Abstract
Aims
The purpose of our study was to evaluate the feasibility and efficacy of cardiac resynchronization therapy (CRT) via left bundle branch pacing (LBBP-CRT) compared with optimized biventricular pacing (BVP) with adaptive algorithm (BVP-aCRT) in heart failure with reduced left ventricular ejection fraction ≤35% (HFrEF) and left bundle branch block (LBBB).
Methods and results
One hundred patients with HFrEF and LBBB undergoing CRT were prospectively enrolled in a non-randomized fashion and divided into two groups (LBBP-CRT, n = 49; BVP-aCRT, n = 51) in four centres. Implant characteristics and echocardiographic parameters were accessed at baseline and during 6-month and 1-year follow-up. The success rate for LBBP-CRT and BVP-aCRT was 98.00% and 91.07%. Fused LBBP had the greatest reduced QRS duration compared to BVP-aCRT (126.54 ± 11.67 vs. 102.61 ± 9.66 ms, P < 0.001). Higher absolute left ventricular ejection fraction (LVEF) and △LVEF was also achieved in LBBP-CRT than BVP-aCRT at 6-month (47.58 ± 12.02% vs. 41.24 ± 10.56%, P = 0.008; 18.52 ± 13.19% vs. 12.89 ± 9.73%, P = 0.020) and 1-year follow-up (49.10 ± 10.43% vs. 43.62 ± 11.33%, P = 0.021; 20.90 ± 11.80% vs. 15.20 ± 9.98%, P = 0.015, P = 0.015). There was no significant difference in response rate between two groups while higher super-response rate was observed in LBBP-CRT as compared to BVP-aCRT at 6 months (53.06% vs. 36.59%, P = 0.016) and 12 months (61.22% vs. 39.22%, P = 0.028) during follow-up. The pacing threshold was lower in LBBP-CRT at implant and during 1-year follow-up (both P < 0.001). Procedure-related complications and adverse clinical outcomes including heart failure hospitalization and mortality were not significantly different in two groups.
Conclusions
The feasibility and efficacy of LBBP-CRT demonstrated better electromechanical resynchronization and higher clinical and echocardiographic response, especially higher super-response than BVP-aCRT in HFrEF with LBBB.
Keywords: Heart failure, Left bundle branch block, Cardiac resynchronization therapy, His bundle pacing, Left bundle branch pacing, Biventricular pacing
What’s new?
We present the first multi-centre comparison of feasibility and safety of biventricular pacing with adaptive algorithm (BVP-aCRT) and cardiac resynchronization therapy via left bundle branch pacing (LBBP-CRT) in patients with left ventricular dysfunction.
The results of research first demonstrated that LBBP-CRT provided better electromechanical resynchronization, higher clinical and echocardiographic response, especially higher super-response than BVP-aCRT in heart failure with reduced left ventricular ejection fraction with typical LBBB.
Introduction
For decades, cardiac resynchronization therapy (CRT) via biventricular pacing (BVP) has been demonstrated to improve heart failure and decrease mortality in reduced left ventricular ejection fraction (LVEF) by large-scale, multi-centre, prospective, and randomized clinical trials, especially in patients of heart failure with reduced LVEF ≤35% (HFrEF) and left bundle branch block (LBBB) with QRS duration (QRSd) ≥150 ms. However, CRT non-response rate is still up to 30% in all CRT candidates.1 With the emerging of a novel algorithm in CRT device in recent years, BVP via adaptive optimization algorithm of CRT (BVP-aCRT) has become a promising alternative to achieve more physiological ventricular activation than conventional BVP and improved clinical outcomes through presetting right ventricular(RV)-synchronized left ventricular (LV) pacing by dynamic adjustment of atrioventricular (AV) delay.2 Nevertheless, combination of pacing from right ventricular endomyocardium and LV epimyocardium was obviously not a physiological conduction system pacing strategy to correct LBBB and recover electrical synchrony fundamentally.
Recently, His bundle pacing (HBP), the most physiological pacing strategy, has been reported to correct LBBB in chronic heart failure patients and improve heart failure in a series of clinical studies and HBP could provide comparable LVEF improvement to BVP.3 However, LBBB correction threshold by HBP was always high and unstable probably due to the pacing site was not beyond the site of the conduction block, which limited HBP to be used in CRT candidates with LBBB.
The more recent left bundle branch pacing (LBBP), pacing at more distal and deeper area than His bundle, has been used as an alternative to deliver CRT by correcting LBBB and improve LVEF with low and stable threshold in several case reports and small observational studies.4–7 Higher clinical and echocardiographic response has been demonstrated in CRT via LBBP (LBBP-CRT) than conventional BVP in CRT candidates in small non-randomized studies.8 However, comparisons of clinical efficacy between LBBP-CRT and optimized BVP (BVP-aCRT) have not been well established. The aim of the present study was to compare the electromechanical effects and clinical efficacy of LBBP-CRT against BVP-aCRT in HFrEF patients with LBBB.
