ABSTRACT
Objective
People with chronic obstructive pulmonary disease (COPD) may suffer from anxiety, depression, low quality of life, and cognitive deficits that could play a role in their clinical conditions. These situations could be worsened during the adaptation process to a new treatment such as noninvasive ventilation (NIV), which is often rejected or inappropriately used. The study aimed to analyze the impact of a brief psychological support intervention on adherence to NIV among patients with COPD.
Methods
A two-branch randomized controlled trial was conducted on 90 patients with COPD who had an indication for NIV. The experimental group received cognitive behavioral therapy support, including counseling, relaxation, and mindfulness-based exercises. Controls received standard care and watched educational videos. The course had been structured for four to eight meetings at the hospital, at home, and/or via telemedicine.
Results
The psychological intervention was related to improvements in both adherence to NIV (F(304) = 19.054, p < .001) and quality of life (F(156) = 10.264, p = .002) after eight meetings from baseline compared with the control group. Results indicated a significant change in the quality of life also over time (F(71.480) = 8.114, p = .006).
Conclusions
The findings suggest that the psychological intervention is an appropriate treatment for acceptance of and adherence to NIV in COPD in clinical practice and highlight the importance of determining the underlying reasons for NIV use.
Trial Registration:ClinicalTrials.gov identifier NCT02499653.
Key words/Abbreviations: chronic obstructive pulmonary disease, noninvasive ventilation, adherence, acceptance, quality of life, ACE-R = Addenbrooke’s Cognitive Examination—Revised, ADL = activities of daily living, CAM = Confusion Assessment Method, CBT = cognitive behavioral therapy, COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, HADS = Hospital Anxiety and Depression Scale, QoL = quality of life, MMSE = Mini-Mental State Examination, NIV = noninvasive ventilation
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the world (1), and it is predicted to be the third by 2030 (2,3). According to the Global Burden of Disease Study, COPD was prevalent in more than 300 million people in 2013 (4). The prevalence of COPD in Europe ranges from about 15% to 20%, and it is higher in men than in women (5,6). Moreover, the disease burden and its impact are predicted to increase because of population aging (7–9).
COPD is characterized by an irreversible obstruction of the airways, which is associated with a progressive inflammation of the lung tissue together with an increased respiratory limitation, punctuated by recurrent episodes of exacerbations (10). Although diagnosed, thanks to spirometry, COPD is recognized as being more than a pulmonary disease. Comorbidities and extrapulmonary aspects such as cardiovascular disease, anemia, decreased muscle mass, diabetes, metabolic syndrome, dysfunctional skeletal myopathies, and osteoporosis are common in COPD people (11,12). Furthermore, psychological problems such as anxiety, depression (13), and cognitive impairment, overall or in specific domains like memory, executive functions, and cognitive flexibility (14), can contribute to complicating the clinical frame. Comorbidities and exacerbations can impair the quality of life (QoL) during the early stage of the illness, increasing mortality in the end stage, the burden of COPD management, and the healthcare costs (15). Anxiety and depression are common psychological comorbidities, which aggravate the clinical situation (16). Psychological aspects, including emotions and expectations (17), could interact with the physical symptoms. A clear example of the role of psychological factors in clinical management is provided by acceptance and adherence to noninvasive ventilation (NIV). NIV refers to the delivery of positive pressure ventilation through a noninvasive interface (e.g., nasal mask, face mask, or nasal plugs), rather than an invasive interface (endotracheal tube, tracheostomy), or postextubating respiratory failure. It can be used as ventilatory support for patients with acute or chronic respiratory failure. NIV works by creating a positive airway pressure—the pressure outside the lungs being greater than the pressure inside of the lungs. This causes air to be forced into the lungs (down the pressure gradient), lessening the respiratory effort and reducing the work of breathing. The number of days of NIV and hours of daily use differs, depending on the severity and course of the respiratory failure and the timing of application. For patients with chronic stable hypercapnic COPD, the use of nocturnal NIV in addition to usual care is recommended (18,19). The major sources of complications in the NIV’s usage are represented by pressure ulcers/necrosis (nasal bridge); facial or ocular abrasions; claustrophobia; anxiety; agitation; air swallowing with gastric/abdominal distension, potentially leading to vomiting and aspiration; hypotension if hypovolemic; aspiration; oronasal mucosal dryness; raised intracranial pressure; increased intraocular pressure; impaired communication; impaired nutrition; and noise and transport, which may also potentially increase patient discomfort (18–20). When properly used, NIV provides significant benefits in clinical outcomes, improving QoL and life expectancy and reducing hospital admissions and length of stay (21,22). Although NIV can produce a significant clinical improvement in COPD people, they often reject it or use it incorrectly, resulting in worse clinical outcomes and healthcare costs. Indeed, patients approaching its use, especially in the long term, face some complications, such as facial injuries due to the pressure exerted by the mask. Many, especially when using NIV for the first time, find it a stressful experience, eliciting emotions such as fear, anxiety, and panic attacks along with feelings of suffocation and claustrophobia, which can undermine acceptance and adherence to treatment if not result in outright rejection (23,24). Moreover, dyspnea during NIV is a complex problem: NIV aims to correct gaseous exchanges, but if it fails to relieve dyspnea—or worse, if dyspnea worsens during NIV—the combination of a life-threatening threat with a feeling of suffocation and thus lack of control leads to an aggravation of both dyspnea and anxiety, creating a traumatic vicious cycle (25,26). Previous studies suggested that the misuse or rejection of medication or NIV can be influenced by some psychological factors such as anxiety, depression, health beliefs, and cognitive impairment (27–29); the unacknowledged recognition of the illness; and the use of different devices and medications due to comorbidities (30–32). On the other hand, it is also important to note that NIV requires some lifestyle and behavioral changes during their routine care, which may affect adherence to the treatment (30). Nevertheless, only a few studies have paid attention to adherence to NIV in COPD people, and most of them have focused only on early NIV failure (33,34) or on the quantification of adherence (35). Moreover, most of the previous studies suggested that the difficult adjustment of the ventilator settings and the influence of NIV on sleep quality with spillover effects on daytime life can have an impact on its usage (36,37). Finally, COPD people who proved a lower survival benefit from the NIV usage exhibit worse average daily NIV adherence, because of the imbalance between the restrains that the device imposes and the improvement that they perceive in their clinical condition (37).
Psychosocial interventions have been suggested to be optimal both as alternative and complementary strategies to reduce physical impairments and psychological distress as well as to promote behavior change (38–40). This approach is supported by recent meta-analyses of controlled studies that emphasize that psychosocial interventions, especially those incorporating cognitive elements, reduce psychological distress (41) and, when based on meditative and relaxation practices, have the potential to improve physical and psychological outcomes (42).
Cognitive behavioral therapy (CBT) is a nonpharmacological, multicomponent therapy that aims to reduce and modify the psychological, behavioral, and physiological processes. It emphasizes helping individuals learn to be their therapists. Through exercises in the session and “homework” exercises outside of sessions, patients are helped to develop coping skills, whereby they can learn to change their thinking, problematic emotions, and behavior. In this way, the psychologist and patient work together, collaboratively, to develop an understanding of the problem and to develop a treatment strategy. Because of its scalability and increased recommendation in the management of COPD, CBT is particularly well positioned for promoting acceptance and adherence to NIV by targeting psychological factors such as anxiety, depression, representation of the disease, representation of the proposed therapies, therapeutic compliance, self-esteem, and cognitive functions as risks factors that could make the adaptation process difficult (43).
Objectives
The study aims to evaluate the impact of a short-term CBT intervention on NIV acceptance and adherence. Changes in QoL were considered as a secondary outcome. Moreover, another secondary outcome consisted of the identification of both psychological (anxiety, depression, representation of the disease, representation of the proposed therapies, therapeutic compliance, self-esteem, cognitive functions) and clinical factors (forced expiratory volume in the first second [FEV1], forced vital capacity [FVC], FEV1/FVC, the potential of hydrogen, partial arterial oxygen pressure, partial carbon dioxide pressure, hydrogen carbonate, fatigue, activities of daily living and instrumental activities of daily living, survival rates) that predicted NIV rejection or misuse.
METHODS
Ethical Considerations
The Ethics Committee of the IRCCS Fondazione Don Carlo Gnocchi (reference: February 15, 2015) and the Ethics Committee of Università Cattolica del Sacro Cuore (reference: January 21, 2016) in Milan (Italy) approved this study.
All participants provided written, informed consent before taking part in the study, and a psychologist explained the information contained in the Information Sheets and the Consent Form.
Study Design
The study was a randomized controlled trial, composed of two arms (experimental and control groups, both involving 4–8 weekly sessions) and four assessments (2 × 4 design) at baseline and at 3, 6, and 12 months after recruitment. The study was registered in the ClinicalTrials.gov repository (ID NCT02499653), and the details of the protocol were previously published (44).
Randomization
Sequence Generation
Participants were randomly allocated to the study groups (sequence generated by the Web site www.random.org) to ensure the internal validity of the results.
Allocation Concealment and Implementation
Allocation concealment was ensured as the automated randomization system did not release the code until the patient had been recruited into the trial, which took place after the completion of the baseline.
Blinding (Masking)
Individuals willing to participate in the trial did not know their assigned group in advance, nor did they know that the experimental and control groups received different treatments.
A debriefing was provided by the psychologist who conducted the intervention, addressing all possible questions. Moreover, a report of the study’s findings was sent to the participants.
The psychologist who led the intervention was blind to the assessment and vice versa. The research assistants involved in the assessments were instructed not to ask patients about their intervention content.
Participants and Setting
This study was conducted in a clinical research center (Respiratory Rehabilitation Unit, IRCCS Santa Maria Nascente, Fondazione Don Carlo Gnocchi, in Milan, Italy).
Inclusion and Exclusion Criteria
Eligible participants were aged at least 18 years, and they were inpatient or outpatient with moderate (Global Initiative for Obstructive Lung Disease 2%–50% ≤ FEV1 < 80% predicted) to severe (Global Initiative for Obstructive Lung Disease 3%–30% ≤ FEV1 < 50% predicted) COPD and were being newly introduced to NIV use at the time of the study.
Participants were excluded if they had an oncological history, were pregnant, or had an immunosuppressive disease as the main condition or a history of psychiatric disorders, except for anxiety and depression, as indicated in the clinical records.
Sample Size
According to preventive inherent power and sample size, the study required between 128 (statistical power of 0.80; α = .05; 1 − β =0.80; t(126) = 1.97) and 172 participants (statistical power equal to 0.90, α = 0.05; 1 − β =0.90; t(179) = 1.97) to detect a two-tailed difference. These statistical analyses were conducted taking into account data from scientific research with comparable primary outcome (45). We aimed to recruit up to 150 patients with COPD to account for a 15% dropout rate.
Time and Duration of the Study
This study was conducted between July 30, 2015, and December 31, 2018. After the pulmonary clinical visit, participants who met the inclusion criteria were invited to join the study. If they agreed, they provided written consent and were given a copy of the consent form along with an information sheet. Later, they underwent a psychological assessment (baseline [T0]). Follow-ups were planned at 3 (time 1 [T1]), 6 (time 2 [T2]), and 12 months (time 3 [T3]) from the recruitment date. Each medical assessment lasted 30 minutes, whereas the psychological one took about 45–50 minutes.
