Abstract
Vagus nerve stimulation (VNS) could potentially facilitate arm function recovery after stroke. The aim of this review was to evaluate the effect of VNS paired with rehabilitation on upper limb function recovery after stroke. We considered randomized controlled trials (RCTs) that used VNS paired with rehabilitation for the improvement of upper limb function after stroke and were published in English. Eligible RCTs were identified by searching electronic databases, including MEDLINE, Web of Science, Embase, CENTRAL and PEDro, from their inception until June 2021. Quality of included studies was assessed using PEDro score and Cochrane’s risk of bias assessment. A meta-analysis was performed on the collected data. Five studies with a total of 178 participants met the inclusion criteria. Overall, the present meta-analysis revealed a significant effect of VNS on Fugl–Meyer Assessment for Upper Extremity (FMA-UE, MD = 3.59; 95% CI, 2.55–4.63; P < 0.01) when compared with the control group. However, no significant difference was observed in adverse events associated with device implantation between the invasive VNS and control groups (RR = 1.10; 95% CI, 0.92–1.32; P = 0.29). No adverse events associated with device use were reported in invasive VNS, and one was reported in transcutaneous VNS. This study revealed that VNS paired with rehabilitation can facilitate the recovery of upper limb function in patients with stroke on the basis of FMA-UE scores, but the long-term effects remain to be demonstrated.
Keywords: stroke, rehabilitation, upper extremity, vagus nerve
Introduction
Stroke is the leading cause of death and years lived with disability globally [1]. Upper limb impairment is one of the most prevalent dysfunctions after stroke, which results in poor health-related quality of life [2,3]. Approximately, 80–85% of patients with acute stroke present with upper limb motor impairment, and 60% of the stroke survivors still experience persistent impaired upper limb function 6 months after stroke [4,5]. Improving upper limb function is a priority for both stroke survivors and caregivers [6]. However, recent studies have revealed that the effects of current interventions for improving upper limb impairment are not satisfactory [3,7,8]. Therefore, novel and more effective methods are required to maximize upper limb recovery and ensure a high quality of life among stroke survivors [9].
Vagus nerve stimulation (VNS), which has been used for the treatment of epilepsy, headache and depression [10–12], can potentially enhance and facilitate the reorganization potential of cortical networks [13–15]. Several studies have investigated the efficacy of VNS paired with rehabilitation for upper limb function improvement in adults with stroke, but the results were conflicting and controversial. A meta-analysis of VNS and stroke published previously reported the potential effect of VNS on stroke [16]. The authors stated that additional high-quality studies, with large sample sizes, were required to validate their findings. Furthermore, the authors did not distinguish between invasive VNS and noninvasive VNS (transcutaneous VNS, tVNS), and the adverse events associated with device implantation and stimulation [16]. A previous study with a large sample size investigated the role of VNS in adults with stroke and was published in the Lancet recently [17]. Consequently, the present systematic review and meta-analysis of randomized controlled trials (RCTs) aimed to integrate new evidence presented in recent years to evaluate the efficacy and safety of VNS paired with rehabilitation for upper limb function improvement and to compare its effect with that of rehabilitation only or with sham VNS in adults with stroke.
Methods
The present systematic review and meta-analysis were performed and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 statement (PRISMA 2020), and Cochrane Handbook for Systematic Reviews of Interventions [18,19]. In addition, the present systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO): CRD42021268269.
Data sources and search strategy
We systematically searched for relevant articles available in English in electronic databases, including MEDLINE (via PubMed), Web of Science, Embase (via Ovid), CENTRAL (Cochrane library) and Physiotherapy Evidence Database (PEDro) from their inception until June 2021. Search terms included keywords associated with stroke, VNS and the upper limb. The specific search strategy of MEDLINE used is presented in Table 1 (see Supplementary Table, Supplemental digital content 1, http://links.lww.com/IJRR/A21 which presents the search strategies of the other four databases). Furthermore, manual screening of reference lists of the articles was performed to identify additional relevant studies. No ethical approval or patient consent was required because all analyses were on the basis of previously published studies.
Table 1.