Methods
Study design and participants
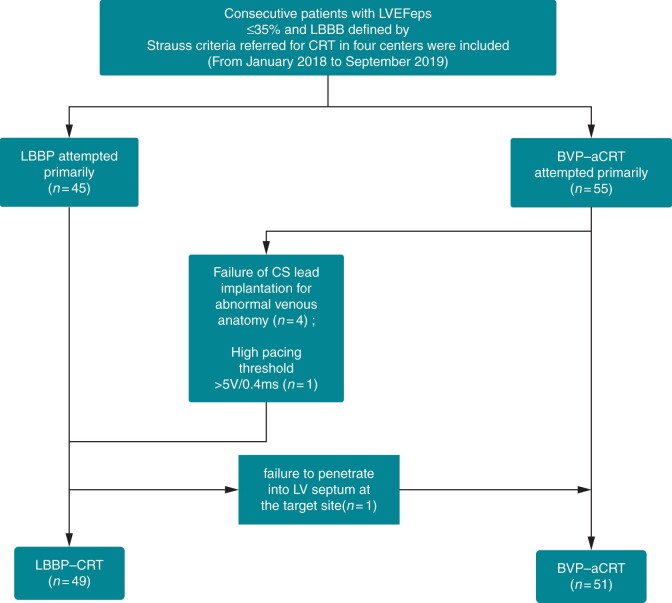
This was a non-randomized, prospective, multi-centre, observational study performed in four centres: Zhongshan Hospital, Fudan University, Shanghai, China; Changhai Hospital affiliated to The Second Military Medical University, Shanghai, China; Sir Run Run Shaw Hospital affiliated to College of Medicine, Zhejiang University, Zhejiang Province, China; and TongRen Hospital, Jiao Tong University, Shanghai, China. Patients indicated for a CRT implantation from January 2018 to September 2019 were consecutive included in the study. Inclusion criteria were (i) symptomatic heart failure [New York Heart Association functional class (NYHA) II–IV and LVEF ≤ 35%] after optimal medical therapy; (ii) sinus rhythm, QRS duration (QRSd) ≥150 ms and presence of typical LBBB in line with Strauss’s criteria9 including QS or rS morphology in V1–V2 along with mid-QRS notching or slurring in ≥2 leads among I, aVL, V1, V2, V5, and V6; and (iii) older than 18 years old, without pregnancy, and with life expectancy more than 1 year. Exclusion criteria included P-R interval >200 ms, persistent atrial fibrillation (AF), and intraventricular conduction defect (IVCD). The enrolled patients were non-randomized divided into two groups (LBBP-CRT and BVP-aCRT) based on a shared decision between the operator and the patients (Figure 1). All the operators at different centres were experienced in successfully implanting both LBBP and BVP ≥50 cases. Written, informed consent was obtained from all of the enrolled participants and this study was approved by Institutional Review Board of Zhongshan Hospital, Fudan University, Shanghai, China.
Figure 1.
Flow chart of our study population. BVP-aCRT, biventricular pacing with adaptive optimization algorithm of CRT; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LBBP, left bundle branch pacing; LBBP-CRT, cardiac resynchronization therapy via left bundle branch pacing; LVEF, left ventricular ejection fraction.
Implantation procedure
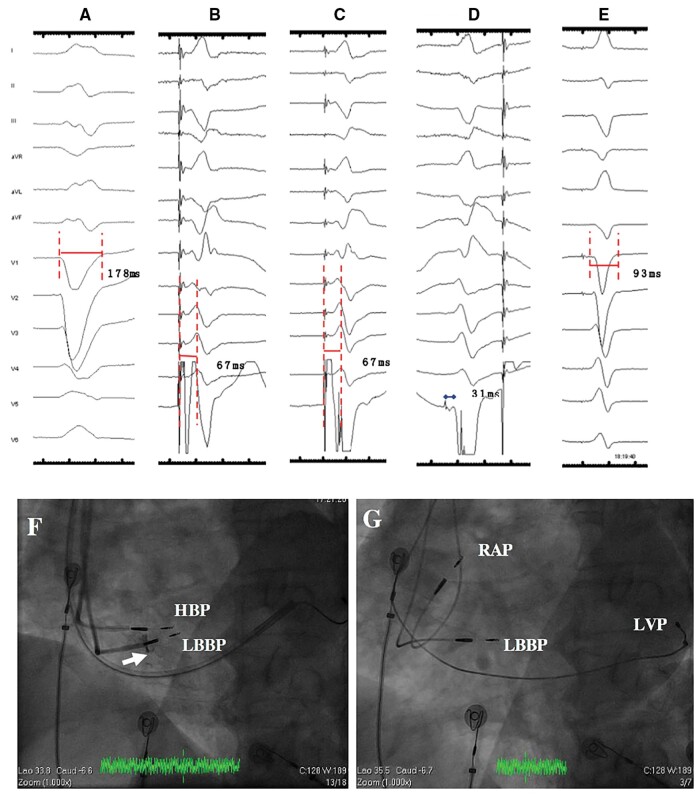
Left bundle branch pacing was attempted according to the literature10,11 in LBBP-CRT group. Briefly, the His bundle was first mapped with the pacing lead through the sheath (Model 3830, C315His, Medtronic Inc., Minneapolis, MN, USA). Then the lead was moved towards the ventricular side ∼1 cm across the tricuspid along the line between His location and RV apex at right anterior oblique (RAO) 30° and was deeply screwed into the interventricular septum. Left bundle branch pacing was achieved and confirmed with paced QRS of a right bundle branch block (RBBB) pattern and confirmation of selective LBBP (paced QRS of a typical RBBB pattern and a discrete component between stimulus and ventricle activation in electrogram(EGM)) or the stimulus to LV activation time in lead V4–6 shortening abruptly by increasing output or remaining shortest and constant at final depth (Figure 2). In LBBP-CRT group, the lead-to-device connection configuration was summarized in a schematic diagram (Supplementary material online, Figure S1). Left ventricular lead was implanted in patients with CRT-P and LBBP lead was connected to the RV port of the generator (Supplementary material online, Figure S1A and D). While in cases with CRT-D, LBBP lead, LV lead, and RV defibrillator lead were implanted with LBBP lead connected to the RV IS-1 port and the RV IS-1 connector of RV defibrillator lead was embedded (Supplementary material online, Figure S1B and E) except for five cases of LBBP lead substituting for LV lead and connecting to LV port due to LV lead implantation failure (Supplementary material online, Figure S1C and F).
Figure 2.
Electrocardiogram and intracardiac electrogram of LBBP during the procedure. (A) Intrinsic rhythm of LBBB with QRSd of 178 ms; non-selective LBBP (B) and selective LBBP (C) with the same Sti-LVAT of 67 ms; (D) LBB potential could be recorded during occurrence of RBBB morphology premature ventricular beat during lead rotation; (E) Paced QRSd of 93 ms during LBBP when fusion with intrinsic RBB after adjustment of AV delay of 130 ms; (F) fluoroscopic image of HBP lead (as a marker) and LBBP lead at LAO 35° (white arrow depicted the depth of LBBP lead inside the septum via angiography through the sheath); (G) fluoroscopic image of final positions of LBBP lead and LV lead at LAO 35° with HBP lead repositioned to RA septum. AV, atrioventricular; HBP, His bundle pacing; LAO, left anterior oblique; LBB, left bundle branch; LBBB, left bundle branch block; LBBP, left bundle branch pacing; LVP, left ventricular pacing; RAP, right atrial pacing; RBBB, right bundle branch block; Sti-LVAT, stimulus to left ventricular activation time.
