Abstract
In order to evaluate the utility of the 3D reconstructed skin micronucleus assay (3DRSMN) to assess clastogenic/aneugenic potential of the fragrance chemicals, a set of 22 fragrance materials were evaluated in 3DRSMN assay. These materials evaluated were also evaluated in an in vitro as well as in vivo micronucleus assay, conducted as per Organisation for Economic Co-operation and Development guidelines. The results of the RSMN assay were in 100% agreement with the in vivo micronucleus assay results. From this dataset, 18 materials were positive in an in vitro micronucleus assay but were negative in an in vivo micronucleus assay. All these 18 materials were also concluded to be negative in 3DRSMN assay, stressing the importance of the assay to help minimize misleading positive outcomes from the in vitro assay. Since the highest exposure for fragrances is through the dermal route, the RSMN assay fits the applicability domain for testing. Thus, RSMN assay is an important alternative to animal testing for characterization of the genotoxicity potential of fragrance materials.
Keywords: 3DRSMN, fragrance materials, animal alternative, genotoxicity
Introduction
As of March 2009, the seventh amendment to the EU Cosmetics Directive banned the use of in vivo genotoxicity studies for cosmetic ingredients [1]. Without in vivo tests, there is no way to determine if a positive in vitro genotoxicity test result represents a real hazard or is a “misleading” positive, as is known to occur with a high frequency in the traditional battery of in vitro genotoxicity studies [2, 3]. Cosmetic and fragrance materials with positive results in the standard in vitro genotoxicity assays would thus be prevented from further development. According to Ates et al. (2014), non-confirmed or “misleading” positive results occurred in up to 93% of in vitro genotoxicity test batteries with cosmetic ingredients [4]. To address this issue, genotoxicity tests in 3D reconstructed human skin models were developed as a non-animal follow-up assay to assess genotoxicity via a dermal route of exposure. 3D reconstructed skin (RS) models consist of a multilayered, differentiated model of the human epidermis that offers more physiologically relevant results for dermally exposed substances when compared with the standard in vitro micronucleus test, as is most common in cosmetic and fragrance products. The metabolizing capacity of RS models is representative of human skin [5–9], therefore is more biologically relevant for assessing hazard from dermal exposures compared to the induced exogenous rat liver S9 used in standard in vitro genotoxicity tests. Importantly, RS models have relatively little or absent Phase I activities, similar to human skin, and have significantly higher and measurable Phase II detoxification activities, both of which contribute to the increased relevance of RS models for genotoxicity assessment with dermal exposure [10, 11].
The 3D reconstructed skin micronucleus assay (RSMN) was developed as a follow-up test for materials that are positive in standard in vitro chromosome aberrations or micronucleus assays. Inter- and intra-laboratory validation studies for the RSMN assay, including a global validation effort by Cosmetics Europe, have shown both good reproducibility and predictivity of in vivo genotoxicity results, making this assay an excellent animal alternative follow-up to traditional in vitro studies [12–14]. The utility of the RSMN assay and development of an Organisation for Economic Co-operation and Development (OECD) guideline was endorsed by the International Working Group on Genetic Toxicology (IWGT) in meetings in 2009 [15] and 2017 [16].
Since the RSMN assay is promising, the Research Institute for Fragrance Materials (RIFM) investigated the performance of 22 fragrance materials in the assay. The Crème RIFM aggregate exposure model demonstrates that dermal exposure is the primary route of exposure for most fragrance products, which further emphasizes the strengths the RSMN assay can offer for this category of materials [17–19]. The intent of this study is to determine whether the experimental dataset for fragrance materials presented here supports the observed high predictivity of the RSMN assay when compared with in vivo genotoxicity also. Another aspect of interest that can be investigated is whether the data support the suggestion to eliminate the 48-hour exposure time to streamline the assay [16].
Materials and methods
Selection of materials
In order to evaluate the utility of the 3D skin micronucleus assay to assess clastogenic/aneugenic potential of the fragrance chemicals, a set of 22 fragrance materials (supplied at market quality by RIFM member companies) were selected (Table 1) based on two criteria.
Table 1.
Summary table describing all genotoxicity data for materials
| Material | CAS # | In vitro MNT | 3D Skin MNT | In vivo MNT |
|---|---|---|---|---|
| sec-Butyl ethyl ether | 2679-87-0 | + | - | - |
| Cadinene | 29350-73-0 | +a | - | |
| 2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propanal | 300371-33-9 | Equivocal | - | |
| 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime | 75147-23-8 | + | - | - |
| 2,2ʹ-(Dithiodimethylene)difuran | 4437-20-1 | + | - | - |
| Ethyl formate | 109-94-4 | + | - | - |
| 2-Ethyl-1,3,3-trimethyl-2-norbornanol | 18368-91-7 | - | - | - |
| Furfuryl thioacetate | 13678-68-7 | + | - | - |
| Isobornyl methyl ether | 5331-32-8 | Equivocal | - | -for read-acrossb |
| Lauric Aldehyde | 112-54-9 | + | - | - |
| p-Methoxy cinnamaldehyde | 1963-36-6 | + | - | - |
| 6-Methoxy-2,6-dimethylheptan-1-al | 62439-41-2 | + | - | - |
| 2-Methyl-2-pentenal | 623-36-9 | + | - | - |
| Methyl beta-phenylglycidate | 37161-74-3 | +∗ | - | - |
| Nona-2 trans- 6-cis-dienal | 557-48-2 | + | - | - |
| 2-Octenoic acid, 4-ethyl-, (2Z) | 60308-75-0 | + | - | - |
| 2-Octen-4-one | 4643-27-0 | + | - | - |
| 4-Phenyl-3-buten-2-ol | 17488-65-2 | + | - | - |
| 5-Phenylhex-3-en-2-one | 60405-50-7 | Equivocal | - | -for read-acrossc |
| 4-Thujanol | 546-79-2 | + | - | - |
| 3,3,5-Trimethylcyclohexaneacetic acid | 3213-73-8 | + | - | - |
| Veratraldehyde | 120-14-9 | + | - | - |
Results did not meet all criteria for a positive.
Read-across analogue is 1-ethyl-3-methoxytricyclo[2.2.1.02,6]heptane (CAS # 31996-78-8).
Read-across analogue is 4-Phenyl-3-buten-2-one (CAS#122-57-6).
(1) Each material was tested in a GLP compliant in vitro micronucleus assay in accordance with OECD TG 487 [20–22] using human peripheral blood lymphocytes in both the presence and absence of an S9 fraction from the livers of male Sprague Dawley rats induced with Aroclor 1254 or phenobarbital intraperitoneally and β-naphthoflavone.
(2) Each material was tested in a GLP compliant in vivo micronucleus assay in accordance with OECD TG 474 [23–25] using groups of male and female Hsd:IRC (CD-1) mice or Han Wistar rats with the test material administered either 2, 3, or 4 times in corn oil or deionized water via oral gavage.
Since the focus of this study was to investigate the ability of the RMSN assay to address “misleading” positive in vitro micronucleus assay results, all of the materials except one had a positive or equivocal result in the in vitro micronucleus assay, and those that were tested in the in vivo micronucleus assay were all negative. Three materials, namely, isobornyl methyl ether, 2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propana, and cadinene, which produced either an equivocal or biologically non-relevant positive outcome in the in vitro micronucleus assay, were not tested in an in vivo micronucleus study. Equivocal was defined as results meeting some but not all the criteria for a positive outcome (such as a statistically significant increase as determined by the Fisher’s Exact test, but negative for dose–response as determined by the Cochran-Armitage test). Biologically non-relevant positive results were defined, for example, if the assay resulted in inconsistent statistically significant increases across repeat assays and steep increases in cytotoxicity at the concentrations with statistically significant increases. Results for these tests are included in the summary Table 1.
RSMN assay
The RSMN assay was conducted according to the protocol described by Dahl et al. [26]. Assays were conducted in compliance with OECD GLP guidelines, most of the studies were conducted at contract research organizations. In the dose range-finding assay, and the first main study, tissues were treated with a 2-day dosing regimen (48 hours harvest). In the confirmatory micronucleus assay, tissues were treated with a 3-day dosing regimen (72 hours harvest). Each chemical was tested in triplicate, using tissues generated from the same batch/skin donor. Cytotoxicity was measured by both relative binucleation and RVCC (relative viable cell count) and whichever was the more sensitive parameter (resulted in 50–60% cytotoxicity first) was used to select the maximum dose for the micronucleus assay. In all the studies, acetone was used as a vehicle control, and mitomycin C (MMC) was used as a positive control and produced a statistically significant response demonstrating the validity of the study.
Results
The results for each material are described below.