Search strategy of MEDLINE
| MEDLINE (via PubMed) |
|---|
| 1. Stroke [mh] or Cerebrovascular disorders [mh] or Basal ganglia cerebrovascular disease [mh] or Brain ischemia [mh] or Carotid artery diseases [mh] or Cerebral small vessel diseases [mh] or Intracranial arterial diseases [mh] or Intracranial embolism and thrombosis [mh] or Intracranial hemorrhages [mh] or Brain infarction [mh] or Stroke, lacunar [mh] or Vasospasm, intracranial [mh] or Vertebral artery dissection [mh] or Hemiplegia [mh] or Paresis [mh] or Brain injuries [mh] or Brain injury, chronic [mh] |
| 2. Stroke* [tiab] or Poststroke [tiab] or Post-stroke [tiab] or Cerebrovasc* [tiab] |
| 3. 1 or 2 |
| 4. Vagus nerve [mh] |
| 5. Vagus nerve [tiab] or Vagal nerve [tiab] or Vagus nerve stimul* [tiab] or Vagal nerve stimul* [tiab] |
| 6. 4 or 5 |
| 7. Upper extremity [mh] |
| 8. Upper limb* [tiab] or upper extremit* [tiab] or arm* [tiab] or shoulder* [tiab] or hand* [tiab] or elbow* [tiab] or forearm* [tiab] or wrist* [tiab] or finger* [tiab] or axilla* [tiab] |
| 9. 7 or 8 |
| 10. 3 and 6 and 9 |
Study selection
Endnote software was used to check for duplicated studies. Two investigators reviewed the studies independently and selected studies on the basis of the predetermined criteria. All potentially relevant articles were retrieved from the databases for the assessment of their full text on the basis of titles and abstracts. Studies that did not meet the inclusion criteria were excluded. Discrepancies between two reviewers were resolved through discussions with a third reviewer and a consensus was reached. The included studies were required to meet the following criteria: (1) studies were RCTs published in English, (2) patients were diagnosed with ischemic or hemorrhagic stroke by computerized tomography or MRI, (3) intervention treatments were VNS (transcutaneous VNS or invasive VNS) paired with rehabilitation versus rehabilitation only and (4) with regard to outcome measures, at least one outcome associated with function of the upper limb was measured.
Data extraction and quality assessment
Two reviewers independently extracted relevant data onto a predeveloped data extraction sheet, and disagreements were adjudicated by a third reviewer. The data extracted from selected studies included basic information (first author, year of publication), study design, demographic characteristics of patients (sample size, age, sex, time from stroke), details of interventions applied to the experimental and control groups, relevant outcome measures and time of evaluation.
Eligible articles were scrutinized for methodological quality by two independent reviewers using PEDro scale. The PEDro scale comprises 11 items with a total score ranging from 0 to 10 (except for item 1). The methodological quality of studies scoring 9–10 was considered to be of ‘excellent’ quality, studies scoring 6–8 were considered to be of ‘good’ quality, studies scoring 4–5 were considered to be of ‘fair’ quality, and studies scoring below 4 were considered to be of ‘poor’ quality [20]. Discrepancies between two reviewers were resolved through discussions with a third reviewer. Additionally, risk of bias assessments was performed using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions [19]. The evaluation entries included the following aspects: random sequence generation, allocation concealment, masking, incomplete outcome data and selective outcome reporting among others. The included articles were evaluated as ‘low risk’, ‘high risk’ or ‘unclear risk’. Quality assessment was not used as a selection or exclusion criterion.
Data synthesis and analysis
The results of all included studies were pooled using standard meta-analytic methods to estimate the effect of VNS paired with rehabilitation versus rehabilitation only for upper limb function improvement after stroke. On the basis of the nature of extracted data, we assessed the mean differences (MDs) and 95% confidence intervals (CIs) for continuous outcomes, and risk ratios (RRs) at 95% CIs for adverse events. A P value <0.05 (two-sided) was considered statistically significant in the estimation of effects. Statistical heterogeneity was evaluated using chi-square test and I2 statistic. P value <0.05 or I2 value >50% was considered high heterogeneity. A fixed-effects model was used when P value was >0.05; otherwise, a random-effects model was used. Sensitivity analyses were performed by excluding each study from the analysis when heterogeneity was detected, and the subgroup analyses were performed on the basis of the different methods of VNS (tVNS or invasive VNS). Publication bias was not assessed due to the limited number of included studies (fewer than ten). All statistical analyses were performed using RevMan software (Version 5.3; Cochrane Collaboration, Copenhagen, Denmark).
