Abstract
Advances in three-dimensional (3D) cell culture technology have led to the development of more biologically and physiologically relevant models to study organ development, disease, toxicology and drug screening. Organoids have been derived from many mammalian tissues, both normal and tumour, from adult stem cells and from pluripotent stem cells. Tissue organoids can retain many of the cell types and much of the structure and function of the organ of origin. Organoids derived from pluripotent stem cells display increased complexity compared with organoids derived from adult stem cells. It has been shown that organoids express many functional xenobiotic-metabolising enzymes including cytochrome P450s (CYPs). This has benefitted the drug development field in facilitating pre-clinical testing of more personalised treatments and in developing large toxicity and efficacy screens for a range of compounds. In the field of environmental and genetic toxicology, treatment of organoids with various compounds has generated responses that are close to those obtained in primary tissues and in vivo models, demonstrating the biological relevance of these in vitro multicellular 3D systems. Toxicological investigations of compounds in different tissue organoids have produced promising results indicating that organoids will refine future studies on the effects of environmental exposures and carcinogenic risk to humans. With further development and standardised procedures, advancing our understanding on the metabolic capabilities of organoids will help to validate their use to investigate the modes of action of environmental carcinogens.
Introduction
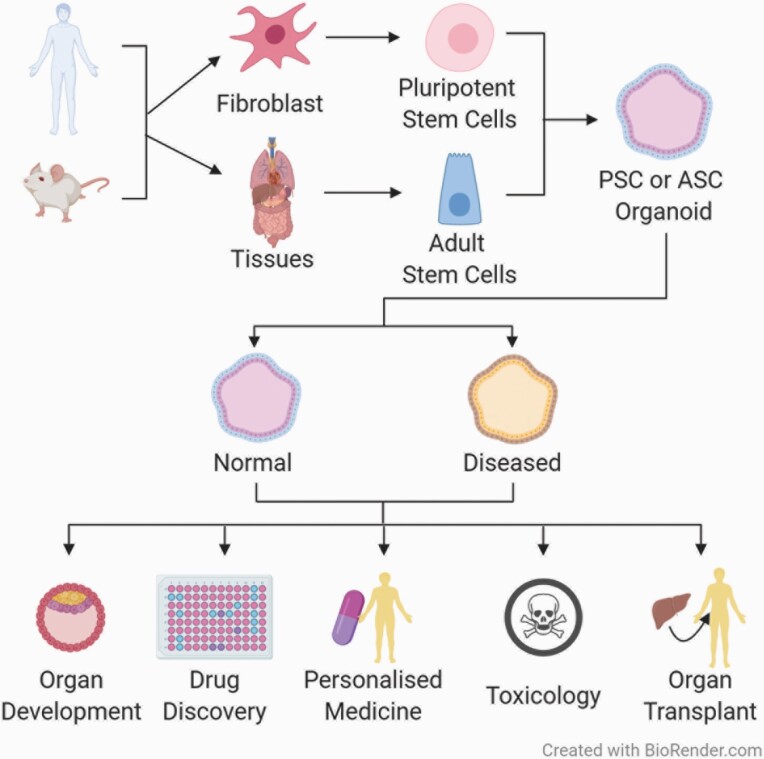
Advances in three-dimensional (3D) cell culture technology in recent years have increased its use in several fields including organ development, disease modelling and drug screening. A number of 3D models have been established, from simple unicellular spheroid cultures to more complex systems, like organoids and microphysiological systems (MPS) (1–3). Organoids are multicellular 3D cultures derived from stem cells that self-assemble into structures that contain organ-specific cell types and that recreate some of the in vivo cell organisation and functions of the organ of origin (4). As shown in Figure 1, organoids can originate from pluripotent stem cells (PSCs), both embryonic and induced pluripotent stem cells (iPSCs), or adult stem cells (ASCs), which can proliferate indefinitely in culture and have been shown to have the ability to differentiate and re-organise to mimic their source organs (5).
Fig. 1.
Scheme showing the origin and application of organoid systems. Organoids can be derived from human and animal (e.g. mouse) pluripotent or adult stem cells. Normal and diseased organoids can be used for many applications such as disease modelling, organ development, drug screening, personalised medicine, toxicology and organ transplant and replacement.
The derivation process of PSC organoids exploits both the ability of these stem cells to differentiate into several cell types and the organ development process, including spatial patterning and morphogenesis (5–8). The ability to generate organoids from ASCs started with the identification of the stem cell marker leucine-rich repeat containing G-protein-coupled receptor 5 (Lgr5) in intestinal epithelium, which allowed the characterisation of multipotent and self-renewing adult stem cell populations in several other tissues, including colon (9), stomach (10), pancreas (11) and liver (12). These Lgr5+ ASCs, from isolated cells or from dissected tissue fragments, can be used to establish organoids by providing an adequate environment to mimic tissue homeostasis and regeneration (5,10,12,13).
The use of human organoids has become increasingly widespread over the past few years as alternatives to more routinely used model systems, such as animals and two-dimensional (2D) mammalian cell cultures. The perceived need for more physiologically relevant in vitro models has been the main reason for this shift towards the use of 3D cultures in different research fields (14–18). Although experimental animals, mainly rodents, have provided substantial amounts of information about many biological processes and have been a key resource for chemical and drug safety studies, these animal models do not always accurately represent the human response due to species-specific disease states and reactions (19–21). Similarly, 2D mammalian cell cultures have been widely used in research for more than a hundred years and have led to the discovery of countless pathways and processes in biology. In spite of this, the dependability of results from studies using 2D cultures for drug and chemical safety and efficacy can been questioned due to the highly artificial nature of their culture environment (22,23). Therefore, the use of human organoids and other 3D cultures may allow the collection of data more relevant to human physiology while contributing to reductions in the use of animals in basic and applied research (24–28).
As mentioned earlier, the application of human organoid systems includes, but is not limited to, organ development, disease modelling and pharmacological studies (Figure 1). PSC-derived human organoids have proved to be very useful for studying organ development, where their propensity to reproduce embryonic stages of development has allowed the replication of key steps in organogenesis, such as spatial organisation of the heart, the brain, the gastrointestinal tract and other organs (29–32). The use of human organoids in disease modelling has led to the establishment of assays and models that assist with diagnosis, drug screening and personalised treatment. This has been achieved for a number of conditions like cystic fibrosis, for which tubuloids from urine, as well as intestinal and airway organoids from patients, have been used to identify treatments that benefit patients with specific mutations (18,33,34). Progress has also been made in cancer research, using biobanked samples of human tumour and normal organoids derived from cancer patients to better understand tumour heterogeneity and responses to chemotherapy, as well as aiding the improvement of personalised therapy (35–38). Drug screening using human organoids is already being carried out to facilitate pre-clinical drug development, as well as to predict an individual patient’s response to treatment (17,18,37). It is anticipated that this will not only maximise clinical benefits of existing therapies, but also enable increased adoption of novel therapeutics (39). Engraftment of organoids in mice has demonstrated the use of organoids in regenerative medicine and transplantation is also very promising, as it appears to minimise the risks of transplant rejection and increase the availability of healthy tissue (40).
Additionally, organoids show great potential in drug toxicology studies (41–46). However, their use in environmental and genetic toxicology is at a relatively early stage. This review will focus on the derivation of organoids and their use in environmental and genetic toxicology, detailing the studies that have been conducted and considering the future potential of organoids in this important field.
Organoid Models: iPSC vs ASC Derived Organoids
Organoid models are characterised by the self-organisation of cells in culture into in vivo-like structures; however, their derivation depends on the starting material. Organoids from both embryonic and induced pluripotent stem cells have been established from several organs, including gut, kidney, liver, lung, intestine and brain (47). Derivation of these PSC organoids utilises knowledge of the cell sorting and lineage commitment pathways, combined with growth in culture under conditions specific for the desired differentiation pathway (4). This process usually takes a few weeks to generate mature organoids (6). In the case of ASC-derived organoids, Wnt signalling pathway activation is key for their establishment (48). These organoids, which originate from Lgr5+ stem cells obtained from single cell sorting or dissected tissue fragments, are grown in organ-specific culture media that contains Wnt activators such as R-spondin 1 and, in some cases, Wnt3A (48). Organoid types that have been derived from ASCs include gut, liver, pancreas, intestine, kidney and also from mammary and salivary glands (47). In contrast to PSC-derived organoids, the process to generate mature organoids from ASCs takes only a few days (11,13).