sec-Butyl ethyl ether (CAS# 2679-87-0)
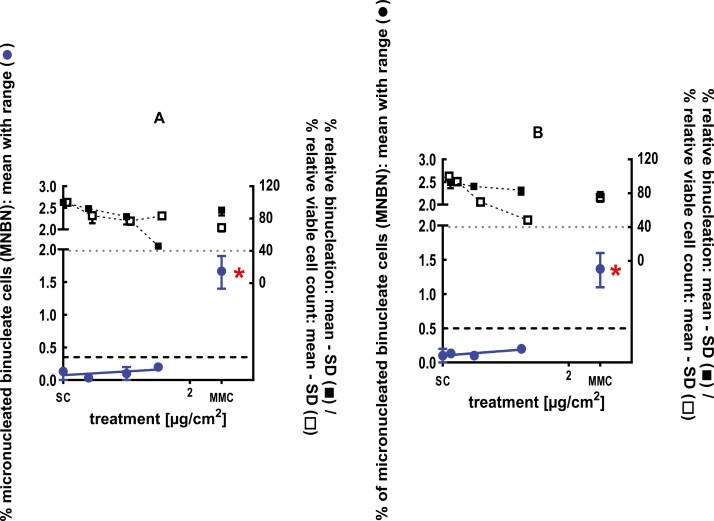
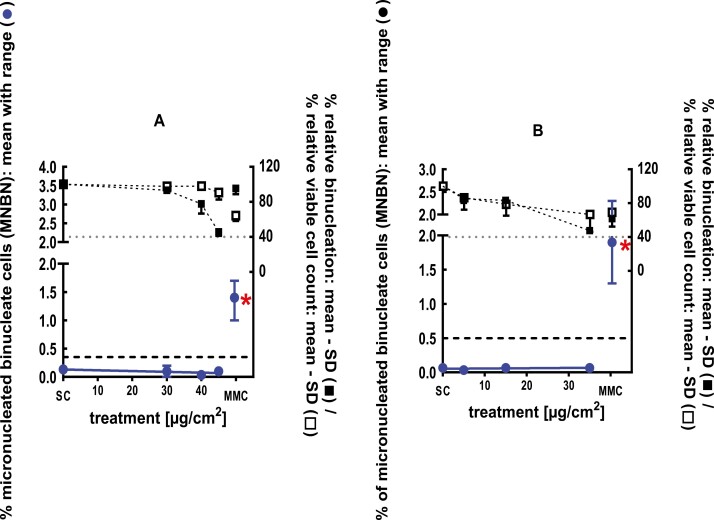
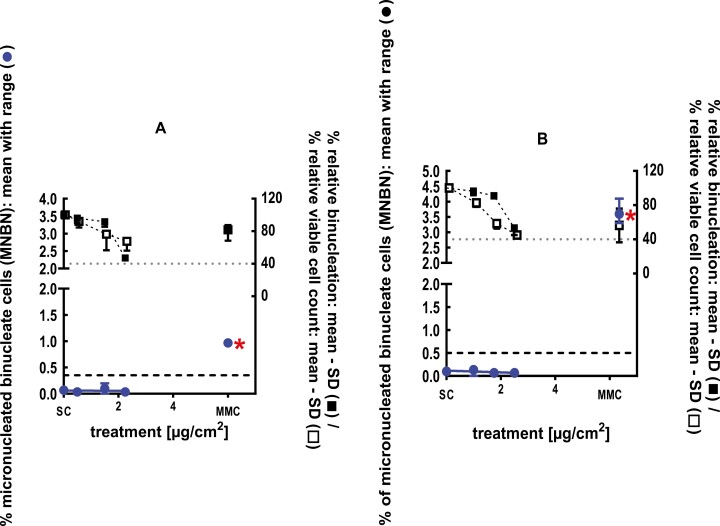
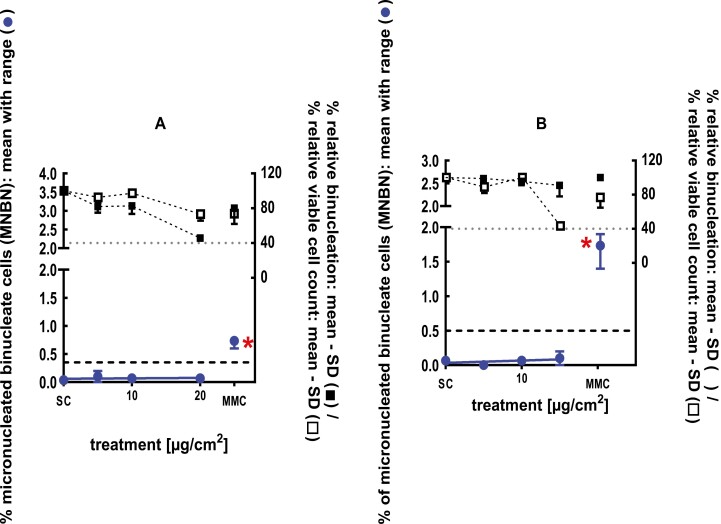
The clastogenic activity of sec-butyl ethyl ether was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1020 µg/ml in ethanol, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses 300 and 1020 µg/ml in the 4-hour treatment in the absence of S9. sec-Butyl ethyl ether was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [27]. A follow-up 3D skin and in vivo study were conducted to further evaluate the biological relevance of the positive in vitro results. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with sec-butyl ethyl ether in acetone for 48 and 72 hours, at concentrations up to 100 mg/ml. sec-Butyl ethyl ether did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [28] (Fig. 1). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [29] (Young, 2018; #75302).
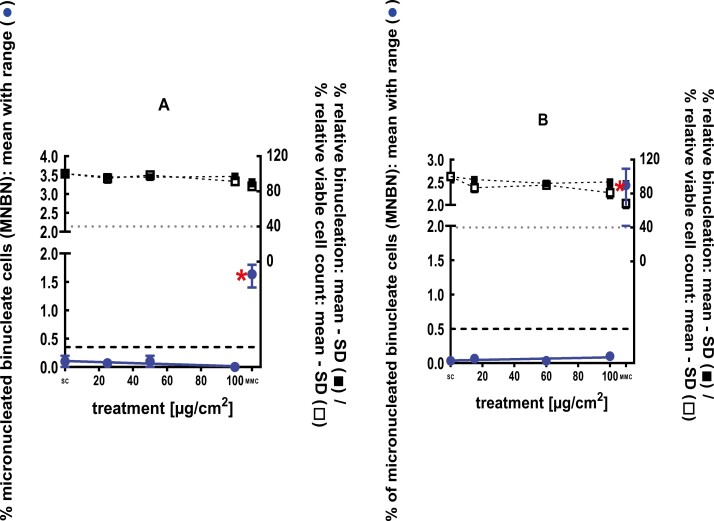
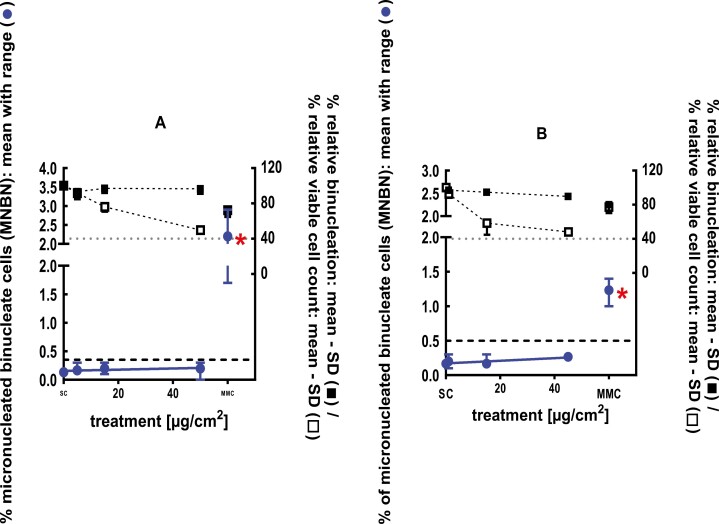
Figure 1.
3Dskin RSMN results for sec-Butyl ethyl ether (CAS # 2679-87-0): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the cutoff for excessive cytotoxicity (40%) is shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Cadinene (CAS# 29350-73-0)
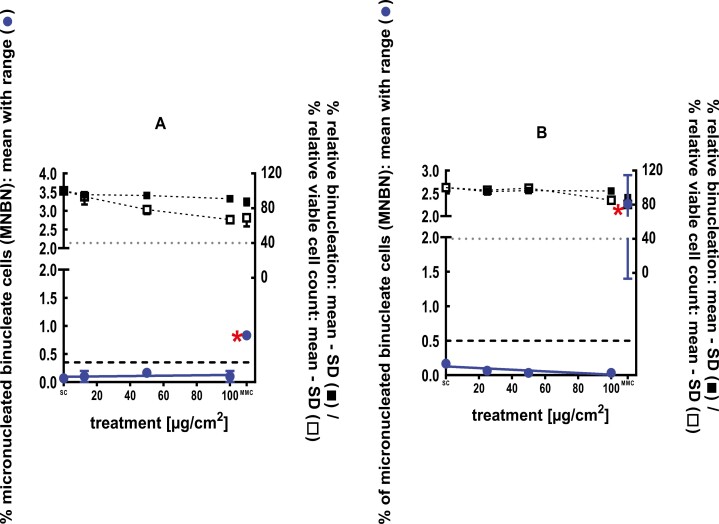
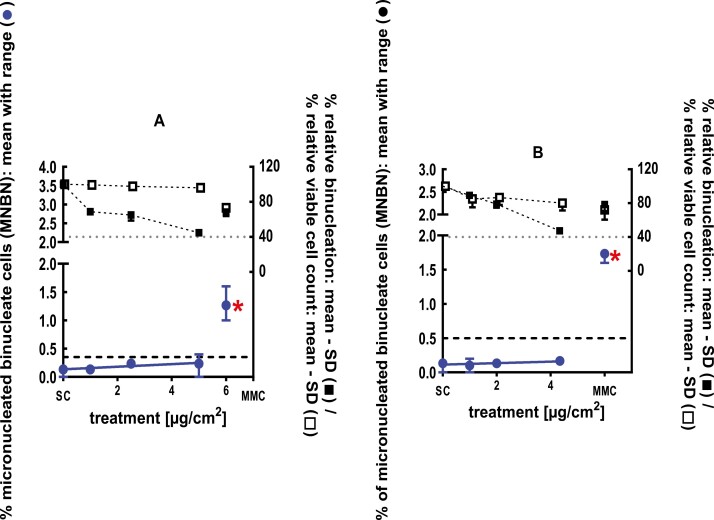
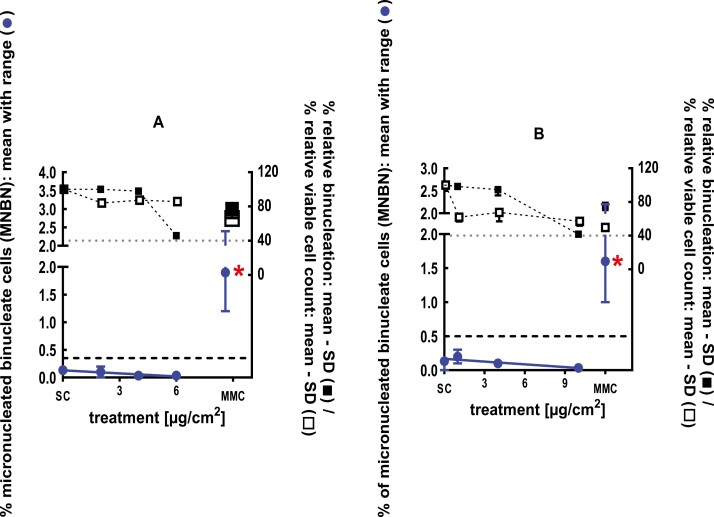
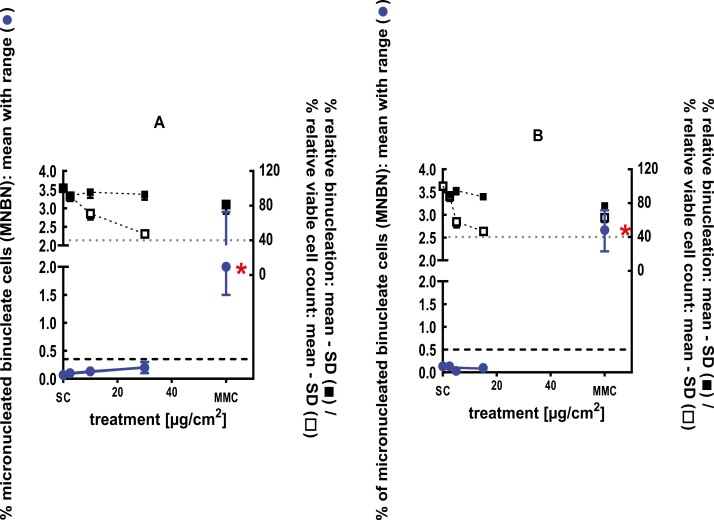
The clastogenic activity of cadinene was evaluated in an in vitro micronucleus test performed with human peripheral blood lymphocytes, at concentrations up to 1000 µg/ml in dimethyl formamide, in the presence and absence of metabolic activation. Cadinene did not induce binucleated cells with micronuclei when tested up to the cytotoxic level concentration in either the presence or absence of an S9 activation system in the 3-hour treatments. Cadinene did induce binucleated cells with micronuclei in the 24-hour treatment at doses of 41.3 and 58.1 µg/ml and was considered to be clastogenic with questionable biological relevance in the in vitro micronucleus test, as the increases observed were statistically significant but they were not dose-dependent or reproducible in every repeat assay [30]. A follow-up 3D skin study was evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with cadinene in acetone for 48 and 72 hours, at concentrations up to 100 mg/ml. Cadinene did not induce binucleated cells with micronuclei when tested up to the maximum cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [31] (Fig. 2).
