1, http://links.lww.com/PSYMED/A820).
FIGURE 3.
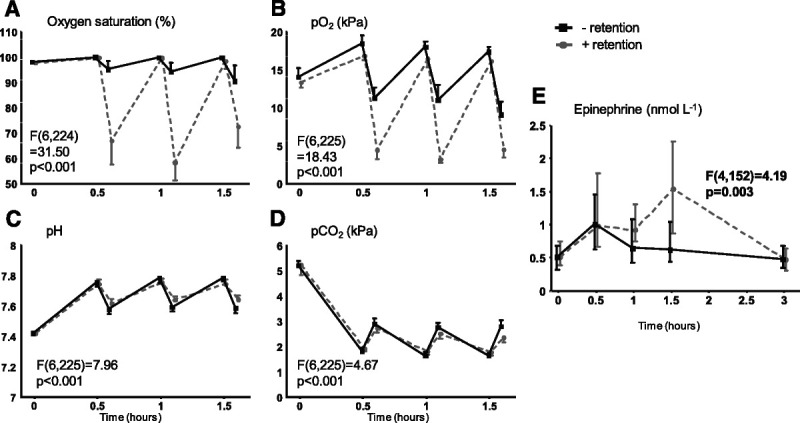
Arterial blood gas parameters and plasma epinephrine levels during the breathing exercises study: influence of breathing exercise. A, Oxygen saturation. B, Oxygen partial pressure (po2). C, pH. D, Carbon dioxide partial pressure (pco2). − retention: data obtained during the first breathing exercise on the experiment day (breathing exercise 1, Figure 1) from participants performing the breathing exercise without prolonged retention of breath. + retention: data obtained during the first breathing exercise on the experiment day (breathing exercise 1, Figure 1) from participants performing the breathing exercise with prolonged retention of breath. Data are presented as mean ± 95% confidence interval (panels A–D) or median and interquartile range (panel E) of 20 participants per group, and p values depicted in the graphs represent the between-group comparison calculated using linear mixed-models analysis (time by column factor). Epinephrine data were log-transformed before analysis. For comparisons that yielded time by column factor p values <.05, results of post hoc analyses performed using the Sidak multiple comparison test are reported in Table S1, http://links.lww.com/PSYMED/A820.
Blood gas parameters and plasma epinephrine levels were not statistically different between the participants trained by an independent trainer compared with participants trained by the creator of the intervention (saturation: F(6,224) = 0.55, p = .76; po2: F(6,225) = 0.26, p = .90; pH: F(6,225) = 0.59, p = .86; pco2: F(6,225) = 1.57, p = .24; epinephrine: F(4,152) = 0.92, p = .46; Figure S2, http://links.lww.com/PSYMED/A820). In addition, no significant differences in these parameters were found between the participants who received the short training versus the long training (saturation: F(6,224) = 0.28, p = .95; po2: F(6,225) = 0.59, p = .74; pH: F(6,225) = 1.30, p = .26; pco2: F(6,210) = 0.83, p = .55; epinephrine: F(4,152) = 0.53, p = .71; Figure S3, http://links.lww.com/PSYMED/A820).
Based on these results, we conclude that the magnitude of the initial increase in epinephrine levels, which we previously showed to be a main determinant of the anti-inflammatory phenotype (1), is not dependent on prolonged breath retention. Furthermore, neither training by the creator of the intervention nor a long training program is required to attain the pronounced epinephrine response. Hence, we used the training modality consisting of a short training by an independent trainer in only the breathing exercise without breath retention for the subsequent experimental human endotoxemia study.
Experimental Human Endotoxemia Study
Demographic Characteristics
Demographic characteristics of the participants are listed in Table 1 and were not different between groups.