Instruments
The evaluation process was characterized by the investigation of the following five areas.
The medical and clinical assessment was performed during baseline, 3, 6, and 12 months from the recruitment (T0, T1, T2, and T3, respectively) and planned to collect data inherent to the primary outcome, acceptance, and adherence to NIV use, detected in terms of prescribed and effective hours and their difference as recorded by the ventilator. This part of the data collection also included examinations in terms of respiratory functions: spirometry (FEV1, FVC, FEV1/FVC); hemogasanalysis (arterial blood gases: potential of hydrogen, partial arterial oxygen pressure, partial carbon dioxide pressure2, hydrogen carbonate). Moreover, the Fatigue Severity Scale was used to assess fatigue (46); activities of daily living and instrumental activities of daily living were used to assess basic self-care tasks (47).
QoL was assessed using the EuroQoL. Three layers, referring to the Italian valuation set, were used to derive utility from the scores (48–53).
Sociodemographic information (age, sex, level of education, weight, height, body mass index, current profession (or previous, if retired), cohabitation, smoking habits, alcohol consumption, current and/or previous level of physical activity, drug therapies, use of psychotropic drugs, years of respiratory illness, the number of comorbidities and hospital admissions during the last year) were gathered at the first assessment (T0).
Psychological assessment was conducted during all the times of the study (T0, T1, T2, and T3, respectively) and consisted of measuring anxiety and depression, using the Hospital Anxiety and Depression Scale (HADS) (54), a 14-item scale; representation of the disease, assessed using the Brief Illness Perception Questionnaire (55), which is an application of the self-regulation theory; the therapeutic compliance, measured using the Questionnaire on Adhesion to Pharmacological and Dietetic Therapy (56); and self-esteem, measured using The Rosenberg Self-esteem Scale (57).
Cognitive functions were assessed using the Addenbrooke’s Cognitive Examination—Revised (ACE-R) (58), the Mini-Mental State Examination (MMSE) scale (59), and the Confusion Assessment Method (CAM), which was chosen to detect delirium (60).
Interventions
Cognitive and Behavioral Therapy
Participants assigned to the experimental group received a CBT intervention during the NIV adaptation process along with standard care. CBT protocol provides a flexible, patient-centered approach to increase patient engagement and adherence while addressing both the mental and physical health needs of patients with COPD who are faced with a new device—the NIV. Patients were guided through the initial receipt of modules focused on increasing awareness and control of physical and emotional symptoms and, subsequently, were supported on skill improvement based on the most pressing needs concerning adaptation. In particular, CBT components included counseling and psychological support, mindfulness-based cognitive restructuring exercises, and relaxation training (61). The intervention also included neuropsychological rehabilitation exercises, if required; this was decided on a case-by-case basis, thanks to the clinical notes and the ACE-R (58) and CAM’s administrations (60). The intervention was delivered by a licensed psychologist, trained in COPD management. The weekly sessions comprised three phases. First of all, each patient’s psychological needs were assessed at the beginning of the psychological intervention, paying special attention to aspects related to the disease (e.g., perception, understanding, awareness of symptoms) (62). Second, a lesson/brief presentation of the intervention components was conducted. The possible intervention components were as follows: information on the disease and its management to facilitate the understanding of how to cope with COPD; providing a supportive environment to address anxieties, doubts, and fears arising from the NIV idea; cognitive reframing exercises; lowering emotional arousal; and cognitive and behavioral coping strategies. Third, specific exercises related to each component were practiced (Figure 1).
FIGURE 1.
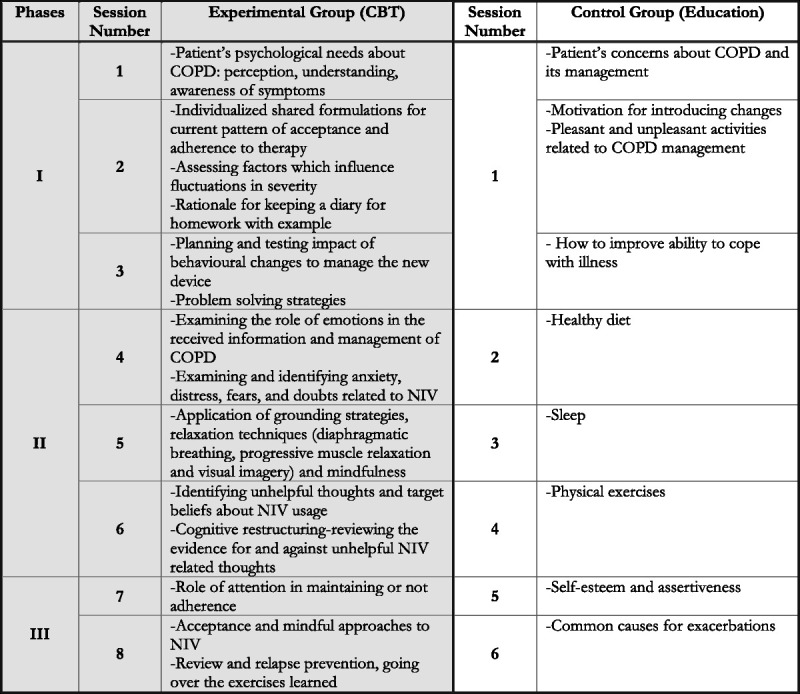
Characteristics of the intervention sessions. CBT = cognitive behavioral therapy; COPD = chronic obstructive pulmonary disease; NIV = noninvasive ventilation.
The duration of the intervention varied from a minimum of four weekly sessions to a maximum of eight according to the needs of the patient. Each session lasted about 45 minutes, and participants received some homework to facilitate daily self-practice. Analogue sessions were conducted in a hospital setting or at the patients’ house or via telemedicine if patients were unable to attend the hospital.
Educational Intervention
The control group spent six sessions watching videos related to COPD management (i.e., improve the ability to cope with illness, benefit from exercise training programs, information about the common causes of exacerbations, smoking cessation, dietary habits; Figure 1). Moreover, participants attended physicians as per usual practice. Each session lasted about 30 minutes, and a psychologist was present for support.
Statistical Analysis
Frequency and descriptive analyses were performed for all the variables considered in the study for the overall sample and both the experimental and control groups. All continuous variables were tested for the normalcy of the distributions at each time. To assess the effects of the intervention immediately and over time, accounting for missing data and a loss of follow-up of at least 15% for 3 months, a 1:1 allocation ratio, primary outcome analysis implemented mixed-effects modeling, including mixed-model analysis of variance for linear data (63). In 2016, slower recruitment than predicted led us to reduce the analyzable sample size to 90, still sufficient to detect an effect size of 45 per group with 99.6% power at 5% (noncentrality parameter λ = 22.5; critical F = 3.94). Pairwise contrasts from the mixed-effects models were used to evaluate between-group differences. Pattern mixture models assessed whether estimates in the mixed-effects models were dependent on missing data patterns. In particular, simple mixed-effects models (also known as multilevel models, random-effects model, and hierarchical models) with a heterogeneous compound symmetry correlation structure among repeated measures were used to compare baseline and 3, 6, and 12 monthly scores on the effectiveness and differences between prescribed and effective hours of NIV, as well as on QoL both with or without considering covariates. This model was first done unconditionally, then with disease and psychological characteristics as covariates. Bonferroni correction was applied. Before model estimation, distributions of all outcome variables and random-effects residuals were inspected and deemed to be close approximations of normality. Using the absolute median deviation method to detect outliers, we found that no data points were deemed to be extreme (64). The analysis was performed using the intention-to-treat procedure. The significance level was 5% (p ≤ .05) and a two-tailed difference. Data analysis used the Statistical Package for Social Science (SPSS) software (version 23).
RESULTS
Participants Characteristics at Baseline and Study Period
A total of 108 patients with COPD met the eligibility criteria (Figure 1). Eight of these patients declined to take part in the study, whereas 10 of them refused to try NIV after it had been imposed on them in the acute phase and they did not even want to start the adaptation.
Ninety participants (mean [standard deviation] age = 76.20 [8.03] years; 51.11% male) were randomly allocated to the psychological support (n = 45, experimental group), or they were called to watch videos related to their illness management (n = 45, control group).
Baseline characteristics were similar by group and are summarized by descriptive comparisons following CONSORT guidelines (Tables 1, 2), notably estimates with 95% confidence interval (CI) representing two-tailed tests at a 5% significant level (65).
TABLE 1.
