ABSTRACT
The purpose of this systematic review is to examine the relationship between dietary patterns and sarcopenia using a protocol developed for use by the 2020 Dietary Guidelines Advisory Committee, and to conduct a meta-analysis to summarize the evidence. Multiple electronic databases were searched for studies investigating sarcopenia risk factors or risk of sarcopenia and dietary patterns. Eligible studies were 1) peer-reviewed controlled trials or observational trials, 2) involving adult or older-adult human subjects who were healthy and/or at risk for chronic disease, 3) comparing the effect of consumption or adherence to dietary patterns (measured as an index/score, factor or cluster analysis; reduced rank regression; or a macronutrient distribution), and 4) reported on measures of skeletal muscle mass, muscle strength, muscle performance, and/or risk of sarcopenia. Thirty-eight publications met all inclusion criteria for qualitative synthesis. Thirteen observational studies met inclusion criteria for meta-analysis. Higher adherence to a healthy dietary pattern was associated with a decreased risk of gait speed reduction (OR = 0.58; 95% CI: 0.18, 0.97). The association between healthy dietary pattern adherence and other intermediate markers or risk of sarcopenia was not statistically significant. The majority of individual studies were judged as “serious” risk of bias and analysis of the collective evidence base was suggestive of publication bias. Studies suggest a significant association between healthy dietary patterns and maintenance of gait speed with age, an intermediate marker of sarcopenia risk, but the evidence base is limited by serious risk of bias, within and between studies. Further research is needed to understand the association between healthy dietary patterns and risk of sarcopenia.
Keywords: dietary pattern, sarcopenia, diet score, diet index, factor analysis, cluster analysis, macronutrient, physical function, muscle, aging
Healthy dietary patterns support maintenance of gait speed with age, an intermediate marker of sarcopenia, but the evidence base is limited by serious within- and between-study risk of bias.
Introduction
Sarcopenia is the progressive loss of skeletal muscle mass leading to reduced muscle strength and function. A common condition in older adults, sarcopenia can negatively impact quality of life and independence (1). It is estimated that 25–45% of US older adults are living with sarcopenia, contributing to an increased risk of falls, reduced activities of daily living, and increased nursing home placement (2). Consensus studies have recommended higher levels of dietary protein (e.g., 1.0–1.2 g/kg body weight per day vs. the recommended dietary allowance of 0.8 g/kg body weight per day) as an appropriate dietary strategy to prevent or delay the onset of sarcopenia (3–6).
Dietary patterns focus on the totality of the diet rather than single foods, beverages, or nutrients (e.g., protein) (1). A dietary pattern can be defined as the quantities, proportions, variety, or combination of different foods and beverages in diets, and the frequency with which they are habitually consumed (7). Adherence to a dietary pattern is often examined using a variety of methods including predefined indexes/scores, data-driven methods such as cluster/factor analysis using observational data, or directly tested in randomized controlled trials (RCTs) (7). Evidence derived from research using dietary pattern methodologies has been used to inform the development of the Dietary Guidelines for Americans (DGA) beginning with the 2010 the Dietary Guidelines Advisory Committee (DGAC) (8). In the most recent DGA (2020–2025) process, the DGAC, supported by the Nutrition Evidence Systematic Review (NESR) team at the United States Department of Agriculture, used systematic reviews to address 8 questions that examined the relation between dietary patterns and health outcomes, including, for the first time, the relation between dietary patterns and sarcopenia (8).
The original NESR systematic review protocol (2019 NESR protocol), initially presented on 10 July 2019, with revisions presented on 24 October 2019, was designed to answer the DGAC question regarding dietary patterns and risk of sarcopenia (9, 10) and included intermediate markers of sarcopenia risk (i.e., skeletal muscle mass, muscle strength, muscle performance) and endpoint outcomes of severe sarcopenia and sarcopenia. In an effort to refine and prioritize the DGAC workflow, the protocol was further revised in early 2020 (2020 NESR protocol) to include only endpoint outcomes (11). Using the 2020 protocol, the NESR systematic review concluded that there was insufficient evidence to determine the relation between dietary patterns and sarcopenia in older adults (12). Subsequently, the 2020–2025 DGA did not provide any specific dietary advice regarding a dietary pattern supportive of muscle health during aging [Tables 1-1 and 6-1 in the 2020–2025 DGA (1)].
The objective of the current systematic review and meta-analysis (MA) was to utilize the original 2019 NESR protocol, which included both intermediate markers and endpoint outcomes of sarcopenia, to examine the relation between dietary patterns and sarcopenia risk. Intermediate outcomes are useful to monitor risk of disease onset and/or disease progression. Clinically meaningful intermediate outcomes, such as muscle strength, muscle mass, and physical function, may provide practical evidence for increased risk or onset of sarcopenia (13). Thus, the present systematic review and meta-analysis is intended to complement and expand upon the NESR systematic review used to inform the 2020–2025 DGA regarding sarcopenia and dietary patterns.
Methods
Search strategy and study selection
The 2019 NESR protocol related to sarcopenia and dietary patterns served as the foundation for this systematic review (9, 10). Our systematic review protocol was prospectively registered in PROSPERO as CRD42020172655. While at the outset of the systematic review it was not expected that sufficient information would be available to conduct a meta-analysis, our PROSPERO registration was updated to reflect the intention to complete a meta-analysis when sufficient data appeared available after the extraction step. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed (14). The PRISMA reporting checklist is available in Supplemental Table 1. Ethics approval and consent to participate verification are not applicable to this systematic review of published literature.
Eligibility criteria
The criteria used to determine study eligibility are presented in Table 1 and Supplemental Table 2.
TABLE 1.
Description of PICOS (Population, Intervention, Comparison, Outcome, Study design) criteria for the research question, “What is the relationship between dietary patterns and risk of sarcopenia?”
| Parameter | Eligibility criteria |
|---|---|
| Population | Human subjects ≥19 y at time of outcome who were healthy and/or at risk for chronic disease, not pregnant or lactating, living in countries ranked as high or higher human development1 |
| Study populations composed of a mixed population of healthy, at risk, and diseased subjects, but not exclusively diagnosed with a disease or with low skeletal muscle mass, low muscle strength, low muscle performance, or sarcopenia were included | |
| Intervention | Consumption of and/or adherence to a dietary pattern (measured as an index/score; factor or cluster analysis; reduced rank regression; having at least 1 macronutrient outside the Acceptable Macronutrient Distribution Range (AMDR);2 or description of dietary pattern, including at a minimum, the foods and beverages included in pattern)3 |
| Comparison | Consumption of and/or adherence to a different dietary pattern, varying levels of adherence to a dietary pattern, or different macronutrient proportions |
| Outcome | Intermediate markers of sarcopenia risk (i.e., skeletal muscle mass, muscle strength, muscle performance) and/or risk of sarcopenia or severe sarcopenia4 |
| Study design | Peer-reviewed controlled trials or observational trials (prospective cohort studies, retrospective cohort studies, nested case-control studies and case-control) published in English |
Human development classification based on Human Development Index rank from the year the study intervention occurred, or data were collected. Available from http://hdr.undp.org/en/data. Rank higher than 110 indicative of medium or lower Human Development Index.
