Abstract
BACKGROUND
Female patients undergoing anticancer treatment are at elevated risk of adverse ovarian outcomes including infertility and premature ovarian insufficiency (POI), which is associated with short- and long-term health risks. Anti-Müllerian hormone (AMH) is a key biomarker of ovarian reserve, but its role prior to and after cancer treatment is less well understood.
OBJECTIVE AND RATIONALE
To conduct a systematic review evaluating AMH as a biomarker of ovarian reserve and POI before and after anticancer treatment, which has become a pressing clinical issue in reproductive medicine. There are a large number of observational studies, but differences in patient groups, cancer diagnoses and study design make this a confusing field that will benefit from a thorough and robust review.
SEARCH METHODS
A systematic literature search for AMH in women with cancer was conducted in PubMed, Embase and Cochrane Central Register of Controlled Trials up to 1 April 2021. Bias review was conducted using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) protocol along with qualitative assessment of quality. Exploratory subgroups were established based on age, cancer type and length of follow-up.
OUTCOMES
Ninety-two publications (N = 9183 patients) were included in this analysis after quality and bias review. Reduced/undetectable AMH was consistently identified in 69/75 studies (92%) following chemotherapy or radiotherapy, with reductions ranging from 42% to concentrations below the limit of detection, and many reporting mean or median declines of ≥90%. Where longitudinal data were analysed (42 studies), a majority (33/42 (79%)) of studies reported at least partial recovery of AMH at follow-up, however, effect estimates were highly variable, reflecting that AMH levels were strongly impacted by anticancer treatment (i.e. the chemotherapy regimen used and the number of treatment cycles need), with recovery and its degree determined by treatment regimen, age and pre-treatment AMH level. In 16/31 (52%) publications, oligo/amenorrhoea was associated with lower post-treatment AMH consistent with impending POI, although menstruation and/or pregnancy were reported in patients with low or undetectable AMH. Long-term (>5 years) follow-up of paediatric patients following cancer treatment also found significantly lower AMH compared with control groups in 14/20 (70%) of studies, with very variable effect sizes from complete loss of AMH to full recovery depending on treatment exposure, as in adult patients.
WIDER IMPLICATIONS
AMH can be used to identify the damaging effect of cancer treatments on ovarian function. This can be applied to individual women, including pre-pubertal and adolescent girls, as well as comparing different treatment regimens, ages and pre-treatment AMH levels in populations of women. While there was evidence for its value in the diagnosis of POI after cancer treatment, further studies across a range of diagnoses/treatment regimens and patient ages are required to clarify this, and to quantify its predictive value. A major limitation for the use of AMH clinically is the very limited data relating post-treatment AMH levels to fertility, duration of reproductive lifespan or time to POI; analysis of these clinically relevant outcomes will be important in further research.
Keywords: anti-Müllerian hormone, AMH, fertility, cancer, gonadotoxicity, ovarian reserve, ovarian insufficiency, chemotherapy
Introduction
Anticancer treatments are known to have substantial impact on ovarian reserve via mechanisms leading to depletion of growing and primordial follicles. This reduction in ovarian reserve may reduce reproductive lifespan and be sufficient in some women to induce premature ovarian insufficiency (POI; Fig. 1; Spears et al., 2019). This has profound implications for women’s health and is associated with infertility, cardiovascular disease (CVD), neurological disease, osteoporosis and mood disorders, culminating in an increased risk of premature mortality (Webber et al., 2016; van Dorp et al., 2018; Panay et al., 2020). Consequently, knowing a patient’s treatment-related risk of POI aids shared decision-making around the choice of anticancer treatment and the need for additional fertility preservation interventions.
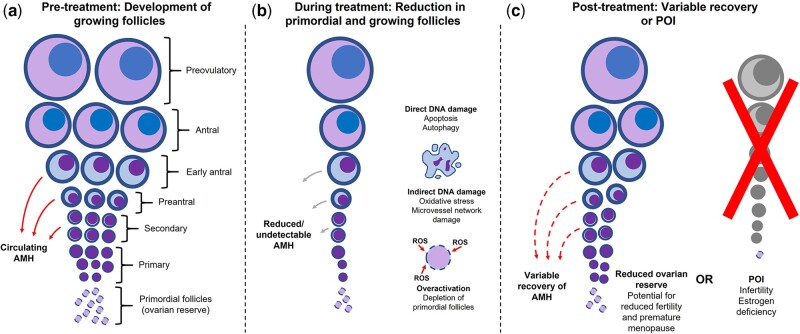
Figure 1.
Putative mechanisms of impact of cancer treatment on ovarian function. (a) In premenopausal individuals, circulating AMH is produced by secondary, preantral and early antral growing follicles, and has been shown in animal models to be one of several molecules which contribute to maintenance of ovarian reserve by inhibiting folliculogenesis; (b) anticancer treatment can reduce the ovarian pool of primordial follicles either by direct or indirect DNA damage, or by overactivation and subsequent depletion of primordial follicles; (c) following treatment, a patient may experience some recovery of the number of AMH-producing growing follicles, depending on the impact of anticancer treatment, or POI. In patients who recover ovarian function, a reduced pool of primordial follicles may still lead to an increased risk of later POI and infertility. AMH, anti-Müllerian hormone; POI, premature ovarian insufficiency; ROS, reactive oxygen species
Measurement of circulating anti-Müllerian hormone (AMH), produced by granulosa cells of growing follicles, is widely used as a surrogate for ovarian reserve and has potential as a diagnostic and predictive biomarker of POI (Anderson and Su 2020). AMH levels are often heavily impacted during anticancer treatment, followed by a highly variable recovery (Anderson and Su 2020). The diagnosis of POI is informed by monitoring a combination of biomarkers, including menstrual function and FSH (Webber et al., 2016; Panay et al., 2020). However, confirming POI is more challenging in patients with cancer, as there is potential for recovery of ovarian/menstrual function, which can occur from several months to years after treatment completion (Silva et al., 2016).
Questions remain about the reliability of AMH for evaluating ovarian reserve in the context of different anticancer treatments, and at what point post-treatment it can provide useful predictive information which may guide later treatment decisions, such as choice of endocrine therapy in women with breast cancer. Our objective was to conduct a systematic review evaluating the value of AMH in assessing ovarian reserve in women with cancer undergoing anticancer treatments, and specifically in the prediction and diagnosis of POI.
Methods
This study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered with PROSPERO (CRD42020161717). PubMed, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) were searched for articles published up to 1 April 2021 (Supplementary Data) and duplicate articles removed.
Search results were screened in stages by title, abstract and full text based on the following inclusion criteria: female, premenopausal patients who have prospectively or retrospectively undergone treatment for any cancer. Exclusion criteria included pathology other than cancer (with the exception of granulosa cell tumours), AMH/ovarian reserve not measured, cohort size <10, duplicate datasets/cohorts, poor quality (e.g. inconsistent reporting of results), review articles and congress abstracts.
Publications requiring evaluation of full text to determine inclusion underwent bias review via the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) protocol (Sterne et al., 2016). Bias conclusions were verified by a second author, with any disagreements mediated by a third author. All authors approved the final selection.
Data were extracted using the Synthesis Without Meta-analysis (SWiM) in systematic reviews reporting guideline (Campbell et al., 2020). The main metrics assessed were differences in AMH following anticancer treatment (change versus pre-treatment or control groups), recovery of AMH (in longitudinal cohorts) and correlation of AMH with menstrual function (regular cycles, oligomenorrhoea or amenorrhoea as reported by patient at follow-up). Certainty of evidence was based on vote counting.
Extracted data also included description of study types (prospective/retrospective, cross-sectional, longitudinal), year of publication, cancer type, treatment, cohort mean/median age, timing of AMH measurement and assay type. Exploratory subgroups were established according to age (paediatric, adult, </≥35 years), diagnosis (breast cancer or lymphoma) and length of follow-up.
The data underlying this article are available in the article and in its online supplementary material, or as referenced publications.
Results
Study characteristics
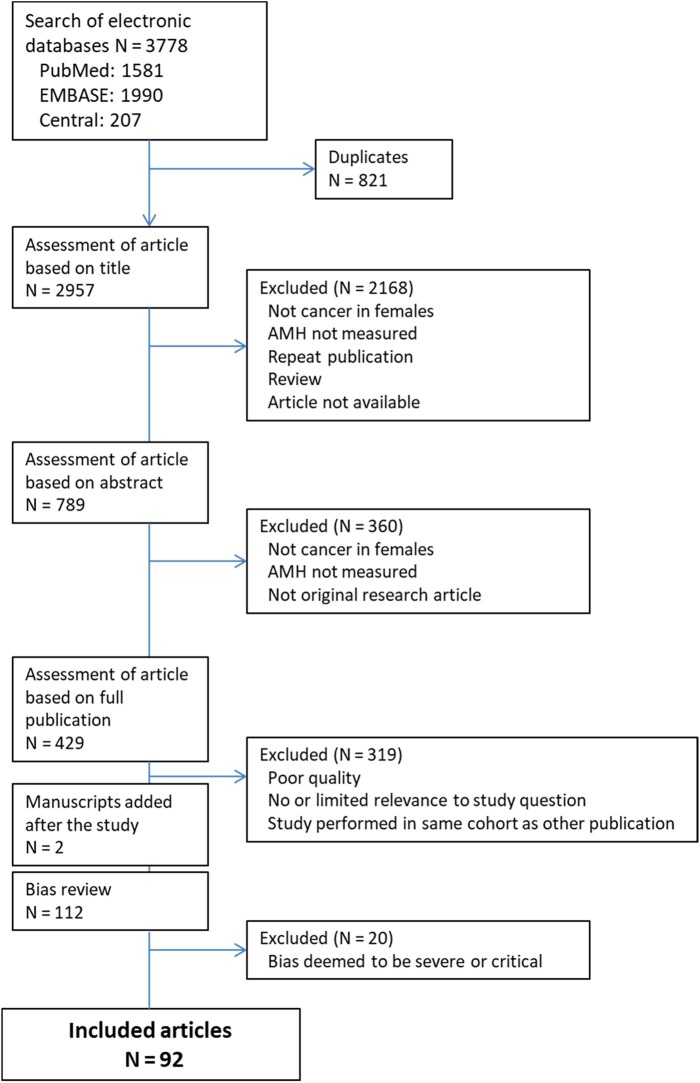
Figure 2 shows the number of citations retrieved, the number after screening and the final number included in the analysis. The searches initially identified over 3700 citations. After removal of duplicates and assessment for quality and relevance to the study question at the title, abstract and full-text level, 110 were included for bias review. Of these, a further 20 were assessed to be at severe or critical risk of bias and excluded (Supplementary Table SI), leaving 90 publications including 8916 patients (N) for systematic review, published between 2003 and 2021. During the publication review process, a further two relevant articles that were published after 1 April 2021 were identified (total 112 for bias review), resulting in a total of 92 publications and 9183 patients. An overview of publications included in this review is summarized in Table I, and details of the individual publications are summarized in Supplementary Table SII.