Results
Search results
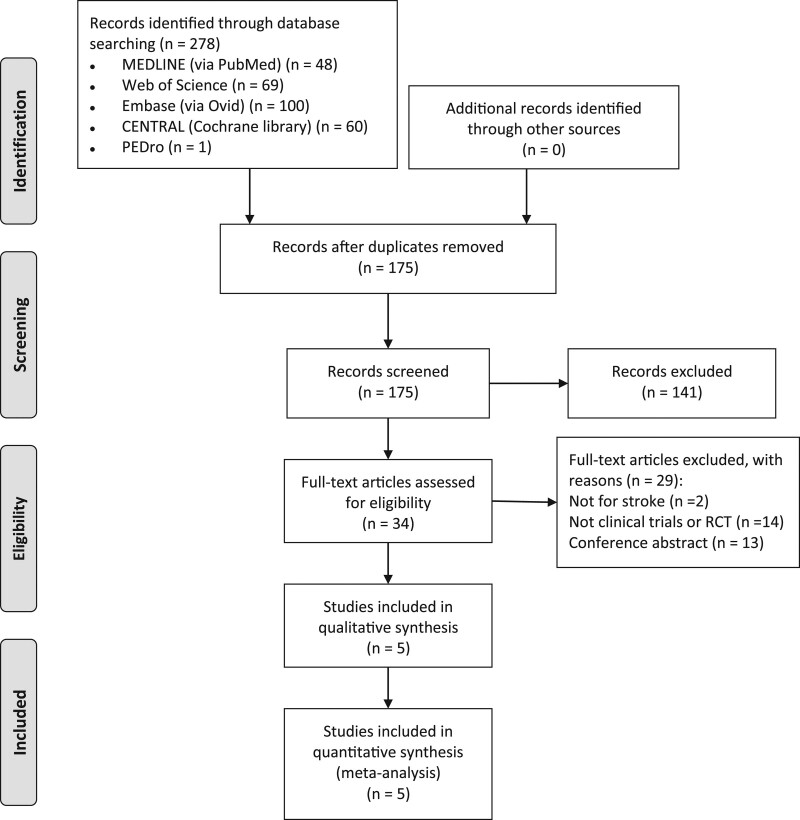
The initial electronic search resulted in a total of 278 studies, of which 175 unique articles were retrieved after duplicates were removed. After screening the titles, abstracts and full text of the articles on the basis of the inclusion and exclusion criteria, five studies [17,21–24] with a total of 178 participants were identified as eligible for the systematic review. The five studies were also used for the meta-analysis. A detailed flowchart of the search process for the studies is included in the systematic review and meta-analysis Fig. 1.
Fig. 1.
PRISMA flow diagram.
Description of studies
The studies included in the analysis were published between 2016 and 2021. The sample size ranged from 12 to 108 participants. The primary characteristics of the selected studies, including study design, baseline characteristics of enrolled participants, details of interventions and outcomes are summarized in Table 2.
Table 2.
Characteristics of included studies
| Study | Design | Participants | Interventions | Outcomes |
|---|---|---|---|---|
| Dawson [21], 2016 | RCT |
N = 20 EG (n = 9) Age: 57.9 ± 17.2 years Onset: 1.8 ± 1.0 years CG (n = 11) Age: 60.7 ± 10.7 years Onset: 1.7 ± 1.3 years |
EG: VNS paired with rehabilitation. (VNS: 0.8 mA, 100 µs, 30 Hz, lasting 0.5 s) CG: Rehabilitation alone (the rehabilitation-only group did not have a device implanted). Both groups: All participants received a 6-week course of 2-h therapy sessions 3× per week. |
FMA-UE ARAT Grip and pinch strength SIS Box and Block test Nine-hole peg test At pre-, and post-Tx (6 weeks) |
| Capone [22], 2017 | RCT |
N = 12 EG (n = 7) Age: 53.71 ± 5.88 years Onset: 93.91 ± 38.81 months CG (n = 5) Age: 55.60 ± 7.12 years Onset: 46.00 ± 21.85 months |
EG: tVNS and robotic-assisted therapy. Electric stimulator was placed in the left external acoustic meatus at the inner side of the tragus. tVNS was delivered as trains lasting 30 s and composed by 600 pulses (pulse frequency = 20 Hz; pulse duration = 0.3 ms) repeated every 5 min for 60 min. CG: Sham tVNS and robotic-assisted therapy. Both groups: Robotic treatment was delivered daily for 10 consecutive working days, immediately after the end of real or sham tVNS. |
FMA-UE At pre-, and post-Tx (10 days) |
| Kimberley [23], 2018 | RCT |
N = 17 EG (n = 8) Age: 59.5 ± 7.4 years Onset: 18 (11-43) months CG (n = 9) Age: 60.0 ± 13.5 years Onset: 18 (6.3–53) months |
EG: VNS paired with rehabilitation. VNS (0.8 mA). CG: Sham VNS paired with rehabilitation. VNS (0 mA) Both groups: Both groups were surgically implanted with the VNS device. All participants received 6-week in-clinic rehabilitation (≈3×a week for 6 weeks) followed by a home exercise program. |
FMA-UE WMFT Box and Block test Nine-hole peg test SIS Motor Activity Log At pre-, and days 1, 7, 30, and 90 days after in-clinical therapy |
| Wu [24], 2020 | RCT |
N = 21 EG (n = 10) Age: 64.50 ± 9.97 years Onset: 36.30 ± 9.23 days CG (n = 11) Age: 61.82 ± 10.63 years Onset: 35.55 ± 6.47 days |
EG: tVNS paired with rehabilitation. Parameters: 600 pulses (pulse frequency = 20 Hz; pulse duration = 0:3 ms), lasting 30 s each time, stimulating once every 5 min. CG: Sham tVNS paired with rehabilitation. Both groups: Rehabilitation training, lasting approximately 30 min, was performed immediately after the end of real or sham tVNS per day for 15 days. |
FMA-UE WMFT FIM BS At pre-, and post-Tx. |
| Dawson [17], 2021 | RCT |
N = 108 EG (n = 53) Age: 59.1 ± 10.2 years Onset: 3.1 ± 2.3 years CG (n = 55) Age: 61.1 ± 9.2 years Onset: 3.3 ± 2.6 years |
EG: VNS paired with rehabilitation (VNS: 0.8 mA, 100µs, 30 Hz stimulation pulses, lasting 0.5 s). CG: Sham VNS paired with rehabilitation. Both groups: Both groups were surgically implanted with the VNS device. Participants received 6 weeks of in-clinic therapy (three times per week; total of 18 sessions) followed by a home exercise program. |
FMA-UE WMFT SIS |
ARAT, arm research arm test; SIS, Stroke Impact Scale; BS, Brunnstrom stage; CG, control group; EG, experimental group; FIM, functional independence measurement; FMA-UE, Fugl-Meyer Assessment for Upper Extremity scale; Tx, treatment; VNS, vagus nerve stimulation; WMFT, Wolf motor function test.