Outside AMDR: <45% or >65% of energy from carbohydrate, <20% or >35% of energy from fat, or <10% or >35% of energy from protein.
Interventions for weight loss or examining dietary supplements or single foods as a macronutrient source (i.e., nuts) were not included.
For detailed description of eligible outcome measures, see Supplemental Table 2.
Information sources and search strategy
Using search strategies made publicly available by NESR in September 2019, we searched PubMed, Cochrane Central Register of Controlled Trials, and Embase from inception through the end date used by NESR (i.e., October 2019) (12). Each search strategy included search terms, both Medical Subject Headings (MeSH) and text words, designed to capture sarcopenia and intermediate markers and dietary pattern concepts. The detailed search strategies are provided in Supplemental File 1.
Screening and study selection
Two investigators (LT and MEVE) independently screened titles and abstracts of studies resulting from the literature search. Studies for which eligibility could not be determined or confirmed from the title and abstract were retrieved and reviewed as full text. Publications were included for qualitative summary if they met all of the eligibility criteria and none of the exclusion criteria. Publications were included for meta-analysis if data necessary for analyses were provided in sufficient detail (see Supplemental File 2 for additional detail of meta-analysis methods). Disagreements were resolved by consensus or via a third reviewer (CSL or CJS).
Data extraction
Three reviewers (CSL, LT, and MEVE) independently extracted relevant data from studies meeting eligibility at full text using a pilot-tested extraction protocol and companion Excel spreadsheet (Microsoft Corporation). Extracted data included, but were not limited to, primary author, title and year of publication, type of study, subject demographics, intervention arms, diet and dietary pattern methodology description, number of subjects per treatment, duration of study, author narrative summary of results, results for individual food components (when available), means and/or effect estimators, measures of variability (highest adherence, multivariable adjusted) for outcomes of interest, and author narrative conclusion.
Risk of bias in individual studies
Risk-of-bias assessments for each included study were conducted independently, in duplicate (LT and MEVE), with each article assessed for the outcome of interest. Assessment responses were compared, and disagreements were discussed and reconciled.
The Cochrane risk-of-bias tool for randomized trials was used to assess the risk of bias for RCTs (15, 16). Risk of bias for each domain (randomization process, intended intervention, missing outcome data, measurement of outcome, selection of reported results) and overall could be judged as having “low,” “some concerns,” or “high” risk of bias, as well as “no information.” A response of “no information” to 1 or more signaling questions expected to be answered with reported data resulted in an overall judgment of “some concerns” for the particular domain. If at least 1 domain was judged to have “some concerns,” but none were high, the overall risk of bias was judged as “some concerns.” A single judgment of “high” resulted in an overall judgment of high risk of bias.
Risk of bias of observational studies was assessed using the Risk of Bias for Nutrition Observational Studies tool [RoB-Nobs; (8)]. The RoB-Nobs tool was developed by the NESR team based on modifications to the commonly used Risk of Bias in Non-randomized Studies-of Interventions tool [ROBINS-I; (17)] and is designed to ensure applicability to observational studies of food and nutrition. Risk of bias for each domain (confounding, selection of participants, classification of exposure, departures from intended exposures, missing data, measurement of outcomes, selection of reported result) and overall could be judged as having “low,” “moderate,” “serious,” or “critical” risk of bias, as well as “no information.” The overall risk of bias was judged to be the highest risk assigned for any individual domain.
Meta-analysis methods
A set of meta-analyses were conducted to complete a quantitative assessment of the available evidence. The effects of higher adherence compared with lower adherence to dietary patterns on risk of sarcopenia or changes in intermediate markers of sarcopenia (13) were evaluated using fully adjusted results. Included studies tested for an association between the odds of an outcome of interest and adherence to a dietary pattern. Studies excluded from the meta-analysis reported either insufficient data (e.g., no or incomplete measure of the outcome of interest), an outcome not accepted to be an intermediate marker of sarcopenia risk [i.e., accepted criteria per revised European consensus (13)], or an outcome or dietary pattern method that was insufficiently represented by the collective evidence base, so as to preclude a meaningful meta-analysis. Weighting and aggregation of each of the qualified studies’ relevant findings were performed to determine the expected strength of association, via an odds ratio (OR), between adherence to a dietary pattern and development of sarcopenia or change in an intermediate marker of sarcopenia risk.
For dietary pattern scores designed to indicate the consumption of healthy food and beverage components (e.g., higher adherence to a healthier dietary pattern leads to a higher score), a reported OR <1.0 is interpreted as having lower odds of developing the outcome of interest (e.g., sarcopenia, low muscle mass, weakness, etc.), whereas an OR >1.0 indicates higher odds of developing the outcome of interest. For certain dietary patterns, such as the Energy-adjusted Dietary Inflammatory Index (E-DII) or the Shivappa's DII, a higher score suggests low adherence to a healthier dietary pattern. Thus, a reported OR >1.0 is interpreted as having lower odds of developing the outcome of interest, whereas an OR <1.0 indicates higher odds of developing the outcome of interest. Accordingly, the inverse of the reported OR for these cases was calculated and used in the meta-analysis calculations.
A random-effects meta-analysis statistical model was used to aggregate the qualified study findings using the approach laid out by DerSimonian and Laird, and built using Stata software (StataCorp) and Microsoft Excel (18). A random-effects model is ideal in cases when the presence of heterogeneity, or variance, in the nature or characteristics of the qualified findings between studies due to differences in study protocols, study duration, diet patterns, and characteristics of the study sample such as demographic make-up is expected. Subgroup analyses were performed for Mediterranean dietary pattern indices and scores, as this was the most common dietary pattern tested in the evidence base. Sensitivity analyses were conducted to explore the impact of Shivappa's DII compared with Tabung's Empirical DII (TEDII) results from Laclaustra et al. (19) on relevant outcomes. Between-study heterogeneity was explored using Cochran's Q test and I2 statistic. Additional statistical details are provided as Supplemental File 2.
Risk of bias across studies
Studies included in the meta-analysis of this review were assessed for risk of publication bias via visual funnel plot assessment and the Egger's test, using a significance of P < 0.05 to indicate significant asymmetry (20).
Results
Study selection
Figure 1 depicts the flow diagram of the study selection process. A total of 8526 unique records were screened at the title/abstract level. Of those, 168 full texts were retrieved to confirm or further assess eligibility, resulting in 38 publications (representing 37 studies) included in the qualitative synthesis of this systematic review [Figure 1; Tables 2 and 3; (19, 21–57)]. The most common reason for exclusion at the full-text level was failing to meet dietary pattern–related criteria [n = 57; i.e., no dietary pattern methodology, missing description of food and beverages in the dietary pattern, intervention as individual foods or supplements rather than dietary patterns, or a macronutrient mix within the Acceptable Macronutrient Distribution Range (AMDR); Supplemental Table 3].