Figure 2.
Database search results and exclusion flow of publications. AMH, anti-Müllerian hormone.
Table I.
Summary of publications included in review.
| Subgroups | Number of publications | Number of patients | AMH reduction reported during/after anticancer treatment | AMH correlated with menstrual function* |
|---|---|---|---|---|
| All publications | 92 | 9183 | 69/75 (92%) | 15/30 (50%) |
| Longitudinal (pre-/post-treatment) | 56 | 4825 | 46/49 (94%) | 11/18 (61%) |
| Cross-sectional (post-treatment data only) | 36 | 4358 | 23/26 (88%) | 4/12 (33%) |
| Cancer type† | ||||
| Breast | 38 | 3600 | 31/31 (100%) | 11/18 (61%) |
| Lymphoma | 11 | 1199 | 9/9 (100%) | 3/43 (75%) |
| Multiple | 33 | 3877 | 21/25 (84%) | 2/8 (25%) |
| Other‡ | 11 | 573 | 9/11 (82%) | 0/1 (0%) |
| Follow-up period (mean/median) | ||||
| <1 year | 11 | 796 | 9/9 (100%) | 4/6 (67%) |
| 1–2 years | 36 | 2943 | 33/33 (100%) | 7/15 (47%) |
| >2–5.5 years | 19 | 2010 | 15/15 (100%) | 3/6 (50%) |
| >5.5 years | 26 | 3434 | 12/18 (67%) | 1/4 (25%) |
| Cohort age | ||||
| Paediatric | 24 | 2392 | 14/19 (74%) | 1/6 (17%) |
| Adult | 61 | 5991 | 48/49 (98%) | 13/22 (59%) |
| Mixed | 7 | 800 | 7/7 (100%) | 1/3 (33%) |
| <35 years old at follow-up§ | 57 | 5917 | 43/47 (91%) | 6/16 (38%) |
| ≥35 years old at follow-up§ | 33 | 2999 | 24/26 (92%) | 8/13 (62%) |
*Regular menstruation, oligomenorrhoea or amenorrhoea at follow-up.
N = 91 as Palinska-Rudzka et al. (2019) included separate analysis for breast cancer and lymphoma.
Acute lymphoblastic leukaemia, differentiated thyroid cancer and gestational trophoblastic neoplasia.
Mean or median as reported.
AMH, anti-Müllerian hormone.
Changes in AMH as a result of anticancer treatments
Papers reporting AMH before and after treatment (N = 46; N = 4117), and a clear majority of cross-sectional papers comparing post-treatment survivors with control groups (23/26, 88%; n/N = 2283/3088) reported a large reduction in AMH following treatment (Table I;Supplementary Table SII). Effect sizes were variable depending on the treatment and diagnosis, but in the 26 papers that reported AMH values at both baseline and ≤3 months from end of treatment (Lutchman Singh et al., 2007; Decanter et al., 2010, 2018, 2021; Rosendahl et al., 2010; Yu et al., 2010; Brougham et al., 2012; Henry et al., 2014; Ben-Aharon et al., 2015; Gharwan et al., 2016; Gupta et al., 2016; Anderson et al., 2017, 2018; Bi et al., 2017; D'Avila et al., 2017; Leonard et al., 2017; Trapp et al., 2017; Cameron et al., 2018; Evranos et al., 2018; Yaish et al., 2018; Passildas et al., 2019; Silva et al., 2019; Loubersac et al., 2020; Berjeb et al., 2021; Demeestere et al., 2021; Martin et al., 2021), reductions ranged from 42% to below the limit of detection (LOD) and 18 reported mean or median declines of ≥90% (Rosendahl et al., 2010; Brougham et al., 2012; Dillon et al., 2013a, b; Henry et al., 2014; Gharwan et al., 2016; Ben-Aharon et al., 2015; D'Avila et al., 2017; Dezellus et al., 2017; Trapp et al., 2017; Cameron et al., 2018; Decanter et al., 2018; Lambertini et al., 2019; Palinska-Rudzka et al., 2019; Passildas et al., 2019; Silva et al., 2019; Oktay et al., 2020; Ruddy et al., 2021; Yu et al., 2010). The six papers that did not detect a significant difference in AMH versus controls after treatment were all in paediatric populations (i.e. under 18 years of age at cancer diagnosis) and represented long-term follow-up periods of 10–30 years in heterogeneous populations of childhood cancer survivors (Lie Fong et al., 2009; Nielsen et al., 2013; van den Berg et al., 2018; Nystrom et al., 2019; Nies et al., 2020; Elitzur et al., 2021). A shorter-term longitudinal study (follow-up of up to 43 months) in this population did, however, show clear reductions in AMH following treatment (Brougham et al., 2012), with large differences in the degree of recovery of AMH by treatment gonadotoxicity: thus in those exposed to high-risk treatment, AMH remained undetectable, while recovering to levels similar to pre-treatment in those exposed to lower risk treatment.
Recovery of AMH in longitudinal studies
In publications examining longitudinal data, post-treatment recovery of AMH was described in 33/42 (79%). With several notable exceptions described below, recovery was typically partial (i.e. to lower than pre-treatment level) or only occurred in a subset of patients. Twelve publications specifically evaluated the association of pre- and post-treatment AMH levels, with all reporting a significant association (Rosendahl et al., 2010; Dillon et al., 2013b; D’Avila et al., 2015; Dezellus et al., 2017; Anderson et al., 2018; Lee et al., 2018, 2020; Palinska-Rudzka et al., 2019; Silva et al., 2019; Celebi et al., 2020; Loubersac et al., 2020; Decanter et al., 2021).
Overall relationship of AMH with measures of POI and menstrual function after anticancer treatment
Ten publications specifically evaluated either the value of post-treatment AMH in the diagnosis of POI, or pre-treatment AMH in predicting the likelihood of POI. These studies were either in breast cancer or across multiple diagnoses, representing 762 patients (Nielsen et al., 2013; Lunsford et al., 2014; Elchuri et al., 2016; Anderson et al., 2017; Nystrom et al., 2019; Passildas et al., 2019; Silva et al., 2019; Zhong et al., 2019; Demeestere et al., 2021; Parissone et al., 2021). Six studies (two of which were in paedia3tric cohorts) reported post-treatment AMH levels <1.0 ng/ml or undetectable in patients with POI (Nielsen et al., 2013; Lunsford et al., 2014; Anderson et al., 2017; Silva et al., 2019; Zhong et al., 2019; Parissone et al., 2021) but the diagnostic accuracy of AMH for post-treatment POI has only been reported in one study (Anderson et al., 2017). Two papers found that lower pre-treatment AMH levels were associated with higher risk of POI (as assessed) post-treatment (Passildas et al., 2019; Zhong et al., 2019), but no studies have assessed post-treatment AMH as a predictor of time to POI.
Of 37 papers that specifically investigated menstrual function after treatment, all used prospective evaluation, and 14 (38%) found that reduced post-treatment AMH was associated with oligomenorrhoea or amenorrhoea (Rosendahl et al., 2008; Su et al., 2010; Lunsford et al., 2014; Ruddy et al., 2014; D'Avila et al., 2015; Gharwan et al., 2016; Palinska-Morarji et al., 2017; Wenners et al., 2017; Decanter et al., 2018, 2021; Kim et al., 2018; Palinska-Rudzka et al., 2019; Silva et al., 2019; Li et al., 2020). In some of these studies, the observations were nuanced, for example Su et al. (2010) found that post-treatment AMH was associated with amenorrhoea but was not associated with recovery of menses (at 5.2 years post-treatment follow-up). Palinska-Rudzka et al. (2019) found that AMH was lower in patients with amenorrhoea, but that some (7/17) patients with AMH <LOD had menses. Gharwan et al. (2016) found AMH was associated with menstrual status only in woman aged 21−25 years old. Dezellus et al. (2017) found an association between AMH and menstrual status at 6 months of post-treatment follow-up, but not at 12 months of follow-up. Further studies are required to assess with rigour whether measurement of AMH adds to or can replace current diagnostic criteria for POI.
Post-treatment AMH and pregnancy
A small number of studies have reported pregnancies in some patients despite low or undetectable AMH levels after cancer treatment (Hamy et al., 2016; Dezellus et al., 2017; Anderson et al., 2018; Loubersac et al., 2020; Demeestere et al., 2021). A recent analysis of AMH in women treated for advanced Hodgkin lymphoma in a randomized controlled trial showed similar pregnancy rates in the two treatment arms after 5 years of follow-up, despite much lower AMH levels and a higher POI rate in the arm receiving higher doses of alkylating agents (Demeestere et al., 2021). This is consistent with the lack of predictive value of AMH for pregnancy in healthy women (Steiner et al., 2017), but these limited data on a clinically highly relevant topic highlight the need for further prospective studies to inform on this subject.
Age at treatment, ovarian reserve and extent of recovery
Of 39 publications that evaluated whether patient age at treatment was associated with post-treatment AMH levels, 30 (77%) found higher post-treatment AMH in younger individuals (Lutchman Singh et al., 2007; Lie Fong et al., 2009; Charpentier et al., 2014; Henry et al., 2014; Ben-Aharon et al., 2015; D'Avila et al., 2015; Acibucu et al., 2016; Elchuri et al., 2016; Anderson et al., 2017, 2018; Dezellus et al., 2017; Morarji et al., 2017; Shandley et al., 2017; Wenners et al., 2017; Al-Janabi et al., 2018; Evranos et al., 2018; Lee et al., 2018; Malisic et al., 2018; Yaish et al., 2018; Cameron et al., 2019; Silva et al., 2019; Celebi et al., 2020; Li et al., 2020; Loubersac et al., 2020; Su et al., 2020; van Velsen et al., 2020; Berjeb et al., 2021; Decanter et al., 2021; Elitzur et al., 2021; Martin 2021). Thirteen of these studies also stratified patient groups by <35 versus ≥35 years old, with 10 (83%) reporting lower AMH and poorer recovery in the older age group (Yu et al., 2010; Ben-Aharon et al., 2015; Acibucu et al., 2016; Dezellus et al., 2017; Trapp et al., 2017; Al-Janabi et al., 2018; Anderson et al., 2018; van den Berg et al., 2018; Yaish et al., 2018; Li et al., 2020; Mittica et al., 2020; van Velsen et al., 2020; Decanter et al., 2021). However, only five publications both stratified these two age groups and evaluated menstrual function, with three also showing a reduced chance of recovery of menstruation in older women (Yu et al., 2010; Dezellus et al., 2017; Decanter et al., 2021).