Participant Characteristics of the Overall Sample and of Both the Experimental and Control Groups at the Baseline
| Sociodemographic Characteristics | ||||
|---|---|---|---|---|
| Variable | Overall Sample (n = 90) | Experimental Group (n = 45) | Control Group (n = 45) | p |
| Sex, M/F (% male) | 46/44 (51.11) | 20/25 (44.4) | 26/19 (57.8) | .21 |
| Age, mean (SD), y | 76.20 (8.03) | 76.71 (7.62) | 75.69 (8.47) | .55 |
| Marital status, n (%) | .73 | |||
| Single | 7 (7.77) | 3 (6.7) | 2 (4.4) | |
| Married | 57 (63.33) | 30 (66.7) | 27 (60) | |
| Widower | 18 (20) | 7 (15.6) | 11 (24.4) | |
| Separated | 3 (3.33) | 3 (6.7) | 0 | |
| Divorced | 1 (1.11) | 0 | 1 (2.2) | |
| Without any family or social support | 2 (2.22) | 1 (2.2) | 1 (2.2) | |
| Cohabitants, n (%) | .28 | |||
| Spouse | 47 (52.22) | 25 (27.77) | 22 (24.44) | |
| Mather | 1 (1.1) | 1 (2.2) | 0 | |
| Father | 0 | 0 | 0 | |
| Sons | 9 (10) | 4 (8.9) | 5 (11.1) | |
| Other relatives | 2 (2.22) | 2 (4.4) | 0 | |
| Carer | 1 (1.1) | 1 (2.2) | 0 | |
| Friends | 0 | 0 | 0 | |
| Senior center | 1 (1.1) | 0 | 1 (2.2) | |
| Alone | 18 (20) | 7 (15.55) | 11 (12.22) | |
| Spouse and carer | 1 (1.1) | 1 (2.2) | 0 | |
| Spouse and sons | 8 (8.88) | 4 (8.9) | 4 (8.9) | |
| Educational level, n (%) | .81 | |||
| None | 6 (6.66) | 4 (8.9) | 2 (4.4) | |
| Primary school | 29 (32.22) | 14 (31.1) | 15 (33.3) | |
| Secondary school | 20 (22.22) | 8 (17.8) | 12 (26.7) | |
| High school | 23 (25.55) | 12 (26.7) | 11 (24.4) | |
| Bachelor’s degree | 1 (1.11) | 1 (2.2) | 0 | |
| Master’s degree | 9 (10) | 5 (11.1) | 4 (8.9) | |
| Working area, n (%) | .81 | |||
| Craft industry | 4 (4.44) | 2 (4.4) | 2 (4.4) | |
| Business sector | 7 (7.77) | 3 (6.67) | 4 (8.9) | |
| Housewives | 9 (10) | 6 (13.3) | 3 (6.7) | |
| Industrial chemistry | 4 (5.55) | 3 (6.7) | 2 (4.4) | |
| Desk jobs | 28 (31.11) | 16 (35.6) | 12 (26.7) | |
| Workers | 17 (18.88) | 6 (13.3) | 11 (24.4) | |
| Food service industry | 2 (2.22) | 1 (2.2) | 1 (2.2) | |
| Health care | 4 (4.44) | 2 (4.4) | 2 (4.4) | |
| Construction industry | 3 (3.33) | 1 (2.2) | 2 (4.4) | |
| Transport | 4 (4.44) | 1 (2.2) | 3 (6.7) | |
| More areas | 4 (4.44) | 3 (6.7) | 1 (2.2) | |
| Mean length of illness, n (%) | .61 | |||
| About 5 y | 31 (34.44) | 15 (33.3) | 16 (35.6) | |
| 6–14 y | 36 (40) | 20 (44.4) | 16 (35.6) | |
| >15 y | 19 (21.11) | 9 (20) | 10 (22.2) | |
| Exacerbations during the last year, n (%) | .15 | |||
| None | 49 (54.4) | 21 (46.7) | 28 (62.2) | |
| 1–3 | 29 (32.22) | 18 (40) | 11 (24.4) | |
| >3 | 9 (10) | 5 (11.1) | 4 (8.9) | |
| Hospitalizations last year, n (%) | .597 | |||
| <1 | 44 (48.88) | 21 (46.7) | 23 (51.1) | |
| 2 | 29 (32.22) | 13 (28.9) | 16 (35.6) | |
| >2 | 14 (15.55) | 9 (20) | 5 (11.1) | |
| Assistance during the last year, n (%) | .49 | |||
| Yes | 15 (16.66) | 9 (20) | 6 (13.3) | |
| None | 70 (77.77) | 35 (77.8) | 35 (77.8) | |
| Type of assistance (if received), n (%) | .50 | |||
| Senior center | 1 (1.1) | 0 | 1 (2.2) | |
| Day care | 1 (1.1) | 1 (2.2) | 0 | |
| Nurse | 7 (7.77) | 4 (9.5) | 3 (7.1) | |
| Physiotherapy | 4 (4.44) | 2 (4.4) | 2 (4.4) | |
| Other (i.e., clean) | 2 (2.22) | 2 (4.4) | 1 (2.2) | |
| Smoking habits, n (%) | .35 | |||
| Yes, active | 13 (14.44) | 4 (8.9) | 9 (20) | |
| No, never | 10 (10) | 7 (15.6) | 3 (6.7) | |
| Ex | 60 (66.66) | 30 (71.1) | 30 (66.7) | |
| Pack/year, n (%) | .123 | |||
| <10 | 4 (4.44) | 4 (8.9) | 0 | |
| 10–20 | 24 (26.66) | 15 (33.3) | 9 (20) | |
| >20 | 47 (52.22) | 17 (37.8) | 30 (66.7) | |
| Alcohol habits, n (%) | .06 | |||
| Never | 37 (41.11) | 22 (48.9) | 15 (33.3) | |
| Rarely | 20 (23.33) | 10 (22.2) | 10 (22.2) | |
| Sometimes | 20 (23.33) | 9 (20) | 11 (24.4) | |
| Quite often | 0 | 0 | 0 | |
| Almost always | 1 (1.1) | 2 (4.4) | 0 | |
| Always | 4 (4.44) | 0 | 4 (8.9) | |
| Physical activity, n (%) | .07 | |||
| Never | 31 (34.44) | 19 (42.2) | 14 (31) | |
| Rarely | 15 (16.66) | 8 (17.8) | 7 (15.6) | |
| Sometimes | 13 (14.44) | 8 (17.8) | 5 (11.11) | |
| Quite often | 16 (17.77) | 8 (17.8) | 8 (17.8) | |
| Almost always | 4 (4.44) | 0 | 4 (8.9) | |
| Always | 2 (2.22) | 0 | 2 (4.4) | |
| Medications, n (%) | ||||
| LABA | 60 (66.66) | 27 (60) | 33 (73.30) | .13 |
| LAMA | 49 (54.44) | 26 (57.8) | 23 (51.1) | .86 |
| ICS | 62 (72.22) | 29 (64.4) | 36 (80) | .06 |
| Anxiolytics | 25 (27.77) | 15 (33.3) | 10 (22.2) | .22 |
| Antidepressants | 20 (23.33) | 14 (31.1) | 7 (15.6) | .10 |
| No. medication, mean (SD) | 7.94 (4.78) | 8.60 (3.87) | 7.29 (5.50) | .19 |
| No. comorbidities, mean (SD) | 2.78 (1.42) | 2.88 (1.31) | 2.69 (1.53) | .63 |
| BMI, mean (SD), kg/m2 | 29.45 (7.91) | 30.06 (8.96) | 28.85 (6.76) | .49 |
n = number of subjects; M = male; F = female; SD = standard deviation; LABA = long-acting β2-agonists; LAMA = long-acting muscarinic antagonist; ICS = inhaled corticosteroids; BMI = body mass index.
TABLE 2.
COPD-Related Medical and Clinical Characteristics and Psychological Measures of the Overall Sample and of Both the Experimental and Control Groups at the Baseline
| Variable | Overall Sample (n = 90) | Experimental Group (n = 45) | Control Group (n = 45) | p Values (ANOVA) |
|---|---|---|---|---|
| Medical and clinical characteristics | ||||
| NIV hours, mean (SD) | ||||
| Prescribed hours | 8.57 (2.21) | 8.90 (1.76) | 8.20 (2.59) | .22 |
| Effective hours | 6.21 (3.30) | 6.86 (3.15) | 5.56 (3.35) | .59 |
| Difference | 1.89 (2.73) | 1.67 (2.56) | 2.11 (2.90) | .39 |
| ADL, mean (SD) | 0.99 (1.74) | 1.18 (1.88) | 0.77 (1.56) | .33 |
| IADL, mean (SD) | 3.16 (1.48) | 3.20 (1.37) | 3.10 (1.61) | .76 |
| FSS, mean (SD) | 45.27 (14.18) | 48.82 (12.55) | 41.37 (14.98) | .45 |
| Spirometry, mean (SD) | ||||
| FEV1 | 1.23 (0.71) | 1.15 (0.69) | 1.20 (0.62) | .75 |
| FEV1% | 50.71 (27.01) | 49.74 (29.48) | 51.74 (25.51) | .32 |
| FVC | 1.88 (0.86) | 1.73 (0.84) | 2.04 (0.86) | .11 |
| FVC% | 63.66 (24.77) | 60.78 (25.39) | 66.62 (24.08) | .30 |
| FEV1/FVC | 0.65 (0.36) | 0.62 (0.17) | 0.69 (0.49) | .51 |
| FEV1/FVC% | 63.95 (18.36) | 62.56 (17.96) | 65.24 (18.29) | .76 |
| EGA, mean (SD) | ||||
| pH | 7.42 (0.04) | 7.41 (0.04) | 7.42 (0.04) | .46 |
| Pao2 | 69.17 (10.78) | 69.66 (9) | 68.65 (12.48) | .67 |
| Paco2 | 47.86 (10.16) | 50.04 (10.86) | 45.57 (8.95) | .14 |
| HCO3 | 31.36 (7.67) | 32.88 (8.63) | 29.77 (6.24) | .07 |
| Psychological and cognitive features | ||||
| HADS, mean (SD) | ||||
| Tot. | 11.92 (7.21) | 13.20 (7.36) | 10.55 (6.86) | .09 |
| HADS-A | 7 (4.81) | 8.07 (4.72) | 5.86 (4.70) | .17 |
| HADS-D | 4.97 (3.48) | 5.13 (3.64) | 4.79 (3.34) | .64 |
| EQ-5D, mean (SD) | ||||
| Algorithm | 0.23 (0.52) | 0.13 (0.57) | 0.34 (0.46) | .06 |
| VAS | 57.29 (21.91) | 53.04 (20.36) | 61.73 (22.82) | .07 |
| QAF, mean (SD) | ||||
| Tot. | 53.61 (7.27) | 49.98 (13.80) | 49.38 (16.51) | .85 |
| B-IPQ, mean (SD) | 37.07 (12.63) | 36.68 (9.76) | 37.46 (13.63) | .43 |
| ROSES, mean (SD) | 30.41 (7.07) | 29.48 (7.13) | 31.37 (6.96) | .23 |
| Cognitive variables | ||||
| MMSE, mean (SD) | 24.49 (7.04) | 24.76 (6.48) | 25.95 (4.11) | .72 |
| MMSE Adj., mean (SD) | 24.72 (3.44) | 24.22 (7.62) | 24.58 (3.95) | .52 |
| ACE-R, mean (SD) | ||||
| Tot. | 72.85 (18.64) | 74.63 (15.34) | 71.07 (21.48) | .38 |
| Tot. Adj. | 76.62 (27.81) | 82.03 (21.87) | 71.21 (32.03) | .06 |
| DI, mean (SD) | 2.91 (3.44) | 3.40 (3.52) | 2.38 (3.31) | .18 |
| CAM, n (%) | ||||
| 0.91 | ||||
| Yes | 28 (31.11) | 18 (40) | 10 (22.2) | |
| No | 53 (58.88) | 24 (57.1) | 29 (64.4) | |
COPD = chronic obstructive pulmonary disease; n = number of subjects; ANOVA = analysis of variance; SD = standard deviation; ADL = activities of daily living; IADL = instrumental activities of daily living; FSS = Fatigue Severity Scale; FEV1 = forced expiratory volume in the first second; FVC = forced vital capacity; FEV1/FVC = ratio between forced expiratory volume in the first second and forced vital capacity or Tiffeneau Index; EGA = hemogasanalysis; pH = potential of hydrogen; Pao2 = partial pressure of oxygen in arterial blood; PaCO2 = partial pressure of carbon dioxide in arterial blood; HCO3 = hydrogen carbonate concentration; HADS = Hospital Anxiety and Depression Scale; Tot. = total score; HADS-A = Hospital Anxiety and Depression Scale—Anxiety; HADS-D = Hospital Anxiety and Depression Scale—Depression; EQ-5D = EuroQoL-5D Questionnaire; VAS = visual analog scale; QAF = Questionnaire on Adhesion to Pharmacological and Dietetic Therapy; B-IPQ = Brief Illness Perception Questionnaire; ROSES = Rosenberg’s Self-Esteem Scale; MMSE = Mini-Mental Status Examination; MMSE Adj. = Mini Mental Status Examination corrected; ACE-R = Addenbrooke’s Cognitive Examination Test—Revised; Tot. Adj. = total score adjusted; DI = Delirium Index; CAM = Confusion Assessment Method.