Both LV lead and RV lead were performed in BVP-aCRT group. The RV lead was placed at RV apex. The LV lead was placed in the branch of coronary sinus with QLV >90 ms, measured as the duration from the onset of the surface QRS in lead II to the first larger positive or negative peak in the LV EGM. The precise location of LV lead in the branch of the coronary sinus was recorded.12 The fluoroscopy time of positioning LBBP lead and LV lead were collected and compared in two groups.
Device programming
After the procedure, LBBP lead was programmed as pacing only or pacing prior to LV or RV lead with interventricular(VV) delay of 80 ms if AV block occurred due to RBB injury during the procedure. Atrioventricular delay was adjusted according to electrocardiogram to fusion with the intrinsic RBB conduction to achieve narrowest QRS duration (Supplementary material online, Figure S2). Once RBB injury was diagnosed to be recovered during follow-up, LBBP lead would be programmed back to pacing only. Adaptive optimization algorithm was programmed on with DDD mode in BVP-aCRT group after procedure.
Data collection and follow-up
Baseline characteristics including age, gender, aetiology, and type of pacemaker were collected among all the enrolled patients. Subjects were then followed in the device clinic at 1-, 3-, and 6-month post-procedure.
Paced QRSd, which was measured from the onset of QRS wave, was documented and compared by 12-lead electrocardiogram at default output of 3.5 V/0.5 ms during LBBP-CRTVVI (LBBP during VVI mode), LBBP-CRTfusion (LBBP during DDD mode with AV delay adjusted to fusion with the intrinsic RBB conduction) (Supplementary material online, Figure S2), BVP-CRT (BVP during DDD mode with default AV delay of 100 ms and VV delay of 0 ms), and BVP-aCRT (BVP with DDD mode and adaptive optimization algorithm).
Lead parameters of LBBP lead and LV lead including pacing thresholds and impedance were compared at implant and follow-up. Ventricular pacing percentage was also collected during follow-up.
New York Heart Association classification and echocardiogram parameters including LVEF, LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV end-systolic volume(LVESV), LV end-diastolic volume (LVEDV), tricuspid annular plane systolic exclusion (TAPSE), left atrial diameter (LAD), pulmonary artery systolic pressure (PASP), mitral regurgitation, and tricuspid regurgitation in two groups were collected and compared. Among them, LVEF was measured by the two-dimensional modified biplane Simpson method. Two experienced echocardiographers blind to the study design were responsible for assessments of individual echocardiograms and the echocardiographic response was defined as an absolute increase in LVEF by ≥5% between baseline and follow-up while a ≥20% absolute increase or LVEF ≥50% was considered as super-response.
Left ventricular mechanical synchronization parameters including interventricular mechanical delay (IVMD), standard deviation of time to regional peak systolic velocity in the 12 segments (TS-12-SD) were measured in cases enrolled in Zhongshan Hospital, Fudan University. Interventricular mechanical delay was defined as the difference between the pre-ejection intervals of left and right ventricles. To obtain TS-12-SD, tissue velocity profile signals as well as the time-to-peak contraction velocity were analysed in each LV segment (except for the apex segments), during which the QRS complex was used as the reference point.
Procedure-related complications (e.g. RBB injury, significant increase of pacing threshold >2.5 V/0.5 ms, dislodgement, infection, embolism, perforation and pericardial effusion), episode of AF and sustained ventricular tachycardia required for anti-tachycardia pacing (ATP) or shock, and adverse clinical outcomes [heart failure hospitalization (HFH) and mortality] were routinely tracked and collected according to hospital records and office visits.
Ethical approval and informed consent
The study was approved by the Institutional Review Board of Zhongshan Hospital, Fudan University, Shanghai, China. Informed consent was obtained from all individual participants included in the study.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and paired Student’s t-test was used to compare the difference between baseline and follow-up in each group. ANOVA test was used to perform comparisons among more than two groups and was followed by least significant difference test for multiple comparisons. Categorical variables were presented as number (percentages) by using the Pearson’s χ2 test or Fisher’s exact test. All analyses were performed by SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) and a two-sided P-value <0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 100 consecutive patients undergoing CRT during the study period were included. The total success rate for LBBP and BVP was 98.00% and 91.07%. Primary BVP-aCRT was attempted in 56 patients (55 primary and 1 secondary to failed LBBP), but 5 of the primary cases switched to LBBP due to failure of LV lead implantation for abnormal venous anatomy (n = 4) and high pacing threshold >5 V/0.4 ms (n = 1). The LBBP procedure was successfully performed in 49 cases (45 primary and 5 secondary to failed BVP-aCRT) while only one patient failed due to unable to penetrate into LV septum at the target site (Figure 1).
The baseline characteristics of patients including age, gender, aetiology, comorbidity, intrinsic QRS duration, and medicine history between two groups were not significantly different (all P > 0.05, Table 1).
Table 1.