Small intestine organoids
Although both PSC and ASC-derived organoids can be grown in culture for a long time, they have distinct differences, not only in the stages of development they represent, but also in the cell types they contain, and, therefore, in their complexity (Figure 2). A clear example of this can be seen with gastrointestinal tract organoids (49). Those derived from ASCs contain only organ-specific epithelial and stem cells, while organoids derived from PSCs contain epithelial cells as well as mesenchymal cells, including fibroblasts and smooth muscle, due to the ability of PSCs to differentiate into any cell type (50). Small intestine organoids were first derived from ASCs by the Clevers lab at the Hubrecht Institute, The Netherlands (13). These organoids established from single Lgr5+ cells form crypts containing Paneth cells as well as Lgr5+ stem cells that surround a central lumen lined by a villus-like epithelium with polarised enterocytes, and goblet and enteroendocrine cells dispersed throughout the organoid (13,51). This has been replicated a number of times from murine and human tissues and adapted for the establishment of intestinal organoids from cystic fibrosis patients (34,52,53). Intestinal tissue organoids originating from human PSCs were first reported by Spence et al. (6). In this study, embryonic intestinal development was mimicked by using a series of culture conditions that included growth factors to induce intestinal growth, morphogenesis and cytodifferentiation. These organoids contained polarised, columnar epithelium resembling villus- and crypt-like structures with all intestinal endothelial cells present, as well as a layer of mesenchymal cells including subepithelial myofibroblasts, smooth muscle and fibroblasts (6). This model has been replicated and used for different purposes such as generating in vivo human organoid engraftment mouse models and viral infection models, amongst others (54,55).
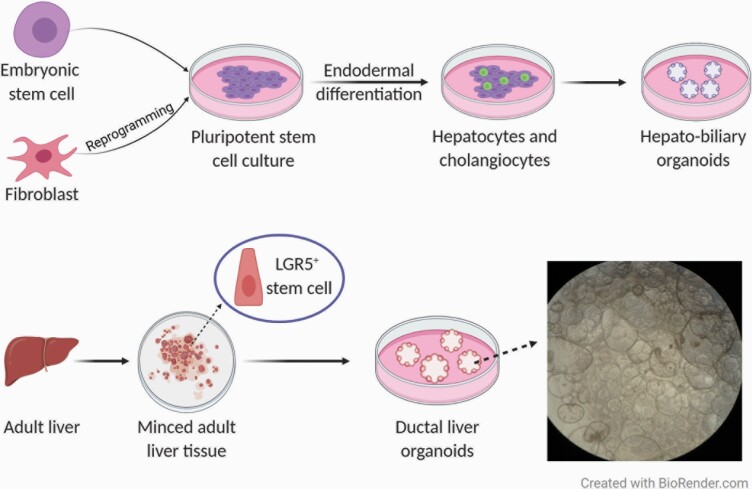
Fig. 2.
Scheme of liver organoid culture formation from pluripotent stem cells or adult stem cells. On the top panel, pluripotent stem cells, embryonic or induced, undergo differentiation towards the desired cell type. PSCs are differentiated into hepatocytes and cholangiocytes which then generate organoid cultures containing both hepatocytes and bile duct cells. The bottom panel shows how organoids can be derived from minced adult tissue which contains Lgr5+ stem cells that in this case can give rise to liver organoid cultures containing ductal cells. Organoid cultures are embedded in basement membrane extract and are grown in media complemented with essential growth factors. Adapted from (66,119). An example of normal human adult liver organoids grown in our lab is shown on the right in the bottom panel.
Colon organoids
As with the small intestine, colon and gut organoids have also been derived both from PSCs and from ASCs. Colon Lgr5+ ASCs were identified in the Clevers lab, where colon organoids were later established from human and murine tissues (9,51). Tissue-derived colonic organoids appeared as cystic or budding structures with proliferative cells; subsequently when culture conditions were modified, goblet and enteroendocrine cells were also present (51,56). Human PSC-derived colon organoids were established by stimulating bone morphogenetic protein (BMP) signalling; these organoids comprised colon-specific goblet and enteroendocrine cells as well as mesenchyme (57). At the same time, others also generated PSC-colonic organoids by using a differentiation method that involved the modulation of the Wnt pathway via the addition of glycogen synthase kinase (GSK)-3β inhibitors as well as BMP regulation (58).
Gastric organoids
Gut or gastric organoids were first derived from mouse Lgr5+ ASCs and they resembled the pyloric epithelium organised around a central lumen (10). Human ASC gastric organoids were later established from different regions of the stomach; these consisted of budding or cystic structures comprised of mainly mucous gland, chief and enteroendocrine cells surrounding a central lumen (59,60). These human tissue gastric organoids can also be further differentiated by altering the culture conditions in order to obtain the various gastric cell lineages (59,60). In a similar way, PSC-derived gut organoids formed simple gland and pit domains and contained Lgr5+ cells as well as mucous and endocrine cells; however, they represented early developmental stages and contained submucosal myofibroblasts and a small population of subepithelial myofibroblasts (32).
Liver organoids
As mentioned earlier, organoids derived from both types of stem cell populations have also been established for the liver, pancreas and kidney (see below for more details on pancreatic and kidney organoids). In adults, Lgr5 expression in these organs is low under normal conditions, however, after injury, the Wnt pathway is activated leading to increased expression of Lgr5 and, therefore, tissue regeneration (11,12). ASC-derived liver organoids were first reported by Huch et al. (11,12); they consisted of large cysts with Lgr5+ cells and bile duct cells, while hepatocyte markers were only weakly expressed. Hepatocyte maturation was achieved by altering culture conditions; however, this led to a concurrent decrease in the expression of proliferative cells (12,61,62). Hepatocyte-derived organoids were later established from murine and human single mature hepatocytes. They resembled the main functions and gene expression patterns of hepatocytes in vivo and could proliferate in culture for at least 6 months (63,64). PSC-derived liver organoids were first generated as vascularised liver buds. These liver organoids originated by co-culturing human hepatic endoderm and stromal cells, expressed early liver-specific markers including alpha-fetoprotein and albumin, and were capable of drug metabolism (65). More recently, hepatobiliary organoids obtained from human PSCs were established. These organoids displayed functions of both hepatocytes (e.g. drug metabolism and albumin production) and bile duct cells (66) (Figure 2). Other investigators have established hepatocyte-like organoids that resemble adult liver tissue-derived organoids (43).
Pancreatic organoids
Pancreatic organoids were first made from ductal fragments of mouse pancreas. These formed cysts that contained duct cells in vitro, some of which differentiated into endocrine cells in vivo (11). This protocol was then adapted for the growth of human tissue organoids that also comprised stem and ductal cells (62,67). Human tissue pancreatic organoids that have the potential to differentiate into endocrine lineage cells in vitro were later produced from digested islet-depleted pancreatic tissue. Initially these formed budding structures with almost all cells displaying a ductal phenotype with progenitor cells at the tip regions, some of which differentiated into endocrine cells and produced insulin after transplantation (68). Organoids that reproduce pancreatic development were also derived from mouse foetal pancreatic progenitors. These were composed of epithelial cells that expressed progenitor markers such as Pdx1, Sox9, Hnf1b and Nkx2.2, which then differentiated into ductal and exocrine lineages (69). Pancreatic organoids from PSCs formed hollow structures surrounded by a layer of polarised epithelium that comprised cells expressing exocrine and progenitor cell markers, as well as cells capable of secreting collagen IV and laminin-α5 (70).