Blood Gas Parameters and Plasma Epinephrine Levels
pco2 levels slightly decreased over time in the groups that did not practice the breathing exercise (CEX and CON groups; Figures 4A–C), which may reflect a small increase in breathing frequency after LPS administration. However, no significant changes in this or any of the other arterial blood gas parameters were observed over time in these groups, and all values remained within the reference ranges (CEX and CON groups; Figures 4A–C). In contrast, blood gas parameters were profoundly altered in the BRT and CBR groups upon initiation of the breathing exercise and normalized quickly after cessation. pH increased from 7.46 ± 0.04 (BRT) and 7.40 ± 0.004 (CBR) at baseline to 7.72 ± 0.02 (BRT) and 7.70 ± 0.01 (CBR) 15 minutes after the start of the breathing exercise, resulting in significant higher pH during the execution of the breathing exercises in both groups compared with the CON group (BRT: F(3,88) = 27.80, p < .01; CBR: F(3,66) = 82.13, p < .01; Figure 4A and Table S2, http://links.lww.com/PSYMED/A820). Oxygen saturation increased from 98% (98%–99%; BRT) and 99% (98%–100%; CBR) at baseline to 100% (100%–100%; BRT) and 100% (100%–100%; CBR) 15 minutes into practicing of the breathing exercise (BRT: F(3,88) = 12.67, p < .001 versus CON; CBR: F(3,88) = 6.49), p < .001 versus CON; Figure 4B and Table S2, http://links.lww.com/PSYMED/A820). pco2 dropped from 5.16 ± 0.1 kPa (BRT) and 5.17 ± 0.01 kPa (CBR) at baseline to 1.74 ± 0.06 kPa (BRT) and 1.99 ± 0.07 kPa (CBR) 15 minutes after the start of the breathing exercise (BRT: F(3,66) = 95.23, p < .001 versus CON; CBR: F(3,66) = 91.04, p < .001 versus CON; Figure 4C and Table S2, http://links.lww.com/PSYMED/A820).
FIGURE 4.
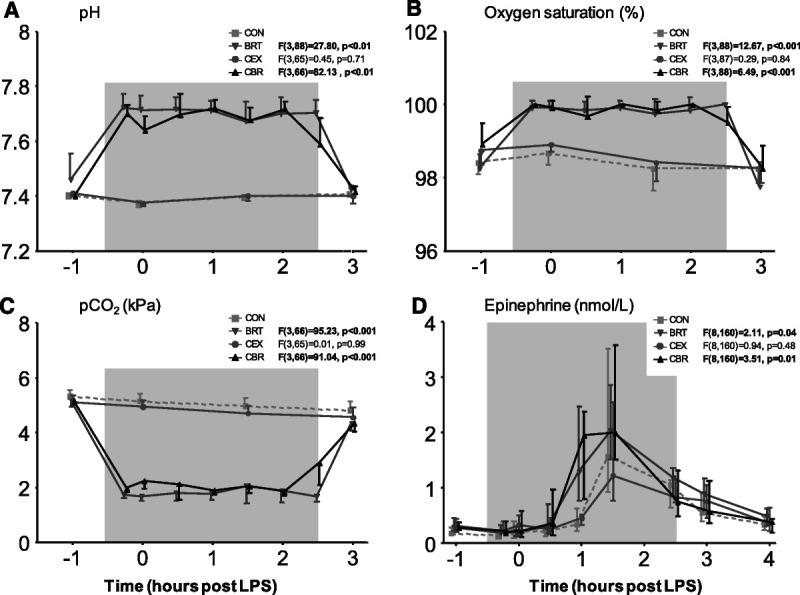
Arterial blood gas parameters and plasma epinephrine levels during human endotoxemia. A, pH. B, Oxygen saturation. C, pco2. D, Plasma epinephrine concentrations. The gray box indicates the period during which the trained participants practiced the breathing exercise (BRT and CBR groups only). Data are presented as mean ± 95% confidence interval (panels A–C) or median and interquartile range of 12 participants per groups. p Values depicted next to the legend represent the comparison of that group with the control group over time, calculated using linear mixed-models analysis on log-transformed data (time by column factor). Epinephrine data were log-transformed before analysis. Significant p values are shown in bold. For comparisons that yielded time by column factor p values <.05, results of post hoc analyses performed using the Sidak multiple comparison test are reported in Table S2, http://links.lww.com/PSYMED/A820. BRT = breathing exercise group; CBR = cold exposure and breathing exercise group; CEX = cold exposure group; CON = control group; pco2 = carbon dioxide partial pressure.
Baseline plasma epinephrine levels were comparable between all four groups (all p values >.05; Figure 4D and Table S2, http://links.lww.com/PSYMED/A820). Concentrations increased during human endotoxemia in all groups, with peak values observed 1.5 hours after administration of LPS (Figure 4D and Table S2, http://links.lww.com/PSYMED/A820). There were no differences in plasma epinephrine levels over time between the CEX and CON groups (F(8,160) = 0.94, p = .48). However, in both groups of participants who practiced the breathing exercises, the increase in plasma epinephrine commenced much earlier and was significantly more pronounced than in the CEX and CON groups that did not exercise the breathing exercise (BRT F(8,160) = 2.11, p = .04 versus CON; CBR: F(8,160) = 3.51 p = .01 versus CON; Figure 4D and Table S2, http://links.lww.com/PSYMED/A820).