Baseline characteristics
| LBBP-CRT (n = 49) | BVP-aCRT (n = 51) | P-value | |
|---|---|---|---|
| Age (years) | 67.14 ± 8.88 | 64.37 ± 8.74 | 0.119 |
| Male, n (%) | 24 (49.98) | 30 (58.82) | 0.323 |
| Hypertension, n (%) | 14 (28.57) | 16 (31.37) | 0.760 |
| Diabetes, n (%) | 12 (24.49) | 10 (19.61) | 0.556 |
| Renal dysfunction, n (%) | 4 (8.16) | 3 (5.88) | 0.712 |
| DCM, n (%) | 36 (73.47) | 41 (80.39) | 0.411 |
| Paroxysmal AF, n (%) | 4 (8.16) | 3 (5.88) | 0.712 |
| Intrinsic QRSd (ms) | 180.12 ± 15.79 | 175.70 ± 11.29 | 0.121 |
| Medicine history | |||
| Beta-blockers, n (%) | 48 (97.96) | 51 (100.00) | 0.490 |
| ACEI/ARB, n (%) | 48 (97.96) | 50 (98.04) | ≥0.999 |
| Diuretics, n (%) | 46 (93.88) | 46 (90.20) | 0.715 |
| Aldosterone antagonist, n (%) | 46 (93.88) | 50 (98.04) | 0.357 |
ACEI, angiotensin-converting enzyme-inhibitors; AF, atrial fibrillation; ARB, Angiotensin Receptor Blocker; BVP-aCRT, biventricular pacing with adaptive optimization algorithm of cardiac resynchronization therapy; DCM, dilated cardiomyopathy; LBBP-CRT, cardiac resynchronization therapy via left bundle branch pacing; QRSd, QRS duration.
QRS duration and pacing parameters
No difference was found concerning QRSd in two groups at baseline (180.12 ± 15.79 vs. 175.70 ± 11.29 ms, P = 0.121). In LBBP-CRTVVI, a paced QRS morphology of an RBBB pattern was obtained, and the mean paced QRSd was measured as 120.96 ± 13.11 ms. The paced QRSd in LBBP-CRTfusion was then further reduced to 102.61 ± 9.66 ms after AV delay optimization (111.82 ± 10.79 ms) to promote fusion with intrinsic RBB conduction to eliminate RBBB shape and achieve narrowest QRSd. Significant difference in QRSd was observed in LBBP-CRTfusion as compared to BVP-aCRTVVI (144.70 ± 14.36 ms, P < 0.001) and BVP-aCRTDDD (126.54 ± 11.67 ms, P < 0.001) (Figure 3A). Maximum reduction in QRSd was also identified in LBBP-CRTfusion (77.51 ± 15.48 ms) and LBBP-CRTVVI (59.16 ± 15.97 ms), followed by BVP-CRT (31.00 ± 11.29 ms) and BVP-aCRT (49.15 ± 11.96 ms) (Figure 3B).
Figure 3.
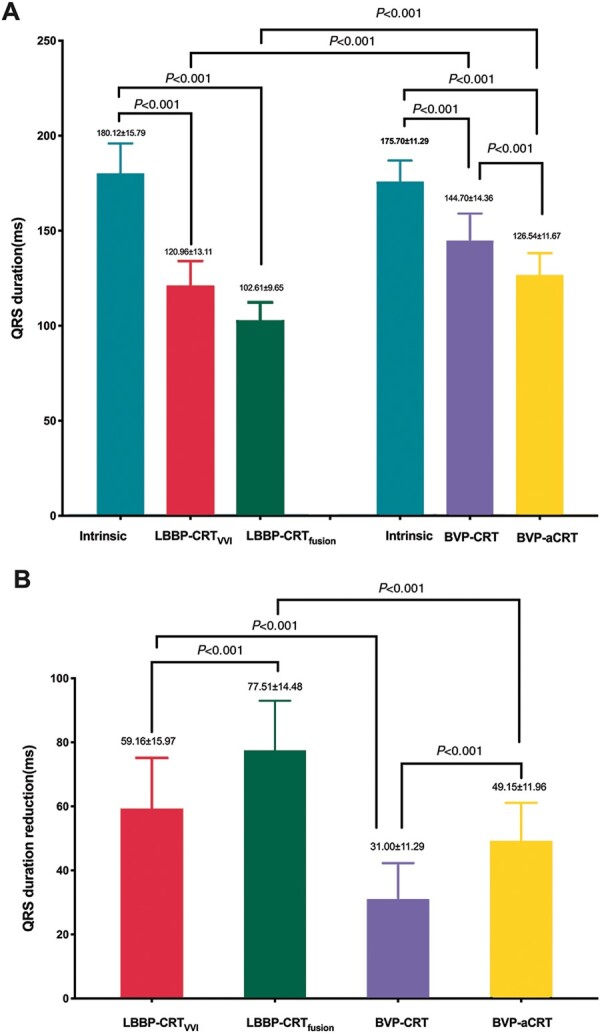
The intrinsic and paced QRSd during LBBP-CRT, BVP-CRT, and BVP-aCRT. (A) Comparison of intrinsic and paced QRSd; (B) reduction in QRSd. BVP-aCRT, BVP with DDD mode, and adaptive optimization algorithm; BVP-CRT, BVP during DDD mode with default AV delay of 100 ms and VV delay of 0 ms; LBBP-CRT, cardiac resynchronization therapy via left bundle branch pacing; LBBP-CRTfusion, LBBP during DDD mode with AV delay adjusted to fusion with the intrinsic RBB conduction; LBBP-CRTVVI, LBBP during VVI mode.
The percentage of CRT-D implanted in two groups was not significantly different (P = 0.760). Pacing threshold at implant was lower in LBBP-CRT than BVP-aCRT (0.92 ± 0.20 vs. 1.45 ± 0.39 V/0.5 ms, P < 0.001) and significantly lower threshold remained in LBBP-CRT at 6-month (0.76 ± 0.17 vs. 1.46 ± 0.37 V/0.5 ms) and 1-year follow-up (0.66 ± 0.17 vs. 1.42 ± 0.33 V/0.5 ms) (all P < 0.001). As shown in Table 2, no significant difference was found in impedance between LBBP in comparison to BVP-aCRT at implantation (P = 0.294), 6-month (P = 0.533), and 12-month (P = 0.899) follow-up. The pacing percentages was 98.40% in LBBP-CRT and 96.10% in BVP-aCRT group (P = 0.006) (Table 2).
Table 2.