Kidney organoids
Kidney organoids have been mainly derived from human PSCs and several protocols have been published for the generation of kidney organoids containing different kidney cell types. The formation of kidney organoids from human embryonic stem cells through stepwise differentiation into the key developmental lineages of the kidney was reported (71). These organoids contain ureteric buds and metanephric mesenchyme, including early nephrons, as well as cells expressing podocyte, proximal tubule and collecting duct genes. Others reported deriving organoids from mouse and human stem cells in which proximal and distal tubules were formed, as well as glomerulus-like structures, and cells with podocyte markers were also seen (72). Like these, many other nephron organoids expressing podocyte, proximal tubule, loops of Henle and distal tubule markers with different degrees of differentiation and complexity have been generated from human and murine embryonic and pluripotent stem cells (14,73–76). ASC-derived kidney organoids have been named ‘tubuloids,’ as they mainly contain tubular epithelial cells, and similarly to organoids derived from adult tissues they reproduce tissue regeneration rather than development (77). Tubuloids from normal human adult tissue were reported consisting of cystic structures containing tubular epithelial cells that resembled proximal tubular cells and that expressed the renal tubule protein Tamm-Horsfall (78). Others established tubuloids from human tissues that showed high expression of proximal tubule markers, as well as some collecting duct, loop of Henle and distal tubule markers (33). Tubuloids were also established from cells isolated from urine of cystic fibrosis patients and from kidney tumours (33).
Organoids in Drug Screening and Toxicology
Due to their structural and functional features, organoid models have great potential in toxicology studies. Table 1 lists studies in which organoids derived from various human and animal tissues from both PSCs and ASCs have been used in toxicological research. Different aspects have been investigated, from the effects and efficiency of drugs to the toxicity of environmental pollutants. The former has been investigated more widely, possibly due to the opportunity that testing of large libraries of therapeutic compounds on patient-derived organoids offers to bridge the gap between monolayer cell culture and animal models, and the relevance of the results for human physiology in pre-clinical trials (46,79).
Table 1.
Studies using organoids in drug screening and toxicology
| Therapeutic drugs | |||
|---|---|---|---|
| Organoid type | Aim of the study | Key findings | Reference |
| Oesophageal adenocarcinoma – ASCs | Establishment and characterisation of oesophageal adenocarcinoma organoids. Evaluation of their sensitivity to 24 anticancer compounds, including approved drugs and preclinical targeted agents. | Organoids recapitulated the features of the primary tumour. A range of compound sensitivities, including drug potency and efficacy, was observed, which was consistent with patient responses to therapy (e.g. resistance). | (17) |
| Ductal pancreatic cancer – ASCs and PSCs | Development of pancreatic tumoroids from PSCs and patient samples that model ductal pancreatic cancer. Assessment of patient tumour organoid responses to gemcitabine and drugs targeting epigenetic regulators. | Patient-derived tumour organoids maintained tumour-specific traits. Treatment with gemcitabine led to poor response with only 30% growth inhibition and treatment with epigenetic regulators decreased proliferation to varied degrees in different organoids. | (70) |
| Pancreatic tumour – ASCs | Studying human pancreatic tumoroid survival in co-culture with liver organoids after treatment with docetaxel. | Pancreatic tumoroid co-culture with CYP-induced liver organoids showed higher survival rate compared with undifferentiated and differentiated liver after treatment with docetaxel. | (46) |
| Colon tumour and normal – ASCs | Establishment and characterisation of colon tumour and normal organoid lines. Assessment of organoids for their sensitivity after treatment with a library of 83 compounds including chemotherapeutics, drugs in clinical use or in clinical trials and experimental compounds. | Colon tumour organoids recapitulated several features of the primary tumour and reflected the heterogeneity of the tumours. Differential responses of the organoids to the compounds were seen measured by the IC50, the slope of the dose–response curve and the area under the curve, as well as organoid-drug interactions. | (37) |
| Rectal – ASCs | Measurement of drug efficacy of the CFTR-modulating treatments (ivacaftor, lumacaftor plus ivacaftor and genistein plus curcumin) in rectal organoids from cystic fibrosis patients and correlation with in vivo effects. | In vitro responses to CFTR modulators (ivacaftor, lumacaftor plus ivacaftor and genistein plus curcumin) and forskolin-induced swelling in the rectal organoids from cystic fibrosis patients correlated with two indicators of therapeutic response in vivo, sweat chloride concentration and pulmonary response. | (39) |
| Rectal – ASCs | Studying CFTR function of rectal organoids from cystic fibrosis patients and the organoid’s response to the drug treatments ivacaftor and lumacaftor. | CFTR function and response of rectal organoids to treatment with ivacaftor and lumacaftor depended on the CFTR mutation and the genetic background of the patient. Results positively correlated with published data from clinical trials. | (84) |
| Kidney cancer – ASCs | Establishment of kidney organoid biobank from paediatric cancer patients. Characterisation of tumour kidney organoids and testing their sensitivity towards drugs used in standard chemotherapy. | Kidney tumour organoids maintain key features of the tumour of origin, including their heterogeneity. Tumour organoid lines were more sensitive, based on a dose–response curve, to some therapies than normal kidney organoids. | (35) |
| Kidney – PSCs | Establishment of kidney organoids and testing their ability to endocytose dextran and to undergo apoptosis in response to cisplatin treatment. | Demonstration of kidney organoid functionality by selective uptake of dextran. The nephrotoxicant cisplatin induced acute apoptosis in kidney organoids. | (76) |
| Kidney – PSCs | Establishment of kidney organoids and assessment of drug nephrotoxicity by treating them with gentamicin or cisplatin, drugs known to cause proximal tubular toxicity. | Nephrotoxicity was observed after organoid treatment with these drugs, as proximal and distal tubule biomarkers for toxicity, like kidney injury molecule-1, were expressed. | (14) |
| Bile tract carcinoma – ASCs | Testing a library of drugs used clinically for their ability to suppress the tumoroids derived from intrahepatic cholangiocarcinoma, gall- bladder cancer and neuroendocrine carcinoma of the ampulla of Vater. | The library screening showed that the antifungal drugs amorolfine and fenticonazole suppress tumour organoid growth while little cytotoxicity was seen in normal cells. | (45) |
| Liver cancer –ASCs | Studying the functional heterogeneity of liver cancer organoids by testing 129 cancer drugs to assess liver cancer heterogeneity. | Liver cancer organoids showed large variability in intra-tumour drug response, with many drugs showing no cytotoxic response and some drugs effective only in certain organoids. A group of drugs used for other cancers rather than liver cancer showed moderate activity in most organoids. | (36) |
| Testicular – ASCs | Evaluation of testicular organoids as a model for reproductive toxicity by treatment with cisplatin, etoposide, doxorubicin and busulfan. Comparison of results to those obtained in 2D culture of the same cell types. | The testicular organoids showed a dose-dependent response in terms of cell viability after treatment with cisplatin, etoposide, doxorubicin and busulfan and had IC50 values higher than those in the same cell types cultured in 2D. | (41) |
| Intestinal – ASCs | Studying toxicity and cell death induction in normal intestinal organoids after exposure to cisplatin, 5-fluorouracil, UV or X-ray radiation. Comparison of results to those obtained in colon carcinoma cell lines. | Intestinal organoids were more sensitive to chemotherapeutic drugs than colon carcinoma cell lines, mimicking the in vivo situation. The organoids also responded much more sensitively to radiation exposure than the immortal cell lines. | (42) |
| Intestinal crypt – ASCs | Studying gene expression of XMEs and activation of xenobiotic nuclear receptors in intestinal organoids after treatment with the anticancer pro-drug camptothecin-11. | Expression of XMEs, such as CYPs, Adh1, Ces, Ugts and Sult and transporters, as well as functional nuclear receptors, like the aryl hydrocarbon receptor, were observed. Organoids metabolised the anticancer prodrug camptothecin-11 by the action of Ces and Ugt1a1. | (44) |
| Liver – PSCs | 1) Characterisation of human PSCs-derived liver organoids and functionality assessment. Study of their toxicity profiles after treatment with compounds such as omeprazole, hepatotoxic compounds and antibiotics, and comparison to a 2D model. 2) Development of a steatosis pathology model and testing L-carnitine, metformin and other compounds for their toxicity and efficiency to treat this condition. |