Studies investigating gonadotoxicity of anticancer treatments
Fifty-two papers specifically investigated gonadotoxicity of treatments either by comparison of groups receiving specific regimens or by cumulative toxicity scores (e.g. cyclophosphamide equivalent dose [CED]; summarized in Supplementary Table SIII). In order to specifically evaluate treatment effect, papers that evaluated patient risk of gonadotoxicity based on other factors, such as diagnosis and disease stage, were not counted. Forty papers (77%) reported a treatment effect on AMH, with higher gonadotoxicity correlating with lower post-treatment AMH. In general, chemotherapy regimens containing alkylating agents, such as cyclophosphamide (e.g. ACVBP, BEACOPP, CHOP, FEC), resulted in lower levels of AMH and poorer recovery of AMH after treatment than non-alkylating treatment regimens (Rosendahl et al., 2008; Gracia et al., 2012; Thomas-Teinturier et al., 2015; Morarji et al., 2017; Anderson et al., 2018; Leiper et al., 2020; Decanter et al., 2021).
Twenty-one publications reported gonadotoxicity scores or ranked treatments as higher/lower toxicity or higher/lower risk, based on treatment type and/or cumulative chemotherapy. In every study where higher versus lower toxicity was assessed, higher toxicity therapies and more treatment cycles resulted in lower post-treatment AMH compared with lower overall toxicity exposure (Rosendahl et al., 2010; Brougham et al., 2012; Gracia et al., 2012; Hamre et al., 2012; Di Paola et al., 2013; Nielsen et al., 2013; Krawczuk-Rybak et al., 2013a, 2013b, 2019; Charpentier et al., 2014; Lunsford et al., 2014; Thomas-Teinturier et al., 2015; Elchuri et al., 2016; van der Kooi et al., 2017, 2019; van den Berg et al., 2018; Cameron et al., 2019; George et al., 2019; Su et al., 2020; van Velsen et al., 2020; Parissone et al., 2021).
The evidence regarding radiotherapy was less conclusive as its use was only cited in 10 publications (van Beek et al., 2007; Lie Fong et al., 2009; Brougham et al., 2012; Gracia et al., 2012; Dillon et al., 2013b; Miyoshi et al., 2013; Elchuri et al., 2016; van den Berg et al., 2018; George et al., 2019; Nies et al., 2020). However, in studies where radiotherapy did result in significantly lower AMH, this was typically when targeted to pelvic/abdominal regions or total body irradiation (Lie Fong et al., 2009; Gracia et al., 2012; Miyoshi et al., 2013; Elchuri et al., 2016; van den Berg et al., 2018; George et al., 2019).
AMH in patients with breast cancer
Thirty-eight papers evaluated AMH levels in women following treatment for breast cancer, representing a total of 3600 patients (Table II). All reported a negative treatment effect on AMH, and in 12 papers with data available, reductions in AMH from pre-treatment to <3 months post-treatment ranged from ∼80% to 99% (Lutchman Singh et al., 2007; Yu et al., 2010; Henry et al., 2014; Ben-Aharon et al., 2015; Anderson et al., 2017; D'Avila et al., 2017; Leonard et al., 2017; Trapp et al., 2017; Decanter et al., 2018; Passildas et al., 2019; Silva et al., 2019; Loubersac et al., 2020). Of those publications with available data, 13/16 found that post-treatment AMH levels were associated with age at baseline (Lutchman Singh et al., 2007; Henry et al., 2014; Ben-Aharon et al., 2015; D'Avila et al., 2015; Anderson et al., 2017; Dezellus et al., 2017; Wenners et al., 2017; Al-Janabi et al., 2018; Lee et al., 2018; Silva et al., 2019; Celebi et al., 2020; Li et al., 2020; Loubersac et al., 2020). Some degree of recovery in average AMH levels during follow-up was reported in 14/20 publications; however, the extent of this recovery was marginal (<15% average recovery from nadir levels as a percentage of baseline values) in 12 publications, and only partial in the remainder (range of follow-up 0–5 years), with none demonstrating a return of AMH to pre-treatment levels (Yu et al., 2010; Henry et al., 2014; Ben-Aharon et al., 2015; Anderson et al., 2017; Dezellus et al., 2017; Leonard et al., 2017; Decanter et al., 2018; Lambertini et al., 2019; Palinska-Rudzka et al., 2019; Silva et al., 2019; Zhong et al., 2019; Loubersac et al., 2020; Oktay et al., 2020; Goldfarb et al., 2021). In all studies reporting no recovery of AMH levels, patients received treatment with alkylating agents, such as cyclophosphamide-based chemotherapy regimens; however, higher pre-treatment AMH and lower age were identified as factors associated with greater recovery after these regimens (Yu et al., 2010; Anderson and Cameron 2011; Anderson et al., 2013; Elgindy et al., 2013; D'Avila et al., 2017; Wenners et al., 2017; Palinska-Rudzka et al., 2019; Zhong et al., 2019).
Table II.
Characteristics of studies in breast cancer cohorts included in the systematic review (N = 38).
| Publication | Study design | Adult/ paediatric | Cohort size | Timing of AMH assessment | AMH assay | LOD (ng/ml) | Reduced/low AMH following anticancer treatment* | Correlation of post-treatment AMH with menstrual function † | Correlation of post-treatment AMH recovery with age |
|---|---|---|---|---|---|---|---|---|---|
| Al-Janabi et al. (2018) | Prospective longitudinal | Adult | 60 | BL, after first menses after 1st dose, after 4th dose | Gen II ELISA (Beckman Coulter) | Not reported | Yes | N/A | Yes |
| Anderson et al. (2011) | Prospective longitudinal | Adult | 42 | BL, PTFU 2, 3, 4, 5 years | ELISA (Beckman Coulter) | 0.05 | Yes | Yes | N/A |
| Anderson et al. (2013) | Prospective longitudinal | Adult | 55 | BL, after 1st and 2nd cycle, PTFU 1 year | Gen II ELISA (Beckman Coulter) | 0.16 | Yes | Yes | N/A |
| Anderson et al. (2017) | Prospective longitudinal | Adult | 101 | BL, PTFU 0, 12, 24 months | Elecsys® AMH (Roche Diagnostics) | 0.01 | Yes | N/A | Yes |
| Ben-Aharon et al. (2015) | Prospective longitudinal | Adult | 20 | BL, PTFU 3–4 days, 6, 12 months | ELISA (Beckman Coulter) | 0.01 | Yes | N/A | Yes |
| Çelebi et al. (2020) | Prospective cross-sectional | Adult | 31 | BL, during/after treatment every 3 months up to 12 months | ELISA | Not reported | Yes | N/A | Yes |
| D'Avila et al. (2015) | Prospective longitudinal | Adult | 52 | BL, PTFU 6 months | ELISA (Beckman Coulter) | Not reported | Yes | Yes | Yes |
| D'Avila et al. (2017) | Prospective longitudinal | Adult | 52 | BL, PTFU 2, 6 months | ELISA (Beckman Coulter—Immunotech) | Not reported | Yes | Yes | N/A |
| Decanter et al. (2018) | Prospective longitudinal | Adult | 32 | BL, 15 days before last cycle, PTFU 3, 6, 9 and 12 months | picoAMH ELISA (Ansh) | 0.0224 (0.16 pmol/l) | Yes | Yes | N/A |
| Dezellus et al. (2017) | Prospective cross-sectional | Adult | 249 | BL, before each of 6 cycles, PTFU 6, 12 and 24 months | ELISA (Beckman Coulter—Immunotech) | 0.14 | Yes | No | Yes |
| Elgindy et al. (2013) | Prospective cross-sectional | Adult | 100 | BL, PTFU 12 months | Not reported | Not reported | Yes | N/A | N/A |
| Goldfarb et al. (2020) | Prospective cross-sectional | Adult | 142 | BL, PTFU 12, 18 and 24 months | picoAMH ELISA (Ansh Labs) | Not reported | Yes | N/A | N/A |
| Hamy et al. (2016) | Prospective cross-sectional | Adult | 134 | BL, during treatment, PTFU 4–66 months | ELISA (Beckman Coulter—Immunotech) | 0.14 | N/A | N/A | N/A |
| Henry et al. (2014) | Prospective cross-sectional | Adult | 27 | BL, mean PTFU 4.9 weeks, 13.6 months | Gen II ELISA (Beckman Coulter) | 0.16 | Yes | No | Yes |
| Kim et al. (2018) | Prospective cross-sectional | Adult | 82 | PTFU <2 months | ELISA (USCN Life Science) | 0.003 | N/A | Yes | N/A |
| Lambertini et al. (2019) | Prospective longitudinal | Adult | 148 | BL, PTFU 1, 3 years | Elecsys AMH (Roche Diagnostics) | 0.01 | Yes | N/A | N/A |
| Lee et al. (2018) | Prospective cross-sectional | Adult | 105 | PTFU 508 days | Gen II ELISA (Beckman Coulter) | 0.16 | N/A | No | Yes |
| Lee et al. (2020) | Prospective cross-sectional | Adult | 67 | BL, PTFU 1 year | Gen II ELISA (Beckman Coulter) | 0.16 | Yes | No | No |
| Leonard et al. (2017) | Prospective cross-sectional | Adult | 202 | BL, during treatment (C3), PTFU 0, 9, 12, 24 months | Elecsys AMH (Roche Diagnostics) | Not reported | Yes | N/A | N/A |
| Li et al. (2020) | Prospective cross-sectional | Adult | 144 | BL, PTFU 6 months | Human AMH ELISA (Cusabio Biotech) | Not reported | Yes | Yes | Yes |
| Lutchman Singh et al. (2007) | Prospective cross-sectional | Adult | 35 | BL, PTFU after resumption of menses | ELISA (Beckman Coulter—Immunotech) | Not reported | Yes | N/A | Yes |
| Malisic et al. (2018) | Prospective cross-sectional | Adult | 40 | PTFU 1–66 months | ELISA (Ansh Labs) | 0.023 | N/A | N/A | Yes |
| Morarji et al. (2017) | Prospective longitudinal | Adult | 100 | PTFU 2 years | Gen II ELISA (Beckman Coulter) | 0.08 | Yes | Yes | Yes |
| Oktay et al. (2020) | Prospective cross-sectional | Adult | 108 | BL, PTFU <24 months | picoAMH ELISA (Ansh Labs) | 0.003 | Yes | N/A | N/A |
| Palinska-Rudzka et al. (2019) | Prospective longitudinal | Adult | 54 | BL, PTFU 1, 5 years | Gen II ELISA (Beckman Coulter) | 0.02 | Yes | Yes | N/A |
| Partridge et al. (2010) | Prospective cross-sectional | Adult | 20 | PTFU 5 years | ELISA (Diagnostic Systems Laboratories) | 0.03 | Yes | N/A | N/A |
| Passildas et al. (2019) | Prospective longitudinal | Adult | 58 | BL, PTFU 1 month | Elecsys AMH (Roche Diagnostics) | 0.03 | Yes | No | N/A |
| Ruddy et al. (2014) | Retrospective cross-sectional | Adult | 124 | BL, PTFU 12, 18 months | ELISA (Diagnostic Systems Laboratories) | Not reported | N/A | Yes | N/A |
| Ruddy et al. (2021) | Prospective longitudinal | Adult | 277 | BL, PTFU 1 year | Ultrasensitive ELISA (Ansh Labs) | Not reported | Yes | N/A | N/A |
| Shandley et al. (2017) | Prospective longitudinal | Adult | 108 | PTFU 7 years | Ultrasensitive ELISA and pico AMH ELISA (Ansh Labs) | 0.006 | N/A | N/A | Yes |
| Silva et al. (2019) | Prospective longitudinal | Adult | 38 | BL, during treatment, PTFU 1, 6 and >9 months up to 18 months | Ultrasensitive ELISA (Ansh Labs) | 0.06 | Yes | Yes | Yes |
| Su et al. (2014) | Prospective longitudinal | Adult | 109 | BL, PTFU 1–1009 days | Gen II ELISA (Beckman Coulter) | 0.17 | N/A | N/A | N/A |
| Su et al. (2010) | Prospective longitudinal | Adult | 127 | PTFU 1–4 years | ELISA (Diagnostic Systems Laboratories) | 0.025 | Yes | Yes | N/A |
| Trapp et al. (2017) | Prospective cross-sectional | Adult | 170 | BL, PTFU 2 years | ELISA (Beckman Coulter) | 0.08 | Yes | No | No |
| Wenners et al. (2017) | Prospective longitudinal | Adult | 51 | BL, during treatment, PTFU 6, 12, 24 months | Elecsys AMH (Roche Diagnostics) | Not reported | Yes | Yes | Yes |
| Yu et al. (2010) | Prospective longitudinal | Adult | 26 | BL, PTFU 6, 12, 24, 36, 52 weeks | ELISA (Diagnostic Systems Laboratories) | Not reported | Yes | No | No |
| Zhong et al. (2019) | Prospective cross-sectional | Adult | 96 | BL, PTFU 6, 12 months | Immunoassay (Beckman Coulter Unicel DX1800) | Not reported | Yes | N/A | N/A |
*Between baseline and follow-up in longitudinal studies, and versus controls in cross-sectional studies.