Participants had similar baseline characteristics to those who declined to try NIV or to take part in the study, and the most common reason for refusal to participate in the trial was time constraints.
Response Rate and Missing Data Patterns
The response rate at each assessment is reported in the flowchart (Figure 2). At 3 months (T1), 62 patients took part in the assessment, with a dropout rate of 31.11% (23 patients from the control group and 5 from the experimental group). The response rate at 6 months (T2) was 44.44% (15 experimental group and 25 control group) and at 12 months (T3) was 32.22% (18 experimental group and 11 control group). Over the three-time point assessments, these dropout rates were mainly due to worsening clinical conditions (40%), missed clinical appointments due to physical ill-health (42.22%), or change in circumstances (1.11%). In six cases (6.66%), we had no information about the patient, and they were not reachable on the phone. Moreover, 17.77% of the COPD patients involved in the study died, whereas others were not yet due for follow-up (Figure 2).
FIGURE 2.
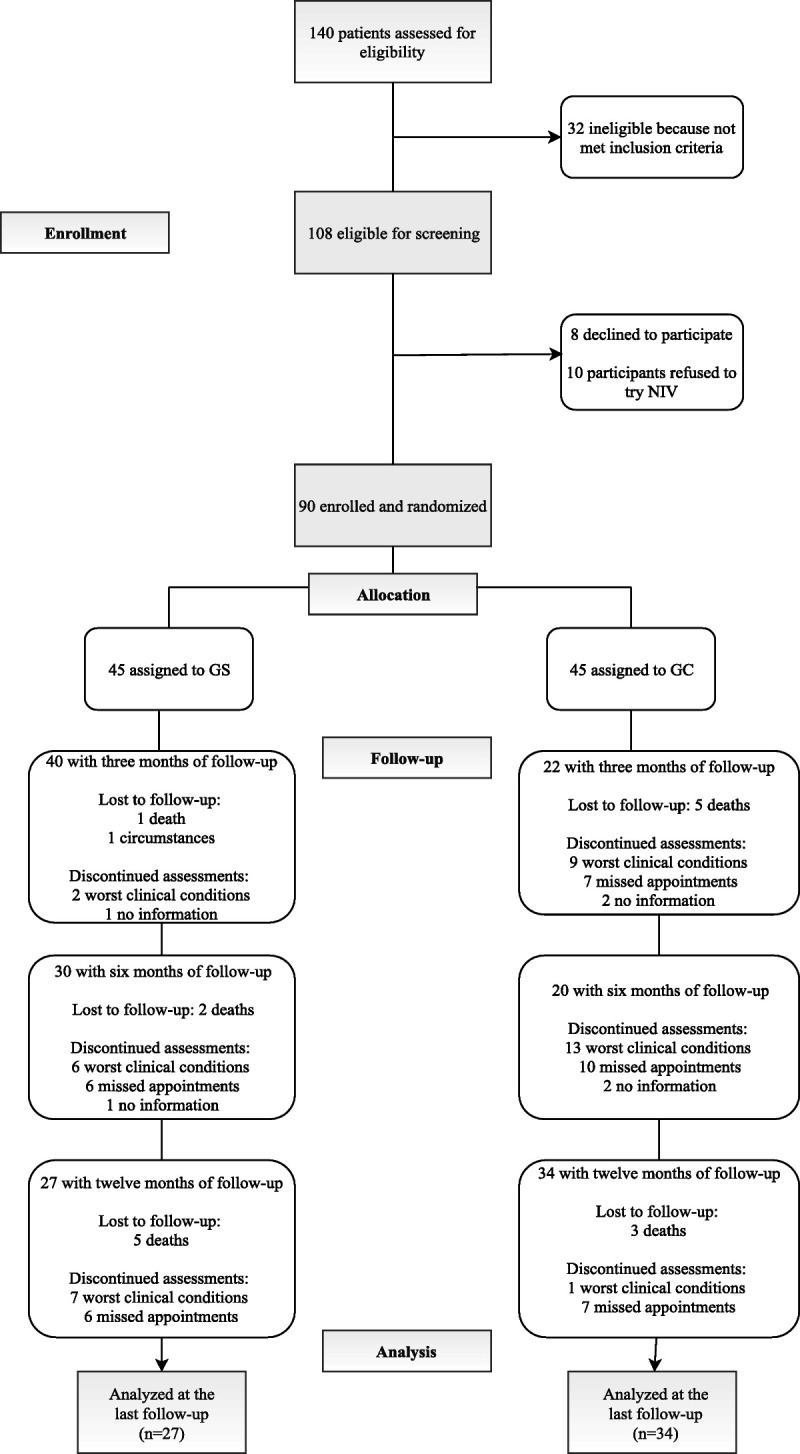
CONSORT flowchart. CONSORT = Consolidated Standards of Reporting Trials; NIV = noninvasive ventilation.
Outcome Analysis
Table 3 shows the means and standard deviations of both patients’ clinical parameters and questionnaire scores at each measurement occasion. Independent mixed-effects models were estimated for each outcome measure. Each model included group (control, intervention) and measurement interval (preassessment, postassessment, follow-up) as fixed effects (i.e., in the form of an interaction predictor [group by interval]) and participant (within measurement Interval) as a random effect. This allowed a unique regression model (i.e., intercept and slope) to be specified for every participant across measurement intervals.
TABLE 3.
Mean Scores on the Outcome Measures Over the Study Period
| Outcome Measures | Follow-Up Assessment | |||||
|---|---|---|---|---|---|---|
| 3 mo (T1) | 6 mo (T2) | 12 mo (T3) | ||||
| Experimental Group (n = 40) | Control Group (n = 22) | Experimental Group (n = 30) | Control Group (n = 20) | Experimental Group (n = 27) | Control Group (n = 34) | |
| Medical and clinical characteristics | ||||||
| NIV hours, mean (SD) | ||||||
| Prescribed hours | 8.63 (1.47) | 7.78 (2.13) | 8.87 (1.45) | 8.52 (1.5) | 8.73 (1.04) | 8.41 (2.06) |
| Effective hours | 6.11 (3.65) | 3.83 (3.44) | 4.16 (4.18) | 2.69 (3.37) | 8.02 (1.78) | 6.15 (2.66) |
| Difference | 0.73 (2.00) | 1.51 (2.41) | 0.64 (1.17) | 0.98 (2.01) | 0.35 (1.50) | 0.84 (2.03) |
| ADL, mean (SD) | 1.20 (1.77) | 0.21 (0.60) | 1.26 (1.94) | 0.33 (0.80) | 0.93 (1.68) | 0.27 (0.57) |
| IADL, mean (SD) | 3.01 (1.80) | 2.91 (1.47) | 3.33 (1.49) | 2.71 (1.62) | 3.18 (1.28) | 2.88 (1.74) |
| FSS, mean (SD) | 41.38 (13.94) | 43.75 (14.45) | 40.78 (13.53) | 33.88 (16.01) | 39.52 (13.76) | 42.70 (12.03) |
| Spirometry, mean (SD) | ||||||
| FEV1 | 0.83 (0.41) | 1.41 (0.77) | 0.93 (0.29) | 1.49 (0.81) | 1.33 (0.77) | 1.41 (1.02) |
| FEV1% | 45.67 (18.4) | 54.04 (23.70) | 46.43 (21.67) | 60.18 (30.05) | 53 (30.58) | 71.71 (43.11) |
| FVC | 1.53 (0.61) | 2.43 (0.85) | 1.15 (1.20) | 1.94 (0.69) | 2.011 (0.85) | 2.27 (1.65) |
| FVC% | 56.24 (19.76) | 71.85 (20.56) | 63.92 (17.38) | 68.90 (23.03) | 68.26 (27.78) | 72.50 (51.17) |
| FEV1/FVC | 0.55 (0.97) | 0.64 (0.20) | 0.68 (0.11) | 0.565 (0.11) | 0.60 (0.18) | 65.56 (10.82) |
| FEV1/FVC% | 66.36 (15.18) | 60.40 (29.08) | 73.64 (23.77) | 74.44 (23.34) | 75.08 (20.93) | 58.15 (28.27) |
| EGA, mean (SD) | ||||||
| pH | 7.40 (0.03) | 7.42 (0.05) | 7.39 (0.55) | 7.44 (0.03) | 7.00 (0.00) | 7.45 (0.03) |
| Pao2 | 65.53 (10.28) | 71.21 (17.23) | 64.90 (5.70) | 63.78 (10.12) | 69.53 (10.66) | 70.20 (5.44) |
| PaCO2 | 52.20 (9.44) | 50.29 (14.53) | 52.00 (7.46) | 58.89 (27.67) | 47.30 (6.31) | 41.60 (5.02) |
| HCO3 | 30.00 (9.62) | 29.31 (5.01) | 31.94 (13.71) | 27.24 (10.14) | 30.572 (4.49) | 38.80 (12.49) |
| Psychological and cognitive features | ||||||
| Psychological variables | ||||||
| HADS, mean (SD) | ||||||
| Tot. | 9.78 (5.81) | 10.88 (8.05) | 10.78 (6.51) | 6.55 (5.27) | 9.66 (6.48) | 9.00 (6.37) |
| HADS-A | 5.83 (4.17) | 5.31 (5.23) | 6.07 (4.12) | 3.11 (3.82) | 6.22 (4.41) | 5.50 (5.01) |
| HADS-D | 4.50 (1.91) | 5.69 (3.53) | 4.71 (2.86) | 3.44 (2.78) | 3.44 (2.89) | 3.50 (2.17) |
| EQ-5D, mean (SD) | ||||||
| Algorithm | 0.29 (0.40) | 0.23 (0.62) | 0.35 (0.349) | 0.70 (0.48) | 0.26 (0.35) | 0.43 (0.51) |
| VAS | 60.83 (20.88) | 59.06 (15.97) | 71.07 (16.54) | 68.50 (15.28) | 64.16 (15.55) | 66 (13.49) |
| QAF, mean (SD) | ||||||
| Tot. | 54.38 (4.10) | 55.26 (5.20) | 53.92 (6.78) | 54.77 (5.33) | 53.47 (6.69) | 53.30 (5.12) |
| B-IPQ, mean (SD) | 43.66 (7.53) | 37.05 (14.80) | 34.92 (14.31) | 33.5 (9.02) | 35.11 (13.02) | 37.80 (13.00) |
| ROSES, mean (SD) | 31.77 (5.96) | 31.93 (6.12) | 35.85 (3.61) | 33.77 (3.52) | 34.20 (3.12) | 29.60 (8.82) |
| Cognitive variables | ||||||
| MMSE, mean (SD) | N/A | N/A | 27.81 (1.60) | 27.75 (1.50) | 27.30 (2.25) | 26.20 (3.56) |
| MMSE Adj., mean (SD) | N/A | N/A | 26.42 (1.51) | 27.33 (1.15) | 26.72 (1.40) | 25.00 (2.58) |
| ACE-R, mean (SD) | ||||||
| Tot. | N/A | N/A | 84.30 (8.43) | 79.5 (8.66) | 78.76 (9.40) | 75.80 (15.97) |
| Tot. ACE-R Adj. | N/A | N/A | 92.70 (7.90) | 96.25 (6.18) | 87.75 (9.22) | 86.40 (11.88) |
| DI, mean (SD) | 2.11 (3.56) | 0.53 (0.99) | 0.88 (1.72) | 0 (0.0) | 0.78 (1.88) | 0.25 (0.70) |
n = number of subjects; NIV = noninvasive ventilation; SD = standard deviation; ADL = activities of daily living; IADL = instrumental activities of daily living; FSS = Fatigue Severity Scale; FEV1 = forced expiratory volume in the first second; FVC = forced vital capacity; FEV1/FVC = ratio between forced expiratory volume in the first second and forced vital capacity or Tiffeneau Index; EGA = hemogasanalysis; pH = potential of hydrogen; Pao2 = partial pressure of oxygen in arterial blood; Paco2 = partial pressure of carbon dioxide in arterial blood; HCO3 = hydrogen carbonate concentration; HADS = Hospital Anxiety and Depression Scale; Tot. = total score; HADS-A = Hospital Anxiety and Depression Scale—Anxiety; HADS-D = Hospital Anxiety and Depression Scale—Depression; EQ-5D = EuroQoL-5D Questionnaire; VAS = visual analog scale; QAF = Questionnaire on Adhesion to Pharmacological and Dietetic Therapy; B-IPQ = Brief Illness Perception Questionnaire; ROSES = Rosenberg’s Self-Esteem Scale; MMSE = Mini-Mental Status Examination; N/A = not applicable; MMSE Adj. = Mini Mental Status Examination corrected; ACE-R = Addenbrooke’s Cognitive Examination Test—Revised; DI = Delirium Index.