Hemodynamic Parameters, Temperature, and Symptoms
Experimental endotoxemia resulted in a gradual increase in heart rate in the CEX and CON groups, with no differences between these two groups (F(18,396) = 0.61, p = .89, Figure 5A). In the two groups that performed the breathing exercise (BRT and CBR groups), a sharp increase in heart rate was observed immediately after the start of the first hyperventilation cycle, and this effect ensued during most of the period that the participants practiced the exercise, resulting in a significant higher heart rate during the experiment compared with the CON group (BRT: F(18,396) = 4.78, p < .001; CBR: F(18,396) = 3.97, p < .001; Figure 5A and Table S3, http://links.lww.com/PSYMED/A820). After cessation of the breathing exercises, the heart rate data of the BRT and CBR groups were similar to that of the CEX and CON groups. Expectedly, mean arterial pressure gradually decreased in all groups (Figure 5B and Table S3, http://links.lww.com/PSYMED/A820), and no clear differences between any of the groups were present. Although there was a statistically significant difference between the BRT and CON groups in mean arterial pressure over time (F(18,396) = 1.93, p = .01, Figure 5B), post hoc analysis did not reveal significance at any of the individual time points (Table S3, http://links.lww.com/PSYMED/A820). An LPS-induced mean increase in tympanic temperature of 1.8°C ± 0.1°C was observed across all groups (Figure 5C). Although peak temperatures were similar between the CON (38.8°C ± 0.1°C) and the three intervention groups (BRT: 38.8°C ± 0.2°C, p = .94; CEX: 38.6°C ± 0.2°C, p = .44; CBR: 38.7°C ± 0.2°C, p = .73), these were attained significantly earlier in the BRT group (F(18,396) = 1.96, p = .01 versus CON; Figure 5C and Table S3, http://links.lww.com/PSYMED/A820). Administration of LPS resulted in flu-like symptoms in all groups (Figure 5D). Peak symptom scores were comparable between the CON (9.3 ± 1.3), BRT (9.4 ± 1.5, p = .70), and CBR (7.04 ± 1.2, p = .21) groups, but significantly lower in the CEX group (5.5 ± 0.8, p = .017). Symptoms resolved significantly more rapidly in all three intervention groups compared with the CON group (BRT: F(18,396) = 4.47, p < .001; CEX: F(18,396) = 1.96, p = .01; CBR: F(18,396) = 2.29, p = .002; Figure 5D and Table S3, http://links.lww.com/PSYMED/A820).
FIGURE 5.
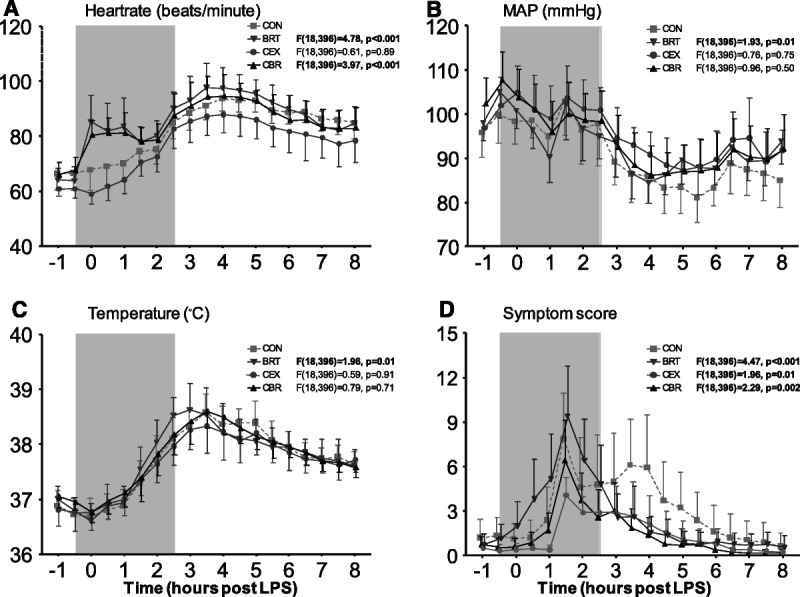
Cardiorespiratory parameters, tympanic temperature, and symptoms during human endotoxemia. A, Heart rate. B, MAP. C, Tympanic temperature. D, Score of self-reported symptoms. The gray box indicates the period during which the trained participants practiced the breathing exercise (BRT and CBR groups only). Data are expressed as mean ± 95% confidence interval of 12 participants per group. p Values depicted next to the legend represent the comparison of that group with the control group over time, calculated using linear mixed-models analysis (time by column factor). Significant p values are shown in bold. For comparisons that yielded time by column factor p values <.05, results of post hoc analyses performed using Sidak multiple comparison test are reported in Table S3, http://links.lww.com/PSYMED/A820. CON = control group; BRT = breathing exercise group; CBR = cold exposure and breathing exercise group; CEX = cold exposure group; MAP = mean arterial pressure.