Presence of regular cycles, oligomenorrhoea or amenorrhoea.
AMH: anti-Müllerian hormone; BL, baseline; LOD, limit of detection; N/A, not applicable; PTFU, post-treatment follow-up.
In patients with breast cancer, post-treatment AMH was also linked to menstrual function at follow-up in 11/18 publications (Su et al., 2010; Ruddy et al., 2014; D'Avila et al., 2015, 2017; Morarji et al., 2017; Wenners et al., 2017; Decanter et al., 2018; Kim et al., 2018; Palinska-Rudzka et al., 2019; Silva et al., 2019; Li et al., 2020). Patients with undetectable AMH generally had a higher risk of amenorrhoea, although several studies reported patients with low or undetectable AMH who did recover menstrual function.
The impact of BRCA status was investigated in two studies (Lambertini et al., 2019; Oktay et al., 2020), with one identifying increased loss of ovarian reserve and greater reduction in AMH post-treatment recovery in BRCA+ individuals (Oktay et al., 2020).
AMH in patients with lymphoma
Of 11 studies in lymphoma (Table III), all nine publications evaluating treatment effect on AMH in patients with lymphoma (including Hodgkin lymphoma), representing a total of 521 patients, found a significant impact (van Beek et al., 2007; Decanter et al., 2010, 2021; Nitzschke et al., 2010; Demeestere et al., 2016, 2021; Gharwan et al., 2016; Anderson et al., 2018; Palinska-Rudzka et al., 2019). Eight papers reported significant decline in AMH values from pre-treatment to ≤3 months’ post-treatment (Decanter et al., 2010, 2021; Demeestere et al., 2016, 2021; Gharwan et al., 2016; Anderson et al., 2018; Palinska-Rudzka et al., 2019). An additional study also reported significant decline in post-treatment AMH compared with controls (Nitzschke et al., 2010). All eight papers evaluating gonadotoxicity found a regimen- or dose-dependent effect on AMH following treatment and during recovery (van Beek et al., 2007; Decanter et al., 2010, 2021; Hamre et al., 2012; Behringer et al., 2013; Anderson et al., 2018; Palinska-Rudzka et al., 2019; Demeestere et al., 2021). Five of these studies identified that patients receiving doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD)-based regimens had at least partial post-treatment recovery of AMH at follow-up compared with those receiving cyclophosphamide-containing regimens (Decanter et al., 2010, 2021; Anderson et al., 2018; Palinska-Rudzka et al., 2019; Demeestere et al., 2021). One paper described limited recovery of AMH in older ABVD-treated patients (Anderson et al., 2018) whereas another found that the impact of age on recovery was only found in women treated with alkylating agents (Decanter et al., 2021).
Table III.
Characteristics of studies in lymphoma cohorts included in the systematic review (N = 11).
| Publication | Study design | Adult/ paediatric | Cohort size | Timing of AMH assessment | AMH assay | LOD (ng/ml) | Reduced/low AMH following anticancer treatment* | Correlation of post-treatment AMH with menstrual function † | Correlation of post-treatment AMH recovery with age |
|---|---|---|---|---|---|---|---|---|---|
| Anderson et al. (2018) | Prospective longitudinal | Adult | 67 | BL, after 2nd cycle, PTFU 0, 1, 2, 3 years | Elecsys AMH (Roche Diagnostics) | 0.01 | Yes | N/A | Yes |
| Behringer et al. (2013) | Prospective cross-sectional | Adult | 562 | PTFU 46 months | Gen II ELISA (Beckman Coulter) | Not reported | N/A | N/A | Yes |
| Decanter et al. (2010) | Prospective longitudinal | Adult | 30 | BL, 15 days after 1st cycle, 15 days before last cycle, PTFU 0, 3, 6, 9, 12 months | Gen II ELISA (Beckman Coulter) | 0.098 (0.7 pmol/L) | Yes | N/A | N/A |
| Decanter et al. (2021) | Prospective longitudinal | Adolescent, Young adult, Adult | 122 | BL, 15 days after 1st cycle, 15 days before last cycle, PTFU 3, 6, 9, 12 months | Gen II ELISA (Beckman Coulter) | Not reported | Yes | Yes | Yes |
| Demeestere et al. (2016) | Prospective longitudinal | Adult | 67 | BL, PTFU 2, 3, 4, 5–7 years | Elecsys AMH (Roche Diagnostics) | Not reported | Yes | N/A | N/A |
| Demeestere et al. (2021) | Prospective longitudinal | Adult | 145 | BL, PTFU 0, 1, 2, 3, 4, 5 years | Elecsys AMH (Roche Diagnostics) | 0.01 ng/ml | Yes | N/A | Yes |
| Gharwan et al. (2016) | Prospective longitudinal | Adult | 28 | BL, PTFU 4–18 months | Gen II ELISA (Beckman Coulter) | Not reported | Yes | Yes (in 21- to 25-year-old group) | N/A |
| Hamre et al. (2012) | Prospective cross-sectional | Paediatric | 62 | PTFU 18.1 years | ELISA (Beckman Coulter) | 0.15 | N/A | No | N/A |
| Nitzschke et al. (2010) | Prospective longitudinal | Adult | 20 | PTFU 30.6–32.2 months | ELISA (Diagnostic Systems Laboratories) | 0.025 | Yes | N/A | N/A |
| Palinska-Rudzka et al. (2019) | Prospective longitudinal | Adult | 12 | BL, PTFU 1, 5 years | Gen II ELISA (Beckman Coulter) | 0.02 | Yes | Yes | N/A |
| van Beek et al. (2007) | Prospective longitudinal | Paediatric | 30 | PTFU 11.6 years | ELISA (Diagnostic Systems Laboratories) | Not reported | Yes (in patients receiving MOPP) | N/A | No |
*Between baseline and follow-up in longitudinal studies, and vs controls in cross-sectional studies.
Presence of regular cycles, oligomenorrhoea or amenorrhoea.
AMH: anti-Müllerian hormone; BL, baseline; LOD, limit of detection; N/A, not applicable; PTFU, post-treatment follow-up.
Paediatric patients
The majority (20/29; 69%) of papers including paediatric cancer patients were cross-sectional studies reporting on adult childhood cancer survivors (Bath et al., 2003; van Beek et al., 2007; Lie Fong et al., 2009; Gracia et al., 2012; Hamre et al., 2012; El-Shalakany et al., 2013; Miyoshi et al., 2013; Nielsen et al., 2013; Krawczuk-Rybak et al., 2013b, 2019; Charpentier et al., 2014; Lunsford et al., 2014; Thomas-Teinturier et al., 2015; Elchuri et al., 2016; van der Kooi et al., 2017; van den Berg et al., 2018; Cameron et al., 2019; George et al., 2019; Nystrom et al., 2019; Nies et al., 2020), of which 19 had follow-up periods of >5 years. Reductions in AMH compared with pre-treatment levels or versus controls were reported by 14/20 (70%) publications (Bath et al., 2003; van Beek et al., 2007; Brougham et al., 2012; El-Shalakany et al., 2013; Krawczuk-Rybak et al., 2013a, 2013b, 2019; Elchuri et al., 2016; Gupta et al., 2016; Morse et al., 2016; van der Kooi et al., 2017, 2019; Cameron et al., 2019; George et al., 2019). As with adult cancer patients, the degree of reduction varied with treatment received, with complete loss of AMH in some patients. Low AMH levels were found in some cancer survivors despite normal FSH levels (e.g. in 20% of survivors (George et al., 2019)), indicating the added value of AMH in detecting partial ovarian damage. Additional studies reported trends to lower AMH, or low AMH levels in patients following childhood cancer that were not compared with a control group or baseline data (Hamre et al., 2012; Miyoshi et al., 2013; Nielsen et al., 2013; Charpentier et al., 2014; Lunsford et al., 2014; van den Berg et al., 2018; George et al., 2019). While these studies support that long-term AMH levels can be reduced in survivors of childhood cancer, important outcomes such as relationships with fertility and age at menopause have not been evaluated.