1) Human PSCs-derived liver organoids maintain liver properties including the ability to metabolise drugs and showed toxic responses to drug treatment. Organoids were more sensitive than 2D hepatocyte monolayers at clinically relevant doses. 2) Human PSCs-derived liver organoids displayed steatosis phenotypes after treatment with lipids. Treatment with L-carnitine ameliorated the disease phenotype and further compounds were identified from an autophagy library. |
(43) |
| Liver, cardiac, lung, brain, testes and colon – PSCs | Treatment of liver, cardiac, lung, brain, testes and colon organoids with 10 drugs that were recalled due to adverse effects, including troglitazone, astemizole and bromfenac, in comparison to a number of non-toxic drugs (aspirin, ibuprofen, loratadine, ascorbic acid and quercetin) used as control. Treatment of liver and cardiac organoids was conducted individually and together with the other 4 organoid types in a ‘body-on-chip’ system. Comparison of results obtained with a 2D model. | Toxic concentrations were lower in the organoids compared with those in 2D primary cells and immortalised cell lines. However, organoids were more sensitive to some drugs like astemizole and more resistant to others like rofecoxib. Results from the cell lines were less significant as they showed greater variability. Most drugs considered as non-toxic did not show any toxicity in organoids. Results from the ‘body-on-chip’ system showed that when organoids are used in combination on a chip, one organoid type influences the activity of another organoid type creating more complex responses. | (116) |
| Environmental and experimental toxicants | |||
| Colon – ASCs | Investigating the effect of ethanol exposure in normal colon organoids on gene expression and chromatin accessibility. | Identification of more than 1500 differentially expressed genes and 2000 differentially accessible chromatin regions in normal colon organoids after ethanol treatment. | (87) |
| Liver and cardiac – PSCs | Viability and cytotoxicity assessment of liver and cardiac organoids after exposure to the environmental pollutants lead, mercury, thallium and glyphosate. | Liver and cardiac organoids showed toxicity after treatment with environmental pollutants (lead, mercury, thallium and glyphosate) with thallium being the most toxic compound tested. All pollutants led to a decrease in cardiac organoid beating activity. | (15) |
| Mammary – ASCs | Studying the effect on organoid formation and morphology in organoids derived from mammoplasty patients treated with physiologically relevant doses of cadmium. | Cadmium negatively affected mammary organoid formation and branching at the highest concentration tested, 2.5 µM. Gene expression analysis showed the up- regulation of metal response genes, like metallothioneins and zinc transporters, and inhibited hypoxia inducible factor-1α activity. | (86) |
| Brain (organoid on chip) – PSCs | Assessment of the effects of nicotine in brain organoids on a chip that recapitulated features of the developing foetal human brain at early stages. | Organoids exposed to nicotine showed premature and abnormal neuronal differentiation. Brain regionalisation and development were also affected. | (106) |
| Intestinal – ASCs | Studying toxicity and cell death induction in normal intestinal organoids after exposure to cisplatin, 5-fluorouracil, UV or X-ray radiation. Comparison of results to those obtained in colon carcinoma cell lines. | Intestinal organoids were more sensitive to chemotherapeutic drugs than colon carcinoma cell lines, mimicking the in vivo situation. The organoids also responded much more sensitively to radiation exposure than the immortal cell lines. | (42) |
| Small intestine and liver – ASCs | Assessment of drug-metabolising enzymes and evaluation of CYP induction after treatment of organoids with the CYP inducers dexamethasone, β- naphthoflavone and 1,4-bis-2-(3,5-dichloropyridyloxy)-benzene in murine intestinal and liver organoids. | Expression of CYP1A1, CYP1A2, CYP2A12, CYP2C37, CYP3A11 and CYP3A13 in both intestinal and liver organoids. These CYPs were differentially induced indicating high drug-metabolic capacity. | (46) |
| Kidney – ASCs | Toxicity assessment of hydroxylated generation-5 PAMAM dendrimer (G5- OH) nanoparticles and gold nanoparticles in kidney organoids. Comparison to in vivo models. | PAMAM nanoparticles induced toxicity biomarkers like Kim-1, neutrophil gelatinase- associated lipocalin, osteopontin, clusterin, vimentin, haem oxygenase-1 and cell toxicity in kidney organoids, mirroring previously published in vivo data. | (117) |
| Lung, liver and mammary –ASCs | Assessment of the in vivo tumorigenicity of organoids after in vitro treatment with ethyl methanesulfonate, acrylamide, diethylnitrosamine and 7,12-dimethylbenz[a]anthracene. | Lung, liver and mammary organoids treated in vitro with ethyl methanesulfonate, acrylamide, diethylnitrosamine and 7,12-dimethylbenz[a]anthracene were injected into mice leading to the formation of subcutaneous nodules in vivo, indicating the carcinogenic potential of the chemicals tested. | (16) |
| Mammary – ASCs | Studying the effects of bisphenol A, mono-n-butyl phthalate and polychlorinated biphenyl 153 on the proteome in mammary organoids. | Treatment of mammary organoids with bisphenol A, mono-n-butyl phthalate and polychlorinated biphenyl 153 induced differential effects on the proteome. Treatment also altered the abundance of protein splice variants. | (85) |
| Endometrial –ASCs | Characterisation of the impact of zinc stearate (plastic additive) on the development of organoids from endometrial cells from domestic cats. | Zinc stearate did not affect morphology, viability or cellular composition of endometrial organoids. The model developed could be used in future studies investigating the effects of plastic additives or drugs. | (118) |
Metabolic potential of organoids
A few studies have focused on investigating the xenobiotic metabolic potential of organoids, primarily in liver and intestinal organoids. These studies have shown that the expression and function of enzymes responsible for the biotransformation of xenobiotic compounds, e.g. cytochrome P450s (CYP), are maintained in the organoids (44,46,61,66,80). CYP3A4 is one of the main xenobiotic-metabolising enzymes (XMEs) in liver and intestine (81). The expression of this enzyme in differentiated liver organoids, i.e. hepatocyte-like organoids, has been shown to be at levels slightly lower than those in adult liver but significantly higher than those seen in foetal liver and stem cells (61,66). The activity of CYP3A4 has also been shown to be significantly higher in differentiated liver organoids than in commonly used cell lines such as human hepatoma HepG2 cells (61,66). Levels of expression of other CYP enzymes important in drug metabolism were compared in mouse small intestine and liver organoids with the respective tissues (46). These investigations showed that mouse Cyp1a2 and Cyp3a11 mRNA levels were higher in intestinal organoids than those in the tissue, whilst only Cyp1a2 mRNA was greater in the liver organoids than in the tissue (46). The ability of organoids to metabolise drugs was also investigated in mouse crypt organoids, which express both Phase I and Phase II XMEs (44). In the latter study, organoid toxicity was also seen after treatment with camptothecin-11, demonstrating that crypt organoids can metabolise this compound. Furthermore, data from our group show a high induction of CYP1A1 mRNA in human pancreatic, gastric and liver organoids after treatment with the environmental carcinogen benzo[a]pyrene (BaP) (Caipa Garcia et al., unpublished results). Collectively, these studies illustrate the utility of organoids for investigations of xenobiotic metabolism and toxicity.
Disease modelling
The use of organoids for disease modelling could provide a very valuable tool in many areas including drug screening and personalised therapy (82). The availability of organoids derived from patient samples has allowed research on these areas by modelling different disease conditions, including cystic fibrosis, infectious diseases and cancer. Cystic fibrosis was one of the first conditions to be modelled; organoids from cystic fibrosis patient ASCs (intestinal, lung and tubuloids), as well as human PSCs have been established (18,33,34,53,83). These organoids have facilitated the assessment of the cystic fibrosis transmembrane conductance regulator (CFTR) function in individual patients in vitro, and in turn the effect of different drugs used to treat cystic fibrosis, including VX-809 (lumacaftor) and VX-770 (ivacaftor) (34). Since CFTR mutations together with the patient’s genetic background are important host factors that contribute towards the patient’s response to therapy, in vitro testing in organoids has proved to be very useful as they retain these host characteristics (84). Positive correlations of in vitro results with clinical data strongly suggest that this approach could be used to identify treatments that will be more beneficial to patients, and at the same time more cost-efficient (39,84).