FIGURE 1.
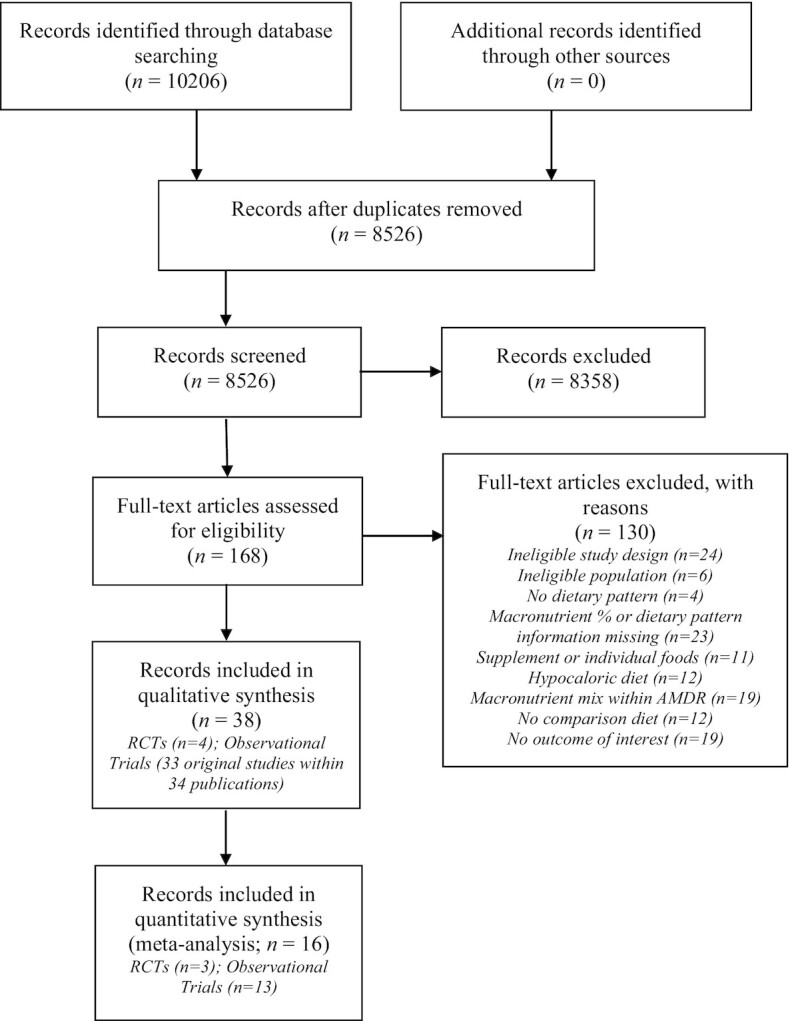
PRISMA flow diagram. AMDR, Acceptable Macronutrient Distribution Range; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
TABLE 2.
Characteristics of randomized controlled trials included in the systematic review1
| First author, year (ref) | Country | Population/cohort | No. enrolled | % Male | Mean age at baseline, y | Dietary pattern(s) | Duration | Sarcopenia | Skeletal muscle mass | Self-reported muscle performance | Objective muscle performance | Muscle strength | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baker et al., 20192 (54) | USA | Recreationally active subjects | 11 | 36 | 28 ± 3 | MEDAS3 | 4 d | NA | NA | NA | 5-km run time | Hand grip | Some concerns |
| Western diet | Vertical jump height | ||||||||||||
| Wingate anaerobic cycle test | |||||||||||||
| Castaneda et al., 1995 (55) | USA | Healthy sedentary to moderately active females | 12 | 0 | P group (71 ± 2) | P group: 1.47g protein/kg body cell mass/d (7% protein) | 9 wk | NA | NA | NA | NA | Chest press | Some concerns |
| 2P group (72 ± 1) | 2P group: 2.94 g protein/kg body cell mass/d (13% protein) | Leg extensor | |||||||||||
| Hand grip | |||||||||||||
| Leg power output | |||||||||||||
| Dipla et al., 20082 (56) | Greece | Healthy recreationally active (low-to-medium intensity) females | 10 | 0 | NR | High protein: 30% CHO; 40% protein; 30% fat for 1 wk | 1 wk | NA | NA | NA | NA | Hand grip | Some concerns |
| Control: 55% CHO; 15% protein; 30% fat for 1 wk | Knee flexor and extensor | ||||||||||||
| Van Zant et al., 20022 (57) | USA | Healthy males either aerobically (AER) or strength (STR) trained or sedentary (SED) | 18 | 100 | AER (32 ± 8.7) STR (32.2 ± 6.9) SED (32 ± 3.9) | Moderate CHO and fat: 42% CHO; 18% protein; 40% fat; included wheat bran for 3 wk (7-d meal rotation) | 3 wk | NA | NA | NA | Isokinetic knee contractions | Knee flexor and extensor | High |
| High CHO and low fat: 62% CHO; 18% protein; 20% fat; included wheat bran for 3 wk (7-d meal rotation) | Bench press repetitions | Bench press |
CHO, carbohydrate; MEDAS, Mediterranean Diet Adherence Screener; NA, not applicable; NR, not reported; PREDIMED, Prevención con Dieta Mediterránea; ref, reference; ROB, risk of bias.
Crossover study design.
MEDAS score was developed to assess compliance with the dietary intervention of the PREDIMED trial.
TABLE 3.