Most studies (10/12 (90.9%)) (van Beek et al., 2007; Lie Fong et al., 2009; Brougham et al., 2012; Hamre et al., 2012; Miyoshi et al., 2013; Krawczuk-Rybak et al., 2013a, 2019; Charpentier et al., 2014; Thomas-Teinturier et al., 2015; van der Kooi et al., 2019) which had data for both pre- and post-menarchal patients did not detect significant differences in post-treatment AMH by this parameter, and one reported better AMH recovery in pre-menarchal patients (van der Kooi et al., 2019). Two publications also reported delayed puberty in patients with low post-treatment AMH (El-Shalakany et al., 2013; Lunsford et al., 2014).
Impact of duration of follow-up
To assess the impact of cancer treatment on AMH levels during long-term follow-up, studies with <1 year of follow-up were excluded owing to the potential for a residual effect of cancer treatment and incomplete recovery. Based on median reported follow-up post-treatment periods, there were 35 studies with 1- to 2-year follow-up (Bath et al., 2003; Rosendahl et al., 2010; Yu et al., 2010; Brougham et al., 2012; Anderson et al., 2013, 2017; Elgindy et al., 2013; Iwase et al., 2013; Dillon et al., 2013b; Henry et al., 2014; Ruddy et al., 2014, 2021; Ben-Aharon et al., 2015; Gharwan et al., 2016; Gupta et al., 2016; Dezellus et al., 2017; Leonard et al., 2017; Morarji et al., 2017; Trapp et al., 2017; Wenners et al., 2017; Decanter et al., 2010, 2018; Evranos et al., 2018; Lee et al., 2018, 2020; Yaish et al., 2018; Silva et al., 2019; van der Kooi et al., 2019; Zhong et al., 2019; Celebi et al., 2020; Loubersac et al., 2020; Oktay et al., 2020; Berjeb et al., 2021; Goldfarb et al., 2021; Martin, 2021). Most (33/35; 96%) found that AMH was reduced after treatment, 19/25 (76%) later then identified some degree of increase in AMH at follow-up. Although only a few studies found a (very modest) increase in AMH after 12 months, several studies with a maximum post-treatment follow-up period of 12 months showed the highest post-treatment AMH value at 12 months, raising the possibility that further recovery could have been observed (Brougham et al., 2012; Ben-Aharon et al., 2015; Gupta et al., 2016; Decanter et al., 2018; Yaish et al., 2018). It therefore appears that 12 months after completion of treatment is a minimum period to allow complete or near-complete recovery of ovarian function: little recovery is seen thereafter and becomes confounded with the normal decline in AMH with increasing age.
Of 18 studies with a median >2–5.5 years of follow-up, 15/15 with available data (100%) identified AMH reductions in cancer survivors (Nitzschke et al., 2010; Partridge et al., 2010; Su et al., 2010; Anderson and Cameron 2011; El-Shalakany et al., 2013; Acibucu et al., 2016; Demeestere et al., 2016, 2021; Morse et al., 2016; Anderson et al., 2018; Cameron et al., 2019; Lambertini et al., 2019; Palinska-Rudzka et al., 2019; Chemerinski et al., 2020; van Velsen et al., 2020), 8/10 (80%) identified at least partial recovery at follow-up in ≥1 patient group (Demeestere et al., 2016, 2021; Morse et al., 2016; Anderson et al., 2018; Malisic et al., 2018; Cameron et al., 2019; Lambertini et al., 2019; Palinska-Rudzka et al., 2019) and 3/5 (60%) reported correlation of post-treatment AMH with menstrual function in some patients (Rosendahl et al., 2008; Su et al., 2010; Palinska-Rudzkaet al., 2019). Of 24 cross-sectional studies with follow-up >5.5 years, 11/17 (65%) found AMH reductions with treatment when compared with controls (van Beek et al., 2007; Gracia et al., 2012; Di Paola et al., 2013; Krawczuk-Rybak et al., 2013a, 2013b, 2019; Thomas-Teinturier et al., 2015; Elchuri et al., 2016; Leiper et al., 2020; Mittica et al., 2020; Roshandel et al., 2021) and 1/4 (25%) linked AMH to menstrual function in some patients (Lunsford et al., 2014). There are very limited data regarding continuing changes in AMH after cancer treatment and whether cancer survivors show an accelerated decline in AMH in the later reproductive years. In a mixed longitudinal/cross-sectional analysis, AMH levels were shown to be reduced according to gonadotoxicity but maintained a plateau for a prolonged period after treatment (Su et al., 2020). Supporting that, cancer survivors showed no evidence of an increased rate of decline in AMH compared to age-matched controls (Cameron et al., 2019).
Discussion
Anticancer treatment has a clear negative impact on women’s ovarian reserve, with reduced AMH levels observed across studies and diagnoses, and reductions of ≥90% commonly identified. Some degree of post-treatment recovery in AMH levels was described in many patient groups, although full- or near-complete restoration to pre-treatment levels was rare and typically occurred in patients receiving milder treatment. Figure 3 gives a representation of the key findings of this review. It is notable that there are relatively few prospective studies, and among those with longitudinal data, several had a maximum 1 year of follow-up. While AMH levels measured 1–2 years after treatment are likely to be reliable at indicating longer-term menstrual outcomes, further research is required to confirm this, especially in older women where recovery is slower and more limited (van Beek et al., 2007; Decanter et al., 2010; Behringer et al., 2013; Anderson et al., 2018; Palinska-Rudzka et al., 2019).
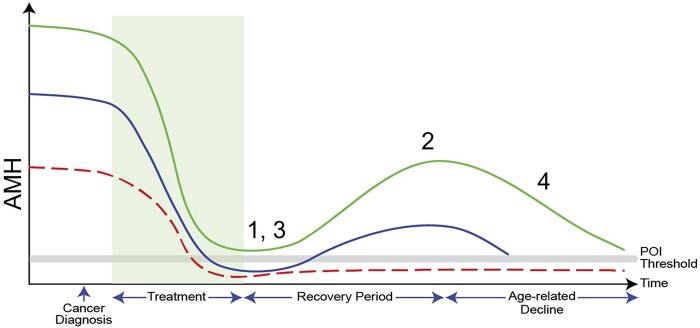
Figure 3.
Summary of findings of systematic review represented graphically. The three lines represent women with high (green), average (blue) and low (red) AMH levels before treatment, with treatment represented by the shaded area and a threshold indicating POI is also represented. (1) AMH concentrations are reduced by cancer treatments. (2) Recovery is variable, depending on patient age, treatment regimen and pre-treatment AMH levels. Recovery can be near complete or absent, with the latter resulting in permanent POI. The relationship of post-treatment AMH to POI needs to be explored further. (3) Prediction of permanent POI at the time of end of treatment may be possible in some situations, but further studies are required to determine the patient groups for which this may and may not be possible. (4) There are insufficient data to be able to predict the duration of post-recovery ovarian function using AMH levels before or after treatment, which will interact with the physiological decline in AMH with increasing age. Reproduced from Jayasinghe et al. (2018) with permission.
Treatments known to be more gonadotoxic, whether this be drug or cumulative exposure related, generally caused a greater reduction in AMH. The clearest evidence comes from lymphoma, where patients receiving ABVD-based regimens showed more complete AMH recovery and resumption of menstruation compared to more severe treatment strategies (van Beek et al., 2007; Decanter et al., 2010, 2021; Behringer et al., 2013; Anderson et al., 2018; Palinska-Rudzka et al., 2019). Studies in breast cancer also identified taxanes as contributing to lower AMH (Hamy et al., 2016; Lambertini et al., 2019; Silva et al., 2019). However, studies evaluating cumulative dose of chemotherapy, regardless of regimen, show this to be a key factor in ovarian outcomes (Charpentier et al., 2014; Elchuri et al., 2016; van den Berg et al., 2018; Cameron et al., 2019; George et al., 2019).
Pre-treatment AMH values were associated with post-treatment AMH in all 11 studies that evaluated this, supporting the predictive value of AMH at baseline. All of these studies were in post-menarchal patients. Several studies also identified low pre-treatment AMH in some patients (Lutchman Singh et al., 2007; Cameron et al., 2018; Palinska-Rudzka et al., 2019; van der Kooi et al., 2019), which was not limited to a particular diagnosis.
An important question is whether the lower AMH levels after cancer treatment then decline at a different rate compared to other women. Three studies found no significant difference in rates between patients and age-matched controls (van der Kooi et al., 2017; Cameron et al., 2019; Elitzur et al., 2021). However, there is a higher rate of decline in older women, both cancer survivors and controls (Dezellus et al., 2017; Cameron et al., 2019). These studies highlight the need for research investigating the relationships between post-treatment AMH, age and age of POI/menopause, which would be of substantial clinical value.
While the picture regarding treatment impact on AMH is clear, there was no clear relationship between AMH and menstrual status at follow-up, with menstruation reported in patients with undetectable AMH across different diagnoses and treatments (Decanter et al., 2010; Rosendahl et al., 2010; Ben-Aharon et al., 2015). The reliability of AMH in predicting menstrual function may be better in older patients, consistent with AMH becoming undetectable in advance of natural menopause in healthy women. In contrast, in younger women, menstrual cycles may continue despite a very limited ovarian reserve and undetectable AMH. Although several studies have reported a clear relationship between low/undetectable AMH and diagnosis of POI, the diagnostic validity of AMH for post-cancer treatment POI has not been sufficiently evaluated, either alone or in combination with established criteria.
Interpretation of AMH in paediatric cohorts is potentially complicated by the natural arc of AMH, which increases during childhood and through the early adult years before a decline to the menopause (Kelsey et al., 2011). With the exception of one publication (van der Kooi et al., 2019), there were no differences in the effect of treatment on post-treatment AMH levels between pre- and post-menarchal patients, suggesting the reliability of AMH as a biomarker in younger populations. It should also be noted that all six studies that did not find a treatment influence on AMH were in adult survivors of childhood cancer (Lie Fong et al., 2009; Nielsen et al., 2013; van den Berg et al., 2018; Nystrom et al., 2019; Nies et al., 2020; Elitzur et al., 2021), whereas other studies did find an effect. Further longitudinal studies, from diagnosis through to adulthood, are required to further explore the value of AMH for predicting late ovarian function shortly after completion of therapy in paediatric and adolescent patients with cancer.