According to the objectives and outcomes explained previously, global scores for the participants were reported using mixed methods.
Test of the Intervention on Primary Outcomes
Results from the estimated mixed-effects models show an overall good effect of intervention compared with control for the primary outcome measures. Point estimates, 95% CIs of effect sizes, and summaries of each model are shown in Tables 4 and 5.
TABLE 4.
Fixed-Effects Estimates (at Postassessment and Follow-Up Assessment Phases) and Per Groups With 95% CIs for Medical and Clinical Outcome Measures
| Outcome Measures | Value | SE | Cls | t | p |
|---|---|---|---|---|---|
| NIV hours | |||||
| Prescribed hours | |||||
| (Intercept) | 8.67 | 0.122 | 8.43 to 8.91 | 71.137 | <.001 |
| 3-mo follow-up | −0.087 | 0.302 | −0.67 to 0.505 | −0.288 | .77 |
| 6-mo follow-up | 0.0509 | 0.341 | −0.618 to 0.720 | 0.149 | .89 |
| 12-mo follow-up | 0.0585 | 0.444 | −0.812 to 0.929 | 0.132 | .91 |
| (Intercept) | 8.639 | 0.115 | 8.41 to 8.86 | 75.03 | <.001 |
| Group | 0.451 | 0.23 | 4.81 to 5 | 1.96 | .05 |
| Effective hours | |||||
| (Intercept) | 7.134 | 0.75 | 5.665 to 8.60 | 10 | .07 |
| 3-mo follow-up | 0.94 | 0.035 | 0.87 to 1.01 | 26.62 | .000 |
| 6-mo follow-up | −1.02 | 0.35 | −1,73 to −0.31 | −2.9 | .006 |
| 12-mo follow-up | 1.283 | 0.52 | 0.235 to 2.27 | 2.412 | 0.026 |
| (Intercept) | 6.261461 | 0.2514 | 5.76 to 6.75 | 24.901 | <.000 |
| Group | 1.5259 | 0.3389 | 0.85 to 2.19 | 4.503 | <.000 |
| Difference | |||||
| (Intercept) | 1.55 | 0.511 | 0.54 to 2.55 | 3.035 | .20 |
| 3-mo follow-up | −0.399 | 0.5 | −0.38 to −1.58 | −0.798 | .49 |
| 6-mo follow-up | −0.34 | 0.483 | −1.28 to −0.60 | −0.704 | .51 |
| 12-mo follow-up | −1.386 | 0.527 | −2.41 to −0.35 | −2.629 | .029 |
| (Intercept) | 1.63 | 0.264 | 1.11 to 2.15 | 6.18 | .013 |
| Group | −1.05 | 0.351 | −1.64 to −0.36 | −3.01 | .007 |
| FSS | |||||
| (Intercept) | 41.73 | 1.7 | 38.4 to 45.06 | 24.534 | .011 |
| 3-mo follow-up | −2.57 | 5.39 | −13.1 to 7.99 | −0.477 | .71 |
| 6-mo follow-up | −7.87 | 3.28 | −14.3 to −1.44 | −2.399 | .023 |
| 12-mo follow-up | −3.84 | 5.65 | −14.9 to 7.24 | −0.679 | .61 |
| (Intercept) | 42.3 | 1.52 | 39.32 to 45.28 | 27.81 | <.001 |
| Group | 2.56 | 2.78 | −2.89 to 8.01 | 0.921 | .40 |
| Spirometry | |||||
| FEV1 | |||||
| (Intercept) | 7.63 | 0.881 | 5.90 to 9.36 | 8.66 | <.001 |
| 3-mo follow-up | −5.77 | 1.413 | −8.54 to −3.00 | −4.08 | <.001 |
| 6-mo follow-up | −6.27 | 0.897 | −8.03 to −4.51 | −6.99 | <.001 |
| 12-mo follow-up | 0 | 0 | 0 | 0 | 0 |
| (Intercept) | 1.73 | 0.36 | 1.01 to 2.45 | 4.71 | .66 |
| Group | 3.67 | 3.39 | −2.97 to 10.32 | 1.08 | .29 |
| FEV1% | |||||
| (Intercept) | 49.96 | 3.69 | 42.7 to 57.2 | 13.5388 | .05 |
| 3-mo follow-up | −0.0906 | 7.72 | −15.2 to 15 | −0.0117 | .99 |
| 6-mo follow-up | −2.6516 | 6.7 | −15.8 to 10.5 | −0.3961 | .72 |
| 12-mo follow-up | 0.9961 | 6.54 | −11.8 to 13.8 | 0.1522 | .88 |
| (Intercept) | 50.44 | 2.04 | 46.4 to 54.4 | 24.67 | <.001 |
| Group | −6.55 | 4.09 | −14.6 to 1.46 | −1.6 | .111 |
| FVC | |||||
| (Intercept) | 2.733 | 0.361 | 2.02 to 3.44 | 7.572 | <.001 |
| 3-mo follow-up | 1.48 | 1.009 | −0.49 to 3.46 | 1.467 | .16 |
| 6-mo follow-up | 1.3 | 1.236 | −1.122 to 3.72 | 1.052 | .30 |
| 12-mo follow-up | 0.153 | 0.747 | −1.310 to 1.62 | 0.205 | .84 |
| (Intercept) | 2.531 | 0.381 | 1.78 to 3.27 | 6.643 | .073 |
| Group | −0.327 | 0.476 | −1.26 to 0.606 | −0.687 | .53 |
| FVC% | |||||
| (Intercept) | 62 | 2.74 | 56.6 to 67.4 | 22.601 | .008 |
| 3-mo follow-up | −1.44 | 2.74 | −15.8 to 12.9 | −0.196 | .87 |
| 6-mo follow-up | −3.38 | 7.11 | −17.3 to 10.5 | −0.476 | .69 |
| 12-mo follow-up | −3.4 | 7.77 | −18.6 to 11.8 | −0.438 | .74 |
| (Intercept) | 63.51 | 1.92 | 59.8 to 67.27 | 33.12 | <.001 |
| Group | −6.46 | 3.83 | 14 to 1.06 | −1.68 | .094 |
| FEV1/FVC | |||||
| (Intercept) | 61.54 | 3.38 | 54.9 to 68.2 | 18.193 | <.001 |
| 3-mo follow-up | −6.85 | 14.24 | −34.8 to 21.1 | −0.481 | .71 |
| 6-mo follow-up | −10.44 | 15.31 | −40.4 to 19.6 | −0.682 | .59 |
| 12-mo follow-up | −4.68 | 13.3 | −30.8 to 21.4 | −0.352 | .78 |
| (Intercept) | 60.99 | 2.38 | 56.3 to 65.66 | 25.625 | <.001 |
| Group | −4.1 | −13.4 | −13.4 to 5.23 | −0.861 | .39 |
| FEV1/FVC% | |||||
| (Intercept) | 70.313 | 2.15 | 66.10 to 74.5 | 32.7048 | <.001 |
| 3-mo follow-up | 8.438 | 6.63 | −4.56 to 21.4 | 1.2722 | .39 |
| 6-mo follow-up | 12.39 | 7.06 | −1.44 to 26.2 | 1.7558 | .23 |
| 12-mo follow-up | 0.555 | 10.94 | −10.89 to 22 | 0.0507 | .97 |
| (Intercept) | 70.53 | 3.14 | 64.4 to 76.68 | 22.463 | <.001 |
| Group | −4.09 | 5.18 | −14.2 to 6.06 | −0.789 | .56 |
| ABG or EGA | |||||
| pH | |||||
| (Intercept) | 7.42 | 0.005 | 7.40 to 7.43 | 1245.11 | <.001 |
| 3-mo follow-up | −0.004 | 0.009 | −0.0236 to 0.0143 | −0.4824 | .64 |
| 6-mo follow-up | 0.001 | 0.022 | −0.04 to 0.0453 | 0.0822 | .95 |
| 12-mo follow-up | 0.001 | 0.024 | −0.04 to 0.0455 | 0.0824 | .95 |
| (Intercept) | 7.42 | 0.003 | 7.41–7.42 | 1905.16 | <.001 |
| Group | −0.017 | 0.01 | −0.03 to 0.003 | −1.62 | .36 |
| Pao2 | |||||
| (Intercept) | 68.01 | 1.06 | 65.94–70.08 | 64.27 | <.001 |
| 3-mo follow-up | 0.528 | 3.55 | 7.48 | 0.1487 | .90 |
| 6-mo follow-up | −4.84 | 2.8 | 0.642 | −1.7305 | .11 |
| 12-mo follow-up | −0.277 | 2.8 | 5.210 | −0.0988 | .92 |
| (Intercept) | 68.671 | 0.991 | 66.73–70.61 | 69.278 | .003 |
| Group | −0.777 | 2.084 | −4.86 to 3.31 | −0.373 | .75 |
| Paco2 | |||||
| (Intercept) | 50.02 | 1.11 | 47.85–52.19 | 45.141 | <.001 |
| 3-mo follow-up | 3.51 | 2.78 | −1.94 to 8.96 | 1.263 | .33 |
| 6-mo follow-up | 8.12 | 6.81 | −5.24 to 21.47 | 1.191 | .47 |
| 12-mo follow-up | −2.84 | 2.95 | −8.63 to 2.95 | −0.961 | .34 |
| (Intercept) | 49.97 | 2.36 | 45.35–54.58 | 21.208 | <.001 |
| Group | 1.37 | 3.28 | −5.06 to 7.80 | 0.418 | .71 |
| HCO3 | |||||
| (Intercept) | 34.5 | 2.6 | 29.40–39.60 | 13.26 | .056 |
| 3-mo follow-up | −2.79 | 2.72 | −8.11 to 2.53 | −1.03 | .44 |
| 6-mo follow-up | −2.44 | 2.18 | −6.72 to 1.84 | 1.12 | .42 |
| 12-mo follow-up | 9.05 | 7.78 | −6.20 to 24.29 | 1.16 | .45 |
| (Intercept) | 34.43 | 2.61 | 29.3–35.55 | 13.198 | .006 |
| Group | −4.11 | 4.16 | −12.3 to 4.04 | −0.988 | .42 |
CIs = confidence intervals; SE = standard error; NIV = noninvasive ventilation; FSS = Fatigue Severity Scale; FEV1 = forced expiratory volume in the first second; FVC = forced vital capacity; FEV1/FVC = ratio between forced expiratory volume in the first second and forced vital capacity or Tiffeneau Index; ABG = arterial blood gas; EGA = hemogasanalysis; pH = potential of hydrogen; Pao2 = partial pressure of oxygen in arterial blood; Paco2 = partial pressure of carbon dioxide in arterial blood; HCO3 = hydrogen carbonate concentration.