Procedure and pacing characteristics
| LBBP-CRT (n = 49) | BVP-aCRT (n = 51) | P-value | |
|---|---|---|---|
| Fluoroscopy time (min) | 9.50 ± 1.99 | 13.84 ± 5.47 | <0.001 |
| CRT-D, n (%) | 35 (71.43) | 35 (68.63) | 0.760 |
| Pacing threshold (V/0.5 ms) | |||
| At implant | 0.92 ± 0.20 | 1.45 ± 0.39 | <0.001 |
| At 6 months | 0.76 ± 0.17a | 1.46 ± 0.37 | <0.001 |
| At 1 year | 0.66 ± 0.17a | 1.42 ± 0.33 | <0.001 |
| Ventricular impedance (Ω) | |||
| At implant | 534.79 ± 125.10 | 488.59 ± 194.77 | 0.294 |
| At 6 months | 443.67 ± 122.66b | 421.00 ± 154.95 | 0.533 |
| At 1 year | 430.62 ± 119.94a | 434.94 ± 142.95 | 0.899 |
| VP (%) | 98.40 | 96.10 | 0.006 |
BVP-aCRT, biventricular pacing with adaptive optimization algorithm of cardiac resynchronization therapy; LBBP-CRT, cardiac resynchronization therapy via left bundle branch pacing; VP, ventricular pacing.
Comparisons of pacing parameters between implantation and during follow-up, P < 0.01.
Comparisons of pacing parameters between implantation and during follow-up, P < 0.05.
Echocardiographic and clinical response
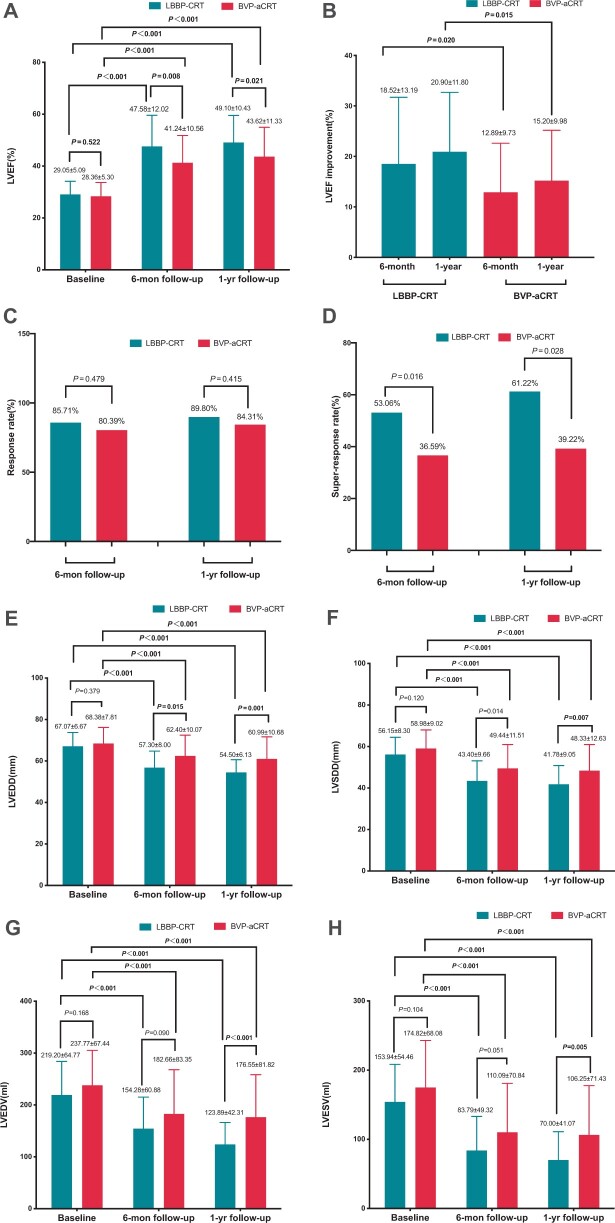
None of the patients in our study population was lost to 12-month follow-up. As shown in Figure 4A, the LVEF was similar at baseline in both groups (29.05 ± 5.09% vs. 28.36 ± 5.30%, P = 0.522) while patients in LBBP-CRT group had a significantly higher LVEF (47.58 ± 12.02%) at 6-month follow-up than BVP-aCRT (41.24 ± 10.56%, P = 0.008). Cardiac resynchronization therapy via LBBP showed more improvement in absolute LVEF at 6-month and 1-year follow-up, in comparison to BVP-aCRT (18.52 ± 13.19% vs. 12.89 ± 9.73%, P = 0.020; 20.90 ± 11.80% vs. 15.20 ± 9.98%, P = 0.015) (Figure 4B). Between the two groups, there was no statistically significant difference in response rate defined as an LVEF improvement >5% during follow-up (85.71% vs. 80.39%, P = 0.479) (Figure 4C). With regard to super-responders including patients with a >20% improvement and a normalization of LVEF (≥50%), the rates were significantly higher in LBBP-CRT than BVP-aCRT at 6 months (53.06% vs. 36.59%) and 1 year after implantation (61.22% vs. 39.22%) (both P < 0.001) (Figure 4D).
Figure 4.
Comparisons of echocardiographic parameters in patients at baseline and during follow-up in LBBP-CRT and BVP-aCRT. *P < 0.05, comparison within the same group at baseline; #P < 0.05, comparison of outcomes at 6-month follow-up between each group. (A) LVEF; (B) LVEF improvement; (C) Response rate; (D) Super-response rate; (E) LVEDD; (F) LVESD; (G) LVEDV; (H) LVESV. LVEF: left ventricular ejection fraction; LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV).