Plasma Cytokines
Because of the absence of an inflammatory response before LPS administration and waning of this response multiple hours after the LPS challenge, 349 of a total of 4224 cytokine measurements (8%) fell below the lower limit of detection. As expected, plasma concentrations of the anti-inflammatory cytokine IL-10 and the proinflammatory cytokines TNF-α, IL-6, IL-8, IP-10, MCP-1, MIP-1α, and MIP-1β increased after LPS administration in all groups (Figure 6 and Table S4, Figure S4 and Table S6, http://links.lww.com/PSYMED/A820). In the CBR group, IL-10 levels were significantly higher compared with the CON group (mean increase in AUC of +44%, F(10,220) = 2.20, p = .02; Figure 6D and Table S4, http://links.lww.com/PSYMED/A820). Furthermore, concentrations of proinflammatory cytokines in this group were significantly attenuated compared with the CON group (mean decrease in AUC of TNF-α: −32%, F(10,220) = 2.09, p = .03; IL-6: −35%, F(10,220) = 2.06, p = .03; IL-8: −30%, F(10,220) = 3.97, p < .001; IP-10: −48%, F(10,220) = 7.99, p < .001; MCP-1: −29%, F(10,220) = 4.64, p < .001; MIP-1α: −35%, F(10,220) = 6.25, p < .001; MIP-1β: −30%, F(10,220), p < .001; Figures 6B–D and Table S4, and Figure S4 and Table S6, http://links.lww.com/PSYMED/A820). When comparing the BRT group with the control group, similar but less pronounced effects on plasma cytokines were observed, reaching statistical significance compared with the CON group for proinflammatory cytokines IL-6 (−34%, F(10,220) = 1.95, p = .04), IL-8 (−14%, F(10,220) = 2.25, p = .02), IP-10 (−48%, F(10,220) = 9.42, p < .001), MCP-1 (−37%, F(10,220) = 6.07, p < .001), MIP-1α (−37%, F(10,220) = 6.59, p < .001, and MIP-1β (−28%, F(10,220) = 2.24, p = .02), but not for IL-10 (+17%, F(10,220) = 1.42, p = .17). In the CEX group, only levels of MCP-1 were significantly lower than in the CON group (−25%, F(10,220) = 4.75, p < .001).
FIGURE 6.
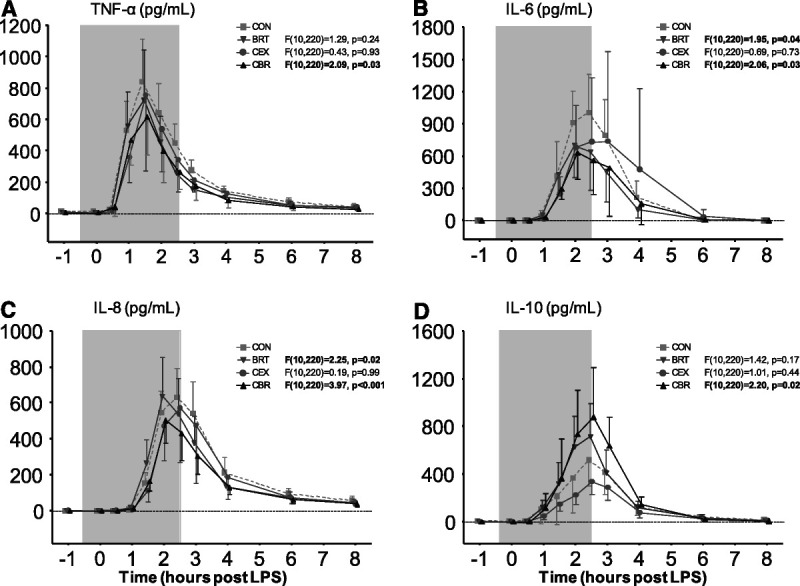
Plasma concentrations of inflammatory cytokines during human endotoxemia. A, TNF-α. B, IL-6. C, IL-8. D, IL-10. The gray box indicates the period during which the trained participants practiced the breathing exercise (BRT and CBR groups only). Data are presented as mean ± 95% confidence interval of 12 participants per group. p Values depicted next to the legend represent the comparison of that group with the control group over time, calculated using linear mixed-models analysis (time by column factor). Significant p values are shown in bold. For comparisons that yielded time by column factor p values <.05, results of post hoc analyses performed using the Sidak multiple comparison test are reported in Table S4, http://links.lww.com/PSYMED/A820. BRT = breathing exercise group; CBR: cold exposure and breathing exercise group; CEX = cold exposure group; CON = control group; IL = interleukin; TNF = tumor necrosis factor.