There have also been several advances in the use of organoids for drug screening for cancer therapies. Cancer organoids or tumoroids have been derived from several primary tumour types from which they retain the genetic and morphologic features (37,45,70). Organoids derived from colorectal cancer patients were used in a proof-of-concept drug screening in which drug sensitivity and its correlation with the genetic background of the organoids were investigated (37). This screening generated thousands of organoid-drug interactions and showed a wide range of sensitivities to the compound library, which included drugs in clinical use, those currently in clinical trials and those under pre-clinical investigation. They have been tested in organoids from different patients and in multiple organoids derived from the same patient (37). Tumour heterogeneity has also been addressed with organoids from other cancer types, including pancreatic ductal adenocarcinoma and liver cancer; different combinations of drugs were used to treat organoids from different patients, resulting in the organoids displaying differential responses (36,70). Liver tumoroids derived from different sections of the same primary tumour showed heterogeneity as some drugs, e.g. belinostat, dasatinib, gemcitabine and ceritinib, were effective only in a subset of the organoids (36). Due to the varied responses seen between patient organoids, this model could aid the field of personalised medicine as patient-derived organoids can be used to test drug efficacy in vitro, and toxicity comparisons between disease and normal organoids could identify therapies with higher efficiency and fewer side effects (45).
Environmental toxicology
Thus far studies investigating the effects of environmental agents in organoids are scarce, although studies using both human and mouse organoids have been reported. In one study, mouse intestinal crypt organoids were exposed to cisplatin, 5-fluorouracil, ultraviolet (UV) and X-ray radiation to examine cell death and survival of intestinal epithelial cells, as well as investigate the role of certain genes in cell death regulation (42). The results obtained were closer to those obtained in primary tissue cells and in vivo than those obtained from immortalised cell lines, thereby demonstrating the usefulness of this model (42). Mammary organoids derived from mice have been used to study the effect of bisphenols and phthalates on the proteome (85), while human mammary organoids were recently utilised to test the effects of cadmium exposure on stem cell proliferation and differentiation by looking at organoid formation and morphology, showing a negative impact on these processes at concentrations relevant to human physiology (86). The treatment of human liver and heart organoids with lead, mercury, thallium and glyphosate led to damaging and toxic effects, such as a decrease in the beating activity of cardiac organoids (15). Human colon organoids have been used to explore the effect of prolonged ethanol exposure in healthy colon cells by analysing the changes in gene expression and chromatin accessibility, identifying almost 2000 gene expression changes (87). Others recently established an organoid-based model in which the carcinogenicity of environmental chemicals could be studied by treating organoids in vitro and then injecting them into nude mice (16). This study showed that the morphological carcinogenic alterations seen in the treated organoids were then also found in the nude mouse model (16). Furthermore, data from our group show that pancreatic and gastric organoids are capable of forming pre-mutagenic DNA adducts after exposure to BaP indicating that normal human tissue organoids could be useful models for genotoxicity assessment (Caipa Garcia et al., unpublished results). Collectively, these first advances in the use of organoids in field of environmental and genetic toxicology indicate that organoids will allow the investigation of the relationship between the effects of environmental exposure and the increased risk of developing adverse human effects, including cancer (87). Organoid models will also allow the study of early molecular events in tumour formation and, therefore, aid the study of the modes of action and mechanisms of different carcinogens (16,87).
Other 3D Culture Models in Toxicology
Various other 3D cell culture models have been used recently in toxicology studies which, like organoids, aim to provide a more representative microenvironment and physiology than monolayer cultures (88–90). For example, genotoxicity assays using 3D skin models have been established and validated, while liver and lung tissue models are at earlier validation stages as more robust protocols are required (91).
Spheroids, which are cellular aggregates made from cell lines, have been used in toxicological studies for different endpoints, including drug toxicity, cytotoxicity and genotoxicity (92–97). For example, liver spheroids from both primary hepatocyte (HepaRG) and tumour (HepG2, JHH1 and Huh7) cell lines have been particularly useful in the modelling of human toxicity. Multiple studies have demonstrated different cellular responses between liver, lung, bladder and mammary cell line spheroids and their monolayer cell line counterparts, showing that 3D structures are more sensitive to damage and present higher expression levels of metabolic enzymes like CYPs (92,93,95,98,99). Although spheroids provide more relevant results due to their 3D microenvironment and are relatively easy to use, they are less complex than organoids as they only contain a single cell type and are unable to replicate the relevant tissue structure (90).
Mini organ cultures (MOCs) have also been employed in toxicological studies. These cultures consist of small tissue fragments that maintain the structure of the tissues of origin and, in the case of those derived from the respiratory tract, ciliary beat activity (100–102). Although MOCs provide the 3D structure of the tissue of origin, they need to be kept at a certain size and cannot be grown for extended periods of time as their structure starts to change and viability decreases (100,101).
More complex models such as MPS have also been developed. These MPS models aim to recreate human physiological systems in vitro by interconnecting multiple organs in the form of organ-on-chips and/or organoids (3). MPS for many organs including the liver, heart, kidney, skeletal muscle and vasculature have been established, and these MPS organs can be connected in different combinations from 2 to 13 organs in one system depending on the interactions to be replicated (3). These systems have been used for the assessment of toxicity of drugs and other xenobiotics, including environmental compounds (103–106). Although it has been demonstrated that MPS are useful in the study of organ–organ interactions and are able to replicate a range of functions such as absorption, metabolism and contractile forces, their complexity limits the development of high throughput assays as there can be too much variability (3).
Lastly, the use of 3D in vitro skin models has been key in various industries to test the toxicity of compounds such as drugs and cosmetics (107). There are several skin models available, which provide different advantages depending on their origin. Due to the increase in regulations on the use of animals and the limited availability of human skin for ex vivo assays, artificial models made from different polymers or skin substitutes such as reconstructed epidermis or full thickness skin have become more widely used (107–109). Reconstructed skin models have been developed for normal and diseased skin, and consist of layers of human cells grown on a polymer matrix and can be of various complexities (107,110). Many different reconstructed epidermis models are commercially available and are being used to test toxicity and irritation, as well as effects of formulations (110). It has been shown that human reconstructed skin and full thickness models express XMEs although at levels that do not replicate those in native tissue (111). CYP enzyme levels are also very low in human skin and 3D skin models, but higher than in immortalised keratinocytes, so the reconstructed skin model is the better option for toxicology studies (112). Due to the ban in animal testing of cosmetics, reconstructed human skin models have been validated and are widely used; however, they are still being improved, e.g. by adding additional layers of complexity like immune cells (109,113–115).
Conclusions
The recent progress of 3D cell culture technology has allowed the development of several assays for the study of organ development and disease, as well as toxicology and drug screens. Different 3D models of various complexities have been established, offering a more biologically relevant environment and results more representative of human physiology. Organoids have become a popular cell culture system in many fields, as they can be easily derived from stem cells (ASCs or PSCs) and retain some of the cell types, structure and function of the organ of origin. Organoids from different tissues have been established from both ASCs and PSCs, with organoids derived from PSCs being slightly more complex than those derived from ASCs.
Organoids have great potential in the study of organ development, disease modelling, drug development and organ regeneration. It has been shown that organoids from different tissues express functional XMEs including CYPs, such as CYP3A4, CYP1A1, CYP1A2 and CYP3A11. Drug development has benefitted from this as organoids have facilitated pre-clinical testing of more personalised treatments and large screens for efficacy and toxicity of a range of compounds.