The studies included in the current systematic review and meta-analysis satisfied specific inclusion and exclusion criteria. All participants in the selected studies were diagnosed with different stages of stroke [25]. One study reported subacute or chronic phase of stroke [23], one study reported acute or subacute phase of stroke [24] and three studies reported the chronic phase of stroke [17,21,22].
All experimental groups received VNS paired with rehabilitation. Two studies used tVNS [22,24] and three studies used surgically implanted VNS [17,21,23]. The intervention period ranged from 10 days to 6 weeks. One study compared VNS paired with rehabilitation to rehabilitation only [21], one study compared tVNS combined with robotic-assisted therapy to sham tVNS combined with robotic-assisted therapy [22], two studies compared VNS paired with rehabilitation to sham VNS combined with rehabilitation [17,23] and one study compared tVNS paired with rehabilitation to sham tVNS combined with rehabilitation [24].
Outcomes were measured at baseline and at the end of the intervention. The Fugl-Meyer Assessment for Upper Extremity (FMA-UE) Score was the main outcome in the evaluation of the effect of intervention and it was measured in five studies [17,21–24]. Additionally, three trials employed the Wolf Motor Function test (WMFT) [17,23,24] and Stroke Impact Scale (SIS) [17,21,23]; two trials used the Box and Block test and Nine-Hole Peg test [21,23], and five trials reported adverse events [17,21–24].
Quality
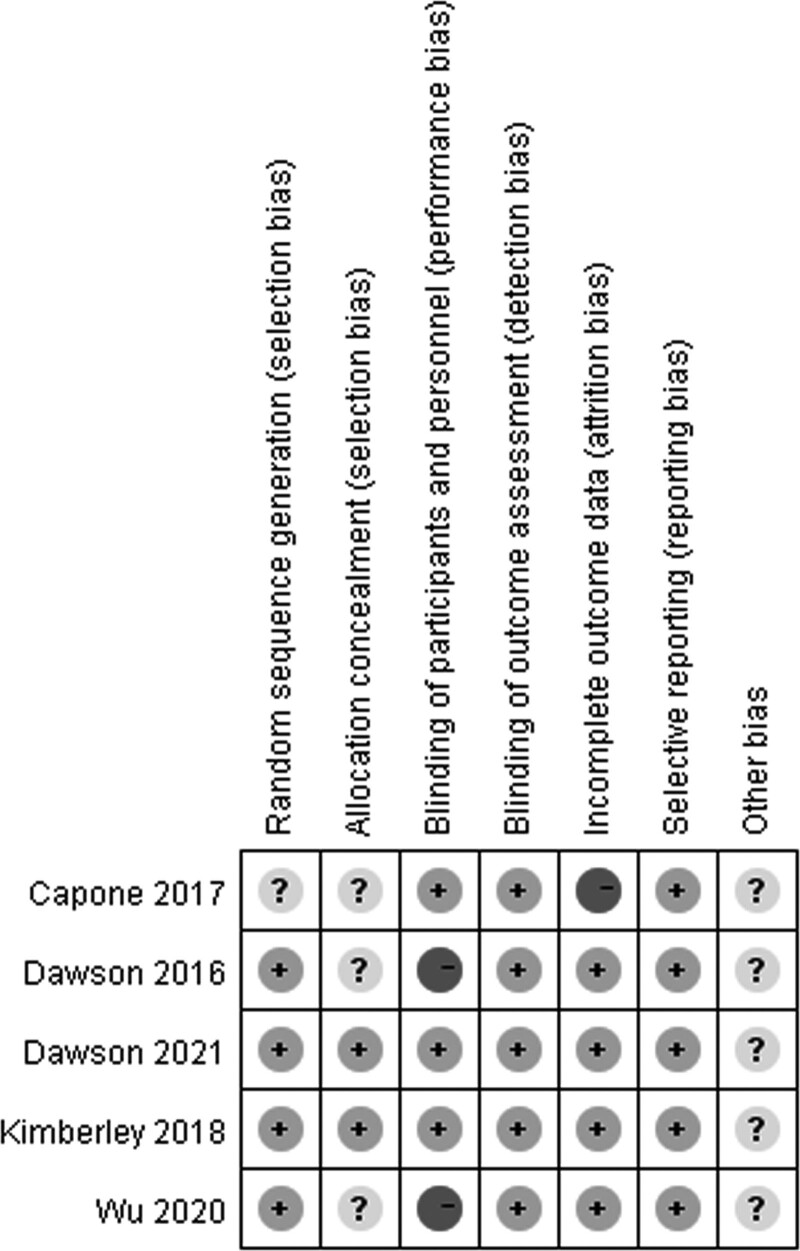
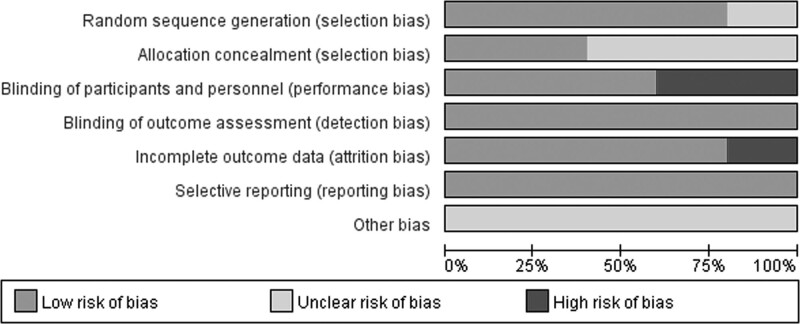
PEDro scores of the included studies ranged from 6 to 10, with a mean score of 8. The methodological quality of two studies was considered to be of ‘excellent’ quality [17, 23], while that of three studies was considered to be of ‘good’ quality [21,22,24]. A detailed evaluation of the PEDro scores is presented in Table 3. All included studies reported adequately with regard to their methods of blinding outcome assessors and random sequence generation, except for one study [22]. Only two studies satisfied the concealed allocation criterion. Subject blinding was satisfied in three of the selected studies [17,22,23]. Risk of bias assessment of the studies included in the present systematic review and meta-analysis is illustrated in Figs. 2, 3.