Across the different publications, spanning nearly two decades, multiple AMH assay types, most commonly ELISAs or electrochemiluminescence immunoassays (ECLIAs), from a variety of manufacturers have been used. There is currently no internationally agreed calibration standardization for AMH, and the improvements in sensitivity afforded by automated assays in comparison with historical methods, as well as inherent variation and non-linear conversion between assays, can complicate interpretation of the data (Li et al., 2021). Whether variations in assay calibration and performance influence the clinical value of AMH assessment was not formally assessed, but the overall consistency of the main findings indicates that this is not a major issue. It is possible though that the use of assays with greater sensitivity (Chai et al., 2014; Decanter et al., 2014) will impact on analysis of AMH as a diagnostic test for POI.
A variety of additional independent factors can influence levels of AMH (Dafopoulos et al., 2010; Dólleman et al., 2013). Although publications featuring potentially AMH-modifying conditions, such as systemic illness, endometriosis, polycystic ovary syndrome and granulosa cell tumours, were excluded, studies with patients who use cigarettes or oral contraceptives (Elitzur et al., 2021), as well as BRCA mutation carriers (Lambertini et al., 2019; Oktay et al., 2020), were included. The studies described here represent highly heterogeneous patient populations, incorporating a wide range of ages, diagnoses and treatment types. While this may limit the ability of this review to make specific recommendations, it reinforces the wider applicability of our observations.
This review confirms AMH as a marker of ovarian reserve but does not specifically address assessment of time to menopause, which may be clinically useful. This has previously been addressed in relation to natural menopause via probability (Finkelstein et al., 2020) and estimated rate of decline-based models (de Kat et al., 2019; Ramezani Tehrani et al., 2020). As AMH has been shown to correlate with patient age and treatment gonadotoxicity and declines at a similar rate to healthy individuals following any post-treatment recovery, it should be possible to develop a model for menopause risk over time based on pre- and post-treatment AMH measurements. Additional longitudinal studies would support such a model and help to further establish the time course of any AMH recovery, and in which patient groups it occurs, especially whether a time point <2 years’ post-treatment can reliably provide a long-term predictive marker.
Conclusion
In this systematic review, we confirm that anticancer treatment substantially impacts AMH, with large reductions during treatment followed by a period of partial recovery in some women, peaking within 1–2 years (Fig. 3). Low post-treatment AMH was predicted by lower pre-treatment AMH, as well as by older age and gonadotoxicity of treatment. Consequently, discussions around treatment and ovarian outcomes should consider the risk of POI and need for family planning and fertility preservation, informed by AMH. While low post-treatment AMH correlated with increased likelihood of POI, the evidence with respect to oligo- or amenorrhoea was less clear and there were very little data relating AMH to fertility or time to/age at POI or menopause. AMH is therefore a clinically useful biomarker of ovarian reserve before and after cancer treatment and it is likely to be of value in the diagnosis of POI after cancer treatment but relationships with post-treatment fertility and reproductive lifespan require further investigation.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
There are no new data associated with this article.
Supplementary Material
Acknowledgements
The authors thank Simon Lott of Elements Communications Limited for editorial assistance with the preparation of the manuscript.
Authors’ roles
All authors developed the strategy for the literature search, reviewed the outputs of the searches and reviewed and approved the manuscript. R.A.A. guided the data analysis and development of the manuscript.
Funding
All authors attended an Advisory Board meeting in December 2019 to discuss the project, for which they or their institution received an honorarium sponsored by Roche Diagnostics. Medical writing support and article processing charges were funded by Roche Diagnostics. M.L. was supported by the Italian Ministry of Health (5 × 1000 funds 2017; no grant number) and the Italian Association for Cancer Research (AIRC; MFAG 2020 ID 24698).
Conflict of interest
D.C.’s institution has received research grants from Roche; payment for participation in independent data monitoring committees from Roche. F.P. has received speaker’s honoraria from Ipsen and Merck. F.C.’s institution has received reagents from Roche Diagnostics. I.D. has received an academic trial grant and reagents (given to the institution) from Roche Diagnostics; an academic trial grant from Ferring; speaker’s honoraria from Novartis; support for attending meetings from Ferring and Theramex. M.L. has received consulting fees and speaker’s honoraria from AstraZeneca, Exact Sciences, Lilly, Novartis, Pfizer, Roche Diagnostics, Roche Pharma and Seagen; speaker's honoraria from Ipsen, Sandoz and Takeda. R.A.A. has received consulting fees from Ferring, NeRRE Therapeutics, Roche Diagnostics and Sojournix Inc; payment from Merck and IBSA for educational events; laboratory materials from Roche Diagnostics. S.M.N. has received grants from CSO, ESHRE and MRC; consulting fees from Access Fertility, Coopers Genomics, Ferring, Merck, Modern Fertility and TFP; speaker’s honoraria from Ferring, Merck and Roche Diagnostics; payment for expert testimony from Medical Defence Work; support for attending meetings from Ferring and Merck. S.M.N. has personally invested in TFP.
R.A.A. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Acibucu F, Acibucu DO, Akkar OB, Dokmetas HS.. Evaluation of ovarian reserve with AMH level in patients with well-differentiated thyroid cancer receiving radioactive iodine ablation treatment. Exp Clin Endocrinol Diabetes 2016;124:593–596. [DOI] [PubMed] [Google Scholar]
- Al-Janabi HT, Al-Taee HA, Alawad AS.. The impact of age on antimullerian hormone serum level in women attending chemotherapy unit for primary breast cancer. Middle East Fertil Soc J 2018;23:126–130. [Google Scholar]
- Anderson RA, Cameron DA.. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab 2011;96:1336–1343. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mansi J, Coleman RE, Adamson DJA, Leonard RCF.. The utility of anti-Mullerian hormone in the diagnosis and prediction of loss of ovarian function following chemotherapy for early breast cancer. Eur J Cancer 2017;87:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Remedios R, Kirkwood AA, Patrick P, Stevens L, Clifton-Hadley L, Roberts T, Hatton C, Kalakonda N, Milligan DW. et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin's lymphoma (RATHL): a secondary analysis of a randomised phase 3 trial. Lancet Oncol 2018;19:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Rosendahl M, Kelsey TW, Cameron DA.. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer 2013;49:3404–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Su HI.. The clinical value and interpretation of anti-Müllerian hormone in women with cancer. Front Endocrinol (Lausanne) 2020;11:574263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA.. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod 2003;18:2368–2374. [DOI] [PubMed] [Google Scholar]
- Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, Wessels C, Wiehlputz M, Rosenbrock J, Halbsguth T. et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol 2013;31:231–239. [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I, Granot T, Meizner I, Hasky N, Tobar A, Rizel S, Yerushalmi R, Ben-Haroush A, Fisch B, Stemmer SM.. Long-term follow-up of chemotherapy-induced ovarian failure in young breast cancer patients: the role of vascular toxicity. Oncologist 2015;20:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjeb KK, Debbabi L, Braham M, Zemni Z, Chtourou S, Hannachi H, Hamdoun M, Ayadi M, Kacem K, Zhioua F. et al. Evaluation of ovarian reserve before and after chemotherapy. J Gynecol Obstet Hum Reprod 2021;50:102035. [DOI] [PubMed] [Google Scholar]
- Bi X, Zhang J, Cao D, Sun H, Feng F, Wan X, Xiang Y, Qiu L, Cheng X, Yang J. et al. Anti-Müllerian hormone levels in patients with gestational trophoblastic neoplasia treated with different chemotherapy regimens: a prospective cohort study. Oncotarget 2017;8:113920–113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH.. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab 2012;97:2059–2067. [DOI] [PubMed] [Google Scholar]
- Cameron K, Sammel MD, Prewitt M, Gracia C.. Differential rates of change in measures of ovarian reserve in young cancer survivors across the reproductive lifespan. J Clin Endocrinol Metab 2019;104:1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KE, Kole MB, Sammel MD, Ginsberg JP, Gosiengfiao Y, Mersereau JE, Su HI, Gracia CR.. Acute menopausal symptoms in young cancer survivors immediately following chemotherapy. Oncology 2018;94:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J. et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebi F, Ordu C, Ilgun S, Ozturk A, Erdogan Iyigun Z, Alco G, Duymaz T, Aktepe F, Soybir G, Baysal B. et al. ; The effect of systemic chemotherapy on ovarian function: a prospective clinical trial. Eur J Breast Health 2020;16:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Howie AF, Cameron DA, Anderson RA.. A highly-sensitive anti-Müllerian hormone assay improves analysis of ovarian function following chemotherapy for early breast cancer. Eur J Cancer 2014;50:2367–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier A-M, Chong AL, Gingras-Hill G, Ahmed S, Cigsar C, Gupta AA, Greenblatt E, Hodgson DC.. Anti-Müllerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. J Cancer Surviv 2014;8:548–554. [DOI] [PubMed] [Google Scholar]
- Chemerinski A, Cameron K, Sammel M, Ginsberg J, Carlson C, Gracia C.. Relationship of menopausal symptoms and ovarian reserve in reproductive-aged cancer survivors. J Cancer Surviv 2020;14:607–613. [DOI] [PubMed] [Google Scholar]
- Dafopoulos A, Dafopoulos K, Georgoulias P, Galazios G, Limberis V, Tsikouras P, Koutlaki N, Maroulis G.. Smoking and AMH levels in women with normal reproductive history. Arch Gynecol Obstet 2010;282:215–219. [DOI] [PubMed] [Google Scholar]