In accordance with the previously described results, a multivariate analysis yielded a significant effect of the breathing exercise, as well as the interaction between cold exposure training and breathing exercise on the integral cytokine response (breathing exercise: F(8,37) = 3.804, p = .002; Wilk’s Λ = 0.549, partial η2 = 0.451; cold exposure by breathing exercise: F(8,37) = 2.571, p = .024; Wilk’s Λ = 0.649, partial η2 = 0.357). Cold exposure alone did not significantly affect the integral cytokine response: F(8,37) = 0.603, p = .769, Wilk’s Λ = 0.885, partial η2 = 0.115.
DISCUSSION
In this study, we investigated the effects of different aspects of a training program, which was previously shown to allow for voluntary activation of the sympathetic nervous system and attenuation of the inflammatory response. First, we showed that, although arterial blood saturation levels and po2 were significantly lower when participants performed the breathing exercise with prolonged breath retention compared with that without, plasma epinephrine levels increased with a similar magnitude shortly after initiation of both breathing exercises. Second, we demonstrated that the previously observed physiological and immunological effects (1) are independent from either the length of training or the individual who provides it. Third, our data signify that the combination of the breathing exercise and cold exposure training is most effective in attenuating the inflammatory response during human endotoxemia.
As the magnitude of the initial increase in plasma epinephrine concentrations was similar for the breathing exercises with and without prolonged breath retention. The cyclic hypoxia caused by the exercise with prolonged breath retention is therefore unlikely to be an important factor in the observed epinephrine response. In accordance, hyperventilation itself and the subsequent shift in acid-base balance have been shown to increase plasma catecholamines in the absence of hypoxia, and an important role for bicarbonate has been implicated (6,13). Nevertheless, because catecholamine release from the adrenal chromaffin cells is dependent on a combination of neural, hormonal, redox, and immune signaling pathways (14,15), the exact mechanism behind the epinephrine release induced by the breathing exercise remains elusive. The finding that neither the duration of the training nor the trainer who provides it affected any of the measured parameters signifies that the breathing exercise is easy to learn within a time frame of 2 hours. These findings may greatly facilitate uncomplicated implementation of the training program in clinical studies.
Our data clearly demonstrate that the breathing exercise plays a pivotal role in the anti-inflammatory effect of the training intervention. Nevertheless, although cold exposure training alone had minimal effects on the cytokine response, it significantly potentiated the breathing exercise-induced anti-inflammatory effects. Because plasma epinephrine levels in our study were comparable between the groups practicing the breathing exercises with or without prior cold exposure training, other mechanisms are likely involved. Noteworthy, despite little effects on the cytokine response, participants in the cold exposure training group reported remarkably less symptoms compared with the control group as well as to the other two groups. In accordance, other studies reporting symptoms during repeated exposures to cold found similar attenuation of symptoms such as discomfort and shivering (16,17). This is possibly part of a stress-induced analgesic response to cold (18) Symptoms, especially headache, were more pronounced during practicing of the breathing exercise, likely resulting from the hyperventilation-induced changes in pco2 and pH. After cessation of the breathing exercise, a sharp decrease of symptoms was observed and flu-like symptoms resolved more rapidly compared with the control group.