Table 3.
PEDro assessment quality results of included studies
| Study | Eligibility* | Random allocation | Concealed allocation | Baseline comparability | Blind subjects | Blind therapists | Blind assessors | Adequate follow-up | Intention-to-treat analysis | Between-group comparisons | Point estimates and variability | Total score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dawson [21], 2016 | YES | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | GOOD |
| Capone[22], 2017 | YES | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 6 | GOOD |
| Kimberley [23], 2018 | YES | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Excellent |
| Wu [24], 2020 | YES | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | GOOD |
| Dawson [17], 2021 | YES | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Excellent |
*Eligibility criteria is not included in the scoring of PEDro scale.
Fig. 2.
Risk of bias summary according to the Cochrane risk of bias tool: ‘−’, ‘+’ and ‘?’ indicate high, low and unclear risk of bias, respectively.
Fig. 3.
Risk of bias graph according to the Cochrane risk of bias tool.
Effect of intervention
Fugl–Meyer assessment for upper extremity scores
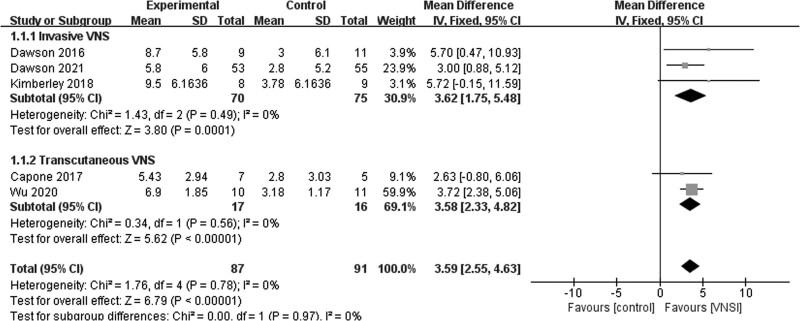
A fixed-effects model was used for the analysis of FMA-UE scores. The variations in FMA-UE scores before and after intervention in five studies [17,21–24] indicated that FMA-UE scores increased significantly as a result of VNS paired with rehabilitation when compared to rehabilitation with or without sham VNS (MD = 3.59; 95% CI, 2.55–4.63; P < 0.01). On the basis of subgroup analyses, three studies [17,21,23] reported that the variations in FMA-UE scores between invasive VNS paired with rehabilitation and the control groups were significantly different (MD = 3.62; 95% CI, 1.75to–5.48; P < 0.01). Furthermore, two studies [22, 24] revealed that the variations in FMA-UE scores between tVNS paired with rehabilitation and control groups were significantly different (MD = 3.58; 95% CI, 2.33–4.82; P < 0.01). No heterogeneity was detected among the studies (I2 = 0%; P = 0.78; Fig. 4).