As shown in Table 3, similar baseline LVEDD, LVSDD, and LAD (P > 0.05) together with a significant decrease was demonstrated in two groups at follow-up (all P < 0.05). Whereas, the means of LVEDD and LVSDD were significantly lower in LBBP-CRT compared with BVP-aCRT at 6-month (57.30 ± 8.00 mm vs. 62.40 ± 10.07 ms, P = 0.015; 43.40 ± 9.66 ms vs. 49.44 ± 11.51 mm, P = 0.014) and 1-year follow-up (54.50 ± 6.13 vs. 60.99 ± 10.68 mm, P = 0.001; 41.78 ± 9.05 vs. 48.33 ± 12.63 mm, P = 0.007) (Figure 4E and F). At 1 year after implantation, the trend towards lower LAD, LVEDV, and LVESV all reached statistical difference in comparison to BVP-aCRT (P = 0.014, P < 0.001, and P = 0.005, respectively) (Figure 4G and H). For other echocardiographic parameters including TAPSE, PASP, and IVMD were significantly different between 1-year post-procedure and baseline in both groups (all P < 0.05). Both groups showed significant improvement in clinical heart function concerning the percentage of NYHA classification III–IV at follow-up as compared to baseline (all P < 0.05) while it was much lower in LBBP-CRT vs. BVP-aCRT (4.08% vs. 19.61%, P = 0.028) at 1-year follow-up.
Table 3.
Echocardiographic and clinical outcomes
| LBBP-CRT (n = 49) | BVP-aCRT (n = 51) | P-value | ||
|---|---|---|---|---|
| LVEF (%) | Baseline | 29.05 ± 5.09 | 28.36 ± 5.30 | 0.522 |
| 6-month follow-up | 47.58 ± 12.02*** | 41.24 ± 10.56*** | 0.008# | |
| 1-year follow-up | 49.10 ± 10.43*** | 43.62 ± 11.33*** | 0.021 | |
| NYHA III–IV, N (%) | Baseline | 45 (91.84) | 45 (88.24) | 0.741 |
| 6-month follow-up | 6 (12.24)*** | 13 (25.49)*** | 0.126 | |
| 1-year follow-up | 2 (4.08)*** | 10 (19.61)*** | 0.028# | |
| PASP (mmHg) | Baseline | 41.22 ± 12.89 | 39.36 ± 12.06 | 0.508 |
| 6-month follow-up | 35.36 ± 8.41** | 33.73 ± 7.19** | 0.443 | |
| 1-year follow-up | 35.52 ± 8.45** | 33.60 ± 7.48** | 0.721 | |
| TAPSE (mm) | Baseline | 16.24 ± 2.47 | 16.62 ± 3.14 | 0.604 |
| 6-month follow-up | 18.32 ± 2.11** | 18.60 ± 2.50** | 0.706 | |
| 1-year follow-up | 17.32 ± 2.43** | 18.51 ± 3.38** | 0.146 | |
| LVEDD (mm) | Baseline | 67.07 ± 6.67 | 68.38 ± 7.81 | 0.379 |
| 6-month follow-up | 57.30 ± 8.00*** | 62.40 ± 10.07*** | 0.015# | |
| 1-year follow-up | 54.50 ± 6.13*** | 60.99 ± 10.68*** | 0.001# | |
| LVSDD (mm) | Baseline | 56.15 ± 8.30 | 58.98 ± 9.02 | 0.120 |
| 6-month follow-up | 43.40 ± 9.66*** | 49.44 ± 11.51*** | 0.014 | |
| 1-year follow-up | 41.78 ± 9.05*** | 48.33 ± 12.63*** | 0.007# | |
| LAD (mm) | Baseline | 45.14 ± 4.81 | 46.40 ± 5.42 | 0.234 |
| 6-month follow-up | 42.36 ± 6.33 | 45.77 ± 8.24 | 0.065 | |
| 1-year follow-up | 40.21 ± 6.39 | 44.88 ± 8.45 | 0.014# | |
| LVEDV (mL) | Baseline | 219.20 ± 64.77 | 237.77 ± 67.44 | 0.168 |
| 6-month follow-up | 154.28 ± 60.88*** | 182.66 ± 83.35*** | 0.090 | |
| 1-year follow-up | 123.89 ± 42.31*** | 176.55 ± 81.82*** | <0.001# | |
| LVESV (mL) | Baseline | 153.94 ± 54.46 | 174.82 ± 68.08 | 0.104 |
| 6-month follow-up | 83.79 ± 49.32*** | 110.09 ± 70.84*** | 0.051 | |
| 1-year follow-up | 70.00 ± 41.07*** | 106.25 ± 71.43*** | 0.005# | |
| MR | Baseline | 1.41 ± 1.03 | 1.68 ± 0.95 | 0.179 |
| 6-month follow-up | 1.18 ± 0.86 | 1.51 ± 0.73 | 0.086 | |
| 1-year follow-up | 1.09 ± 0.82 | 1.54 ± 0.92 | 0.099 | |
| TR | Baseline | 1.17 ± 0.86 | 1.45 ± 0.69 | 0.180 |
| 6-month follow-up | 0.86 ± 0.73 | 1.14 ± 0.39 | 0.089 | |
| 1-year follow-up | 1.00 ± 0.52 | 1.11 ± 0.40 | 0.402 | |
| IVMD (ms) | Baseline | 60.42 ± 21.07 | 65.71 ± 22.81 | 0.414 |
| 6-month follow-up | 13.92 ± 20.85*** | 48.75 ± 24.48*** | <0.001# | |
| 1-year follow-up | 13.00 ± 21.01*** | 46.88 ± 22.94*** | <0.001# | |
| TS-12-SD (ms) | Baseline | 59.40 ± 20.66 | 59.10 ± 23.51 | 0.963 |
| 6-month follow-up | 51.25 ± 16.27 | 50.96 ± 16.60 | 0.960 | |
| 1-year follow-up | 43.92 ± 11.09** | 51.63 ± 17.28 | 0.156 | |
| HFH, N (%) | 6-month follow-up | 1 (2.04) | 3 (5.88) | 0.618 |
| 1-year follow-up | 2 (4.08) | 5 (9.80) | 0.437 | |
| AF, N (%) | 6-month follow-up | 0 | 3 (5.89) | 0.243 |
| 1-year follow-up | 0 | 5 (9.80) | 0.057 | |
AF, atrial fibrillation; BVP-aCRT, biventricular pacing with adaptive optimization algorithm of cardiac resynchronization therapy; HFH, heart failure hospitalization; IVMD, interventricular mechanical delay; LAD, left atrial diameter; LBBP-CRT, cardiac resynchronization therapy via left bundle branch pacing; LVEDD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVSDD, left ventricular end-diastolic diameter; MR, mitral regurgitation; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic exclusion; TR, tricuspid regurgitation; TS-12-SD, standard deviation of time to peak myocardial velocities.