TABLE 5.
Fixed-Effects Estimates (at Postassessment and Follow-Up Assessment Phases) With 95% CIs for Psychological and Cognitive Outcome Measures
| Outcome Measures | Value | SE | Cls | t | p |
|---|---|---|---|---|---|
| Psychological variables | |||||
| HADS | |||||
| Tot. | |||||
| (Intercept) | 10.09 | 0.91 | 8.31 to 11.87 | 11.09 | 0.042 |
| 3-mo follow-up | −1.33 | 2.078 | −5.41 to 2.73 | −0.642 | 0.62 |
| 6-mo follow-up | −3.09 | 1.708 | −6.44 to 0.259 | −1.808 | 0.16 |
| 12-mo follow-up | −2.27 | 1.772 | −5.45 to 1.198 | −1.284 | 0.35 |
| (Intercept) | 10.41 | 0.706 | 9.02 to 11.80 | 14.74 | <.001 |
| Group | 1.4 | 1.089 | −0.730 to 3.54 | 1.29 | 0.23 |
| HADS-A | |||||
| (Intercept) | 5.715 | 0.823 | 4.10 to 7.32 | 6.943 | 0.088 |
| 3-mo follow-up | −1.278 | 1.184 | −3.60 to 1.043 | −1.079 | 0.042 |
| 6-mo follow-up | −2.293 | 1.09 | −4.43 to −0.157 | −2.104 | 0.079 |
| 12-mo follow-up | −0.978 | 1.18 | −3.29 to 1.334 | −0.829 | 0.50 |
| (Intercept) | 5.9 | 0.492 | 4.93 to 6.86 | 11.98 | <.001 |
| Group | 1.54 | 0.702 | 0.167 to 2.92 | 2.2 | 0.039 |
| HADS-D | |||||
| (Intercept) | 4.43 | 0.277 | 3.89 to 4.97 | 15.99 | <.001 |
| 3-mo follow-up | 0.061 | 0.914 | −1.73 to 1.85 | 0.0675 | 0.96 |
| 6-mo follow-up | −0.082 | 0.835 | −2.47 to 0.80 | −0.9935 | 0.40 |
| 12-mo follow-up | −1.3592 | 0.748 | −2.83 to 0.107 | −1.81 | 0.15 |
| (Intercept) | 4.56 | 0.327 | 3.92 to 5.202 | 13.967 | 0.005 |
| Group | −0.058 | 0.496 | −1.03 to 0.914 | −0.117 | 0.91 |
| EQ-5D | |||||
| Algorithm | |||||
| (Intercept) | 1.07 | 0.901466 | −0.70 to 2.84 | 1,188 | 0.23 |
| 3-mo follow-up | 0.1108 | 1.01 | −1.93 to 2.15 | 0.109 | 0.91 |
| 6-mo follow-up | −0.41 | 1.02 | −2.42 to 1.59 | −0.405 | 0.68 |
| 12-mo follow-up | −0.31 | 1.01 | −2.31 to 1.68 | −0.31 | 0.75 |
| (Intercept) | 0.58 | 0.114137 | 0.36 to 0.81 | 5.15 | <.000 |
| Group | -0.40 | 0.1535 | −0.70 to −0.10 | −2.631 | <.009 |
| VAS | |||||
| (Intercept) | 62.68 | 1.72 | 59.30 to 66 | 36.46 | <.001 |
| 3-mo follow-up | 2.58 | 6.03 | −9.23 to 14.4 | 0.428 | 0.73 |
| 6-mo follow-up 6-mo follow-up | 12.46 | 6.68 | −0.62 to 25.6 | 1.866 | 0.28 |
| 12-mo follow-up | 6.19 | 5.72 | −5.02 to 17.4 | 1.082 | 0.44 |
| (Intercept) | 62.21 | 3.85 | 57.12 to 67.30 | 23.96 | <.001 |
| Group | −1.57 | 3.85 | −9.13 to 5.98 | −0.408 | 0.70 |
| QAF | |||||
| Tot. | |||||
| (Intercept) | 54.10 | 0.494 | 53.14 to 55.08 | 109.53 | <.001 |
| 3-mo follow-up | 0.986 | 1.159 | −1.29 to 3.26 | 0.851 | 0.40 |
| 6-mo follow-up | 0.325 | 1.327 | −2.28 to 2.93 | 0.244 | 0.81 |
| 12-mo follow-up | −0.448 | 1.248 | −2.89 to 2 | −0.359 | 0.72 |
| (Intercept) | 54.09 | 0.437 | 53.24 to 54.95 | 123.83 | <.001 |
| Group | −0.66 | 0.874 | −2.38 to 1.05 | −0.762 | 0.45 |
| B-IPQ | |||||
| (Intercept) | 38.51 | 1.93 | 34.73 to 42.29 | 19.984 | 0.026 |
| 3-mo follow-up | −2.09 | 2.55 | −7.08 to 2.90 | −0.822 | 0.046 |
| 6-mo follow-up | −7.95 | 4.78 | −17.32 to 1.42 | −1.663 | 0.33 |
| 12-mo follow-up | −5.79 | 6.42 | −18.36 to 6.79 | −0.902 | 0.53 |
| (Intercept) | 351.153 | 1.8192 | 31.51 to 38.72 | 19.302 | .000 |
| Group | 73.10 | 2.4761 | 2.40 to 12.21 | 2.953 | .004 |
| ROSES | |||||
| (Intercept) | 32.92 | 0.419 | 32.10 to 33.74 | 78.65 | <.001 |
| 3-mo follow-up | 1.53 | 1.329 | −1.072 to 4.14 | 1.15 | 0.41 |
| 6-mo follow-up | 3.72 | 1.494 | 0.796 to 6.65 | 2.49 | 0.16 |
| 12-mo follow-up | 0.055 | 2.55 | −4.94 to 5.06 | 0.02 | 0.99 |
| (Intercept) | 32.81 | 0.759 | 31.32 to 34.3 | 43.2 | <.001 |
| Group | 0.803 | 1.25 | −1.65 to 3.25 | 0.64 | 0.56 |
| Cognitive variables | |||||
| MMSE | |||||
| (Intercept) | 26.7 | 0.909 | 25.18 to 28.75 | 29.66 | <.001 |
| 3-mo follow-up | 1.06 | 3.441 | −5.68 to 7.8 | 0.308 | 0.76 |
| 6-mo follow-up | 2 | 0.932 | 0.17 to 3.82 | 2.141 | 0.034 |
| 12-mo follow-up | 1.06 | 0.888 | −0.8 to 2.8 | 1.193 | 0.23 |
| (Intercept) | 26.73 | 0.581 | 25.59 to 27.87 | 45.97 | <.001 |
| Group | 0.183 | 0.636 | −2.49 | 0.287 | 0.77 |
| MMSE Adj. | |||||
| (Intercept) | 26.973 | 0.944 | 25.12 to 28.82 | 28.568 | 0.995 |
| 3-mo follow-up | 1.591 | 3.711 | −5.68 to 8.86 | 0.429 | 1 |
| 6-mo follow-up | 1.583 | 2.102 | −2.54 to 5.7 | 0.753 | 0.46 |
| 12-mo follow-up | −0.949 | 2.406 | −5.66 to 3.77 | −0.394 | 0.76 |
| (Intercept) | 26.29 | 0.69 | 24.93 to 27.65 | 37.96 | <.001 |
| Group | 1.32 | 1.58 | −1.79 to 4.43 | 0.83 | 0.51 |
| ACE-R | |||||
| Tot. | |||||
| (Intercept) | 78.49 | 4.13 | 70.41 to 86.6 | 19.02 | 0.37 |
| 3-mo follow-up | 4.11 | 15.59 | −26.44 to 34.7 | 0.26 | 0.90 |
| 6-mo follow-up | 10.24 | 4.34 | 1.73 to 18.7 | 2.36 | 0.02 |
| 12-mo follow-up | 4.05 | 4.02 | −3.83 to 11.9 | 1.007 | 0.32 |
| (Intercept) | 77.53 | 2.85 | 71.95 to 83.12 | 27.21 | 0.002 |
| Group | 1.71 | 2.94 | −11.52 | 0.582 | 0.57 |
| DI | |||||
| (Intercept) | 1.27 | 0.494 | 0.305 to 2.24 | 2.58 | 0.23 |
| 3-mo follow-up | −1.54 | 0.638 | −2.78 to −0.28 | −2.41 | 0.099 |
| 6-mo follow-up | −2.39 | 0.629 | −3.62 to −1.61 | −3.81 | <.001 |
| 12-mo follow-up | −2.41 | 0.669 | −3.72 to −1.10 | −3.61 | 0.022 |
| (Intercept) | 1.307 | 0.607 | 0.11 to 2.50 | 2.15 | 0.11 |
| Group | 0.93 | 0.439 | 0.07 to 1.79 | 2.12 | 0.036 |
CIs = confidence intervals; CG=Control Group; SE = standard error; HADS = Hospital Anxiety and Depression Scale; Tot. = total score; HADS-A = Hospital Anxiety and Depression Scale—Anxiety; HADS-D = Hospital Anxiety and Depression Scale—Depression; EG= Experimental Group; EQ-5D = EuroQoL-5D Questionnaire; VAS = visual analog scale; QAF = Questionnaire on Adhesion to Pharmacological and Dietetic Therapy; B-IPQ = Brief Illness Perception Questionnaire; ROSES = Rosenberg’s Self-Esteem Scale; MMSE = Mini Mental Status Examination; MMSE Adj. = Mini Mental Status Examination corrected; ACE-R = Addenbrooke’s Cognitive Examination Test—Revised; DI = Delirium Index.
Bold indicates statistically significant.