In the endotoxemia study, the increase in plasma epinephrine concentrations observed after initiation of the breathing exercises described in the present work was similar in magnitude to that in our previous endotoxemia study (1). Nevertheless, epinephrine levels before the start of the breathing exercises were higher in the past work (1). Effects on the cytokine response in the combined cold exposure and breathing group in the current study were largely comparable to our previous work, in which participants were also trained in both exercises (1), although the magnitude of the immunomodulatory effects was less pronounced, with the anti-inflammatory IL-10 response augmented by 44% instead of 194% in Ref. (1) and proinflammatory cytokines attenuated by approximately 30% as opposed to more than 50% in Ref. (1). There are several possible explanations for this discrepancy. First, the previously mentioned higher baseline plasma epinephrine concentrations could play a role (1), which may in turn have triggered a more pronounced early IL-10 release and subsequent stronger attenuation of the proinflammatory response. Second, our data from the breathing exercises study show that, although the initial increase in epinephrine levels was similar in response to the exercise with and without breath retention, it was more prolonged in the former. Despite that fact that we previously showed that the initial epinephrine increase is a main determinant of the anti-inflammatory phenotype (1), we cannot exclude the possibility that a more prolonged increase has a more pronounced effect. Third, the hypoxia induced by breath retention in our previous study may have directly (i.e., independently from epinephrine) modulated the inflammatory response, as our group has recently demonstrated that hypoxia enhances IL-10 release and attenuates the proinflammatory response via enhanced adenosine release (19). In this light, future studies into the training intervention should still consider including the exercise with prolonged breath retention. This exercise may nevertheless be less preferable from a safety perspective, as the profound cyclic decreases in oxygen saturation may present risks for patients with for instance cardiovascular conditions.
A striking finding from the breathing exercises study was that the profound increase in plasma epinephrine levels only occurred during the first session in the morning, not during the second session performed in the afternoon after a 1.5-hour resting period. Nevertheless, the saturation, po2, pco2, and pH were identical between the morning and the afternoon sessions. Therefore, the lack of a profound increase of plasma epinephrine levels during the afternoon session may be due to adaptation of the stress response, resulting in lower epinephrine release by the adrenal gland in response to repeated application of the same stressor, a phenomenon that has been described in animals (20). Alternatively, because the synthesis and storage of catecholamines mainly take place within chromaffin cells of the adrenal medulla, it may be speculated that the breathing exercises deplete the intravesicular stores in the cytoplasm of these cells (14,15). Although animal experiments have shown that fully depleted catecholamine stores can be replenished within 2 hours (21), this may take longer in humans. In any case, if stores are indeed depleted by the breathing exercise, replenishment must occur within a relatively short time frame (<24 hours), as participants of this study, as well as our previous study (1) practiced the breathing exercise daily in the week leading up to the experiment in which the plasma epinephrine concentrations were measured.
Several limitations of our work need to be addressed. First, we studied groups of healthy young male adults, not (older) patients with possible comorbidities, who represent the intended target group for this intervention. We only included male participants because there are considerable differences in the cytokine response to LPS between the sexes (22). This could be due to menstrual cycle–related hormonal variations that affect immunity. Because human endotoxemia studies are very labor-intensive and expensive, and for ethical reasons (to expose as few volunteers as possible to endotoxemia), nearly all of our LPS studies are restricted to males to increase homogeneity and reduce sample size. Of note, there are no data available regarding the influence of sex differences on training-induced modulation of the immune response. Furthermore, the creator of the intervention has trained both men and women, with no apparent differences in competence regarding completion of the training exercises (observational data). Although upcoming studies into this intervention should also include females, this study provides essential information in terms of designing the most safe and optimal training protocol for use in these future investigations. Second, the autoimmune response observed in patients with chronic inflammatory conditions clearly differs from that elicited by LPS administration, which models an acute inflammatory response to a bacterial infection. However, several drugs currently used in patients with inflammatory conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis are aimed at reducing the release of several proinflammatory cytokines (23), on which the studied intervention has a substantial suppressive effect. Furthermore, in vivo human efficacy of many biologics used in the treatment of auto-inflammatory disorders, such as anakinra and infliximab, was first established in the experimental human endotoxemia model (24,25), illustrating that it has value for these diseases. Third, in the breathing exercises study, we did not include a control group that was not trained. We chose not to because the breathing exercises study was designed to primarily investigate what specific exercise caused the increase in epinephrine during the combination of breathing exercises performed by the participants in our original study (1) and whether training by the creator of the intervention is required. Therefore, we compared different breathing exercises and training modalities head-to-head. Furthermore, the participants acted as their own controls by measuring epinephrine levels before the start of the breathing exercise on the experiment day. Fourth, although psychological, social, or behavioral factors may also be influenced by this intervention, the focus of the present study was on physical outcomes. Because the intervention is ultimately aimed at alleviating symptoms among individuals with chronic disease, future studies should also evaluate whether the beneficial physiological effects of the intervention are offset, for example, by anxiety. Fifth, the reliability of the cytokine assay could not be confirmed because samples were not run in duplicate. Finally, the control group in the current study did not undergo any form of training. It must be acknowledged that a training program or other intervention guaranteed not to influence the sympathetic nervous system and immune response, but that does result in matching expectations compared with the intervention groups, would represent a more optimal control condition. Possibilities may entail mindfulness training, self-affirmation, educational materials, and placebo injection of a purported beneficial substance. Furthermore, in future studies, a dismantling design could be considered in which the component conditions are compared with the full protocol. Another point related to the control conditions is that we did not record the frequency of spontaneous (deep) breaths in the endotoxemia study. Because endotoxemia has been shown to increase deep breath frequency (26), this may have introduced bias in the control and cold exposure training groups. However, our pco2 data indicate that the LPS-induced increase in (deep) breathing frequency was limited at most. Furthermore, if anything, it would have led to an underestimation of the effect of the learned breathing exercises and therefore does not compromise the validity of our findings.