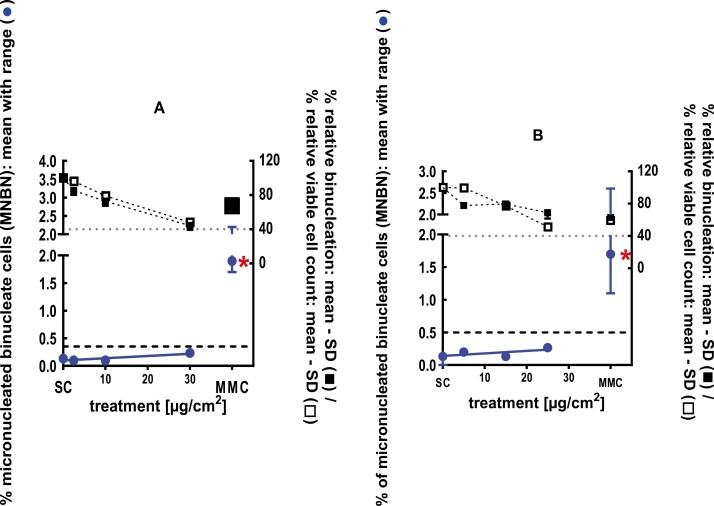
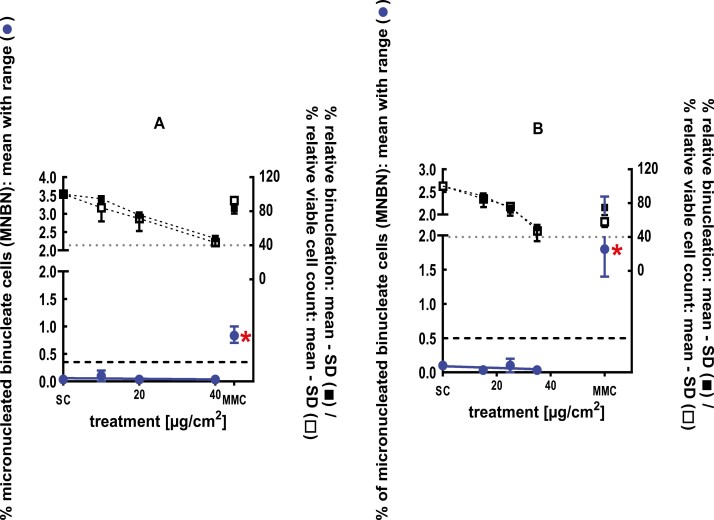
Figure 2.
3Dskin RSMN results for Cadinene (CAS#29350-73-0): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the cutoff for excessive cytotoxicity (40%) is shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propanal (CAS# 300371-33-9)
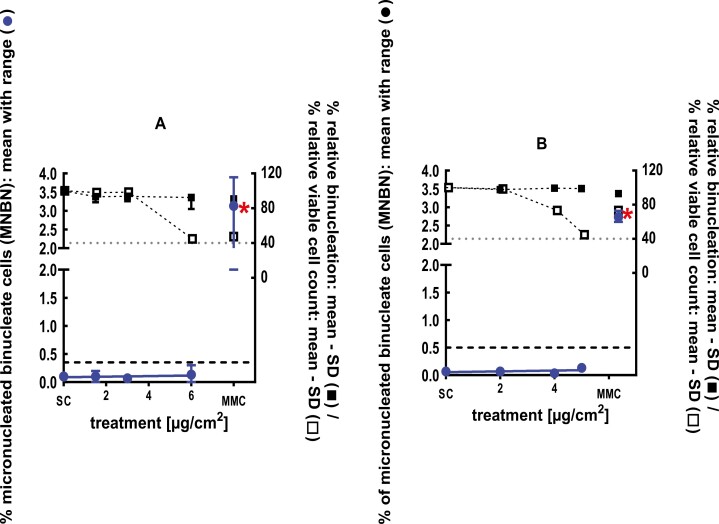
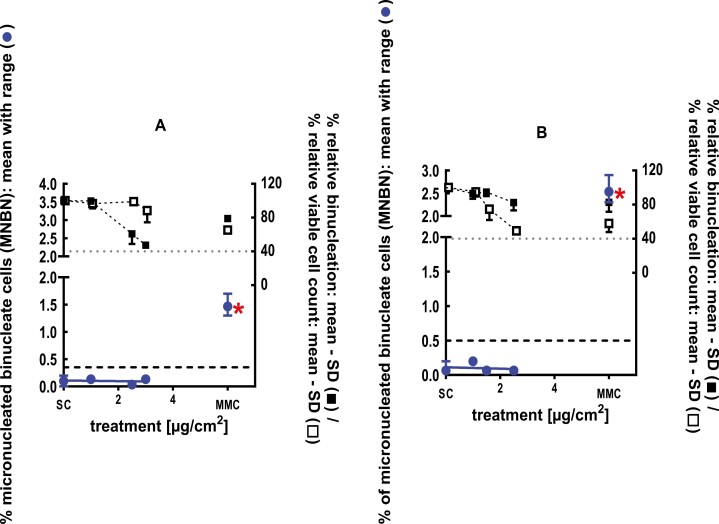
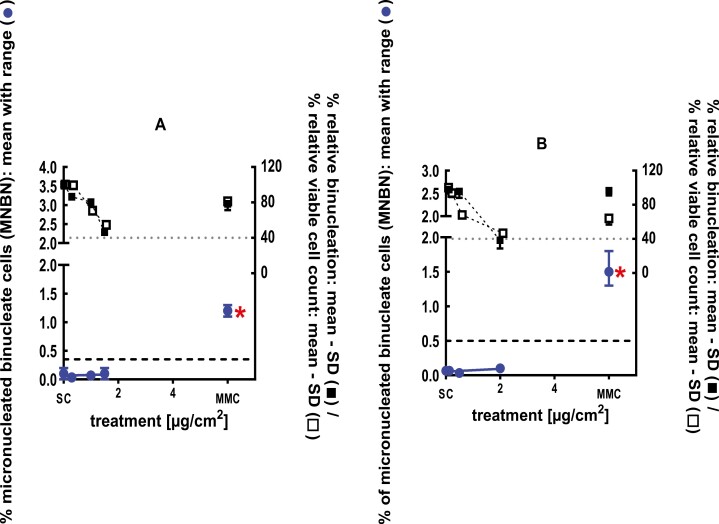
The clastogenic activity of 2,3-dihydro-1,1-dimethyl-1H-indene-ar-propanal was evaluated in an in vitro micronucleus performed with human peripheral blood lymphocytes, at concentrations up to 1300 µg/ml in dimethyl sulfoxide (DMSO), in the presence of and absence of metabolic activation. 2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propanal did not induce binucleated cells with micronuclei when tested up to cytotoxic levels in non-activated 24-hour test systems. However, a statistically significant increase in micronuclei was observed at the 4-hour treatment period in the presence and absence of S9 metabolic activation at doses of 15 µg/ml in the presence of S9 and at doses of 7.5 and 35 µg/ml in the absence of S9. Despite these increases, a dose–response was not observed, and the study was concluded to be equivocal [32]. A follow-up 3D skin study was evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 2,3-dihydro-1,1-dimethyl-1H-indene-ar-propanal in acetone for 48 and 72 hours, at concentrations up to 12 mg/ml. 2,3-dihydro-1,1-dimethyl-1H-indene-ar-propanal did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [33] (Fig. 3).