Fig. 4.
Fugl–Meyer assessment for upper extremity scores.
Wolf motor function test scores
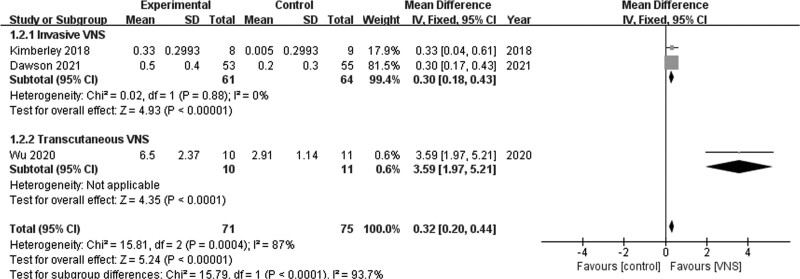
A fixed-effects model was used to analyze WMFT scores. Two studies [17,23] revealed a significant difference in the variations of WMFT scores between invasive VNS and control groups (MD = 0.30; 95% CI, 0.18–0.43; P < 0.01), and no heterogeneity was observed among the studies (I2 = 0%; P = 0.88; Fig. 5). One study [24] revealed a significant difference in the variations of WMFT scores between the tVNS and control groups (MD = 3.59; 95% CI, 1.97–5.21; P < 0.01; Fig. 5).
Fig. 5.
Wolf motor function test scores.
Stroke impact scale (hand function)
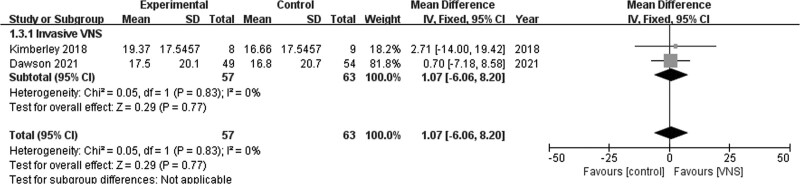
Pooling data from two studies in the fixed-effects model [17,23] revealed no significant difference in SIS (Hand function) scores between the invasive VNS and control groups (MD = 1.07; 95% CI, −6.06 to 8.20; P = 0.77). Pooled studies were homogenous (I2 = 0%; P = 0.83; Fig. 6).
Fig. 6.
Stroke Impact Scale (hand function).
Box and block test
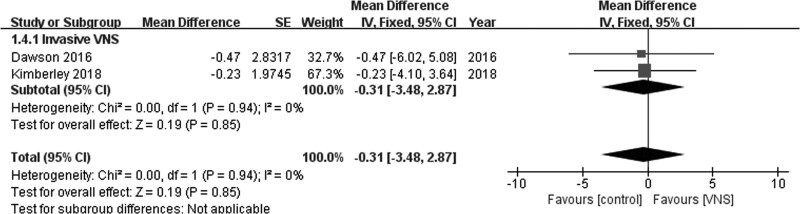
No significant difference was observed between invasive VNS and control groups on the basis of Box and Block test scores (MD = −0.31; 95% CI, −3.48 to 2.87; P = 0.85) in the fixed-effects model when data from two studies were pooled [21,23]. Pooled studies were homogenous (I2 = 0%; P = 0.94; Fig. 7).
Fig. 7.
Box and Block test.
Nine-hole peg test
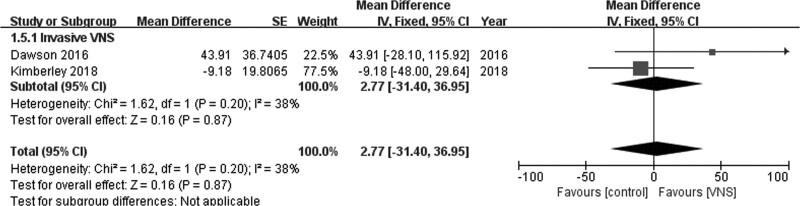
No significant difference was observed in the Nine-Hole Peg test scores between invasive VNS and control groups (MD = 2.77; 95% CI, −31.40 to 36.95; P = 0.87) in the fixed-effects model when data from two studies were pooled [21,23]. Pooled studies were homogenous (I2 = 38%; P = 0.20; Fig. 8).