In conclusion, the present study corroborates previous findings that voluntary activation of the sympathetic nervous system, attenuation of the proinflammatory response, and alleviation of symptoms during experimental human endotoxemia are possible after following a training program consisting of cold exposure and a breathing exercise. Furthermore, these interventions can be provided by an independent trainer and acquired within a short time frame. Although these results provide an important next step in the clinical development of this intervention, they will need to be replicated and generalized before this intervention can be considered appropriate for application in clinical populations with chronic disease.
Supplementary Material
Acknowledgments
We thank the independent trainer Rogier van Groenendael for his contribution to this study and Remi Beunders for editing of the video illustrating the training procedures.
Source of Funding and Conflicts of Interest: The authors report no conflicts of interest. This study was internally funded by the Department of Intensive Care Medicine, Radboud university medical center, Nijmegen, the Netherlands.
Author contributions: study design/planning: J.Z., A.E.v.H., P.P., M.K.; study conduct: J.Z., R.N., P.P., M.K.; data analysis: J.Z., M.K.; writing the paper: J.Z., M.K.; critical revision: A.E.v.H., P.P., M.K.; revising the paper: all authors. All authors gave final approval of the version to be published.
Previous Posting: An earlier version of this article was previously posted to the Research Square preprint server: https://doi.org/10.21203/rs.2.20192/v1.
Footnotes
Supplemental Digital Content
Contributor Information
Jelle Zwaag, Email: jelle.zwaag@radboudumc.nl.
Rick Naaktgeboren, Email: rick.naaktgeboren@radboudumc.nl.
Antonius E. van Herwaarden, Email: teun.vanHerwaarden@radboudumc.nl.
Peter Pickkers, Email: peter.pickkers@radboudumc.nl.
REFERENCES
- 1.Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FC, van der Hoeven JG, Pickkers P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A 2014;111:7379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kox M, Stoffels M, Smeekens SP, van Alfen N, Gomes M, Eijsvogels TM, Hopman MT, van der Hoeven JG, Netea MG, Pickkers P. The influence of concentration/meditation on autonomic nervous system activity and the innate immune response: a case study. Psychosom Med 2012;74:489–94. [DOI] [PubMed] [Google Scholar]
- 3.Staubli M, Vogel F, Bartsch P, Fluckiger G, Ziegler WH. Hyperventilation-induced changes of blood cell counts depend on hypocapnia. Eur J Appl Physiol Occup Physiol 1994;69:402–7. [DOI] [PubMed] [Google Scholar]
- 4.Mantysaari M, Joutsi-Korhonen L, Siimes MA, Siitonen S, Parkkola K, Lemponen M, Lassila R. Unaltered blood coagulation and platelet function in healthy subjects exposed to acute hypoxia. Aviat Space Environ Med 2011;82:699–703. [DOI] [PubMed] [Google Scholar]
- 5.Oltmanns KM, Gehring H, Rudolf S, Schultes B, Hackenberg C, Schweiger U, Born J, Fehm HL, Peters A. Acute hypoxia decreases plasma VEGF concentration in healthy humans. Am J Physiol Endocrinol Metab 2006;290:E434–9. [DOI] [PubMed] [Google Scholar]
- 6.Krapf R, Caduff P, Wagdi P, Staubli M, Hulter HN. Plasma potassium response to acute respiratory alkalosis. Kidney Int 1995;47:217–24. [DOI] [PubMed] [Google Scholar]
- 7.Sperber D. The guru effect. Rev Philos Psychol 2010;1:583–92. [Google Scholar]
- 8.Martin JS, Summerville A, Wickline VB. Persuasion and pragmatics: an empirical test of the guru effect model. Rev Philos Psychol 2017;8:219–34. [Google Scholar]