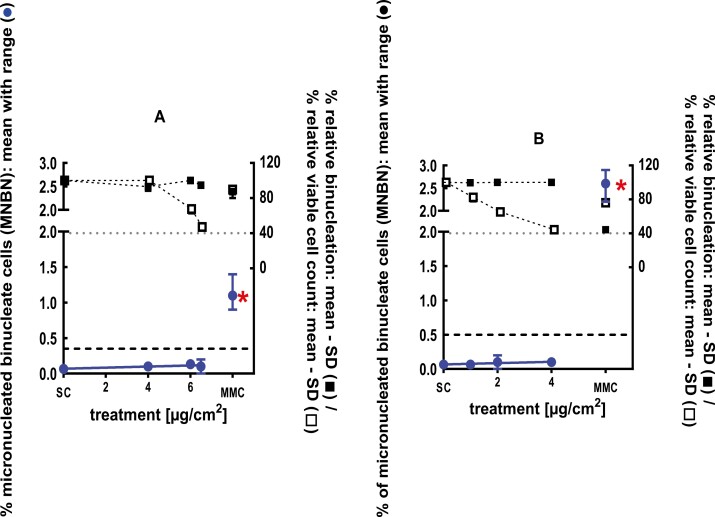
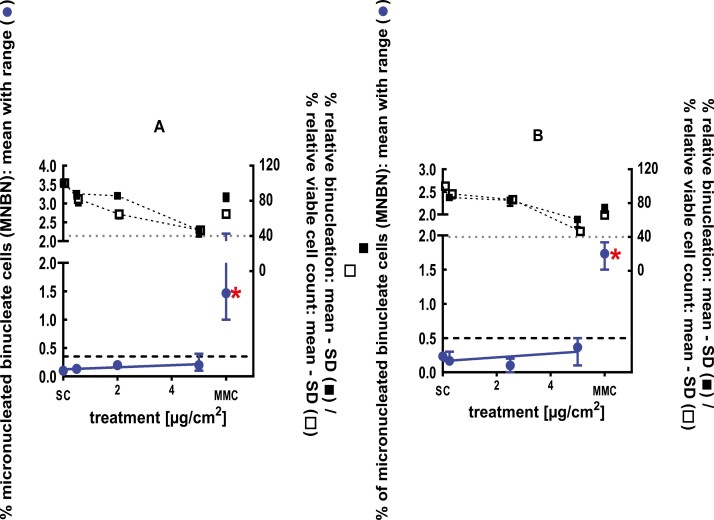
Figure 3.
3Dskin RSMN results for 2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propanal (CAS#300371-33-9): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime (CAS# 75147-23-8)
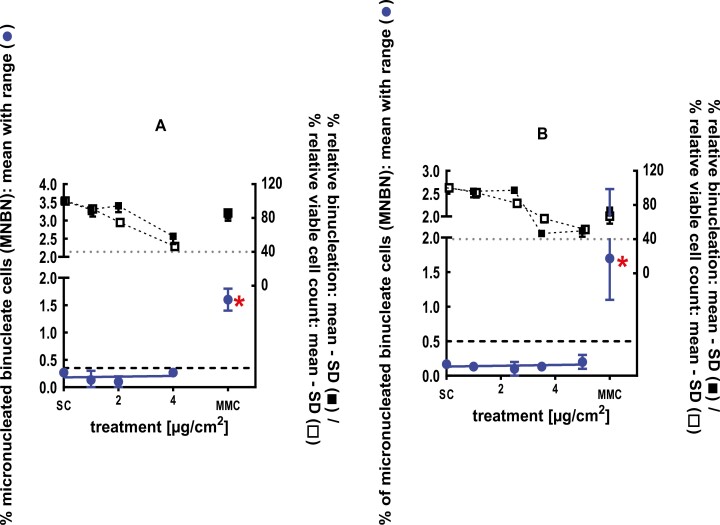
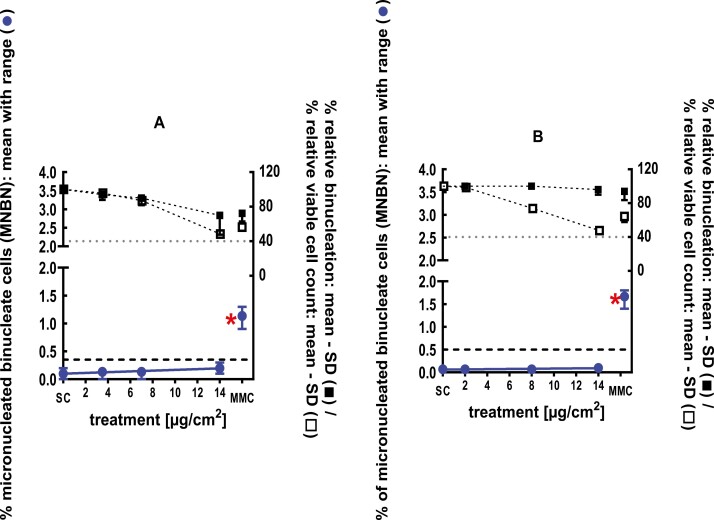
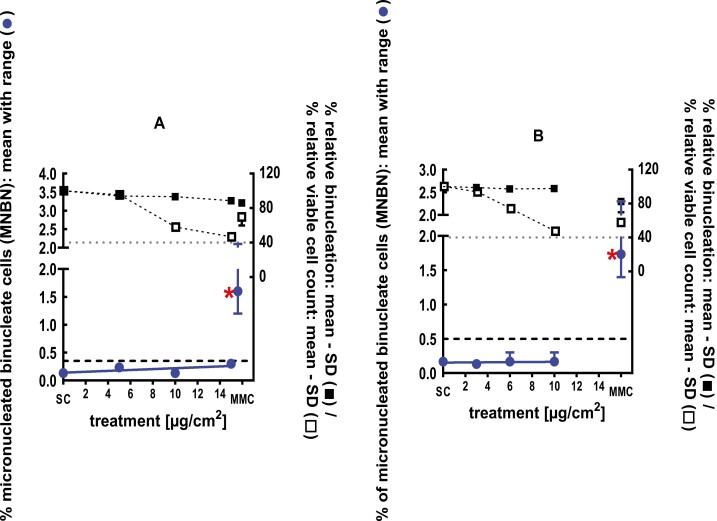
The clastogenic activity of 1,5-dimethylbicyclo[3.2.1]octan-8-one-oxime was evaluated in an in vitro micronucleus test performed with human peripheral blood lymphocytes, at concentrations up to 1482 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 402 µg/ml in the 3-hour treatment in the presence of S9, 300 and 425 µg/ml in the 3-hour treatment in the absence of S9, and 47.3 µg/ml in the 24-hour treatment in the absence of S9. 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [34]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP-compliant RSMN assay, EpiDermTM tissues were treated with 1,5-dimethylbicyclo[3.2.1]octan-8-one-oxime for 48 and 72 hours, at concentrations up to 90 mg/ml. 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime ether did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [35] (Fig. 4). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 125, 250, or 500 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [36].
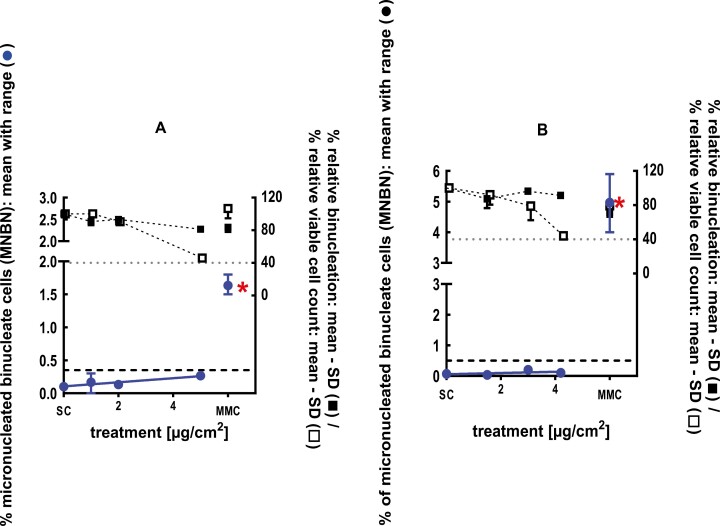
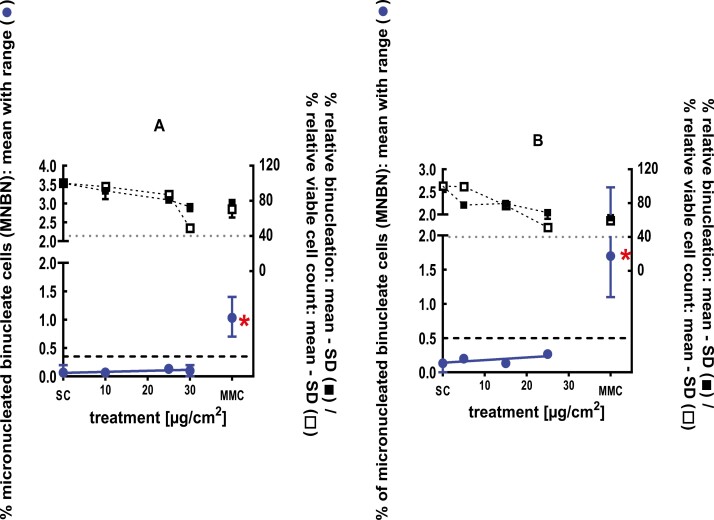
Figure 4.
3Dskin RSMN results for 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime (CAS# 75147-23-8): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
2,2ʹ-(Dithiodimethylene)difuran (CAS# 4437-20-1)
The clastogenic activity of 2,2ʹ-(dithiodimethylene)difuran was performed using human peripheral blood lymphocytes at concentrations up to 1000 µg/ml in DMSO. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 29.5 and 32.8 µg/ml in the 3-hour treatment in the presence of S9 and at doses of 14.3, 19.5, and 29.4 µg/ml on the 3-hour treatment in the absence of S9 [37]. 2,2ʹ-(dithiodimethylene)difuran was considered to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP-compliant RSMN assay, EpiDermTM tissues were treated with 2,2ʹ-(dithiodimethylene)difuran in DMSO for 48 and 72 hours, at concentrations up to 1.25 mg/ml. 2,2ʹ-(dithiodimethylene)difuran did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative for the induction of micronuclei in the RSMN assay [38] (Fig. 5). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil via oral gavage on two consecutive days at doses of 62.5, 125, or 250 mg/kg body weight to groups of male and female HSD:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was considered to be negative in the in vivo micronucleus test [39].
Figure 5.
3Dskin RSMN results for 2,2ʹ-(Dithiodimethylene)difuran (CAS#4437-20-1): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Ethyl formate (CAS# 109-94-4)
The clastogenic activity of ethyl formate was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 741 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 596 and 741 µg/ml in the 3-hour treatment in the absence of S9, doses of 479, 596, and 741 µg/ml in the 3-hour treatment in the presence of S9, and doses of 554 µg/ml in the 24-hour treatment in the absence of S9. Ethyl formate was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [40]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with ethyl formate in ethanol for 48 and 72 hours, at concentrations up to 100 mg/ml. Ethyl formate did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [41] (Fig. 6). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [42].