- D’Avila ÂM, Biolchi V, Capp E, Corleta HVE.. Age, anti-mullerian hormone, antral follicles count to predict amenorrhea or oligomenorrhea after chemotherapy with cyclophosphamide. J Ovarian Res 2015;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avila ÂM, Capp E, Corleta HVE.. Antral follicles count and anti-Mullerian hormone levels after gonadotoxic chemotherapy in patients with breast cancer: cohort study. Rev Bras Ginecol Obstet 2017;39:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kat AC, van der Schouw YT, Eijkemans MJC, Broer SL, Verschuren WMM, Broekmans FJM.. Can menopause prediction be improved with multiple AMH measurements? Results from the prospective Doetinchem cohort study. J Clin Endocrinol Metab 2019;104:5024–5031. [DOI] [PubMed] [Google Scholar]
- Decanter C, Cloquet M, Dassonneville A, D'Orazio E, Mailliez A, Pigny P.. Different patterns of ovarian recovery after cancer treatment suggest various individual ovarian susceptibilities to chemotherapy. Reprod Biomed Online 2018;36:711–718. [DOI] [PubMed] [Google Scholar]
- Decanter C, Delepine J, Behal H, Manier S, Bruno B, Barbatti M, Robin C, Labreuche J, Morschhauser F, Pigny P.. Longitudinal study of AMH variations in 122 adolescents and young adults (AYA) and non-AYA lymphoma patients to evaluate the chemo-induced ovarian toxicity to further personalise fertility preservation counselling. Hum Reprod 2021;36:2743–2752. [DOI] [PubMed] [Google Scholar]
- Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D.. Anti-Mullerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online 2010;20:280–285. [DOI] [PubMed] [Google Scholar]
- Decanter C, Peigne M, Mailliez A, Morschhauser F, Dassonneville A, Dewailly D, Pigny P.. Toward a better follow-up of ovarian recovery in young women after chemotherapy with a hypersensitive antimüllerian hormone assay. Fertil Steril 2014;102:483–487. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, Casasnovas O, Van Den Neste E, Dechene J, De Maertelaer V. et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol 2016;34:2568–2574. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Racape J, Dechene J, Dupuis J, Morschhauser F, De Wilde V, Lazarovici J, Ghesquieres H, Touati M, Sibon D. et al. Gonadal function recovery in patients with advanced Hodgkin lymphoma treated with a PET-adapted regimen: prospective analysis of a randomized phase III trial (AHL2011). J Clin Oncol 2021;39:3251–3260. [DOI] [PubMed] [Google Scholar]
- Dezellus A, Barriere P, Campone M, Lemanski C, Vanlemmens L, Mignot L, Delozier T, Levy C, Bendavid C, Debled M. et al. Prospective evaluation of serum anti-Mullerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer 2017;79:72–80. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Costantini C, Tecchio C, Salvagno GL, Montemezzi R, Perandini A, Pizzolo G, Zaffagnini S, Franchi M.. Anti-Mullerian hormone and antral follicle count reveal a late impairment of ovarian reserve in patients undergoing low-gonadotoxic regimens for hematological malignancies. Oncologist 2013;18:1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR.. Pregnancy after cancer: results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer 2013a;60:2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, Gosiengfiao Y, Gracia CR.. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril 2013b;99:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dólleman M, Verschuren WMM, Eijkemans MJC, Dollé MET, Jansen EHJM, Broekmans FJM, van der Schouw YT.. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab 2013;98:2106–2115. [DOI] [PubMed] [Google Scholar]
- Elchuri SV, Patterson BC, Brown M, Bedient C, Record E, Wasilewski-Masker K, Mertens AC, Meacham LR.. Low anti-Mullerian hormone in pediatric cancer survivors in the early years after gonadotoxic therapy. J Pediatr Adolesc Gynecol 2016;29:393–399. [DOI] [PubMed] [Google Scholar]
- Elgindy EA, El-Haieg DO, Khorshid OM, Ismail EI, Abdelgawad M, Sallam HN, Abou-Setta AM.. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol 2013;121:78–86. [DOI] [PubMed] [Google Scholar]
- Elitzur S, Frank S, Goshen-Lago T, Barzilai-Birenboim S, Gilad G, Avrahami G, Goldberg T, Litichever N, Masarwa A, Oron G. et al. Long-term ovarian reserve and fertility outcomes in female survivors of childhood acute lymphoblastic leukemia. Leuk Lymphoma 2021;62:2211–2218. [DOI] [PubMed] [Google Scholar]
- El-Shalakany AH, Ali MS, Abdelmaksoud AA, Abd El-Ghany S, Hasan EA.. Ovarian function in female survivors of childhood malignancies. Pediatr Hematol Oncol 2013;30:328–335. [DOI] [PubMed] [Google Scholar]
- Evranos B, Faki S, Polat SB, Bestepe N, Ersoy R, Cakir B.. Effects of radioactive iodine therapy on ovarian reserve: a prospective pilot study. Thyroid 2018;28:1702–1707. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Karlamangla A, Neer RM, Sluss PM, Burnett-Bowie SM, Darakananda K, Donahoe PK, Harlow SD, Prizand SH. et al. Antimullerian hormone and impending menopause in late reproductive age: the study of women's health across the nation. J Clin Endocrinol Metab 2020;105:e1862–e1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Williamson Lewis R, Schirmer DA, Effinger KE, Spencer JB, Mertens AC, Meacham LR.. Early detection of ovarian dysfunction by anti-Mullerian hormone in adolescent and young adult-aged survivors of childhood cancer. J Adolesc Young Adult Oncol 2019;8:18–25. [DOI] [PubMed] [Google Scholar]
- Gharwan H, Lai C, Grant C, Dunleavy K, Steinberg SM, Shovlin M, Fojo T, Wilson WH.. Female fertility following dose-adjusted EPOCH-R chemotherapy in primary mediastinal B-cell lymphomas. Leuk Lymphoma 2016;57:1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb SB, Turan V, Bedoschi G, Taylan E, Abdo N, Cigler T, Bang H, Patil S, Dickler MN, Oktay KH.. Impact of adjuvant chemotherapy or tamoxifen-alone on the ovarian reserve of young women with breast cancer. Breast Cancer Res Treat 2021;185:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP.. Impact of cancer therapies on ovarian reserve. Fertil Steril 2012;97:134–140.e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AA, Lee Chong A, Deveault C, Traubici J, Maloney AM, Knight S, Lorenzo A, Allen L.. Anti-Mullerian hormone in female adolescent cancer patients before, during, and after completion of therapy: a pilot feasibility study. J Pediatr Adolesc Gynecol 2016;29:599–603. [DOI] [PubMed] [Google Scholar]
- Hamre H, Kiserud CE, Ruud E, Thorsby PM, Fossa SD.. Gonadal function and parenthood 20 years after treatment for childhood lymphoma: a cross-sectional study. Pediatr Blood Cancer 2012;59:271–277. [DOI] [PubMed] [Google Scholar]
- Hamy AS, Porcher R, Eskenazi S, Cuvier C, Giacchetti S, Coussy F, Hocini H, Tournant B, Perret F, Bonfils S. et al. Anti-Mullerian hormone in breast cancer patients treated with chemotherapy: a retrospective evaluation of subsequent pregnancies. Reprod Biomed Online 2016;32:299–307. [DOI] [PubMed] [Google Scholar]
- Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF.. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist 2014;19:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Sugita A, Hirokawa W, Goto M, Yamamoto E, Takikawa S, Nakahara T, Nakamura T, Kondo M, Kikkawa F.. Anti-Mullerian hormone as a marker of ovarian reserve following chemotherapy in patients with gestational trophoblastic neoplasia. Eur J Obstet Gynecol Reprod Biol 2013;167:194–198. [DOI] [PubMed] [Google Scholar]
- Jayasinghe YL, Wallace WHB, Anderson RA.. Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Rev Endocrinol Metab 2018;13:125–136. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB.. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Choi J, Park CS, Seong MK, Hong SE, Kim JS, Park IC, Lee JK, Noh WC; The ASTRRA trial investigators. Post-chemotherapy serum anti-Mullerian hormone level predicts ovarian function recovery. Endocr Connect 2018;7:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Leszczynska E, Poznanska M, Zelazowska-Rutkowska B, Wysocka J.. Anti-Mullerian hormone as a sensitive marker of ovarian function in young cancer survivors. Int J Endocrinol 2013a;2013:125080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Leszczynska E, Poznanska M, Zelazowska-Rutkowska B, Wysocka J.. The progressive reduction in the ovarian reserve in young women after anticancer treatment. Horm Metab Res 2013b;45:813–819. [DOI] [PubMed] [Google Scholar]
- Krawczuk-Rybak M, Płonowski M, Leszczyńska E, Latoch E, Sawicka-Żukowska M, Muszyńska-Rosłan K, Skalska-Sadowska J, Wachowiak J, Sga-Pondel D, Kazanowska B. et al. The influence of different intensity of treatment on hormonal markers of gonadal function in acute lymphoblastic leukemia survivors. Hematol Oncol 2019;37:609–616. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Olympios N, Lequesne J, Calbrix C, Fontanilles M, Loeb A, Leheurteur M, Demeestere I, Di Fiore F, Perdrix A. et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-mullerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol 2019;9:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Kim JY, Yu J, Kim SW.. Prediction of successful ovarian protection using gonadotropin-releasing hormone agonists during chemotherapy in young estrogen receptor-negative breast cancer patients. Front Oncol 2020;10:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Park YH, Lee JE, Choi D.. Prediction of ovarian function recovery in young breast cancer patients after protection with gonadotropin-releasing hormone agonist during chemotherapy. Breast Cancer Res Treat 2018;171:649–656. [DOI] [PubMed] [Google Scholar]
- Leiper A, Houwing M, Davies EG, Rao K, Burns S, Morris E, Laven J, van der Kooi AL, van den Heuvel Eibrink M, Nussey S.. Anti-Mullerian hormone and Inhibin B after stem cell transplant in childhood: a comparison of myeloablative, reduced intensity and treosulfan-based chemotherapy regimens. Bone Marrow Transplant 2020;55:1985–1995. [DOI] [PubMed] [Google Scholar]
- Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, Thomas GA, Coleman RE, Anderson RA. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol 2017;28:1811–1816. [DOI] [PubMed] [Google Scholar]
- Li HWR, Robertson DM, Burns C, Ledger WL.. Challenges in measuring AMH in the clinical setting. Front Endocrinol (Lausanne) 2021;12:691432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu S, Ma L, Chen X, Weng H, Huang R, Yu Y, Zong X.. Can anti-Müllerian hormone be a reliable biomarker for assessing ovarian function in women postchemotherapy. Cancer Manag Res 2020;12:8171–8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie Fong S, Laven JSE, Hakvoort-Cammel FGAJ, Schipper I, Visser JA, Themmen APN, de Jong FH, van den Heuvel-Eibrink MM.. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod 2009;24:982–990. [DOI] [PubMed] [Google Scholar]
- Loubersac S, Dezellus A, Lefebvre T, Reignier A, Barriere P, Masson D, Freour T; RESOVA Investigators Group. Evolution of serum Anti-Mullerian Hormone (AMH) level in young women treated with chemotherapy for breast cancer according to basal AMH level. Eur J Obstet Gynecol Reprod Biol 2020;254:132–137. [DOI] [PubMed] [Google Scholar]