Although the use of organoids in environmental and genetic toxicology has been more limited, the treatment of organoids with various environmental compounds has generated results close to those previously obtained in primary tissue and in vivo models, demonstrating the biological relevance of this model. Other studies have investigated the toxicity of some compounds on different tissue organoids, providing promising results that indicate organoids will facilitate the study of the effects of environmental exposure and the increased risk of developing diseases like cancer. More in-depth analyses of the metabolic capabilities of organoids would help to validate the use of this system to investigate the modes of action of environmental carcinogens. Organoids show great potential for environmental and genetic toxicology research and they will allow us to expand our knowledge of the molecular mechanisms of carcinogenesis, contributing to risk assessment. Initially such advances are likely to emerge from bespoke investigations of the mechanisms of action of specific agents in target organs. Current barriers to their more widespread use, e.g. in routine safety testing are the cost and complexity of organoid culture. Establishing organoid ‘lines’ with stable karyotypes and prolonged growth potential will also be required in the longer term.
Acknowledgements
The authors thank Jill E. Kucab and Halh Al-Serori for many valuable discussions during the preparation of this review and for critical review of the final manuscript.
Funding
This work was supported by the UK Medical Research Council [MR/N013700/1 to C.G.A.L.] and King’s College London, which is a member of the MRC Doctoral Training Partnership in Biomedical Sciences. Work at King’s College London is further supported by the Cancer Research UK Grand Challenge Award ‘Mutographs of Cancer’ [C98/A24032]. D.H.P. is a member of the Health Protection Research Unit in Chemical and Radiation Threats and Hazards, a partnership between Public Health England and Imperial College London which is funded by the National Institute for Health Research (NIHR).
Conflict of Interest: None declared.
References
- 1. Lin, R. Z., Lin, R. Z. and Chang, H. Y. (2008) Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J., 3, 1172–1184. [DOI] [PubMed] [Google Scholar]
- 2. Knight, E. and Przyborski, S. (2015) Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat., 227, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Truskey, G. A. (2018) Human microphysiological systems and organoids as in vitro models for toxicological studies. Front. Public Health, 6, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lancaster, M. A. and Knoblich, J. A. (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science, 345, 1247125. [DOI] [PubMed] [Google Scholar]
- 5. Clevers, H. (2016) Modeling development and disease with organoids. Cell, 165, 1586–1597. [DOI] [PubMed] [Google Scholar]
- 6. Spence, J. R., Mayhew, C. N., Rankin, S. A., et al. (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T. and Sasai, Y. (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 472, 51–56. [DOI] [PubMed] [Google Scholar]
- 8. Lancaster, M. A., Renner, M., Martin, C. A., et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature, 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker, N., Van Es, J. H., Kuipers, J., et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- 10. Barker, N., Huch, M., Kujala, P., et al. (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell, 6, 25–36. [DOI] [PubMed] [Google Scholar]
- 11. Huch, M., Bonfanti, P., Boj, S. F., et al. (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J., 32, 2708–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huch, M., Dorrell, C., Boj, S. F., et al. (2013) In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature, 494, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sato, T., Vries, R. G., Snippert, H. J., et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 459, 262–265. [DOI] [PubMed] [Google Scholar]
- 14. Morizane, R., Lam, A. Q., Freedman, B. S., Kishi, S., Valerius, M. T. and Bonventre, J. V. (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol., 33, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forsythe, S. D., Devarasetty, M., Shupe, T., Bishop, C., Atala, A., Soker, S. and Skardal, A. (2018) Environmental toxin screening using human-derived 3D bioengineered liver and cardiac organoids. Front. Public Health, 6, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naruse, M., Masui, R., Ochiai, M., Maru, Y., Hippo, Y., and Imai, T. (2020) An organoid-based carcinogenesis model induced by in vitro chemical treatment. Carcinogenesis, 20, 1–10. [DOI] [PubMed] [Google Scholar]
- 17. Li, X., Francies, H. E., Secrier, M., et al. (2018) Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun., 9, 2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sachs, N., Papaspyropoulos, A., Ommen, D. D. Z., et al. (2019) Long‐term expanding human airway organoids for disease modeling. EMBO J., 38, e100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Webb, D. R. (2014) Animal models of human disease: inflammation. Biochem. Pharmacol., 87, 121–130. [DOI] [PubMed] [Google Scholar]
- 20. Ruggeri, B. A., Camp, F. and Miknyoczki, S. (2014) Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol., 87, 150–161. [DOI] [PubMed] [Google Scholar]
- 21. Olson, H., Betton, G., Robinson, D., et al. (2000) Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol., 32, 56–67. [DOI] [PubMed] [Google Scholar]
- 22. Kolenda, T., Kapałczyńska, M., Przybyła, W., et al. (2018) 2D and 3D cell cultures-a comparison of different types of cancer cell cultures. Arch Med Sci, 14, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antoni, D., Burckel, H., Josset, E. and Noel, G. (2015) Three-dimensional cell culture: a breakthrough in vivo. Int. J. Mol. Sci., 16, 5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim, J., Koo, B. K. and Knoblich, J. A. (2020) Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol., 21, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark, J. M. (2018) The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br. J. Nutr., 120, S1–S7. [DOI] [PubMed] [Google Scholar]
- 26. Pampaloni, F., Reynaud, E. G. and Stelzer, E. H. (2007) The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol., 8, 839–845. [DOI] [PubMed] [Google Scholar]
- 27. Lou, Y. R. and Leung, A. W. (2018) Next generation organoids for biomedical research and applications. Biotechnol. Adv., 36, 132–149. [DOI] [PubMed] [Google Scholar]
- 28. Silva-Almeida, C., Ewart, M. A. and Wilde, C. (2020) 3D gastrointestinal models and organoids to study metabolism in human colon cancer. Semin. Cell Dev. Biol., 98, 98–104. [DOI] [PubMed] [Google Scholar]
- 29. Rossi, G., Boni, A., Guiet, R., Girgin, M., Kelly, R. G., and Lutolf, M. P. (2019) Embryonic organoids recapitulate early heart organogenesis. bioRxiv, 802181. doi: 10.1101/802181 [DOI] [Google Scholar]
- 30. Renner, M., Lancaster, M. A., Bian, S., Choi, H., Ku, T., Peer, A., Chung, K. and Knoblich, J. A. (2017) Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J., 36, 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finkbeiner, S. R., Hill, D. R., Altheim, C. H., et al. (2015) Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Rep., 4, 1140–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCracken, K. W., Catá, E. M., Crawford, C. M., et al. (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature, 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schutgens, F., Rookmaaker, M. B., Margaritis, T., et al. (2019) Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol., 37, 303–313. [DOI] [PubMed] [Google Scholar]
- 34. Dekkers, J. F., Wiegerinck, C. L., de Jonge, H. R., et al. (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med., 19, 939–945. [DOI] [PubMed] [Google Scholar]
- 35. Calandrini, C., Schutgens, F., Oka, R., et al. (2020) An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat. Commun., 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li, L., Knutsdottir, H., Hui, K., et al. (2019) Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI insight, 4, e121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van De Wetering, M., Francies, H. E., Francis, J. M., et al. (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell, 161, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weeber, F., Ooft, S. N., Dijkstra, K. K. and Voest, E. E. (2017) Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem. Biol., 24, 1092–1100. [DOI] [PubMed] [Google Scholar]