Figure 6.
3Dskin RSMN results for Ethyl formate (CAS# 109-94-4): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
2-ethyl-1,3,3-trimethyl-2-norbornanol (CAS# 18368-91-7)
The clastogenic activity of 2-ethyl-1,3,3-trimethyl-2-norbornanol was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1823 µg/ml in DMSO, in the presence and absence of metabolic activation. No statistically significant and dose-dependent increases in micronuclei induction were observed when tested up to cytotoxic levels concentration in either the presence or absence of an S9 activation system. 2-ethyl-1,3,3-trimethyl-2-norbornanol was concluded to be negative for the induction of micronuclei in the in vitro mammalian cell micronucleus test [43]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 2-ethyl-1,3,3-trimethyl-2-norbornanol in acetone for 48 and 72 hours, at concentrations up to 6 mg/ml. 2-ethyl-1,3,3-trimethyl-2-norbornanol did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [44] (Fig. 7). A follow-up combined in vivo COMET/micronucleus study was also conducted in mice. The test material was administered in corn oil on four consecutive days at doses of 125, 250, or 500 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the blood or induce a significant increase in DNA damage in the liver and was concluded to be negative in the combined in vivo COMET/micronucleus test [45].
Figure 7.
3Dskin RSMN results for 2-ethyl-1,3,3-trimethyl-2-norbornanol (CAS#18368-91-7): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Furfuryl thioacetate (CAS# 13678-68-7)
The clastogenic activity of furfuryl thioacetate was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1000 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 48.4 and 69.8 µg/ml in the 3-hour treatment in the absence of S9 and doses of 48.4, 59.7, and 69.8 µg/ml in the 3-hour treatment in the presence of S9. Furfuryl thioacetate was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [46]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with furfuryl thioacetate in acetone for 48 and 72 hours, at concentrations up to 5 mg/ml. Furfuryl thioacetate did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [47] (Fig. 8). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 31.3, 62.5, and 125 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [48].
Figure 8.
3Dskin RSMN results for Furfuryl thioacetate (CAS#13678-68-7): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Isobornyl methyl ether (CAS# 5331-32-8)
The clastogenic activity of isobornyl methyl ether was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1680 µg/ml in ethanol, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 70 and 80 µg/ml in the 4-hour treatment in the presence of S9. Although the induced values (1.0 and 0.8%, respectively) were within the historical control range (0.0–1.5%), they fell outside of the 95% control range of historical control data (upper limit of 95% control range = 0.0–0.78%). In a repeat assay, statistically significant increases in micronuclei induction were observed at a dose of 80 µg/ml in the 4-hour treatment in the presence of S9. While the increase (1.1%) was outside the 95% control range of historical control data, the increase was not dose-dependent. Due to this, isobornyl methyl ether was concluded to be equivocal for the induction of micronuclei in the in vitro mammalian cell micronucleus test [49]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with isobornyl methyl ether in acetone for 48 and 72 hours, at concentrations up to 70 mg/ml. Isobornyl methyl ether did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [50] (Fig. 9). As an additional weight of evidence, a follow-up in vivo micronucleus study from read-across material 1-ethyl-3-methoxytricyclo[2.2.1.02,6]heptane (CAS # 31996-78-8) was also investigated. 1-ethyl-3-methoxytricyclo[2.2.1.02,6]heptane was administered in propylene glycol as a single dose of 3519 mg/kg body weight via intra gastric gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 24, 48, or 72 hours. 1-ethyl-3-methoxytricyclo[2.2.1.02,6]heptane did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the bone marrow and was concluded to be negative in the in vivo micronucleus test, and this result can be extended to isobornyl methyl ether [51].
Figure 9.
3Dskin RSMN results for Isobornyl methyl ether (CAS#5331-32-8): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Lauric aldehyde (CAS # 112-54-9)
The clastogenic activity of lauric aldehyde was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1840 µg/ml in tetrahydrofuran, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at a dose of 30 µg/ml in the 4-hour treatment in the absence of S9 and doses of 20 and 35 µg/ml in the 4-hour treatment in the presence of S9. Lauric aldehyde was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [52]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with lauric aldehyde in acetone for 48 and 72 hours, at concentrations up to 100 mg/ml. Lauric aldehyde did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [53] (Fig. 10). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [54].
Figure 10.
3Dskin RSMN results for Lauric Aldehyde (CAS # 112-54-9): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
p-Methoxy cinnamaldehyde (CAS# 1963-36-6)
The clastogenic activity of p-methoxy cinnamaldehyde was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1624 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 75., 100, and 110 µg/ml in the 3-hour treatment in the absence of S9, a dose of 160 µg/ml in the 3-hour treatment in the presence of S9, and a dose of 110 µg/ml in the 24-hour treatment in the absence of S9. p-Methoxy cinnamaldehyde was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [55]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with p-methoxy cinnamaldehyde in acetone for 48 and 72 hours, at concentrations up to 5 mg/ml. p-Methoxy cinnamaldehyde did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [56] (Fig. 11). A follow-up combined in vivo COMET/micronucleus study was also conducted in mice. The test material was administered in 1% Methylcellulose (400cPS) in deionized water on four consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the blood or induce a significant increase in DNA damage in the liver and was concluded to be negative in the combined in vivo COMET/micronucleus test [57].
Figure 11.
3Dskin RSMN results for p-Methoxy cinnamaldehyde (CAS#1963-36-6): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
6-Methoxy-2,6-dimethylheptan-1-al (CAS# 62439-41-2)
The clastogenic activity of 6-methoxy-2,6-dimethylheptan-1-al was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1723 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 1245, 1465, and 1723 µg/ml in the 3-hour treatment in the absence of S9 and a doses of 1723 µg/ml in the 3-hour treatment in the presence of S9. 6-Methoxy-2,6-dimethylheptan-1-al was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [58]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 6-methoxy-2,6-dimethylheptan-1-al in acetone for 48 and 72 hours, at concentrations up to 45 mg/ml. 6-Methoxy-2,6-dimethylheptan-1-al did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [59] (Fig. 12). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [60].
Figure 12.
3Dskin RSMN results for 6-Methoxy-2,6-dimethylheptan-1-al (CAS # 62439-41-2): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
2-Methyl-2-pentenal (CAS# 623-36-9)
The clastogenic activity of 2-methyl-2-pentenal was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 981 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at a dose of 500 µg/ml in the 4-hour treatment in the presence and absence of S9 and a dose of 125 µg/ml in the 24-hour treatment in the absence of S9. 2-Methyl-2-pentenal was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [61]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 2-methyl-2-pentenal in acetone for 48 and 72 hours, at concentrations up to 45 mg/ml. 2-Methyl-2-pentenal did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [62] (Fig. 13). A follow-up combined in vivo COMET/micronucleus study was also conducted in mice. The test material was administered in corn oil on three consecutive days at doses of 350, 700, or 1400 mg/kg body weight via oral gavage to groups of male and female Han Wistar rats and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the bone marrow or induce a significant increase in DNA damage in the liver and was concluded to be negative in the combined in vivo COMET/micronucleus test [63].
Figure 13.
3Dskin RSMN results for 2-Methyl-2-pentenal (CAS#623-36-9): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Methyl beta-phenylglycidate (CAS# 37161-74-3)
The clastogenic activity of methyl beta-phenylglycidate was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1782 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant increases in micronuclei induction were observed at doses of 581, 646, and 886 µg/ml in the 3-hour treatment in the presence of S9; however, no clear concentration related increase was observed. Methyl beta-phenylglycidate was concluded to be positive with questionable biological relevance for the induction of micronuclei in the in vitro mammalian cell micronucleus test [64]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with methyl beta-phenylglycidate in acetone for 48 and 72 hours, at concentrations up to 4.34 mg/ml. Methyl beta-phenylglycidate did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [65] (Fig. 14). A follow-up combined in vivo COMET/micronucleus study was also conducted in mice. The test material was administered in corn oil on four consecutive days at doses of 250, 500, or 1000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood or induce a significant increase in DNA damage in the liver and was concluded to be negative in the combined in vivo COMET/micronucleus test [66].
Figure 14.
3Dskin RSMN results for Methyl beta-phenylglycidate (CAS#37161-74-3): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Nona-2 trans- 6-cis-dienal (CAS# 557-48-2)
The clastogenic activity of nona-2-trans-6-cis-dienal was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 60 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 15 and 20 µg/ml in the 4-hour treatment in the absence of S9, at a dose of 40 µg/ml in the 4-hour treatment in the presence of S9, and at doses of 20 and 30 µg/ml in the 24-hour treatment in the absence of S9. Nona-2-trans-6-cis-dienal was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [67]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with nona-2-trans-6-cis-dienal in acetone for 48 and 72 hours, at concentrations up to 3 mg/ml. Nona-2-trans-6-cis-dienal did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [68] (Fig. 15). A follow-up combined in vivo COMET/micronucleus study was also conducted in rats. The test material was administered in corn oil on three consecutive days at doses of 175, 350, or 700 mg/kg body weight via oral gavage to groups of male and female Han Wistar rats and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the bone marrow or induce a significant increase in DNA damage in the liver and was concluded to be negative in the combined in vivo COMET/micronucleus test [69].
Figure 15.
3Dskin RSMN results for Nona-2 trans- 6-cis-dienal (CAS# 557-48-2): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
2-Octenoic acid, 4-ethyl-, (2E)- (CAS# 60308-76-1)
The clastogenic activity of 2-octenoic acid, 4-ethyl-, (2E)- was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1704 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 564, 658, and 692 µg/ml in the 3-hour treatment in the absence of S9 and at doses of 570 and 600 µg/ml in the 3-hour treatment in the presence of S9. 2-Octenoic acid, 4-ethyl-, (2E)- was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [70]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 2-octenoic acid, 4-ethyl-, (2E)- in acetone for 48 and 72 hours, at concentrations up to 14 mg/ml. 2-Octenoic acid, 4-ethyl-, (2E)- did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [71] (Fig. 16). A follow-up combined in vivo COMET/micronucleus study was also conducted in mice. The test material was administered in corn oil on four consecutive days at doses of 250, 500, and 1000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the blood for male or female mice or induce a significant increase in DNA damage in the liver for male mice. However, a statistically significant increase in DNA damage in the liver for female mice was observed at 1000 mg/ kg body weight. 2-Octenoic acid, 4-ethyl-, (2E)- was concluded to be negative in the combined in vivo COMET/micronucleus test in males and positive in the in vivo COMET assay for females [72].