P-value: comparisons between LBBP-CRT group and BVP-aCRT group at baseline, 6-month follow-up, and 1-year follow-up:
P < 0.05.
Compared with baseline in the same group:
P < 0.01.
P < 0.001.
Complications and adverse clinical outcomes
With regard to procedure-related complications, RBB injury occurred in 10 cases during placing LBBP lead and 9 of them recovered before discharge. Only one case was found to recover at 3 months follow-up. Micro dislodgement of LV lead was identified in one patient in BVP-aCRT group with pacing threshold of 2.8 V/0.5 ms and phrenic nerve stimulation occurred, which was relieved by increasing pulse width.
Of our study population, a total of two cases in LBBP-CRT group and five cases in BVP-aCRT group reported HFH. The occurrence of AF was only observed in five patients in BVP-aCRT (Table 3). Sustained ventricular tachycardia required for ATP or shock was not reported in both groups. Moreover, no mortality was identified during the study period in our patients.
Discussions
In this prospective, multi-centre, observational study, we investigated LBBP, a novel physiological pacing strategy used in CRT as compared to BVP-aCRT in patients with LBBB and HFrEF in a largest cohort to date. The major findings of the present study are as follows: (i) LBBP was feasible in CRT candidates and the pacing threshold was low and stable during follow-up; (ii) the mean QRSd in LBBP-CRT was significantly decreased, showing better electrical resynchronization than BVP-aCRT; and (iii) clinical and echocardiographic response rate were significantly increased in LBBP-CRT as compared to BVP-aCRT.
Feasibility of cardiac resynchronization therapy via left bundle branch pacing in cardiac resynchronization therapy candidates
Cardiac resynchronization therapy via LBBP was successfully achieved in 49 cases, which could be confirmed by the criteria as we previously reported.10,11 The left conduction system is a wide network and it is easy to be captured by screwing the lead deep enough to the endomyocardium of the LV septum.13 On the contrary, the anatomy of coronary sinus varies between individuals, resulting in difficulties in placing LV lead into the optimal vein branches. In the present study, even by experienced operators, the percentage of LV lead implanted into the ideal branch (lateral, posterolateral, or posterior vein) accounted for <90%. Furthermore, LBBP lead was also successfully performed in secondary five cases transferred from BVP-aCRT to LBBP-CRT due to LV lead failure, showing LBBP was more feasible at implant and might act as a rescue for LV lead failure. Moreover, the fluoroscopy time in placing LBBP lead was significantly shortened as compared to conventional LV lead since the latter might need more procedures including placing LV delivery sheath into coronary sinus and performing angiography. As for pacing parameters, pacing threshold in LBBP-CRT was stable at implant and during follow-up, in consistent with previous studies.14,15 The safety of LBBP-CRT during 1-year follow-up was also confirmed in our present study, for complications and adverse clinical outcomes, comparable to BVP-aCRT as we described before.16
Better electrical and mechanical resynchronization in cardiac resynchronization therapy via left bundle branch pacing
Heart failure with reduced LVEF with sinus rhythm, QRSd >150 ms, and LBBB is Class I indication for CRT implantation to improve heart failure and reduce mortality. Left bundle branch pacing leads to electrical and mechanical dyssynchrony in LV contraction due to delayed LV lateral wall activation. Thus, the mechanism of BVP is to improve electrical synchrony of the left ventricle through placing the lead in the branch of coronary sinus to activate the lateral wall of LV earlier. It does not correct LBBB but only improves electrical dyssynchrony by decreasing QRSd. The adaptive CRT algorithm offered a dynamic and automatic AV timing optimization, which was demonstrated to decrease RV pacing and paced QRSd and further increase CRT efficacy.2 However, theoretically, LBBP captured the left conduction system and activated the LV faster than epicardial pacing by a conventional LV lead. Thus, QRSd decreased significantly in LBBP-CRT as compared to BVP-aCRT both during the VVI mode and DDD mode with fixed AV delay in LBBP-CRT and adaptive algorithm in BVP-aCRT (P < 0.001) (Figure 3). It has been demonstrated that a narrower QRSd might lead to better mechanical synchronization of the ventricle. Interventricular mechanical delay evaluate the mechanical synchronization between LV and RV and is recognized as a predictive factor in CRT response.17 Our study showed comparable IVMD in two groups at baseline and significantly lower IVMD in LBBP-CRT than BVP-aCRT at follow-up. Mechanical synchrony of LBBP has been demonstrated in several studies using echocardiography, single-photon emission computed tomography myocardial perfusing imaging(MPI) imaging, and non-invasive global epicardial electrogram imaging.18 The underlying mechanism for improvement with LBBP leads may lead to faster and earlier LV contraction through the LV conduction system and decreases the mechanical dyssynchrony between LV and RV, while BVP-aCRT only partly improves mechanical dyssynchrony through pacing from LV epicardium though it could be further improved by dynamically optimizing AV and VV delay through aCRT algorithm to decrease RV pacing as compared to conventional BVP with a fixed AV delay.
Higher clinical and echocardiographic response in cardiac resynchronization therapy via left bundle branch pacing
Although an LV lead was implanted in the majority of LBBP-CRT group, the improvement of cardiac function was attributed to LBBP rather than LV pacing or LV pacing together with LBBP due to our programming with pacing only or pacing prior to LV or RV lead with VV delay of 80 ms merely in the case of RBB injury during the procedure or the concern of the lead safety at follow-up. Moreover, though LBBP-CRT and BVP-aCRT showed comparable echocardiographic response (89.80% vs. 84.31%), significant higher super-response was achieved in LBBP-CRT than BVP-aCRT (61.22% vs. 39.22%, P < 0.001). Super-response in CRT was reported as 10–30% similar to prior studies1 (e.g. MADIT-CRT: 24% at 12-month follow-up),19 while it was relatively high in both groups in our study. It is likely due to the characteristics of our study population: all cases were typical LBBB meeting the Strauss’s criteria and the predominant aetiology was dilated cardiomyopathy, which demonstrated higher CRT response rate than atypical LBBB or IVCD and ischaemic cardiomyopathy.20 Hence, LBBP-CRT with completely correction of LBBB led to even higher super-response than BVP-aCRT in the study.