- 39. Berkers, G., van Mourik, P., Vonk, A. M., et al. (2019) Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep., 26, 1701–1708.e3. [DOI] [PubMed] [Google Scholar]
- 40. Xu, H., Jiao, Y., Qin, S., Zhao, W., Chu, Q. and Wu, K. (2018) Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp. Hematol. Oncol., 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pendergraft, S. S., Sadri-Ardekani, H., Atala, A. and Bishop, C. E. (2017) Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol. Reprod., 96, 720–732. [DOI] [PubMed] [Google Scholar]
- 42. Grabinger, T., Luks, L., Kostadinova, F., Zimberlin, C., Medema, J. P., Leist, M. and Brunner, T. (2014) Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis., 5, e1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mun, S. J., Ryu, J. S., Lee, M. O., et al. (2019) Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol., 71, 970–985. [DOI] [PubMed] [Google Scholar]
- 44. Lu, W., Rettenmeier, E., Paszek, M., Yueh, M. F., Tukey, R. H., Trottier, J., Barbier, O. and Chen, S. (2017) Crypt organoid culture as an in vitro model in drug metabolism and cytotoxicity studies. Drug Metab. Dispos., 45, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saito, Y., Muramatsu, T., Kanai, Y., et al. (2019) Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep., 27, 1265–1276.e4. [DOI] [PubMed] [Google Scholar]
- 46. Park, E., Kim, H. K., Jee, J., Hahn, S., Jeong, S. and Yoo, J. (2019) Development of organoid-based drug metabolism model. Toxicol. Appl. Pharmacol., 385, 114790. [DOI] [PubMed] [Google Scholar]
- 47. Rossi, G., Manfrin, A. and Lutolf, M. P. (2018) Progress and potential in organoid research. Nat. Rev. Genet., 19, 671–687. [DOI] [PubMed] [Google Scholar]
- 48. Clevers, H., Loh, K. M. and Nusse, R. (2014) Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 346, 1248012. [DOI] [PubMed] [Google Scholar]
- 49. Min, S., Kim, S. and Cho, S. W. (2020) Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp. Mol. Med., 52, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin, Y. and Zhou, D. (2018) Organoid and enteroid modeling of salmonella infection. Front. Cell. Infect. Microbiol., 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sato, T., Stange, D. E., Ferrante, M., et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology, 141, 1762–1772. [DOI] [PubMed] [Google Scholar]
- 52. Fujii, M., Matano, M., Toshimitsu, K., Takano, A., Mikami, Y., Nishikori, S., Sugimoto, S. and Sato, T. (2018) Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell, 23, 787–793.e6. [DOI] [PubMed] [Google Scholar]
- 53. Schwank, G., Koo, B. K., Sasselli, V., et al. (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell, 13, 653–658. [DOI] [PubMed] [Google Scholar]
- 54. Watson, C. L., Mahe, M. M., Múnera, J., et al. (2014) An in vivo model of human small intestine using pluripotent stem cells. Nat. Med., 20, 1310–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finkbeiner, S. R., Zeng, X. L., Utama, B., Atmar, R. L., Shroyer, N. F. and Estes, M. K. (2012) Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio, 3, e00159–e00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mohammad, S., Kashfi, H., Almozyan, S., Jinks, N., Koo, B.-K., and Nateri, A. S. (2018) Morphological alterations of cultured human colorectal matched tumour and healthy organoids. Oncotarget, 9, 10572–10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Múnera, J. O., Sundaram, N., Rankin, S. A., et al. (2017) Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell, 21, 51–64.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Crespo, M., Vilar, E., Tsai, S. Y., et al. (2017) Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med., 23, 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bartfeld, S., Bayram, T., van de Wetering, M., Huch, M., Begthel, H., Kujala, P., Vries, R., Peters, P. J. and Clevers, H. (2015) In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology, 148, 126–136.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schlaermann, P., Toelle, B., Berger, H., Schmidt, S. C., Glanemann, M., Ordemann, J., Bartfeld, S., Mollenkopf, H. J. and Meyer, T. F. (2016) A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut, 65, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huch, M., Gehart, H., van Boxtel, R., et al. (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell, 160, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Broutier, L., Andersson-Rolf, A., Hindley, C. J., Boj, S. F., Clevers, H., Koo, B. K. and Huch, M. (2016) Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc., 11, 1724–1743. [DOI] [PubMed] [Google Scholar]
- 63. Hu, H., Gehart, H., Artegiani, B., et al. (2018) Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell, 175, 1591–1606.e19. [DOI] [PubMed] [Google Scholar]
- 64. Peng, W. C., Logan, C. Y., Fish, M., et al. (2018) Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell, 175, 1607–1619.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takebe, T., Sekine, K., Enomura, M., et al. (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature, 499, 481–484. [DOI] [PubMed] [Google Scholar]
- 66. Wu, F., Wu, D., Ren, Y., et al. (2019) Generation of hepatobiliary organoids from human induced pluripotent stem cells. J. Hepatol., 70, 1145–1158. [DOI] [PubMed] [Google Scholar]
- 67. Boj, S. F., Hwang, C. I., Baker, L. A., et al. (2015) Organoid models of human and mouse ductal pancreatic cancer. Cell, 160, 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Loomans, C. J. M., Williams Giuliani, N., Balak, J., et al. (2018) Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Rep., 10, 1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Greggio, C., De Franceschi, F., Figueiredo-Larsen, M., Gobaa, S., Ranga, A., Semb, H., Lutolf, M. and Grapin-Botton, A. (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development, 140, 4452–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang, L., Holtzinger, A., Jagan, I., et al. (2015) Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med., 21, 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takasato, M., Er, P. X., Becroft, M., Vanslambrouck, J. M., Stanley, E. G., Elefanty, A. G. and Little, M. H. (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol., 16, 118–126. [DOI] [PubMed] [Google Scholar]
- 72. Taguchi, A., Kaku, Y., Ohmori, T., Sharmin, S., Ogawa, M., Sasaki, H. and Nishinakamura, R. (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell, 14, 53–67. [DOI] [PubMed] [Google Scholar]
- 73. Li, Z., Araoka, T., Wu, J., et al. (2016) 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell, 19, 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tanigawa, S., Taguchi, A., Sharma, N., Perantoni, A. O., and Nishinakamura, R. (2016) Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. CellReports, 15, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Takasato, M., Er, P. X., Chiu, H. S., and Little, M. H. (2016) Generating kidney organoids from human pluripotent stem cells. Nat. Protoc., 11, 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takasato, M., Er, P. X., Chiu, H. S., et al. (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature, 526, 564–568. [DOI] [PubMed] [Google Scholar]
- 77. Ooms, A. H. A. G., Calandrini, C., de Krijger, R. R. and Drost, J. (2020) Organoid models of childhood kidney tumours. Nat. Rev. Urol., 17, 311–313. [DOI] [PubMed] [Google Scholar]
- 78. Jun, D. Y., Kim, S. Y., Na, J. C., Lee, H. H., Kim, J., Yoon, Y. E., Hong, S. J. and Han, W. K. (2018) Tubular organotypic culture model of human kidney. PLoS One, 13, e0206447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Langhans, S. A. (2018) Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol., 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schneeberger, K., Sánchez-Romero, N., Ye, S., et al. (2020) Large-scale production of LGR5-positive bipotential human liver stem cells. Hepatology, 72, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zanger, U. M. and Schwab, M. (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther., 138, 103–141. [DOI] [PubMed] [Google Scholar]
- 82. Lancaster, M. A. and Huch, M. (2019) Disease modelling in human organoids. Dis. Model. Mech., 12, dmm039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mithal, A., Capilla, A., Heinze, D., et al. (2020) Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun., 11, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dekkers, J. F., Berkers, G., Kruisselbrink, E., et al. (2016) Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med., 8, 344ra84. [DOI] [PubMed] [Google Scholar]