Figure 16.
3Dskin RSMN results for 2-Octenoic acid, 4-ethyl-,(CAS# 60308-76-1): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
2-Octen-4-one (CAS# 4643-27-0)
The clastogenic activity of 2-octen-4-one was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1265 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 13.4, 16.5, and 20.4 µg/ml in the 3-hour treatment in the absence of S9 and at doses of 31.5 and 38.9 µg/ml in the 3-hour treatment in the presence of S9. 2-Octen-4-one was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [73]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 2-octen-4-one in acetone for 48 and 72 hours, at concentrations up to 2.5 mg/ml. 2-Octen-4-one did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [74] (Fig. 17). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 250, 500, or 1000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [75].
Figure 17.
3Dskin RSMN results for 2-Octen-4-one (CAS#4643-27-0): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
4-Phenyl-3-buten-2-ol (CAS# 17488-65-2)
The clastogenic activity of 4-phenyl-3-buten-2-ol was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1000.00 mM (1482 µg/ml) in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 666.67 mM (988 µg/ml) in the 3-hour treatment in the presence of S9. 4-Phenyl-3-buten-2-ol was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [76]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 4-phenyl-3-buten-2-ol in acetone for 48 and 72 hours, at concentrations up to 14 mg/ml. 4-Phenyl-3-buten-2-ol did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [77] (Fig. 18). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [78].
Figure 18.
3Dskin RSMN results for 4-Phenyl-3-buten-2-ol (CAS#17488-65-2): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
5-Phenylhex-3-en-2-one (CAS# 60405-50-7)
The clastogenic activity of 5-phenylhex-3-en-2-one was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1740 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 20, 22.5, and 25 µg/ml in the 24-hour treatment in the absence of S9 in an initial and confirmatory assay. However, while the initial assay was outside the 95% historical control range and the confirmatory assay was within the 95% historical control range, both assays were within the historical control range. Due to this, 5-Phenylhex-3-en-2-one was concluded to be equivocal for the induction of micronuclei in the in vitro mammalian cell micronucleus test [79]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 5-phenylhex-3-en-2-one in acetone for 48 and 72 hours, at concentrations up to 2 mg/ml. 5-Phenylhex-3-en-2-one did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [80] (Fig. 19). As an additional weight of evidence, a combined in vivo COMET/micronucleus study from read-across material 4-phenyl-3-buten-2-one (CAS#122-57-6) was also available. The test material was administered in corn oil on three consecutive days at doses of 250, 500, or 1000 mg/kg body weight via oral gavage to groups of male and female Han Wistar rats and euthanized at 3–4 hours post-last dose. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the bone marrow or induce a significant increase in DNA damage in the liver and was concluded to be negative in the combined in vivo COMET/micronucleus test [81], and this result can be extended to 5-phenylhex-3-en-2-one due to the structural similarity with 4-phenyl-3-buten-2-one.
Figure 19.
3Dskin RSMN results for 5-Phenylhex-3-en-2-one (CAS#60405-50-7): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
4-Thujanol (CAS# 546-79-2)
The clastogenic activity of 4-thujanol was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1540 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at a dose of 630 µg/ml in the 4-hour treatment in the absence of S9. 4-Thujanol was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [82]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 4-thujanol in acetone for 48 and 72 hours, at concentrations up to 2 mg/ml. 4-Thujanol did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [83] (Fig. 20). A follow-up combined in vivo COMET/micronucleus study was also conducted in mice. The test material was administered in corn oil on four consecutive days at doses of 250, 500, and 1000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 3–4 hours post-last dose. The test material did induce a statistically significant increase in the incidence of micronucleated reticulocytes in the blood at 500 and 1000 mg/kg/day in males and did induce a significant increase in DNA damage in the liver at 500 and 1000 mg/kg/day in males and at 1000 mg/kg/day in females. However, these increases were within the historical control range, and therefore not considered to be biologically significant. 4-Thujanol was concluded to be negative in the combined in vivo COMET/micronucleus test [84].
Figure 20.
3Dskin RSMN results for 4-Thujanol (CAS# 546-79-2): A) 48-hour treatment and B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
3,3,5-Trimethylcyclohexaneacetic acid (CAS# 3213-73-8)
The clastogenic activity of 3,3,5-trimethylcyclohexaneacetic acid was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1840 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at doses of 809 and 851 µg/ml in the 3-hour treatment in the presence of S9. 3,3,5-Trimethylcyclohexaneacetic acid was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [85]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with 3,3,5-trimethylcyclohexaneacetic acid in acetone for 48 and 72 hours, at concentrations up to 40 mg/ml. 3,3,5-Trimethylcyclohexaneacetic acid did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [86] (Fig. 21). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 125, 250, or 500 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [87].
Figure 21.
3Dskin RSMN results for 3,3,5-Trimethylcyclohexaneacetic acid (CAS#3213-73-8): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Veratraldehyde (CAS# 120-14-9)
The clastogenic activity of veratraldehyde was evaluated in an in vitro micronucleus test conducted using human peripheral blood lymphocytes, at concentrations up to 1662 µg/ml in DMSO, in the presence and absence of metabolic activation. Statistically significant and dose-dependent increases in micronuclei induction were observed at a dose of 496 µg/ml in the 24-hour treatment in the absence of S9. Veratraldehyde was concluded to be positive for the induction of micronuclei in the in vitro mammalian cell micronucleus test [88]. A follow-up 3D skin and in vivo study were evaluated to verify the in vitro results and their biological relevance. In a GLP compliant RSMN assay, EpiDermTM tissues were treated with veratraldehyde in acetone for 48 and 72 hours, at concentrations up to 30 mg/ml. Veratraldehyde did not induce binucleated cells with micronuclei when tested up to cytotoxic levels, and therefore was concluded to be negative in the RSMN assay [89] (Fig. 22). A follow-up in vivo micronucleus study was also conducted in mice. The test material was administered in corn oil on two consecutive days at doses of 500, 1000, or 2000 mg/kg body weight via oral gavage to groups of male and female Hsd:ICR (CD-1) mice and euthanized at 48 hours. The test material did not induce a statistically significant increase in the incidence of micronucleated reticulocytes in the peripheral blood and was concluded to be negative in the in vivo micronucleus test [90].
Figure 22.
3Dskin RSMN results for Veratraldehyde (CAS#120-14-9): (A) 48-hour treatment and (B) 72-hour treatment condition. The long-dashed black line shows the lab-specific 95%-quantile of the mean of the historical control range for the MNBN data, and the thresholds for excessive cytotoxicity (40%) are shown by the black dotted line. A red asterisk indicates a statistically significant increase in % MNBN vs. the SC (Fisher’s exact test; P < .05). Error bars shown depict the standard error of the mean.
Overall, the results for the 48-hour exposure were in agreement with the 72-hour exposure; in this set of materials both 48- and 72-hour results were negative. It is important to note that similar concentrations were evaluated in both the 48- and 72-hour exposures in this study, indicating that the 48-hour exposure does not result in the ability to test significantly higher concentrations as may be thought to be important for greater detection of genotoxicity. In fact, the 72-hour exposure has been reported as having greater sensitivity compared to the 48-hour exposure (16).
The results of the RSMN assay were in 100% agreement with the in vivo micronucleus assay results, where 18 chemicals positive in an in vitro micronucleus assay were negative in an in vivo micronucleus assay (Table 1).
Discussion
The current data set comparing the standard in vitro and in vivo micronucleus assays to the RSMN assay demonstrates that the RSMN assay is an ideal model for the assessment of genotoxicity of fragrance materials. In our study, 18 fragrance materials that were positive or equivocal in the in vitro micronucleus test were negative in the RSMN assay which agrees 100% with the negative results in the in vivo micronucleus test for these materials. In addition, one material (2-ethyl-1,3,3-trimethyl-2-nobornanol) was negative in an in vitro micronucleus assay, negative in the RSMN, and negative in the in vivo micronucleus assay. Overall, the RSMN assay agreed with the in vivo micronucleus for 19 fragrance materials in our study. In the majority of the in vivo studies, the target tissue exposure was evident from the toxicity and CNS-related effects, bone marrow toxicity, or both which confirm systemic exposure.
These results support the conclusion that the RSMN assay is useful for follow-up testing of materials that are positive in standard in vitro tests. Positive results in in vitro tests for materials that are negative in vivo, i.e. “misleading positive result,” have been discussed extensively in the literature and are likely due to a number of factors including differences in the exogenous S9 metabolism in standard in vitro tests compared to in vivo metabolism. For example, in our dataset, the misleading positive result for 2-octen-4-one (CAS # 4643-27-0) in the in vitro micronucleus study in human peripheral blood lymphocytes (HPBL) could be attributed to the lack of phase-II metabolic enzyme activity which may help in detoxification of the parent and metabolite of 2-octen-4-one resulting in metabolic overload [91], or may be due to formation of a reactive oxygen species as is known to occur in alpha, beta unsaturated ketones [92], whereas 3D skin and the in vivo micronucleus study have phase II detoxification capability to handle oxygen species [11]. The misleading positive result in the in vitro micronucleus assay for 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime may be due to the fact that oximes can form reactive sulfate conjugates that aminate DNA in the absence of appropriate metabolic transformation pathway. For example, 2-Butanone oxime (MEKO) can be directly conjugated with sulfate. Oxidation of MEKO to 2-nitrobutane has been demonstrated to occur in liver microsomal preparation [93], but likely is only a minor pathway of MEKO-biotransformation in rats in vivo. In rats, hydrolysis of MEKO to 2-butanone is the major pathway of biotransformation and 2-butanone is further catabolized to CO2 [94]. A final example from our dataset is for 2,2ʹ-(Dithiodimethylene)difuran and furfuryl thioacetate, where the positive outcomes in the in vitro micronucleus assays were only seen in shorter treatment conditions both with and without metabolic activation treatment, but it was negative in the long-term (24-hour) treatment condition in the absence of metabolic activation. For thiol compounds, the metabolism pathway seems to follow this chronology: S-oxidation, S-methylation, and fission of the disulfide bond proceeding to oxidation at the SH group of the resulting hydrolyzed compounds [95] (EFSA 2011). These can further form glutathione conjugates or undergo glucuronidation leading to its elimination. The enzymes involved in the biotransformation are primarily cytochrome P450 monooxygenase families, glycine-, glucuronide- methyl-, and glutathione transferases [95] (EFSA 2011). All these are present at sufficient level in the 3D RSMN tissue [11], hence negative results were observed in the RSMN assay and were in agreement with more biologically relevant in vivo studies which also has these enzymes present at sufficient levels. Taken together, our results indicate that the RSMN study generates more biologically relevant outcomes to determine genotoxic potential of fragrance materials. As such, the RSMN assay is useful for following up positive results from the standard in vitro cytogenetic assays.