Characteristics of observational studies included in the systematic review1
| First author, year (ref) | Country | Population/cohort | No. enrolled | % Male | Mean age at baseline, y | Diet pattern type | Dietary pattern(s) | Duration | Sarcopenia | Skeletal muscle mass | Self-reported muscle performance | Objective muscle performance | Muscle strength | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al., 20192 (21) | USA | >40 y, MAP | 809 | 26 | 80.7 ± 7.2 | Diet score/index | MIND | 5.3 y | NA | NA | Rosow–Breslau | NA | Ser | |
| MedDietScore | ||||||||||||||
| DASH | ||||||||||||||
| Birnie et al., 20122 (22) | United Kingdom | 45–59 y, CAPS | 2512 | 100 | NR | Macronutrient mix | CHO: 49.3%; Protein: 13.7%; Fat: 37.0%3,4 | 19–25 y | NA | NA | NA | TUG | NA | Ser |
| Standing balance | ||||||||||||||
| Bishop et al., 20185 (23) | USA | ≥65 y, HRS HCNS | 8073 | 41 | NR | Macronutrient mix | High CHO (HC): 69.38% CHO; 13.92% protein; 23.72% fat3 | 2 y | NA | NA | Mobility limitation | NA | NA | Ser |
| Moderate with fat (MF): 49.35% CHO; 17.03% protein; 37.30% fat3 | ||||||||||||||
| Moderate (MOD): 58.65% CHO; 15.78 protein%; 30.80% fat3 | ||||||||||||||
| Low CHO/high fat (LCFH): 39.43% CHO; 18.39 protein%; 45.46% fat3 | ||||||||||||||
| Cervo et al., 20202 (24) | Tasmania | TASOAC | 1098 | 51 | 63.0 ± 7.5 | Diet scores/index | Energy-adjusted-DII | 10 y | NA | NA | NA | NA | Hand grip | Ser |
| Knee extension | ||||||||||||||
| Whole lower-limb muscle strength | ||||||||||||||
| Chan et al., 20162 (25) | Hong Kong | ≥65 y, Mr. OS & Ms. OS (Hong Kong) Study | 3957 | 50 | NR | Diet score/index | DQI-I | 3.9 y | AWGFS | NA | NA | NA | NA | Ser |
| MDS | ||||||||||||||
| Factor/cluster | Vegetables-fruits | |||||||||||||
| Snacks-drinks-milk products | ||||||||||||||
| Meat-fish | ||||||||||||||
| Germain et al., 20132 (26) | France | 45–60 y, SU.VI.MAX | 4407 | 53 | NR | Diet score/index | PNNS-GS | 12 y | NA | NA | SF-36 PCS | NA | NA | Ser |
| Gopinath et al., 20142 (27) | Australia | ≥49 y, BMES | 3654 | 41 | NR | Diet score/index | TDS | 10 y | NA | NA | SF-36 PCS | NA | NA | Ser |
| Granic et al., 20162 (28) | United Kingdom | Newcastle 85+ Study | 791 | 38.2 | 85 | Factor/cluster | High red meat | 5 y | NA | NA | NA | TUG | Hand grip | Ser |
| Low meat | ||||||||||||||
| High butter | ||||||||||||||
| Granic et al., 20202(29) | United Kingdom | Newcastle 85+ Study | 757 | 38.8 | 85 | Factor/cluster | Low red meat | 3 y | EWGSOP | NA | NA | NA | NA | Ser |
| Low butter | ||||||||||||||
| Traditional British | ||||||||||||||
| Hagan et al., 20162 (30) | USA | Registered nurses, NHS | 54,762 | 0 | NR | Diet score/index | AHEI | 18 y | NA | NA | SF-36 PCS | NA | NA | Ser |
| Hagan and Grodstein, 20192 (31) | USA | ≥40 y, HPFS | NR | 100 | NR | Diet score/index | AHEI | 4 y | NA | NA | SF-36 PCS | NA | NA | Ser |
| Isanejad et al., 20182 (32) | Finland | ≥65 y, OSTPRE-FPS—control group of RCT | 282 | 0 | NR | Diet score/index | BSD6 | 3 y | EWGSOP | RSMI | NA | Chair rises | Hand grip | Ser |
| MED | Gait speed (maximal) | Squat test | ||||||||||||
| SPPB | ||||||||||||||
| Standing balance | ||||||||||||||
| Karlsson et al., 20202 (33) | Sweden | 50 y, ULSAM | 1221 | 100 | 70.9 | Diet score/index | Modified HDI | 16 y | EWGSOP | LMM | NA | Gait speed (self-chosen speed) | Hand grip | Ser |
| Modified MDS | SMI | |||||||||||||
| Laclaustra et al., 20202 (19) | Spain | ≥60 y, ENRICA | 2614 | NR | NR | Diet score/index | DII | 3 y | NA | NA | NA | Chair rises | Hand grip (weakness) | Cri |
| TEDII | Gait speed (slow) | |||||||||||||
| SPPB | ||||||||||||||
| Standing balance | ||||||||||||||
| León-Muñoz et al., 20152 (34) | Spain | ≥60 y, ENRICA | 2614 | NR | NR | Factor/cluster | PP: Prudent pattern | 3.5 y | NA | NA | NA | Gait speed (slow) | Hand grip (weakness) | Ser |
| WP: Westernized pattern | ||||||||||||||
| León-Muñoz et al., 20142 (35) | Spain | ≥60 y, ENRICA | 2519 | NR | NR | Diet score/index | MDS | 3.5 y | NA | NA | NA | Gait speed (slow) | Hand grip (weakness) | Ser |
| MEDAS7 | ||||||||||||||
| Mangano et al., 20172 (36) | USA | Non-Hispanic Whites 19–72 y, Framingham Third-Generation Study | 3800 | NR | 40.6 ± 8.7 | Protein cluster | Fast food, full-fat dairy | 8 y | NA | ALM | NA | NA | Knee extension | Ser |
| Fish | ||||||||||||||
| Red meat | ||||||||||||||
| Chicken | ||||||||||||||
| Low-fat milk | ||||||||||||||
| Legumes | ||||||||||||||
| Meng et al., 20092 (37) | Australia | 70–85 y, participants of RCT for oral calcium to prevent osteoporotic fractures | 1500 | 0 | 75 ± 3 | Macronutrient mix | T1 protein (<66 g/d): 44.7% (± 5.9) CHO; 17.7% (± 2.7) protein; 32.1% (± 6.1) fat3 | 5 y | NA | ALM | NA | NA | NA | Cri |
| T2 protein (66–87 g/d): 42.8% (± 4.6) CHO; 19.0% (± 2.3) protein; 33.4% (±4.6) fat3 | ||||||||||||||
| T3 protein (>87 g/d): 42.5% (±5.2) CHO; 20.4% (±3.2) protein; 33.2% (±5.0) fat4 | ||||||||||||||
| Milaneschi et al., 20112 (38) | Italy | ≥65 y, InCHIANTI | 935 | 44.4 | 74.1 ± 6.8 | Diet score/index | MDS | 9 y | NA | NA | NA | SPPB | NA | Ser |
| Mulla et al., 20132,8 (39) | United Kingdom | MRC NSHD 1946 birth cohort | 1771 | 49 | 36 | Macronutrient mix | Low CHO, high fat: Men, 36 y: 41.7% CHO; 14.4% protein 38.3% fat3 | 17 y | NA | NA | NA | Chair rises | Hand grip | Ser |
| Low CHO, high fat: Men, 43 y: 40.9% CHO; 14.6% protein; 38.6% fat3 | Standing balance | |||||||||||||
| Low CHO, high fat: Women, 36 y: 42.3% CHO; 15.7% protein; 40.0% fat3 | ||||||||||||||
| Low CHO, high fat: Women, 43 y: 42.9% CHO; 15.5% protein; 39.2% fat3 | ||||||||||||||