- 9.Suffredini AF, Noveck RJ. Human endotoxin administration as an experimental model in drug development. Clin Pharmacol Ther 2014;96:418–22. [DOI] [PubMed] [Google Scholar]
- 10.Lasselin J, Lekander M, Benson S, Schedlowski M, Engler H. Sick for science: experimental endotoxemia as a translational tool to develop and test new therapies for inflammation-associated depression. Mol Psychiatry 2020;26:3672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Lier D, Geven C, Leijte GP, Pickkers P. Experimental human endotoxemia as a model of systemic inflammation. Biochimie 2019;159:99–106. [DOI] [PubMed] [Google Scholar]
- 12.Willemsen JJ, Ross HA, Jacobs MC, Lenders JW, Thien T, Swinkels LM, Benraad TJ. Highly sensitive and specific HPLC with fluorometric detection for determination of plasma epinephrine and norepinephrine applied to kinetic studies in humans. Clin Chem 1995;41:1455–60. [PubMed] [Google Scholar]
- 13.Messan F, Tito A, Gouthon P, Nouatin KB, Nigan IB, Blagbo AS, Lounana J, Medelli J. Comparison of catecholamine values before and after exercise-induced bronchospasm in professional cyclists. Tanaffos 2017;16:136–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne CJ, Khurana S, Kumar A, Tai TC. Inflammatory signaling in hypertension: regulation of adrenal catecholamine biosynthesis. Front Endocrinol (Lausanne) 2018;9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 2004;56:331–49. [DOI] [PubMed] [Google Scholar]
- 16.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013;123:3395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazaitis M, Eimantas N, Daniuseviciute L, Baranauskiene N, Skrodeniene E, Skurvydas A. Time course of physiological and psychological responses in humans during a 20-day severe-cold-acclimation programme. PLoS One 2014;9:e94698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muzik O, Reilly KT, Diwadkar VA. “Brain over body”—a study on the willful regulation of autonomic function during cold exposure. Neuroimage 2018;172:632–41. [DOI] [PubMed] [Google Scholar]
- 19.Kiers D, Wielockx B, Peters E, van Eijk LT, Gerretsen J, John A, Janssen E, Groeneveld R, Peters M, Damen L, Meneses AM, Kruger A, Langereis JD, Zomer AL, Blackburn MR, Joosten LA, Netea MG, Riksen NP, van der Hoeven JG, Scheffer GJ, Eltzschig HK, Pickkers P, Kox M. Short-term hypoxia dampens inflammation in vivo via enhanced adenosine release and adenosine 2B receptor stimulation. EBioMedicine 2018;33:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav 1990;47:1117–24. [DOI] [PubMed] [Google Scholar]
- 21.Wakade AR, Wakade TD, Malhotra RK. Restoration of catecholamine content of previously depleted adrenal medulla in vitro: importance of synthesis in maintaining the catecholamine stores. J Neurochem 1988;51:820–9. [DOI] [PubMed] [Google Scholar]
- 22.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med 2007;35:1464–9. [DOI] [PubMed] [Google Scholar]
- 23.Smolen JS, Emery P. Infliximab: 12 years of experience. Arthritis Res Ther 2011;13(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granowitz EV, Porat R, Mier JW, Orencole SF, Callahan MV, Cannon JG, Lynch EA, Ye K, Poutsiaka DD, Vannier E. Hematologic and immunomodulatory effects of an interleukin-1 receptor antagonist coinfusion during low-dose endotoxemia in healthy humans. Blood 1993;82:2985–90. [PubMed] [Google Scholar]
- 25.Suffredini AF, Reda D, Banks SM, Tropea M, Agosti JM, Miller R. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol 1995;155:5038–45. [PubMed] [Google Scholar]
- 26.Lasselin J, Lekander M, Paues-Goranson S, Olsson MJ, Axelsson J. Communication of health in experimentally sick men and women: a pilot study. Psychoneuroendocrinology 2018;87:188–95. [DOI] [PubMed] [Google Scholar]