The RSMN assay is also useful in cases where there is an equivocal or positive result that is considered non-biologically relevant in the traditional in vitro micronucleus assay. In the current dataset, 2,3-dihydro-1,1-dimethyl-1H-indene-ar-propanal, 5-phenylhex-3-en-2-one, and isobornyl methyl ether induced an equivocal result, whereas methyl beta-phenylglycidate and cadinene induced an increase in micronuclei in HBPL that was considered not biologically relevant (as discussed above in Results). Negative results were obtained in the RSMN assay for all of these materials, and these results agree with the negative in vivo micronucleus result for methyl beta-phenylglycidate and the negative read-across in vivo micronucleus result for isobornyl methyl ether.
Use of the RSMN assay earlier in a test battery could avoid time consuming, costly repeat testing and additional analyses that occur for those materials that induce weak or questionable effects. This is illustrated by a detailed discussion of the results of the in vitro micronucleus assay in HBPL for isobornyl methyl ether. Isobornyl methyl ether induced a statistically significant increase in micronuclei in the S9-activated 4-hour exposure that was dose-dependent [49]. Micronucleus induction was within the historical control range but outside the 95% historical control range, however, the Cochran-Armitage test was negative for dose–response (P > .05). Overall, isobornyl methyl ether was concluded to be equivocal. By contrast, isobornyl methyl ether was clearly negative in the RSMN assay. It can be argued that conducting an RSMN assay earlier in this evaluation, instead of repeating the standard in vitro micronucleus assay, may have resulted in an expedited, definitive assessment of a lack of genotoxicity. In vivo, alicyclic ethers like isobornyl methyl ether are expected to undergo either ring hydroxylation or side-chain oxidation followed by conjugation with glucuronic acid and excretion in the urine [96, 97], and therefore do not present a genotoxicity hazard.
The current study design for the RSMN assay [26] involves an initial assay using 2-day dosing regimen (48-hour treatment), and if the result is negative then a follow-up confirmatory assay using 3-day dosing regimen (72-hour treatment) is conducted [98]. In the studies reported here, consistent outcome (lack of micronuclei and similar cytotoxicity) was observed in both treatment regimens. We support the recent recommendation from the Cosmetics Europe validation of the RSMN assay by Pfuhler et al. to directly conduct the 72-hour treatment exclusively [16]. Considering the data pulished on the RSMN assay to date, there does not appear to be an advantage or need to routinely conduct a 2-day (48-hour) dosing regimen when there is an appropriately conducted 3-day (72-hour) RSMN assay. The RSMN study design can therefore be streamlined by conducting 3-day dosing directly and if the results are negative, the material can be concluded to be non-clastogenic. This is further supported by recent dataset comparison published by Pfuhler et al. which has discussed the advantages to doing a 72-hour dosing regimen [16].
Conclusion
The RSMN assay is an important alternative to animal testing for characterization of the genotoxicity potential of fragrance materials. In the context of the results obtained here, the RSMN assay was a powerful tool to address potential misleading positive outcomes from the standard in vitro genotoxicity battery as it showed 100% concordance with in vivo outcomes. Importantly, since the primary route of exposure for fragrances is by the dermal route, the RSMN assay fits the applicability domain for testing fragrance materials.
Conflict of interest statement: None declared.
References
- 1. European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Off J Eur Union 2003;66:26–35. [Google Scholar]
- 2. Kirkland D, Aardema M, Henderson Let al. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat Res 2005;584:1–256. [DOI] [PubMed] [Google Scholar]
- 3. Kirkland D, Aardema M, Müller Let al. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens II. Further analysis of mammalian cell results, relative predictivity and tumour profiles. Mutat Res 2006;608:29–42. [DOI] [PubMed] [Google Scholar]
- 4. Ates G, Doktorova TY, Pauwels Met al. Retrospective analysis of the mutagenicity/genotoxicity data of the cosmetic ingredients present on the Annexes of the Cosmetic EU legislation (2000-12). Mutagenesis 2014;29:115–21. [DOI] [PubMed] [Google Scholar]
- 5. Gibbs S, van de Sandt JJ, Merk HFet al. Xenobiotic metabolism in human skin and 3D human skin reconstructs: a review. Curr Drug Metab 2007;8:758–72. [DOI] [PubMed] [Google Scholar]
- 6. Hu T, Khambatta Z, Hayden Pet al. Xenobiotic metabolism gene expression in the EpiDerm™ in vitro 3D human epidermis model compared to human skin. Toxicology in Vitro 2010;24:1450–63. [DOI] [PubMed] [Google Scholar]
- 7. Götz C, Pfeiffer R, Tigges Jet al. Xenobiotic metabolism capacities of human skin in comparison with a 3D-epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: phase II enzymes. Exp Dermatol 2012;21:364–9. [DOI] [PubMed] [Google Scholar]
- 8. Wiegand C, Hewitt NJ, Merk HFet al. Dermal xenobiotic metabolism: a comparison between native human skin, four in vitro skin test systems and a liver system. Skin Pharmacol Physiol 2014;27:263–75. [DOI] [PubMed] [Google Scholar]
- 9. Kazem S, Linssen EC, Gibbs S.. Skin metabolism phase I and phase II enzymes in native and reconstructed human skin: a short review. Drug Discov Today 2019;24:1899–910. [DOI] [PubMed] [Google Scholar]
- 10. Aardema MJ, Barnett BB, Mun GCet al. Evaluation of chemicals requiring metabolic activation in the EpiDerm™ 3D human reconstructed skin micronucleus (RSMN) assay. Mutat Res 2013;750:40–9. [DOI] [PubMed] [Google Scholar]
- 11. Hewitt NJ, Edwards RJ, Fritsche Eet al. Use of human in vitro skin models for accurate and ethical risk assessment: metabolic considerations. Toxicol Sci 2013;133:209–17. [DOI] [PubMed] [Google Scholar]
- 12. Hu T, Kaluzhny Y, Mun GCet al. Intralaboratory and interlaboratory evaluation of the EpiDerm 3D human reconstructed skin micronucleus (RSMN) assay. Mutat Res 2009;673:100–8. [DOI] [PubMed] [Google Scholar]
- 13. Aardema MJ, Barnett BC, Khambatta Zet al. International prevalidation studies of the EpiDerm 3D human reconstructed skin micronucleus (RSMN) assay: transferability and reproducibility. Mutat Res 2010;701:123–31. [DOI] [PubMed] [Google Scholar]
- 14. Pfuhler S, Downs TR, Hewitt NJet al. Validation of the 3D reconstructed human skin micronucleus (RSMN) assay: an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis 2021;36:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfuhler S, Fellows M, van Benthem Jet al. In vitro genotoxicity test approaches with better predictivity: summary of an IWGT workshop. Mutat Res 2011;723:101–7. [DOI] [PubMed] [Google Scholar]
- 16. Pfuhler S, van Benthem J, Curren Ret al. Use of in vitro 3D tissue models in genotoxicity testing: strategic fit, validation status and way forward. Report of the working group from the 7th International Workshop on Genotoxicity Testing (IWGT). Mutat Res Genet Toxicol Environ Mutagen 2020;850–851, 503135. [Google Scholar]
- 17. Safford B, Api AM, Barratt Cet al. Use of an aggregate exposure model to estimate consumer exposure to fragrance ingredients in personal care and cosmetic products. Regul Toxicol Pharmacol 2015;72:673–82. [DOI] [PubMed] [Google Scholar]
- 18. Safford B, Api AM, Barratt Cet al. Application of the expanded Creme RIFM consumer exposure model to fragrance ingredients in cosmetic, personal care and air care products. Regul Toxicol Pharmacol 2017;86:148–56. [DOI] [PubMed] [Google Scholar]
- 19. Comiskey D, Api AM, Barrett Cet al. Integrating habits and practices data for soaps, cosmetics and air care products into an existing aggregate exposure model. Regul Toxicol Pharmacol 2017;88:144–56. [DOI] [PubMed] [Google Scholar]
- 20. OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, 2010.
- 21. OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, 2014.
- 22. OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, 2016.
- 23. OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, 1997.
- 24. OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, 2014.
- 25. OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, 2016.
- 26. Dahl EL, Curren R, Barnett BCet al. The reconstructed skin micronucleus assay (RSMN) in EpiDerm™: detailed protocol and harmonized scoring atlas. Mutat Res 2011;720:42–52. [DOI] [PubMed] [Google Scholar]
- 27. RIFM (Research Institute for Fragrance Materials). sec-Butyl ethyl ether: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 75303. 2019.
- 28. RIFM (Research Institute for Fragrance Materials). sec-Butyl ethyl ether: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2016. [Google Scholar]
- 29. RIFM (Research Institute for Fragrance Materials). sec-Butyl ethyl ether: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 75302. 2018.
- 30. RIFM (Research Institute for Fragrance Materials). Cadinene: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72347. 2016.
- 31. RIFM (Research Institute for Fragrance Materials). Cadinene: in vitro micronucleus test using reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 72544. 2017.
- 32. RIFM (Research Institute for Fragrance Materials). 2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propanal: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 69958. 2014.
- 33. RIFM (Research Institute for Fragrance Materials). 2,3-Dihydro-1,1-dimethyl-1H-indene-ar-propanal: in vitro micronucleus test using reconstructed skin micronucleus (RSMN) assay in Ep RIFM Report number 69959. 2016.