| Parsons et al., 20192 (40) | United Kingdom | 58–79 y, BRHS | 4252 | 100 | NR | Diet score/index | HDI | 15 y | NA | NA | Mobility limitations | NA | NA | Ser |
| Modified EDI | ||||||||||||||
| Factor/cluster | High-fat/low-fiber (HF/LF) | |||||||||||||
| Prudent (PP) | ||||||||||||||
| High sugar (HS) | ||||||||||||||
| Perälä et al., 20162; Perälä et al., 20172 (41, 42) | Finland | Helsinki Birth Cohort | 2003 | 46 | 61 | Diet score/index | NDS6 | 10 y | NA | NA | NA | 6-minute walk | Arm curl | Ser |
| Chair rises | Hand grip | |||||||||||||
| Knee extension | ||||||||||||||
| Pérez-Tasigchana et al., 20162 (43) | Spain | ≥60 y, UAM | UAM: 4008 | NR | NR | Diet score/index | UAM-MDP | 2.3 y | NA | NA | SF-36 PCS | NA | NA | Ser |
| ≥60 y, ENRICA | ENRICA: 2519 | MDS and PREDIMED | 2–4 y | SF-12 PCS | ||||||||||
| Pilis et al., 20189 (44) | Poland | Cases: healthy males with low-CHO diet for ≥3 y (n = 12) | 24 | 100 | NR | Macronutrient mix | Low CHO/very high fat (LCD): 22.5% CHO; 12.29% protein; 65.21% fat3 | 7 d | NA | NA | NA | Maximal workload | NA | Cri |
| Matched controls: (n = 12) | Mixed diet (MD): 48.88% CHO; 14.29% protein; 36.83% fat3 | Total workload | ||||||||||||
| Pilleron et al., 20182 (45) | France | ≥65 y, Three-City Study, Bordeaux Sample | 1328 | 47 | 75.7 | Factor/cluster | HealthySmall eaters Biscuits, snackingCharcuterie, meat, alcohol (men)Charcuterie, starchy food, (women)Pasta (men)Pizza, sandwich (women) | 9 y | NA | NA | Rosow–Breslau | NA | NA | Ser |
| Rahi et al., 20182 (46) | France | ≥65 y, Three-City Study, Bordeaux Sample | 725 | NR | NR | Diet score/index | MDS | 2 y | NA | NA | Slowness | NA | Poor muscle strength (hand grip at baseline; chair rises at 2 y) | Ser |
| Robinson et al., 20182,8 (47) | United Kingdom | MRC NSHD 1946 birth cohort | 2229 | NR | 36 | Diet score/index | ADQ | 28 y | NA | NA | NA | Chair rises | NA | Cri |
| Standing balance | ||||||||||||||
| TUG | ||||||||||||||
| Shahar et al., 20122 (48) | USA | 70–79 y, Whites and all age Blacks, Health ABC | 2225 | 50.1 | 74.6 | Diet score/index | MDS | 8 y | NA | NA | NA | Gait speed (usual and rapid) | NA | Ser |
| Stefler et al., 20182 (49) | Russia, Poland, Czech Republic | 45–69 y, HAPIEE | 28,783 | 46.7 | 58 | Diet score/index | MED | 10 y | NA | NA | SF-36 PF | NA | NA | Ser |
| Struijk et al., 20182 (50) | Spain | ≥60 y, ENRICA | 2614 | NR | NR | Diet score/index | MDS | 3.5 y | NA | NA | Impaired agility | NA | NA | Ser |
| MEDAS7 | Impaired mobility (Rosow-Breslau) | |||||||||||||
| SF-12 PCS | ||||||||||||||
| Talegawkar et al., 20122 (51) | Italy | ≥65 y, InCHIANTI | 1155 | 48.3 | 73.0 ± 6.24 | Diet score/index | MDS | 6 y | NA | NA | NA | Gait speed (slow) | Hand grip (weakness) | Ser |
| Yokoyama et al., 20172 (52) | Japan | Hatoyama cohort and Kusatsu Longitudinal Study | 1407 | 53.4 | NR | Diet score/index | DVS | 4 y | NA | ALM | NA | Gait speed (usual) | Hand grip | Ser |
| Zhu et al., 20182 (53) | China | Females 40–70 y, SWHS, and Males 40–74 y, SMHS | 136,421 | 45 | M 77.7 | Diet score/index | Modified AHEI | 14.4 y | NA | NA | Independent walking capability | NA | NA | Ser |
| F 78.1 | CHFP | |||||||||||||
| Modified DASH |
ADQ, Adult Diet Quality Score; AHEI, Alternative Healthy Eating Index-2010; ALM, appendicular lean mass; AWGFS, Asian Working Group for Sarcopenia; BMES, Blue Mountains Eye Study; BRHS, British Regional Heart Study; BSD, Baltic Sea Diet; CaPS, Caerphilly Prospective Study; CHFP, Chinese Food Pagoda; CHO, carbohydrate; Cri, Critical; DASH, Dietary Approaches to Stop Hypertension diet score; DII, Shivappa's Dietary Inflammatory Index; DQI-I, Dietary Quality Index-International; DVS, Dietary Variety Score; EDI, Elderly Dietary Index; ENRICA, Study on Nutrition and Cardiovascular Risk in Spain; EWGSOP, European Working Group On Sarcopenia; HAPIEE, Health Alcohol & Psychosocial factors in Eastern Europe Study; HDI, Healthy Diet Indicator; Health ABC, Health, Aging & Body Composition Study; HPFS, Health Professionals Follow-Up Study; HRS HCNS, 2013 Health & Retirement Study (Health Care & Nutrition Study); InCHIANTI, Invecchiare in Chianti study; LMM, lean muscle mass; MAP, Rush Memory & Aging Project cohort; MDS, Mediterranean Diet Scale (67); MED, Mediterranean Diet adherence score (68); MEDAS, Mediterranean Diet Adherence Screener; MedDietScore, Mediterranean Diet Score (69); MIND, Mediterranean–DASH Intervention for Neurodegenerative Delay diet score; MRC NSHD, Medical Research Council National Survey of Health & Development Study; NA, not applicable; NDS, Nordic diet score (aka Baltic Sea Diet); NHS, Nurses’ Health Study; NR, not reported; OSTPRE-FPS, Osteoporosis Risk Factor and Prevention-Fracture Prevention Study; PCS, physical component summary; PF, physical functioning; PNNS-GS, French Programme National Nutrition Santé Guidelines Score dietary component; PREDIMED, Prevención con Dieta Mediterránea; RCT, randomized controlled trial; ref, reference; ROB, risk of bias; RSMI, relative skeletal muscle index; Ser, serious; short form, SF-12, SF-36; SMHS, Shanghai Men's Health Study; SMI, skeletal muscle index; SPPB, Short Physical Performance Battery; SU.VI.MAX, Supplementation en Vitamines et Mineraux Antioxydants Study; SWHS, Shanghai Women's Health Study; T, tertile; TASOAC, Tasmanian Older Adult Cohort Study; TDS, Total Diet Score; TEDII, Tabung's Empirical Dietary Inflammatory Index; TUG, Timed Up and Go Test; UAM-MDP, Universidad Autónoma de Madrid-Mediterranean Diet; ULSAM, Uppsala Longitudinal Study of Adult Men.