Fig. 8.
Nine-Hole Peg test.
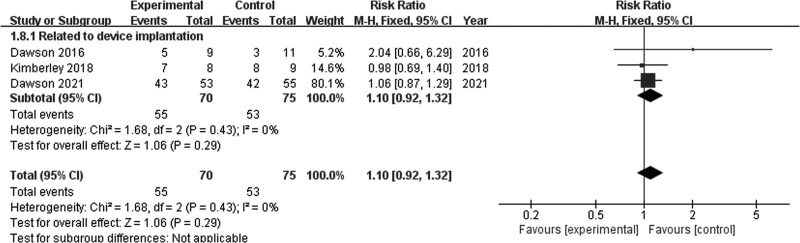
Adverse events
The invasive VNS and control groups did not differ significantly in terms of adverse events associated with device implantation (RR = 1.10; 95% CI, 0.92–1.32; P = 0.29) in the fixed-effects model when data from three studies were pooled [17,21,23]. Pooled studies were homogenous (I2 = 0%; P = 0.43; Fig. 9). Moreover, no adverse events associated with device use were reported in three studies with regard to invasive VNS [17,21,23]. One study [22] regarding tVNS did not report adverse events, while one study [24] reported that one patient in the tVNS group developed skin redness at the point of contact of the auricular skin with electrodes.
Fig. 9.
Adverse events.
Discussion
The present systematic review and meta-analysis reviewed the findings of previous studies to evaluate the safety and determine the effect of VNS paired with rehabilitation on upper limb function recovery in patients with stroke. The outcome measures were evaluated on the basis of the difference in performance between the baseline and immediately after the intervention. The results of the present meta-analysis revealed that the increases in FMA-UE and WMFT scores of patients in the VNS group were significantly greater than those in the control group. However, the increases in SIS (hand function), Box and Block test and Nine-Hole Peg test scores were similar in both groups. The results are consistent with the findings of a previous review [16]. Our findings have presented moderate statistical evidence for improved efficacy of VNS paired with rehabilitation when compared to the efficacy of convenient rehabilitation on the basis of FMA-UE and WMFT scores.
With regard to the FMA-UE, an increase of 3.59 was recorded across all included trials on average. One study revealed that the clinically important difference (CID) for FMA-UE in individuals with minimal to moderate impairment due to chronic stroke ranged from 4.25 to 7.25 points [26]. However, the variations in scores observed in the present systematic review were lower than the CID threshold, which suggest that there was no clinical significance. One study that investigated invasive VNS defined a clinically meaningful response as a 6-point or greater improvement in FMA-UE score and reported that more participants in the VNS group reached a threshold of clinically meaningful response when compared with the control group (23 [47%] of 53 vs. 13 [24%] of 55, P = 0.0098) [17]. Similarly, an increase of 0.3 was observed in invasive VNS on average on the basis of WMFT scores and an increase of 3.59 was observed in tVNS on average. Lin et al. reported that the CID of WMFT in patients with stroke varied from 0.2 to 0.4 points [27]. Both variations reached the CID threshold, which indicated a clinical significance.
The primary safety outcome measure was the number of adverse events associated with device implantation or stimulation. The results of the present meta-analysis revealed no significant difference in adverse events associated with device implantation between the invasive VNS and control groups. Only one study reported that one patient in the tVNS group developed skin redness at the point of stimulation [24]. In addition, no adverse events associated with therapy were reported. tVNS is a relatively safe intervention as a result of surgical-related complications caused by invasive VNS, such as left vocal cord palsy and dysphagia; however, no study has compared the effect of invasive VNS to that of tVNS. Although there is increasing interest in tVNS, concerns regarding the degree of activation of vagal fibers, optimal stimulation site and stimulation parameters, and potential effects of stimulation on other nerves in the region have been raised.
The patients in the selected studies were diagnosed with stroke in the subacute or chronic phase, which suggests that the mechanism of VNS improvement occurs through the upregulation of neuroplasticity. Furthermore, VNS could have a potential benefit in improving acute stroke performance due to its participation in pathophysiological processes associated with anti-glutamate effects, anti-inflammatory activity, attenuating spreading depolarizations and decreasing intracranial pressure [28]. Further studies are required to elucidate the mechanisms and therapeutic effects of VNS.
The stimulation parameters of invasive VNS for three studies that were included in the present systematic review and meta-analysis were the same; that is, burst of 500 ms with a constant current of 0.8 mA, pulse duration of 100 μs, and frequency of 30 Hz, which were derived from hypothesis-driven research in human and animal models [14,15,29,30]. The stimulations of invasive VNS were delivered to the left vagus nerve to avoid activation of the sinoatrial node. The stimulation site for tVNS was the left external acoustic meatus on the inner side of the tragus, and the stimulation intensities for two studies were adjusted independently (above the detection threshold and below the pain threshold) to a pulse duration of 0.3 ms and frequency of 20 Hz repeated every 5 min for 60 min. However, there was no relevant basis for the stimulation parameters of tVNS, and the specific range of parameters that influence cortical plasticity remain unknown. Therefore, further studies regarding tVNS should be conducted.