- 85. Williams, K. E., Lemieux, G. A., Hassis, M. E., Olshen, A. B., Fisher, S. J. and Werb, Z. (2016) Quantitative proteomic analyses of mammary organoids reveals distinct signatures after exposure to environmental chemicals. Proc. Natl Acad. Sci. U.S.A., 113, E1343–E1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rocco, S. A., Koneva, L., Middleton, L. Y. M., et al. (2018) Cadmium exposure inhibits branching morphogenesis and causes alterations consistent with HIF-1α inhibition in human primary breast organoids. Toxicol. Sci., 164, 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Devall, M., Jennelle, L. T., Bryant, J., Bien, S., Peters, U., Powell, S. and Casey, G. (2020) Modeling the effect of prolonged ethanol exposure on global gene expression and chromatin accessibility in normal 3D colon organoids. PLoS One, 15, e0227116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lynch, S., Pridgeon, C. S., Duckworth, C. A., Sharma, P., Park, B. K. and Goldring, C. E. P. (2019) Stem cell models as an in vitro model for predictive toxicology. Biochem. J., 476, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mazzoleni, G., Di Lorenzo, D. and Steimberg, N. (2009) Modelling tissues in 3D: the next future of pharmaco-toxicology and food research? Genes Nutr., 4, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Augustyniak, J., Bertero, A., Coccini, T., Baderna, D., Buzanska, L. and Caloni, F. (2019) Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol., 39, 1610–1622. [DOI] [PubMed] [Google Scholar]
- 91. Pfuhler, S., van Benthem, J., Curren, R., et al. (2020) Use of in vitro 3D tissue models in genotoxicity testing: strategic fit, validation status and way forward. Report of the working group from the 7th International Workshop on Genotoxicity Testing (IWGT). Mutat. Res., 850-851, 503135. [DOI] [PubMed] [Google Scholar]
- 92. Shah, U. K., Mallia, J. O., Singh, N., Chapman, K. E., Doak, S. H. and Jenkins, G. J. S. (2018) A three-dimensional in vitro HepG2 cells liver spheroid model for genotoxicity studies. Mutat. Res. Genet. Toxicol. Environ. Mutagen., 825, 51–58. [DOI] [PubMed] [Google Scholar]
- 93. Zhang, C., Zhang, Q., Li, J., et al. (2020) Integration of in vitro data from three dimensionally cultured HepaRG cells and physiologically based pharmacokinetic modeling for assessment of acetaminophen hepatotoxicity. Regul. Toxicol. Pharmacol., 114, 104661. [DOI] [PubMed] [Google Scholar]
- 94. Amaral, R. L. F., Miranda, M., Marcato, P. D. and Swiech, K. (2017) Comparative analysis of 3d bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front. Physiol., 8, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Štampar, M., Tomc, J., Filipič, M. and Žegura, B. (2019) Development of in vitro 3D cell model from hepatocellular carcinoma (HepG2) cell line and its application for genotoxicity testing. Arch. Toxicol., 93, 3321–3333. [DOI] [PubMed] [Google Scholar]
- 96. Elje, E., Hesler, M., Rundén-Pran, E., Mann, P., Mariussen, E., Wagner, S., Dusinska, M. and Kohl, Y. (2019) The comet assay applied to HepG2 liver spheroids. Mutat. Res., 845, 403033. [DOI] [PubMed] [Google Scholar]
- 97. David, R. M. and Gooderham, N. J. (2016) Using 3D MCF-7 mammary spheroids to assess the genotoxicity of mixtures of the food-derived carcinogens benzo[a]pyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Toxicol. Res. (Camb)., 5, 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Terashima, J., Goto, S., Hattori, H., Hoshi, S., Ushirokawa, M., Kudo, K., Habano, W. and Ozawa, S. (2015) CYP1A1 and CYP1A2 expression levels are differentially regulated in three-dimensional spheroids of liver cancer cells compared to two-dimensional monolayer cultures. Drug Metab. Pharmacokinet., 30, 434–440. [DOI] [PubMed] [Google Scholar]
- 99. Conway, G. E., Shah, U. K., Llewellyn, S., Cervena, T., Evans, S. J., Al Ali, A. S., Jenkins, G. J., Clift, M. J. D. and Doak, S. H. (2020) Adaptation of the in vitro micronucleus assay for genotoxicity testing using 3D liver models supporting longer-term exposure durations. Mutagenesis, 35, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ginzkey, C., Friehs, G., Koehler, C., Hackenberg, S., Voelker, H. U., Richter, E. and Kleinsasser, N. H. (2010) Nicotine and methyl methane sulfonate in mini organ cultures of human parotid gland tissue. Toxicol. Lett., 197, 69–74. [DOI] [PubMed] [Google Scholar]
- 101. Hackenberg, S., Zimmermann, F. Z., Scherzed, A., et al. (2011) Repetitive exposure to zinc oxide nanoparticles induces DNA damage in human nasal mucosa mini organ cultures. Environ. Mol. Mutagen., 52, 582–589. [DOI] [PubMed] [Google Scholar]
- 102. Kleinsasser, N. H., Juchhoff, J., Wallner, B. C., Bergner, A., Harréus, U. A., Gamarra, F., Bührlen, M., Huber, R. M. and Rettenmeier, A. W. (2004) The use of mini-organ cultures of human upper aerodigestive tract epithelia in ecogenotoxicology. Mutat. Res., 561, 63–73. [DOI] [PubMed] [Google Scholar]
- 103. Chang, S.-Y., Weber, E. J., Sidorenko V. S., et al. (2017) Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight, 2, e95978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baudy, A. R., Otieno, M. A., Hewitt, P., Gan, J., Roth, A., Keller, D., Sura, R., Van Vleet, T. R. and Proctor, W. R. (2020) Liver microphysiological systems development guidelines for safety risk assessment in the pharmaceutical industry. Lab Chip, 20, 215–225. [DOI] [PubMed] [Google Scholar]
- 105. Rajan, S. A. P., Aleman, J., Wan, M., et al. (2020) Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater., 106, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang, Y., Wang, L., Zhu, Y., and Qin, J. (2018) Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip, 18, 843–990. [DOI] [PubMed] [Google Scholar]
- 107. Abd, E., Yousef, S. A., Pastore, M. N., Telaprolu, K., Mohammed, Y. H., Namjoshi, S., Grice, J. E. and Roberts, M. S. (2016) Skin models for the testing of transdermal drugs. Clin. Pharmacol., 8, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Netzlaff, F., Lehr, C.-M., Wertz, P. W., and Schaefer, U. F. (2005) The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm., 60, 167–178. [DOI] [PubMed] [Google Scholar]
- 109. Chau, D. Y., Johnson, C., MacNeil, S., Haycock, J. W. and Ghaemmaghami, A. M. (2013) The development of a 3D immunocompetent model of human skin. Biofabrication, 5, 035011. [DOI] [PubMed] [Google Scholar]
- 110. Flaten, G. E., Palac, Z., Engesland, A., Filipović-Grčić, J., Vanić, Ž. and Škalko-Basnet, N. (2015) In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci., 75, 10–24. [DOI] [PubMed] [Google Scholar]
- 111. Jäckh, C., Blatz, V., Fabian, E., Guth, K., van Ravenzwaay, B., Reisinger, K. and Landsiedel, R. (2011) Characterization of enzyme activities of Cytochrome P450 enzymes, Flavin-dependent monooxygenases, N-acetyltransferases and UDP-glucuronyltransferases in human reconstructed epidermis and full-thickness skin models. Toxicol. In Vitro, 25, 1209–1214. [DOI] [PubMed] [Google Scholar]
- 112. Götz, C., Pfeiffer, R., Tigges, J., et al. (2012) Xenobiotic metabolism capacities of human skin in comparison with a 3D epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: activating enzymes (Phase I). Exp. Dermatol., 21, 358–363. [DOI] [PubMed] [Google Scholar]
- 113. Reisinger, K., Blatz, V., Brinkmann, J., et al. (2018) Validation of the 3D Skin Comet assay using full thickness skin models: transferability and reproducibility. Mutat. Res. Genet. Toxicol. Environ. Mutagen., 827, 27–41. [DOI] [PubMed] [Google Scholar]
- 114. Pfuhler, S., Pirow, R., Downs, T. R., et al. (2020) Validation of the 3D reconstructed human skin Comet assay, an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis, 20, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pupovac, A., Senturk, B., Griffoni, C., Maniura-Weber, K., Rottmar, M. and McArthur, S. L. (2018) Toward immunocompetent 3D skin models. Adv. Healthc. Mater., 7, e1701405. [DOI] [PubMed] [Google Scholar]
- 116. Skardal, A., Aleman, J., Forsythe, S., et al. (2020) Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication, 12, 025017. [DOI] [PubMed] [Google Scholar]
- 117. Astashkina, A. I., Jones, C. F., Thiagarajan, G., Kurtzeborn, K., Ghandehari, H., Brooks, B. D. and Grainger, D. W. (2014) Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials, 35, 6323–6331. [DOI] [PubMed] [Google Scholar]
- 118. Dundon, M., Madden, O. and Comizzoli, P. (2019) Three-dimensional culture of endometrial cells from domestic cats: a new in vitro platform for assessing plastic toxicity. PLoS One, 14, e0217365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kretzschmar, K. and Clevers, H. (2016) Organoids: modeling development and the stem cell niche in a dish. Dev. Cell, 38, 590–600. [DOI] [PubMed] [Google Scholar]