Prospective cohort study.
One macronutrient calculated from information provided in study.
Baseline data for macronutrient mix met inclusion criteria.
Retrospective cohort study.
Nordic diet score is based on the Baltic Sea Diet.
MEDAS score was developed to assess compliance with the dietary intervention of the PREDIMED trial.
Birth cohort study.
Case-control.
Thirteen observational studies (19, 24, 25, 32, 33, 35, 38, 42, 46–48, 51, 52) and 3 RCTs (54–56) were included in the meta-analysis (Figure 1).
Study characteristics
The majority of evidence regarding the relation between dietary patterns and markers of sarcopenia risk was provided by observational studies, mainly PC (prospective cohort) studies (Table 3; Supplemental Table 4). The majority of individual observational studies were conducted in Europe and represented cohorts of fewer than 4000 subjects (Supplemental Table 4). Subjects eligible at baseline were typically at least 60 y of age (Supplemental Table 4). Of the intermediate markers considered, measures of muscle performance were most commonly reported (n = 30 studies), whereas measures of skeletal muscle mass were the least studied (n = 5 studies) (Supplemental Table 4). Dietary patterns were primarily assessed using indices and scores, for which there were 26 unique methods (Supplemental Table 5). The most commonly utilized method was a score designed to assess adherence to a Mediterranean dietary pattern (Supplemental Table 4), which was assessed using 6 different scoring methods ranging from 0 to 55 points for varying levels of adherence, some with population-specific modifications (Supplemental Table 5). Mediterranean dietary pattern scores in the current evidence base awarded points for plant-based protein sources and generally subtracted points for animal-based protein sources (meat and dairy), with the exception of fish (Supplemental Table 5). Two scores, the Dietary Quality Index-International (DQI-I) and the Healthy Diet Indicators (HDI), specifically considered protein intake as a percentage of energy, awarding points for either protein ≥10% of energy or between 10% and 15%, respectively (Supplemental Table 5). The Elderly Dietary Index awarded points for intake of meat 1–2 times/wk and fish 1–2 times/wk, while subtracting points for more or less intake of either (Supplemental Table 5). Five studies considered dietary patterns using a factor/cluster analysis approach or a macronutrient distribution pattern (i.e., low carbohydrate) (Supplemental Table 5).
Risk of bias within studies
The overall risk of bias for 3 of 4 included RCTs was judged to have “some concerns” (Table 2), resulting primarily from risk of bias in the randomization process (Supplemental Table 6) (54–56). Specifically, all 4 RCTs provided either insufficient information, or no information at all, to determine if baseline differences between intervention groups were suggestive of a problem with the randomization process (54–57).
The overall risk of bias for the majority of included observational studies was judged to be “serious” (Table 3). The most common reason for a judgment of serious risk of bias resulted from risk of bias due to confounding (Supplemental Table 7)—specifically, lack of appropriate methodology to control for important confounders (e.g., sex, age, race/ethnicity, anthropometry, physical activity and/or disability, or socioeconomic status). While the majority of studies adjusted results for sex, age, anthropometry, and physical activity (as measured using standardized self-report questionnaires or a single question), the minority adjusted for race/ethnicity (data not shown).
Intermediate and endpoint outcome results
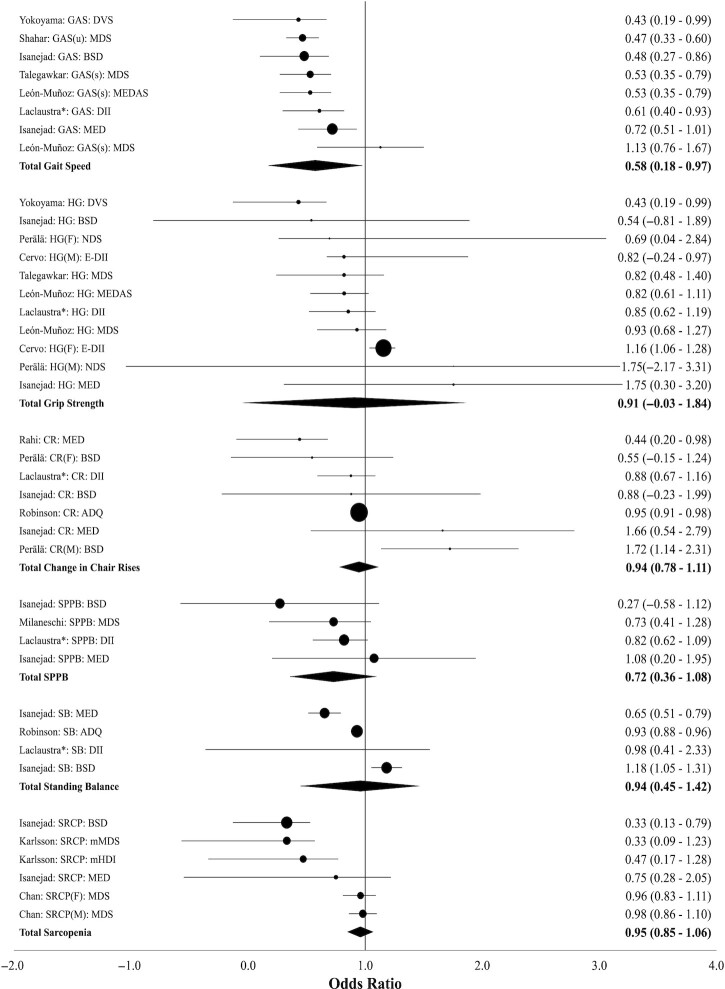
Details of studies included in the meta-analyses are provided in Supplemental Table 8. The association between adherence to a healthy dietary pattern, as proxied by a variety of diet pattern indices/scores, and sarcopenia or change in an intermediate marker of sarcopenia risk is summarized in Figure 2.
FIGURE 2.
Meta-analysis of observational studies reporting the association between healthy dietary pattern indices/scores and the risk of sarcopenia or risk of change in intermediate markers of sarcopenia. Note: Effect sizes show the ratio of the odds of a probable sarcopenic outcome among individuals in the high-adherence diet cohort to a relative to the low-adherence diet cohort. Solid circle sizes indicate the variance-based study weight. Solid diamond markers indicate aggregated weighted results. See Supplemental Table 9 for reported association between all dietary pattern types and risk of sarcopenia. See Supplemental Table 10 for heterogeneity statistics and results of subgroup and sensitivity analyses. *Only Shivappa's DII results from Laclaustra et al. (19) included. ADQ, Adult Diet Quality Score; BSD, Baltic Sea Diet; CR, change in chair rises per minute; DII, Dietary Inflammatory Index; DVS, Dietary Variety Score; E-DII, Energy-adjusted Dietary Inflammatory Index; (F), women; GAS, gait speed; HG, change in hand grip; (M), men; MDS, Mediterranean Diet Scale; MED, Mediterranean Diet adherence score; MEDAS, Mediterranean Diet Adherence Screener; mHDI, modified Healthy Diet Indicator; NDS, Nordic diet score; SB, standing balance; SPPB, Short Physical Performance Battery; (s), slow speed; SRCP, sarcopenia diagnosis; (u), usual speed.