Consequently, on the basis of the evidence provided by the current systematic review and meta-analysis, invasive VNS and tVNS paired with rehabilitation are effective in improving upper limb performance in patients with stroke. VNS could be used as adjuvant therapy for patients with subacute or chronic stroke in clinics. However, further research regarding the adverse events associated with device implantation in invasive VNS should be conducted.
Study limitations
The limitations of the current systematic review and meta-analysis were as follows. First, studies published in languages other than English were excluded. Second, quality assessment was not used as a selection or exclusion criterion. Third, the lack of concealed allocation and blinding in a few of the studies selected could have influenced the results. Fourth, outcomes of selected studies were measured immediately after treatment without any long-term follow-up. Finally, the number of included studies and patients were relatively small and may not provide sufficient statistical power to support the results.
Conclusion
VNS paired with rehabilitation is a promising strategy to promote upper limb function recovery for patients with stroke. The results of this systematic review and meta-analysis indicate that VNS paired with rehabilitation could improve upper limb function in patients with stroke on the basis of FMA-UE and WMFT scores. More studies with a focus on the long-term effect are needed.
Acknowledgements
This study was supported by grants from the National Key R&D Plan of China (2017YFC1308504) and the Key Project of the Science and Technology Department in Sichuan province (2021YJ0184). These funding sources were not involved in the literature review, systematic review, meta-analysis and writing of the report.
K.Z., J.Y. and Y.Q. helped with conceptualization. K.Z. and J.Y. helped with the design search. K.Z. helped with writing. Z.Z. and J.H. helped with data extraction/quality assessment. K.Z. helped with data analysis. K.Z., J.Y. and Y.Q. helped with the consultation and project management.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Kehong Zhao and Jiaen Yang are co-first authors.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.editorialmanager.com/ijrr.
References
- 1.Collaborators GBDS. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al.; Collaborators GBDLRoS. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018; 379:2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009; 8:741–754. [DOI] [PubMed] [Google Scholar]
- 4.Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurol 2012; 11:209. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 1994; 75:394–398. [DOI] [PubMed] [Google Scholar]
- 6.Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke–consensus from stroke survivors, caregivers, and health professionals. Int J Stroke 2014; 9:313–320. [DOI] [PubMed] [Google Scholar]
- 7.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010; 362:1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, et al.; Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Investigative Team. Effect of a Task-Oriented Rehabilitation Program on Upper Extremity Recovery Following Motor Stroke: the ICARE Randomized Clinical Trial. JAMA 2016; 315:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res 2017; 8:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CH, Yang MH, Zhang GZ, Wang XX, Li B, Li M, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation 2020; 17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous Vagus Nerve Stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimul 2016; 9:356–363. [DOI] [PubMed] [Google Scholar]
- 12.Gaul C, Diener HC, Silver N, Magis D, Reuter U, Andersson A, et al.; PREVA Study Group. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia 2016; 36:534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted vagus nerve stimulation for rehabilitation after stroke. Front Neurosci 2019; 13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 2013; 207:275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 2011; 189:207–214. [DOI] [PubMed] [Google Scholar]
- 16.Wei-Jiang Zhang C, Wang JX, Sun FH, Xie YJ, Ou X, Yang SB. The effect of VNS on the rehabilitation of stroke: a meta-analysis of randomized controlled studies. J Clin Neurosci 2020; 81:421–425. [DOI] [PubMed] [Google Scholar]
- 17.Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet 2021; 397:1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10:1–7. [PubMed] [Google Scholar]
- 21.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016; 47:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capone F, Miccinilli S, Pellegrino G, Zollo L, Simonetti D, Bressi F, et al. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plast 2017; 2017:7876507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke 2018; 49:2789–2792. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G. Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients: a randomized pilot study. Neural Plast 2020; 2020:8841752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke 2017; 12:444–450. [DOI] [PubMed] [Google Scholar]
- 26.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012; 92:791–798. [DOI] [PubMed] [Google Scholar]
- 27.Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Liu JS. Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabil Neural Repair 2009; 23:429–434. [DOI] [PubMed] [Google Scholar]
- 28.Kumaria A, Tolias CM. Is there a role for vagus nerve stimulation therapy as a treatment of traumatic brain injury? Br J Neurosurg 2012; 26:316–320. [DOI] [PubMed] [Google Scholar]
- 29.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011; 470:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, II, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017; 289:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]