NIV Hours
Using the mean of effective weekly NIV hours as a dependent variable in the mixed-effects model, without adding any covariate, the difference between preintervention and postintervention scores was significant (F(64.410) = 8.558, p = .005). The intervention had a significant impact (F(304) = 19.054, p < .001) between groups, which was not maintained when both the mentioned covariates were added. However, in this case, both the prescribed hours (F(205.787) = 22.434, p < .001) and the difference between them and the effective NIV hours practiced (F(71.476) = 8.991, p = .004) significantly impacted the mean of the effective hours. Moreover, in the model, if the difference between prescribed and effective NIV hours is used as a dependent variable, a significant difference between preintervention and postintervention scores appears (F(209.744) = 208.480, p < .001), and there was also a significant difference between groups (F(71.476) = 8.991, p = .004).
Test of the Intervention on Secondary Outcomes
Quality of Life
In the mixed-effects model without any covariates, QoL, assessed using the algorithm of the EuroQoL, showed significant difference in both groups (F(156) = 10.264, p = .002) and over time (F(71.480) = 8.114, p = .006). However, none of the covariate results or their interactions were significant.
Psychological Variables
Results from the mixed-effects models showed that, compared with control, the intervention resulted in a slight improvement in the HADS-A score (F(170) = 5.226, p = .023), which was not maintained over time (F(77.416) = 2.747, p = .101). Depression, as assessed by the subscale of the HADS, did not differ significantly between groups (F(50.101) = .042, p = .838) or over time (F(45.099) = .006, p = .939). Other relevant results are represented by the impact of the intervention on the Brief Illness Perception Questionnaire score over time (F(64.444) = 5.418, p = .023) and between groups (F(140) = 6.172, p = .014; compare with Table 5 for specific models). On the other hand, anxiety significantly influenced NIV effective hours overtime (F(75.783) = 2.764, p = .001) as well as between groups (F(102.946) = 2.410, p < .001). For depression, there was also an impact on the number of effective NIV hours (F(112.593) = 2.513, p = .003) between groups and over time (F(75.250) = 2.673, p = .003).
Cognitive Functions
Overall cognitive function scores significantly influenced both acceptance and adherence to NIV. In particular, the total scores of the ACE-R (F(14.453) = 5.140, p = .039), the MMSE (F(18.180) = 5.741, p = .028), and the Delirium Index (F(26.698) = 5.336, p = .029) significantly influenced the difference between the prescribed hours and the effective NIV hours over time, whereas the total score of the ACE-R had an impact on NIV adherence between groups (F(51.540) = 4.704, p = .035). In this context, their interactions were also significant. Moreover, the interaction between the MMSE and the ACE-R (F(49.675) = 5.123, p = .028) as well as between the ACE-R and the Delirium Index derived from the CAM (F(49.157) = 5.980, p = .018) influenced the number of effective NIV hours practiced.
The fixed-effects estimates at postassessment and follow-up assessment phases and per group with 95% CIs for medical and clinical outcome measures are shown in Table 4. Table 5 shows fixed-effects estimates (at postassessment and follow-up assessment phases) with 95% CIs for psychological and cognitive outcome measures.
DISCUSSION
Our study assessed the effectiveness of a short-term CBT intervention on NIV acceptance and adherence in patients with COPD. The intervention resulted in a significant and clinically important impact on groups in terms of NIV adherence and QoL, with a slight short-term improvement for anxiety. In this respect, it is relevant to note that there are no standard interventions to promote acceptance and adherence to medication or NIV in COPD people. There are only a few studies regarding this aspect, maybe because of its complexity and because previous research confirmed the importance of the combination of different components such as information, self-care management, counseling, family therapy, supportive care, or telephone follow-up. Therefore, the results are limited and mainly focused on the possible factors that influence acceptance or adherence (66). This, although representing an important strength of the study and opening the need for further research, does not allow for comparisons with other studies, also considering the cultural and social aspects of other realities.
Despite this limitation, the study allowed us to note that adherence to NIV is a very complex construct, influenced by several factors. These include a significant influence by cognitive function, the number of hours of NIV prescribed, and the difference between these hours and the hours performed. On the other hand, the difference between the prescribed and the effective hours of NIV and the weekly mean NIV hours had an impact on both anxiety and depression over time. It is important to note that the psychological intervention did not specifically aim to improve anxiety and depression, as they operate via different mechanisms. Moreover, their initial means were not clinically elevated at baseline. Therefore, it could be expected that the regression means are not significantly different.
In addition to its primary clinical effectiveness outcomes, our data show the potential for CBT to improve adaptation and adherence to NIV in patients with COPD as well as the access and engagement with psychotherapy in primary care. The majority of patients of the experimental group found the strategies to overcome the difficulties related to the NIV’s usage (i.e., mask fit, air leakage, and claustrophobia), thanks to the psychological intervention, which integrated them into their healthcare routine. These results stress the relevance of paying attention to the patients’ needs during the adaptation to a new device process that is suddenly perceived as another sign of limitation (67). Indeed, expectations are driven by not only medication but also the disease itself and the elaboration process of the information collected by the patients about it (68,69).
An integrated approach, focusing on the overall perceptions of the NIV’s impact on daily life and considering lifestyle factors, demographic characteristics (age, comorbidities, physical limitations, psychological and cognitive status), and pharmacological factors (polypharmacy regimens), should be promoted to optimize treatment adherence and perseverance as well as to prevent worsening clinical and economic outcomes.
Finally, it is interesting to note that the illness beliefs and perceptions influenced the intervention, underlying how it is relevant to provide a different perspective about the illness as well as reinforce the link between mind and body and their mutual relationship, as seen in other studies on medication adherence (70,71). Further studies might investigate if adherence is more connected to the beliefs regarding the illness than to the respiratory parameters, and therefore, the different trajectories between QoL, adherence, and functional and emotional status can be derived from a complex interaction of different variables such as perceived dyspnea levels expectations, beliefs, levels of an activity practice, motivation, cognitive functioning, and mood.
Strengths and Limitations
The study presents certain limitations. First, some characteristics of the design are important in interpreting the results. The randomized controlled trial, which is a meticulous study design, is in contrast with the patient-centered approach used in the intervention to pay attention to their needs. In particular, all the experimental members were biased regarding telemedicine, worrying about their lower levels of confidence with technology and condoning a personal and face-to-face relationship. In this regard, telemedicine can facilitate access to specialists and information avoiding difficult transportations, but the broadband connections can malfunction or run into other technological problems, reducing the practicability and the naturalness of the relationship. Difficult tradeoffs often have to be made in choosing a control or comparison group. In our study, a group of usual care could have been useful to control the confounding factors even better. The choice of an active control group was to draw causal conclusions about the efficacy of a psychological intervention. Second, both the psychological monitoring of adherence and clinical conditions and the statistical analysis can be impacted by the dropout during the follow-up assessments, which was especially difficult to avoid. Reasons for missing appointments were attributed to death or the general worsening of the patient’s health status. Previous psychological and rehabilitation studies that involved people with COPD confirmed similar dropout rates. As previous studies confirm, dropout rates or discontinuity in following rehabilitation programs in patients with COPD tend to be high and frequent. Depression, somatization, smoking, living alone, a lower fat-free mass, and lower confidence in treatment were found to be positively associated with discontinuity in continuing treatment (72–76). Finally, the sample size is underestimated and smaller than expected by the initial potential power analysis. Therefore, the results of the analysis, in particular those of T3, are to be interpreted with caution. It is also relevant to consider that the patients with COPD involved in our study represent only a narrow range of the overall population in need of NIV and of the COPD who use NIV in the world, limiting the generalizability of the data.
Notwithstanding these limitations, this study has the value of bringing attention to the psychological factors that can be involved during the adaptation’s process to an external and life-limiting device like NIV, taking into consideration the patient’s needs and health conditions. Moreover, therapists and staff were not influenced by their participation in the evaluation study (the so-called Hawthorne effect).
Future Directions
Further studies are needed to improve and extend the intervention proposed during the adaptation to the NIV process. Future research should focus on replication of our findings and dissemination in routine care in different settings. Our dissemination efforts for the introduction of psychological support during the adaptation’s process to NIV include the launching of an online platform for those who are interested in learning about the treatment and collaborations. It should be tested in other populations in need of NIV, comparing it with alternative models and considering the role of the caregivers and the healthcare professionals. Finally, the evaluation of the introduction of psychological support during this complicated process in terms of healthcare costs should be implemented (49,76).
Acknowledgments
We would like to give special thanks to our participants who graciously gave their time to take part in this study.
Source of Funding and Conflicts of Interest: This work was supported by Vivisol S.r.l. The authors declare that this work was supported by Vivisol S.r.l.
Author Contributions: E.V. drafted the report, and all authors reviewed and approved it. F.P., P.B., and E.V. were responsible for the design of the trial; E.V. was responsible for the intervention content and data gathering instruments; F.P. and P.B. were responsible for trial conduct; F.P. and E.V. were responsible for database design and management; and F.P. and E.V. were responsible for analyses. The funder of the study had no role in study design, data collection, data analysis, or writing of the report.
Contributor Information
Paolo Banfi, Email: pabanfi@dongnocchi.it.
Francesco Pagnini, Email: Francesco.pagnini@unicatt.it.