Sarcopenia
Four studies measured the association between healthy dietary patterns and risk of sarcopenia (25, 29, 32, 33). The prevalence of sarcopenia at baseline ranged from 7.3% to 24% (Supplemental Table 9). Three studies (25, 32, 33) specifically measured the association between adherence to a variety of healthy dietary patterns examined using an index or score and the risk of sarcopenia. The aggregated findings showed no association between a healthy dietary pattern and reduced risk of sarcopenia (OR = 0.95; 95% CI: 0.85, 1.06) (Figure 2; Supplemental Table 10).
Muscle strength
There was no association between preservation of hand-grip strength and the adherence to a healthy dietary pattern, tracked by 7 observational studies reporting 11 mutually exclusive findings (OR = 0.91; 95% CI: 0.12, 1.69) (19, 24, 32, 35, 42, 51, 52) (Figure 2; Supplemental Table 8). No association was detected between hand-grip strength and adherence to a healthy dietary pattern when subgroup analyses were used to compare results of including either of the 2 inflammatory dietary indices reported by Laclaustra et al. (19) and when limiting the analysis to only Mediterranean dietary patterns (Supplemental Table 10).
In a separate analysis of RCTs, no association between macronutrient distribution patterns or a Mediterranean dietary pattern and muscle strength was found (mean difference in change in hand grip strength = 0.029 kg; 95% CI: −0.50, 0.56) (Supplemental Table 10). The calculated Q statistic is 35.01 (P = 0.00) and I2 statistic is 0.91 (Supplemental Table 10). Two additional studies (28, 34) reported no association between a variety of dietary patterns examined by factor/cluster analyses and hand-grip strength (Supplemental Table 11).
Muscle performance
Objective measures of muscle performance in the current review include gait speed, chair rises, standing balance, and the Short Physical Performance Battery (SPPB). Six studies reported 8 mutually exclusive effect findings that specifically measured the degree of association between gait speed preservation and adherence to a healthy dietary pattern as measured by a variety of indices/scores (19, 32, 35, 48, 51, 52) (Supplemental Table 8). Higher adherence to a healthy dietary pattern was associated with a decreased risk of reduced gait speed (OR = 0.58; 95% CI: 0.18, 0.97) (Figure 2). Findings remained statistically significant when subgroup analyses were used to compare results of including only 1 of 2 inflammatory dietary indices reported by Laclaustra et al. (19) and when limiting the analysis to only Mediterranean dietary patterns (Supplemental Table 10). There was no association between change in the number of chair rises within a set period of time and adherence to a healthy dietary pattern resulting from the analysis of 5 studies reporting 7 mutually exclusive ORs (19, 32, 35, 48, 51, 52) (OR = 0.94; 95% CI: 0.78, 1.11) (Supplemental Table 8; Figure 2). Similarly, the association between standing balance and the adherence to a healthy dietary pattern, tracked by 3 studies reporting 4 mutually exclusive findings (Supplemental Table 8), was not confirmed (OR = 0.94; 95% CI: 0.45, 1.42) (19, 24, 32, 35, 42, 51, 52) (Figure 2). With respect to the SPPB, a common assessment tool used to evaluate older people's lower extremity functionality, analysis of 4 mutually exclusive findings that measured the association between a change in SPPB score and adherence to a healthy dietary pattern (19, 32, 38) resulted in no association (OR = 0.72; 95% CI: 0.36, 1.08) (Supplemental Table 8; Figure 2). Furthermore, no association was confirmed when subgroup analyses were used to compare results of including only 1 of 2 inflammatory dietary indices reported by Laclaustra et al. (19) and when limiting the analysis to only Mediterranean dietary patterns (Supplemental Table 10). Two additional studies (28, 34) examined dietary patterns using factor/cluster analyses; of these, 1 study (34) reported an increased risk of slow gait speed with higher adherence to a Western-style dietary pattern (Supplemental Table 12). One additional study considered macronutrient-mix dietary patterns and reported significantly decreased maximal workload and total workload among subjects reporting adherence to a low-carbohydrate, high-fat diet for 3 y prior to a 7-d observation period (Supplemental Table 12). Finally, 1 RCT reported significant findings of faster run time for 5 km when subjects (mean age: 28 y) consumed a Mediterranean dietary pattern for 4 d compared with a Western-style diet in a crossover design study (54) (Supplemental Table 13). Findings from 13 studies (21, 23, 26, 27, 30, 31, 40, 43, 45, 46, 49, 50, 53) investigating associations between adherence to dietary patterns and self-reported measures of muscle performance are summarized in Supplemental Table 14. Several studies reported at least 1 significant finding with at least 1 of several dietary pattern methods, but findings for specific dietary patterns were varied and inconsistent.
Skeletal muscle mass
Two of 3 studies (32, 33) examining dietary patterns using indices/scores and skeletal muscle mass–related outcomes reported a significant association (Supplemental Table 15). Specifically, Isanejad et al. (32), reported that higher adherence to a Mediterranean dietary pattern decreased skeletal muscle loss in women, whereas Karlsson et al. (33) reported that higher adherence to a Mediterranean dietary pattern increased skeletal muscle mass in men. Isanejad et al. (32) also reported a significant association between adherence to the Baltic Sea Diet (BSD) and decreased skeletal muscle loss. Two additional studies considered dietary patterns derived by factor cluster analysis and/or macronutrient distribution (36, 37). Higher intake of total protein was associated with higher appendicular lean mass in both studies, whereas none of the protein clusters identified by cluster analysis (i.e., “fast food, full-fat dairy,” “fish,” “red meat,” “chicken,” “low-fat milk,” and “legumes”) were associated (36) (Supplemental Table 15).
Between-study heterogeneity
With regard to all of the observational study meta-analyses, low levels of heterogeneity were observed. The calculated Q-statistics were small and not statistically significant (Supplemental Table 10). Furthermore, the reported I2 statistics in all the observational study analyses were zero (Supplemental Table 10).
Risk of bias across studies
Supplemental Figure 1 shows the funnel chart of the findings of the included prospective cohort studies, which suggests the presence of both heterogeneity of study protocols and possible publication bias. An Egger test assessing the presence of publication bias was statistically significant among the prospective cohort studies (PC-MA Egger bias: –0.951; P = 0.01). There are also visual signs of publication bias in the funnel chart as evident by the relatively low number of studies present in the right-hand side of the funnel plot area (right of the null line, OR = 1). This suggests the possibility that smaller studies that could have reported null or negative results are missing in the literature. Among the 3 RCTs (54–56), consisting of 4 mutually exclusive sets of results, an Egger test also shows bias across the included studies but there were too few studies to provide a high level of confidence in this finding (RCT-MA Egger bias: –17.52; P = 0.469).