- 34. RIFM (Research Institute for Fragrance Materials). 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 69943. 2016.
- 35. RIFM (Research Institute for Fragrance Materials). 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 69962. 2016.
- 36. RIFM (Research Institute for Fragrance Materials). 1,5-Dimethylbicyclo[3.2.1]octan-8-one-oxime: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 69963. 2016.
- 37. RIFM (Research Institute for Fragrance Materials). 2,2’-(Dithiodimethylene)difuran: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72512. 2017.
- 38. RIFM (Research Institute for Fragrance Materials). 2,2’-(Dithiodimethylene)difuran: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 77703. 2021.
- 39. RIFM (Research Institute for Fragrance Materials). 2,2’-(Dithiodimethylene)difuran: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 74445. 2017.
- 40. RIFM (Research Institute for Fragrance Materials). Ethyl formate: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72515. 2017.
- 41. RIFM (Research Institute for Fragrance Materials). Ethyl formate: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2017. [Google Scholar]
- 42. RIFM (Research Institute for Fragrance Materials). Ethyl formate: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report Draft. 2019. [Google Scholar]
- 43. RIFM (Research Institute for Fragrance Materials). 2-ethyl-1,3,3-trimethyl-2-norbornanol: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72513. 2017.
- 44. RIFM (Research Institute for Fragrance Materials). 2-ethyl-1,3,3-trimethyl-2-norbornanol: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2016. [Google Scholar]
- 45. RIFM (Research Institute for Fragrance Materials). 2-ethyl-1,3,3-trimethyl-2-norbornanol: combined in vivo COMET and micronucleus assay. RIFM Report number 72598. 2017.
- 46. RIFM (Research Institute for Fragrance Materials). (Furfuryl thioacetate: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 71455. 2017.
- 47. RIFM (Research Institute for Fragrance Materials). Furfuryl thioacetate: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 72332. 2017.
- 48. RIFM (Research Institute for Fragrance Materials). Furfuryl thioacetate: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 71611. 2017.
- 49. RIFM (Research Institute for Fragrance Materials). Isobornyl methyl ether: in vitro mammalian cell micronucleus assay in human peripheral blood lymphocytes (HPBL). RIFM Report number 72542. 2017.
- 50. RIFM (Research Institute for Fragrance Materials). Isobornyl methyl ether: in vitro micronucleus test using reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 72543. 2017.
- 51. RIFM (Research Institute for Fragrance Materials). 1-Ethyl-3-methoxytricyclo[2.2.1.02,6]heptane: in vivo mammalian erythrocyte micronucleus assay in mouse bone marrow. RIFM Report number 47022. 1988.
- 52. RIFM (Research Institute for Fragrance Materials). Lauric Aldehyde: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 63992. 2016.
- 53. RIFM (Research Institute for Fragrance Materials). Lauric Aldehyde: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 69864. 2016.
- 54. RIFM (Research Institute for Fragrance Materials). Lauric Aldehyde: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 69960. 2016.
- 55. RIFM (Research Institute for Fragrance Materials). p-Methoxy cinnamaldehyde: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72325. 2017.
- 56. RIFM (Research Institute for Fragrance Materials). p-Methoxy cinnamaldehyde: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2017. [Google Scholar]
- 57. RIFM (Research Institute for Fragrance Materials). p-Methoxy cinnamaldehyde: combined in vivo COMET and micronucleus assay. RIFM Report number 72927. 2017.
- 58. RIFM (Research Institute for Fragrance Materials). 6-Methoxy-2,6-dimethylheptan-1-al: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 71354. 2016.
- 59. RIFM (Research Institute for Fragrance Materials). 6-Methoxy-2,6-dimethylheptan-1-al: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 71356. 2016.
- 60. RIFM (Research Institute for Fragrance Materials). 6-Methoxy-2,6-dimethylheptan-1-al: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 69968. 2016.
- 61. RIFM (Research Institute for Fragrance Materials). 2-Methyl-2-pentenal: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72918. 2017.
- 62. RIFM (Research Institute for Fragrance Materials). 2-Methyl-2-pentenal: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2016. [Google Scholar]
- 63. RIFM (Research Institute for Fragrance Materials). 2-Methyl-2-pentenal: combined in vivo COMET and micronucleus assay. RIFM Report number 73576. 2016.
- 64. RIFM (Research Institute for Fragrance Materials). Methyl beta-phenylglycidate: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72486. 2017.
- 65. RIFM (Research Institute for Fragrance Materials). Methyl beta-phenylglycidate: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2017. [Google Scholar]
- 66. RIFM (Research Institute for Fragrance Materials). Methyl beta-phenylglycidate: combined in vivo COMET and micronucleus assay. RIFM Report number 69960. 2016.
- 67. RIFM (Research Institute for Fragrance Materials). Nona-2 trans- 6-cis-dienal: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72917. 2017.
- 68. RIFM (Research Institute for Fragrance Materials). Nona-2 trans- 6-cis-dienal: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2016. [Google Scholar]
- 69. RIFM (Research Institute for Fragrance Materials). Nona-2 trans- 6-cis-dienal: combined in vivo COMET and micronucleus assay. RIFM Report number 73536. 2015.
- 70. RIFM (Research Institute for Fragrance Materials). 2-Octenoic acid, 4-ethyl-, (2E)-: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72916. 2017.
- 71. RIFM (Research Institute for Fragrance Materials). 2-Octenoic acid, 4-ethyl-, (2E)-: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report Draft. 2018. [Google Scholar]
- 72. RIFM (Research Institute for Fragrance Materials). 2-Octenoic acid, 4-ethyl-, (2E)-: combined in vivo COMET and micronucleus assay. RIFM Report Draft. 2017. [Google Scholar]
- 73. RIFM (Research Institute for Fragrance Materials). 2-Octen-4-one: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 69944. 2016.
- 74. RIFM (Research Institute for Fragrance Materials). 2-Octen-4-one: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 69782. 2016.
- 75. RIFM (Research Institute for Fragrance Materials). 2-Octen-4-one: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 69781. 2016.
- 76. RIFM (Research Institute for Fragrance Materials). 4-Phenyl-3-buten-2-ol: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 76207. 2018.
- 77. RIFM (Research Institute for Fragrance Materials). 4-Phenyl-3-buten-2-ol: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 76243. 2018.
- 78. RIFM (Research Institute for Fragrance Materials). 4-Phenyl-3-buten-2-ol: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 76268. 2018.
- 79. RIFM (Research Institute for Fragrance Materials). 5-Phenylhex-3-en-2-one: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 76188. 2020.
- 80. RIFM (Research Institute for Fragrance Materials). 5-Phenylhex-3-en-2-one: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 76189. 2020.
- 81. RIFM (Research Institute for Fragrance Materials). 5-Phenylhex-3-en-2-one: combined in vivo COMET and micronucleus assay. RIFM Report number 73067. 2014.
- 82. RIFM (Research Institute for Fragrance Materials). 4-Thujanol: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72343. 2017.
- 83. RIFM (Research Institute for Fragrance Materials). 4-Thujanol: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 77702. 2021.
- 84. RIFM (Research Institute for Fragrance Materials). 4-Thujanol: combined in vivo COMET and micronucleus assay. RIFM Report number 76271. 2019.
- 85. RIFM (Research Institute for Fragrance Materials). 3,3,5-Trimethylcyclohexaneacetic acid: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 70050. 2016.
- 86. RIFM (Research Institute for Fragrance Materials). 3,3,5-Trimethylcyclohexaneacetic acid: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 69871. 2015.
- 87. RIFM (Research Institute for Fragrance Materials). 3,3,5-Trimethylcyclohexaneacetic acid: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 69964. 2016.
- 88. RIFM (Research Institute for Fragrance Materials). Veratraldehyde: in vitro micronucleus assay in human peripheral blood lymphocytes. RIFM Report number 72398. 2017.
- 89. RIFM (Research Institute for Fragrance Materials). (2017) Veratraldehyde: in vitro reconstructed skin micronucleus (RSMN) assay in EpiDerm. RIFM Report number 72349. [Google Scholar]
- 90. RIFM (Research Institute for Fragrance Materials). Veratraldehyde: in vivo mammalian erythrocyte micronucleus assay in mouse peripheral blood with flow cytometry analysis. RIFM Report number 72919. 2017.
- 91. Kirkland DJ, Aardema M, Banduhn Net al. In vitro approaches to develop weight of evidence (WoE) and mode of action (MoA) discussions with positive in vitro genotoxicity results. Mutagenesis 2007;22:161–75. [DOI] [PubMed] [Google Scholar]
- 92. Janzowski C, Glaab V, Mueller Cet al. Alpha,beta-unsaturated carbonyl compounds: induction of oxidative DNA damage in mammalian cells. Mutagenesis 2003;18:465–70. [DOI] [PubMed] [Google Scholar]
- 93. Völkel W, Wolf N, Derelanko Met al. Slow oxidation of acetoxime and methylethyl ketoxime to the corresponding nitronates and hydroxy nitronates by liver microsomes from rats, mice, and humans. Toxicol Sci 1999;47:144–50. [DOI] [PubMed] [Google Scholar]
- 94. Burka LT, Black SR, Mathews JM.. Disposition of methyl ethyl ketoxime in the rat after oral, intravenous and dermal administration. Xenobiotica 1998;28:1005–15. [DOI] [PubMed] [Google Scholar]
- 95. Hogstrand C. Safety and efficacy of furfuryl and furan derivatives belonging to chemical group 14 when used as flavourings for all animal species and categories. EFSA J 2016;14:4389. [Google Scholar]
- 96. Madyastha KM, Chadha A.. Metabolism of 1,8-cineole in rat: its effects on liver and lung microsomal cytochrome P-450 systems. Bull Environ Contam Toxicol 1986;37:759–66. [DOI] [PubMed] [Google Scholar]
- 97. Asakawa Y, Toyota M, Ishida T.. Biotransformation of 1,4-cineole, a monoterpene ether. Xenobiotica 1988;18:1129–34. [DOI] [PubMed] [Google Scholar]
- 98. Curren RD, Mun GC, Gibson DPet al. Development of a method for assessing micronucleus induction in a 3D human skin model (EpiDerm). Mutat Res 2006;607:192–204. [DOI] [PubMed] [Google Scholar]