REFERENCES
- 1.WHO . The World Health Report 2000: Health Systems: Improving Performance. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 2.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397–412. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD. Uncertainty and Data Availability for the Global Burden of Disease Estimates 2000–2002. Evidence and Information for Policy Working Paper. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 4.GBD 2015 Chronic Respiratory Disease Collaborators . Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atsou K, Chouaid C, Hejblum G. Variability of the chronic obstructive pulmonary disease key epidemiological data in Europe: systematic review. BMC Med 2011;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson J, Loddenkemper R, Yves S, Lundbäck B, Fletcher M. Lung health in Europe, facts and figures. Sheffield, United Kingdom: European Lung Foundation; 2013. [Google Scholar]
- 7.Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet 2015;385:549–62. [DOI] [PubMed] [Google Scholar]
- 9.Muka T, Imo D, Jaspers L, Colpani V, Chaker L, van der Lee SJ, Mendis S, Chowdhury R, Bramer WM, Falla A, Pazoki R, Franco OH. The global impact of non-communicable diseases on healthcare spending and national income: a systematic review. Eur J Epidemiol 2015;30:251–77. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, López Varela MV, Martinez F, Montes de Oca M, Papi A, Pavord ID, Roche N, Sin DD, Stockley R, Vestbo J, Wedzicha JA, Vogelmeier C. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164. [DOI] [PubMed] [Google Scholar]
- 11.Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op ’t Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728–35. [DOI] [PubMed] [Google Scholar]
- 13.Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract 2016;65:246–56. [PubMed] [Google Scholar]
- 14.Cleutjens FA, Franssen FM, Spruit MA, Vanfleteren LE, Gijsen C, Dijkstra JB, Ponds RW, Wouters EF, Janssen DJ. Domain-specific cognitive impairment in patients with COPD and control subjects. Int J Chron Obstruct Pulmon Dis 2016;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koskela J, Kilpeläinen M, Kupiainen H, Mazur W, Sintonen H, Boezen M, Lindqvist A, Postma D, Laitinen T. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchmanowicz I, Jankowska-Polanska B, Motowidlo U, Uchmanowicz B, Chabowski M. Assessment of illness acceptance by patients with COPD and the prevalence of depression and anxiety in COPD. Int J Chron Obstruct Pulmon Dis 2016;11:963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagnini F. The potential role of illness expectations in the progression of medical diseases. BMC Psychol 2019;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macrea M, Oczkowski S, Rochwerg B, Branson RD, Celli B, Coleman JM, 3rd, Hess DR, Knight SL, Ohar JA, Orr JE, Piper AJ, Punjabi NM, Rahangdale S, Wijkstra PJ, Yim-Yeh S, Drummond MB, Owens RL. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020;202:e74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006;32:361–70. [DOI] [PubMed] [Google Scholar]
- 20.Rochwerg B Brochard L Elliott MW Hess D Hill NS Nava S Navalesi P Antonelli M Brozek J Conti G Ferrer M Guntupalli K Jaber S Keenan S Mancebo J Mehta S, Members of the Steering Committee Raoof S Members of The Task Force . Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017. 50:1602426. [DOI] [PubMed] [Google Scholar]
- 21.AlYami MA, AlAhmari MD, Alotaibi H, AlRabeeah S, AlBalawi I, Mubasher M. Evaluation of efficacy of non-invasive ventilation in non-COPD and non-trauma patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Ann Thorac Med 2015;10:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;7:CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M, Boutmy-Deslandes E, Perbet S, Mongardon N, Dres M, Razazi K, Guerot E, Terzi N, Andrivet P, Alves M, Sonneville R, Cracco C, Peigne V, Collet F, Sztrymf B, Rafat C, Reuter D, Fabre X, Labbe V, Tachon G, Minet C, Conseil M, Azoulay E, Similowski T, Demoule A. Differential perceptions of noninvasive ventilation in intensive care among medical caregivers, patients, and their relatives: a multicenter prospective study—the PARVENIR study. Anesthesiology 2016;124:1347–59. [DOI] [PubMed] [Google Scholar]
- 24.Christensen HM, Huniche L, Titlestad IL. Involvement of patients’ perspectives on treatment with noninvasive ventilation in patients with chronic obstructive pulmonary disease-a qualitative study. J Clin Nurs 2018;27:e61–9. [DOI] [PubMed] [Google Scholar]
- 25.Linde P, Hanke G, Voltz R, Simon ST. Unpredictable episodic breathlessness in patients with advanced chronic obstructive pulmonary disease and lung cancer: a qualitative study. Support Care Cancer 2018;26:1097–104. [DOI] [PubMed] [Google Scholar]
- 26.Tan Y, Van den Bergh O, Qiu J, von Leupoldt A. The impact of unpredictability on dyspnea perception, anxiety and interoceptive error processing. Front Physiol 2019;10:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonelli Incalzi R, Marra C, Giordano A, Calcagni ML, Cappa A, Basso S, Pagliari G, Fuso L. Cognitive impairment in chronic obstructive pulmonary disease—a neuropsychological and SPECT study. J Neurol 2003;250:325–32. [DOI] [PubMed] [Google Scholar]
- 28.Pierobon A, Sini Bottelli E, Ranzini L, Bruschi C, Maestri R, Bertolotti G, Sommaruga M, Torlaschi V, Callegari S, Giardini A. COPD patients’ self-reported adherence, psychosocial factors and mild cognitive impairment in pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis 2017;12:2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis 2010;5:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R, Vines DL, Sikkema-Ortiz J, Gardner DD, Wilkins RL. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis 2008;3:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax 2008;63:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogliani P, Ora J, Puxeddu E, Matera MG, Cazzola M. Adherence to COPD treatment: myth and reality. Respir Med 2017;129:117–23. [DOI] [PubMed] [Google Scholar]
- 33.Ko BS, Ahn S, Lim KS, Kim WY, Lee Y-S, Lee JH. Early failure of noninvasive ventilation in chronic obstructive pulmonary disease with acute hypercapnic respiratory failure. Intern Emerg Med 2015;10:855–60. [DOI] [PubMed] [Google Scholar]
- 34.Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess DR. The growing role of noninvasive ventilation in patients requiring prolonged mechanical ventilation. Respir Care 2012;57:900–18. [DOI] [PubMed] [Google Scholar]
- 36.Borel JC, Pepin JL, Pison C, Vesin A, Gonzalez-Bermejo J, Court-Fortune I, Timsit JF. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology 2014;19:857–65. [DOI] [PubMed] [Google Scholar]
- 37.Adler D, Perrig S, Takahashi H, Espa F, Rodenstein D, Pépin JL, Janssens JP. Polysomnography in stable COPD under non-invasive ventilation to reduce patient-ventilator asynchrony and morning breathlessness. Sleep Breath 2012;16:1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev 2014;23:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Leupoldt A, Janssens T. Could targeting disease specific fear and anxiety improve COPD outcomes? Expert Rev Respir Med 2016;10:835–7. [DOI] [PubMed] [Google Scholar]
- 40.Norweg A, Collins EG. Evidence for cognitive-behavioral strategies improving dyspnea and related distress in COPD. Int J Chron Obstruct Pulmon Dis 2013;8:439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farver-Vestergaard I, Jacobsen D, Zachariae R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Psychother Psychosom 2015;84:37–50. [DOI] [PubMed] [Google Scholar]
- 42.Volpato E, Banfi P, Rogers SM, Pagnini F. Relaxation techniques for people with chronic obstructive pulmonary disease: a systematic review and a meta-analysis. Evid Based Complement Alternat Med 2015;2015:628365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis 2020;15:903–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpato E, Banfi P, Pagnini F. A psychological intervention to promote acceptance and adherence to non-invasive ventilation in people with chronic obstructive pulmonary disease: study protocol of a randomised controlled trial. Trials 2017;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leiva-Fernández J, Leiva-Fernández F, García-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulm Med 2014;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: a new instrument. J Psychosom Res 1993;37:753–62. [DOI] [PubMed] [Google Scholar]
- 47.Kempen GI, Suurmeijer TP. The development of a hierarchical polychotomous ADL-IADL scale for noninstitutionalized elders. Gerontologist 1990;30:497–502. [DOI] [PubMed] [Google Scholar]
- 48.Szende A, Oppe M, Devlin N, editors. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Berlin, Germany: Springer; 2007. [Google Scholar]
- 49.Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht, the Netherlands: Springer Netherlands; 2014. [PubMed] [Google Scholar]
- 50.Scalone L, Cortesi PA, Ciampichini R, Belisari A, D’Angiolella LS, Cesana G, Mantovani LG. Italian population-based values of EQ-5D health states. Value Health 2013;16:814–22. [DOI] [PubMed] [Google Scholar]
- 51.Balestroni G, Bertolotti G. EuroQol-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis 2015;78:155–9. [DOI] [PubMed] [Google Scholar]
- 52.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med 2001;33:337–43. [DOI] [PubMed] [Google Scholar]
- 53.Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy 2017;15:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 55.Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res 2006;60:631–7. [DOI] [PubMed] [Google Scholar]
- 56.Gerbino G, Dimonte V, Albasi C, Lasorsa C, Vitale C, Marangella M. Adherence to therapy in patients on hemodialysis. G Ital Nefrol 2011;28:416–24. [PubMed] [Google Scholar]
- 57.Robins RW, Hendin HM, Trzesniewski KH. Measuring global self-esteem: construct validation of a single-item measure and the Rosenberg Self-Esteem Scale. Pers Soc Psychol Bull 2001;27:151–61. [Google Scholar]
- 58.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21:1078–85. [DOI] [PubMed] [Google Scholar]
- 59.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 60.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- 61.Lau MA, McMain SF. Integrating mindfulness meditation with cognitive and behavioural therapies: the challenge of combining acceptance- and change-based strategies. Can J Psychiatry 2005;50:863–9. [DOI] [PubMed] [Google Scholar]
- 62.Pagnini F, Zanini S, Gislon MC. Patient’s needs and psychotherapy integration. Int J Psychother 2013;17:20–3. [Google Scholar]
- 63.Khuri AI, Mathew T, Sinha BK. Statistical Tests for Mixed Linear Models. New York, NY: John Wiley & Sons; 1998. [Google Scholar]
- 64.Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol 2013;49:764–6. [Google Scholar]
- 65.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Intervenciones para mejorar la adherencia a la medicación (Revisión Cochrane traducida). In: Library TC, editor. A Biblioteca Cochrane Plus. Chichester, United Kingdom: John Wiley & Sons, Ltd; 2008. [Google Scholar]
- 67.Pozzar M, Volpato E, Valota C, Pagnini F, Banfi PI. How people with chronic obstructive pulmonary disease perceive their illness: a qualitative study between mind and body. BMC Pulm Med 2020;20:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blasini M, Corsi N, Klinger R, Colloca L. Nocebo and pain: an overview of the psychoneurobiological mechanisms. Pain Rep 2017;2:e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pagnini F, Cavalera C, Volpato E, Banfi P. Illness expectations predict the development of influenza-like symptoms over the winter season. Complement Ther Med 2020;50:102396. [DOI] [PubMed] [Google Scholar]
- 70.Kale MS, Federman AD, Krauskopf K, Wolf M, O’Conor R, Martynenko M, Leventhal H, Wisnivesky JP. The association of health literacy with illness and medication beliefs among patients with chronic obstructive pulmonary disease. PLoS One 2015;10:e0123937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krauskopf K, Federman AD, Kale MS, Sigel KM, Martynenko M, O’Conor R, Wolf MS, Leventhal H, Wisnivesky JP. Chronic obstructive pulmonary disease illness and medication beliefs are associated with medication adherence. COPD 2015;12:151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrison SL, Robertson N, Graham CD, Williams J, Steiner MC, Morgan MDL, Singh SJ. Can we identify patients with different illness schema following an acute exacerbation of COPD: a cluster analysis. Respir Med 2014;108:319–28. [DOI] [PubMed] [Google Scholar]
- 73.Fischer MJ, Scharloo M, Abbink JJ, van ’t Hul AJ, van Ranst D, Rudolphus A, Weinman J, Rabe KF, Kaptein AA. Drop-out and attendance in pulmonary rehabilitation: the role of clinical and psychosocial variables. Respir Med 2009;103:1564–71. [DOI] [PubMed] [Google Scholar]
- 74.Tselebis A, Kosmas E, Bratis D, Pachi A, Ilias I, Harikiopoulou M, Theodorakopoulou E, Velentzas K, Dumitru S, Moussas G, Siafakas N, Siafakas N. Contribution of psychological factors in dropping out from chronic obstructive pulmonary disease rehabilitation programs. Biomed Res Int 2014;2014:401326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. COPD 2010;7:315–22. [DOI] [PubMed] [Google Scholar]
- 76.van Reenen M, Janssen B. EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument. Rotterdam, the Netherlands: EuroQol Research Foundation; 2015. [Google Scholar]