Limitations
This is a non-randomized, prospective, multi-centre, short-term, observational study, in a small cohort. Although the baseline characteristics in two groups were comparable, assignment to LBBP-CRT or aCRT depending on the choice of the physician as well as the patients’ preference could possibly result in considerable selection bias. In addition, individual experience and skills from different operators in different centres might also influenced the results of our research. Since the study included consecutive patients with typical LBBB meeting Strauss criteria and the majority had a dilated cardiomyopathy, the results of our study cannot be generalized to heart failure patients with atypical LBBB and other aetiologies. On the other hand, patients with large infarcts are also known to have lower responder rate to CRT, hence, it is also recommended that cardiac magnetic resonance imaging should be performed to further compare the baseline characteristics between two groups in future.
Conclusions
The feasibility and efficacy of LBBP-CRT has been demonstrated to achieve better electromechanical resynchronization and higher clinical and echocardiographic response, especially higher super-response than BVP-aCRT in HFrEF with typical LBBB in the present study. Large-scale, long-term, and randomized controlled clinical trials are needed to further estimate the clinical benefits and safety of LBBP with comparison to BVP in CRT candidates.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We would like to thank Dr Weijian Huang (Department of Cardiology, The First Affiliated Hospital of Wenzhou Medical University) for his technical assistance in the LBBP procedure.
Funding
This research was funded by the Clinical Research Special Fund of Zhongshan Hospital, Fudan University (2020ZSLC08).
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ellenbogen KA, Huizar JF.. Foreseeing super-response to cardiac resynchronization therapy: a perspective for clinicians. J Am Coll Cardiol 2012;59:2374–7. [DOI] [PubMed] [Google Scholar]
- 2. Birnie D, Hudnall H, Lemke B, Aonuma K, Lee KL, Gasparini M. et al. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the Adaptive Cardiac Resynchronization Therapy trial. Heart Rhythm 2017;14:1820–5. [DOI] [PubMed] [Google Scholar]
- 3. Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS. et al. ; His-SYNC Investigators. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol 2019;74:157–9. [DOI] [PubMed] [Google Scholar]
- 4. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X. et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–3. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Wu S, Su L, Su Y, Huang W.. The characteristics of the electrocardiogram and the intracardiac electrogram in left bundle branch pacing. J Cardiovasc Electrophysiol 2019;30:1096–101. [DOI] [PubMed] [Google Scholar]
- 6. Huang W, Wu S, Vijayaraman P, Su L, Chen X, Cai B. et al. Cardiac resynchronization therapy in patients with nonischemic cardiomyopathy using left bundle branch pacing. JACC Clin Electrophysiol 2020;6:849–58. [DOI] [PubMed] [Google Scholar]
- 7. Ji W, Chen X, Shen J, Zhu D, Chen Y, Li F, Left bundle branch pacing improved heart function in a 10-year-old child after a 3-month follow-up. Europace 2020;22:1234–9. [DOI] [PubMed] [Google Scholar]
- 8. Wu S, Su L, Vijayaraman P, Zheng R, Cai M, Xu L. et al. Left bundle branch pacing for cardiac resynchronization therapy: non-randomized on treatment comparison with His bundle pacing and biventricular pacing. Can J Cardiol 2021;37:319–28. [DOI] [PubMed] [Google Scholar]
- 9. Mascioli G, Padeletti L, Sassone B, Zecchin M, Lucca E, Sacchi S. et al. Electrocardiographic criteria of true left bundle branch block: a simple sign to predict a better clinical and instrumental response to CRT. Pacing Clin Electrophysiol 2012;35:927–34. [DOI] [PubMed] [Google Scholar]
- 10. Wu S, Chen X, Wang S, Xu L, Xiao F, Huang Z. et al. Evaluation of the criteria to distinguish left bundle branch pacing from left ventricular septal pacing. JACC Clin Electrophysiol 2021;S2405-500X(21)00202-4. [DOI] [PubMed] [Google Scholar]
- 11. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P.. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm 2019;16:1791–6. [DOI] [PubMed] [Google Scholar]
- 12. Gold MR, Birgersdotter-Green U, Singh JP, Ellenbogen KA, Yu Y, Meyer TE. et al. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J 2011;32:2516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Jin Q, Li B, Jia J, Sharma PS, Huang W. et al. Electrophysiological parameters and anatomical evaluation of left bundle branch pacing in an in vivo canine model. J Cardiovasc Electrophysiol 2020;31:214–9. [DOI] [PubMed] [Google Scholar]
- 14. Chen X, Jin Q, Bai J, Wang W, Qin S, Wang J. et al. The feasibility and safety of left bundle branch pacing vs. right ventricular pacing after mid-long-term follow-up: a single-centre experience. Europace 2020;22:ii36–44. [DOI] [PubMed] [Google Scholar]
- 15. Su L, Wang S, Wu S, Xu L, Huang Z, Chen X. et al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol 2021;14:e009261. [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Wei L, Bai J, Wang W, Qin S, Wang J. et al. Procedure-related complications of left bundle branch pacing: a single-center experience. Front Cardiovasc Med 2021;8:645947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Risum N. Assessment of mechanical dyssynchrony in cardiac resynchronization therapy. Dan Med J 2014;61:B4981. [PubMed] [Google Scholar]
- 18. Chan JYS, Huang WJ, Yan B.. Non-invasive electrocardiographic imaging of His-bundle and peri-left bundle pacing in left bundle branch block. Europace 2019;21:837. [DOI] [PubMed] [Google Scholar]
- 19. Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P. et al. Predictors of response to cardiac resynchronization therapy in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT). Circulation 2011;124:1527–36. [DOI] [PubMed] [Google Scholar]
- 20. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M. et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation 2011;123:1061–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.