- Lunsford AJ, Whelan K, McCormick K, McLaren JF.. Antimullerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril 2014;101:227–231. [DOI] [PubMed] [Google Scholar]
- Lutchman Singh K, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, Groome NP, Davies MC, Chatterjee R.. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer 2007;96:1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisic E, Susnjar S, Milovanovic J, Todorovic-Rakovic N, Kesic V.. Assessment of ovarian function after chemotherapy in women with early and locally advanced breast cancer from Serbia. Arch Gynecol Obstet 2018;297:495–503. [DOI] [PubMed] [Google Scholar]
- Martin HL, Ullah S, Abbas N, Scott‐Hoy A, Kichenadasse G, Karapetis CS, Roy A, Sukumaran S, Ross DM, Khattak MA. et al. Predicting chemotherapy-induced menopause using baseline and post-chemotherapy anti-Müllerian hormone levels: Results of a pilot study. Cancer Rep 2021;4:e11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittica M, Dotto A, Comina M, Teliti M, Monti E, Giusti M.. Cross-sectional and prospective study on anti-Mullerian hormone changes in a cohort of pre-menopausal women with a history of differentiated thyroid cancer. Thyroid Res 2020;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Ohta H, Namba N, Tachibana M, Miyamura T, Miyashita E, Hashii Y, Oue T, Isobe A, Tsutsui T. et al. Low serum concentrations of anti-Mullerian hormone are common in 53 female childhood cancer survivors. Horm Res Paediatr 2013;79:17–21. [DOI] [PubMed] [Google Scholar]
- Morarji K, McArdle O, Hui K, Gingras-Hill G, Ahmed S, Greenblatt EM, Warner E, Sridhar S, Ali AMF, Azad A. et al. Ovarian function after chemotherapy in young breast cancer survivors. Curr Oncol 2017;24:e494–e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H, Elfving M, Turkiewicz A, Andersen CY, Ora I.. Severe gonadotoxic insult manifests early in young girls treated for Ewing sarcoma. Medicine (Baltimore) 2016;95:e4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SN, Andersen AN, Schmidt KT, Rechnitzer C, Schmiegelow K, Bentzen JG, Larsen EC.. A 10-year follow up of reproductive function in women treated for childhood cancer. Reprod Biomed Online 2013;27:192–200. [DOI] [PubMed] [Google Scholar]
- Nies M, Cantineau AEP, Arts E, van den Berg MH, van Leeuwen FE, Muller Kobold AC, Klein Hesselink MS, Burgerhof JGM, Brouwers AH, van Dam E. et al. Long-term effects of radioiodine treatment on female fertility in survivors of childhood differentiated thyroid carcinoma. Thyroid 2020;30:1169–1176. [DOI] [PubMed] [Google Scholar]
- Nitzschke M, Raddatz J, Bohlmann MK, Stute P, Strowitzki T, von Wolff M.. GnRH analogs do not protect ovaries from chemotherapy-induced ultrastructural injury in Hodgkin's lymphoma patients. Arch Gynecol Obstet 2010;282:83–88. [DOI] [PubMed] [Google Scholar]
- Nystrom A, Morse H, Nordlof H, Wiebe K, Artman M, Ora I, Giwercman A, Henic E, Elfving M.. Anti-mullerian hormone compared with other ovarian markers after childhood cancer treatment. Acta Oncol 2019;58:218–224. [DOI] [PubMed] [Google Scholar]
- Oktay KH, Bedoschi G, Goldfarb SB, Taylan E, Titus S, Palomaki GE, Cigler T, Robson M, Dickler MN.. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency. Fertil Steril 2020;113:1251–1260.e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinska-Rudzka KE, Ghobara T, Parsons N, Milner J, Lockwood G, Hartshorne GM.. Five-year study assessing the clinical utility of anti-Mullerian hormone measurements in reproductive-age women with cancer. Reprod Biomed Online 2019;39:712–720. [DOI] [PubMed] [Google Scholar]
- Panay N, Anderson RA, Nappi RE, Vincent AJ, Vujovic S, Webber L, Wolfman W.. Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric 2020;23:426–446. [DOI] [PubMed] [Google Scholar]
- Parissone F, Di Paola R, Balter R, Garzon S, Zaffagnini S, Neri M, Vitale V, Tridello G, Cesaro S.. Female adolescents and young women previously treated for pediatric malignancies: assessment of ovarian reserve and gonadotoxicity risk stratification for early identification of patients at increased infertility risk. J Pediatr Endocrinol Metab 2021;34:25–33. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, Ginsburg E.. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 2010;94:638–644. [DOI] [PubMed] [Google Scholar]
- Passildas J, Collard O, Savoye AM, Dohou J, Ginzac A, Thivat E, Durando X, Kwiatkowski F, Penault-Llorca F, Abrial C. et al. Impact of chemotherapy-induced menopause in women of childbearing age with non-metastatic breast cancer - preliminary results from the MENOCOR study. Clin Breast Cancer 2019;19:e74–e84. [DOI] [PubMed] [Google Scholar]
- Ramezani Tehrani F, Bidhendi Yarandi R, Solaymani-Dodaran M, Tohidi M, Firouzi F, Azizi F.. Improving prediction of age at menopause using multiple anti-Mullerian hormone measurements: the Tehran lipid-glucose study. J Clin Endocrinol Metab 2020;105:dgaa083. [DOI] [PubMed] [Google Scholar]
- Rosendahl M, Andersen CY, Ernst E, Westergaard LG, Rasmussen PE, Loft A, Andersen AN.. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow-up study. Hum Reprod 2008;23:2475–2483. [DOI] [PubMed] [Google Scholar]
- Rosendahl M, Andersen CY, la Cour Freiesleben N, Juul A, Lossl K, Andersen AN.. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril 2010;94:156–166. [DOI] [PubMed] [Google Scholar]
- Roshandel R, van Dijk M, Overbeek A, Kaspers G, Lambalk C, Beerendonk C, Bresters D, van der Heiden-van der Loo M, van den Heuvel-Eibrink M, Kremer L. et al. ; LATER‐VEVO Study Group. Female reproductive function after treatment of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2021;68:e28894. [DOI] [PubMed] [Google Scholar]
- Ruddy KJ, O'Neill A, Miller KD, Schneider BP, Baker E, Sparano JA, Dang C, Northfelt DW, Sledge GW Jr, Partridge AH.. Biomarker prediction of chemotherapy-related amenorrhea in premenopausal women with breast cancer participating in E5103. Breast Cancer Res Treat 2014;144:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy KJ, Schaid DJ, Batzler A, Cecchini RS, Partridge AH, Norman A, Fehrenbacher L, Stewart EA, Trabuco E, Ginsburg E. et al. Anti-mullerian hormone as a serum biomarker for risk of chemotherapy-induced amenorrhea. J Natl Cancer Inst 2021;113:1105–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandley LM, Spencer JB, Fothergill A, Mertens AC, Manatunga A, Paplomata E, Howards PP.. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril 2017;107:243–252.e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC.. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod 2016;31:2737–2749. [DOI] [PubMed] [Google Scholar]
- Silva C, Ribeiro Rama AC, Reis Soares S, Moura-Ramos M, Almeida-Santos T.. Adverse reproductive health outcomes in a cohort of young women with breast cancer exposed to systemic treatments. J Ovarian Res 2019;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG.. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD.. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017;318:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Kwan B, Whitcomb BW, Shliakhsitsava K, Dietz AC, Stark SS, Martinez E, Sluss PM, Sammel MD, Natarajan L.. Modeling variation in the reproductive lifespan of female adolescent and young adult cancer survivors using AMH. J Clin Endocrinol Metab 2020;105:2740–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, Freeman EW, Gracia CR, DeMichele A.. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer 2010;116:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Teinturier C, Allodji RS, Svetlova E, Frey MA, Oberlin O, Millischer AE, Epelboin S, Decanter C, Pacquement H, Tabone MD. et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod 2015;30:1437–1446. [DOI] [PubMed] [Google Scholar]
- Trapp E, Steidl J, Rack B, Kupka MS, Andergassen U, Juckstock J, Kurt A, Vilsmaier T, de Gregorio A, de Gregorio N. et al. Anti-Mullerian hormone (AMH) levels in premenopausal breast cancer patients treated with taxane-based adjuvant chemotherapy - a translational research project of the SUCCESS A study. Breast 2017;35:130–135. [DOI] [PubMed] [Google Scholar]
- van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, van den Bos C, van den Berg H, Pieters R, de Muinck Keizer-Schrama SM.. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab 2007;92:3869–3874. [DOI] [PubMed] [Google Scholar]
- van den Berg MH, Overbeek A, Lambalk CB, Kaspers GJL, Bresters D, van den Heuvel-Eibrink MM, Kremer LC, Loonen JJ, van der Pal HJ, Ronckers CM. et al. ; DCOG LATER-VEVO study group. Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Hum Reprod 2018;33:1474–1488. [DOI] [PubMed] [Google Scholar]
- van der Kooi ALF, van den Heuvel-Eibrink MM, van den Berg SAA, van Dorp W, Pluijm SMF, Laven JSE.. Changes in anti-Mullerian hormone and inhibin B in children treated for cancer. J Adolesc Young Adult Oncol 2019;8:281–290. [DOI] [PubMed] [Google Scholar]
- van der Kooi ALF, van den Heuvel-Eibrink MM, van Noortwijk A, Neggers SJ, Pluijm SM, van Dulmen-den Broeder E, van Dorp W, Laven JS.. Longitudinal follow-up in female Childhood Cancer Survivors: no signs of accelerated ovarian function loss. Hum Reprod 2017;32:193–200. [DOI] [PubMed] [Google Scholar]
- van Dorp W, Haupt R, Anderson RA, Mulder RL, van den Heuvel-Eibrink MM, van Dulmen-den Broeder E, Su HI, Winther JF, Hudson MM, Levine JM. et al. Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol 2018;36:2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velsen EFS, Visser WE, van den Berg SAA, Kam BLR, van Ginhoven TM, Massolt ET, Peeters RP.. Longitudinal analysis of the effect of radioiodine therapy on ovarian reserve in females with differentiated thyroid cancer. Thyroid 2020;30:580–587. [DOI] [PubMed] [Google Scholar]
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F. et al. ; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Wenners A, Grambach J, Koss J, Maass N, Jonat W, Schmutzler A, Mundhenke C.. Reduced ovarian reserve in young early breast cancer patients: preliminary data from a prospective cohort trial. BMC Cancer 2017;17:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish I, Azem F, Gutfeld O, Silman Z, Serebro M, Sharon O, Shefer G, Limor R, Stern N, Tordjman KM.. A single radioactive iodine treatment has a deleterious effect on ovarian reserve in women with thyroid cancer: results of a prospective pilot study. Thyroid 2018;28:522–527. [DOI] [PubMed] [Google Scholar]
- Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA, Hershman DL.. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer 2010;116:2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Lin Y, Cheng X, Huang X, Zhou Y, Mao F, Wang Y, Guan J, Shen S, Xu Y. et al. GnRHa for ovarian protection and the association between AMH and ovarian function during adjuvant chemotherapy for breast cancer. J Cancer 2019;10:4278–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no new data associated with this article.