Discussion
In the current review, it was anticipated that, by expanding the evidence base for sarcopenia and dietary patterns beyond that considered by the 2020 DGAC to include intermediate markers, sufficient evidence may be available to determine a relation between adherence to healthy dietary patterns and reduced risk of sarcopenia. While the resulting evidence base was expanded beyond the 4 studies considered by the 2020 DGAC, the resulting expanded body of evidence had several risks of bias, including lack of adjustment for all potential confounders in observational studies and, in particular, lack of adjustment for race and ethnicity. The 2020 DGAC scientific report notes that race and ethnicity are associated with differential intakes of food groups, nutrients, and food components (8). Nearly all observational studies included in the current review failed to adjust for race and/or ethnicity. There were signs of publication bias suggesting that there is a possibility that smaller studies that would have reported null or negative results are missing in the literature. Given the risk of bias evident in the current evidence base, findings should be interpreted with caution.
Study design protocols explored the relation between dietary pattern scores, constructed using a variety of approaches, and outcomes of interest measured using a variety of methods (58, 59). However, statistical heterogeneity among observational studies was low. This finding is not surprising considering that outcomes in qualified observational studies were individually considered in outcome-specific meta-analyses; original observational study results were consistently expressed as ORs, and dietary indices and scores shared common characteristics including higher intake of foods and nutrients considered healthful versus limitation of those considered by the authors as detrimental for health (60). Low heterogeneity should be interpreted with caution as it does not necessarily suggest no heterogeneity exists but rather that which does exist may not be an important factor when interpreting results (61).
Gait speed was the only intermediate maker of sarcopenia risk found to be significantly associated with consumption of a healthy dietary pattern as examined using an index or score. Significant findings for gait speed, but none of the other markers of sarcopenia, may reflect a broader association of gait speed to overall health and mortality with dietary patterns that are also broad in nature. In fact, many dietary pattern indices and scores were developed to better understand the associations between dietary patterns with mortality or cardiovascular diseases (58, 59). In addition to being an intermediate marker of sarcopenia risk, gait speed is also considered by some as the “sixth vital sign,” reflecting its potential to predict functional decline, risk of falls, and risk of hospitalization but also mortality (62).
The 2020 DGAC report notes that “Older adults have low intakes of protein when compared with the EAR (Estimated Average Requirement). Given the high prevalence of sarcopenia and reduced muscle strength, dietary protein should be further examined” (8). In the current study, 26 indices/scores designed to assess adherence to a healthy dietary pattern are represented, but only a few contributed to the evidence base regarding gait speed. Three Mediterranean dietary pattern scores, a BSD score, 2 scores of diet-induced inflammation, and 1 of dietary variety collectively contributed to the observed maintenance of gait speed in older adults. With regard to protein, Mediterranean dietary pattern scores in the current review typically subtracted points for some protein-rich foods, including red meat, poultry, and dairy (as seen with the Mediterranean Diet Scale, MDS); red meat, poultry, dairy, and eggs (as seen with the Mediterranean Diet Adherence Score, MED); or red meat only (as seen with the Mediterranean Diet Adherence Screener, MEDAS), while awarding points for other protein-rich foods such as nuts and fish/seafood. None of the Mediterranean dietary pattern scores distinguished between lean cuts and higher-fat cuts of red meat or poultry. While 2 of the 3 Mediterranean dietary patterns scores in the gait speed analysis subtracted points for any dairy foods, others like the BSD score, awarded points for low-fat dairy. The BSD also awarded points for higher intake of Nordic cereal grains (e.g., rye, oats, barley), Nordic vegetables (e.g., roots, legumes, cabbages, and peas), and Nordic fish (e.g., salmon and freshwater fishes) (Supplemental Table 5). The Dietary Variety Score is neutral with regard to protein source, awarding points for both animal- and plant-source proteins. Of the 2 inflammatory indices, one addressed micronutrients and bioactives rather than food groups while the other subtracted points for red meat, processed meat, and organ meat but did not otherwise award points to any protein-rich foods.
Examination of the contribution of dietary protein to intermediate markers of sarcopenia was constrained by the design of the current systematic review protocol. To explore the association between dietary patterns of varying macronutrient distribution, the 2020 DGAC sarcopenia protocol required that included studies representing this type of dietary pattern must have at least 1 macronutrient proportion outside of the age-appropriate AMDR in an effort “…to examine the entire distribution of macronutrients in the diet, and not one macronutrient in isolation” (8). In the results of both the current and 2020 DGAC systematic review, studies meeting this AMDR criterion were limited in number as many times studies labeled as “high-protein” compared graded and distinct levels of protein but within the AMDR range (8). In this review, 15% of studies investigating macronutrient distribution dietary patterns were excluded at full-text screening because macronutrients were within the AMDR range. Design of future systematic review protocols to examine dietary patterns and sarcopenia risk might be done in a manner that allows inclusion of higher versus lower protein macronutrient distribution patterns within the AMDR. For example, in a systematic review and meta-analysis of older adults and protein intake, adults with protein intake ≥1.0 g protein/kg per day showed better walking speed performance in comparison to individuals with lower protein intake (<0.80 g/kg per day) (63). Recently, multivariant regression analyses of the association between dietary intake and muscle mass, measured by both dual x-ray absorptiometry and deuterated creatine dilution, in a cohort of older US men found every incremental increase in percentage of nondairy animal protein was associated with increased muscle mass while each incremental increase in plant protein was associated with lower muscle mass (64). A dietary pattern index/score specific to risk of sarcopenia and intake of protein may be warranted.
The current study has limitations. First, while testing of intermediate disease markers within RCTs can provide evidence and support determinations of biological causality where relations for chronic disease endpoints are still emerging, the evidence base regarding sarcopenia and dietary patterns is predominantly observational. The 2020 DGAC report recognizes that few studies evaluated the relations between specific dietary pattern methods and health outcomes with RCTs of adequate sample size in diverse populations. Of the 4 RCTs that met our dietary pattern inclusion criteria, all had small sample sizes and were of short duration. Second, few indices/scores analyzed “healthy” diets that promote consumption of high-quality protein (7), making it potentially difficult to use existing dietary patterns scores to make protein-related recommendations to reduce the risk of sarcopenia. It has been recommended that indices designed to assess dietary pattern adherence should include evaluation of 2 macronutrients to ensure balance (58). A dietary pattern index/score specific to risk of sarcopenia and intake of protein may be warranted. Third, not all intermediate markers of sarcopenia risk are well represented by the current evidence base. Low muscle mass is a required criterion for diagnosis of sarcopenia (13) but was the least studied in the current evidence base. Cost, availability, and ease of use for various muscle mass measurements may limit their application in various settings (13). A relatively inexpensive and precise novel marker of total muscle mass, the deuterated creatine dilution method, has been reported as strongly related to physical performance in a cohort of older men (65). Awareness and application of precise, inexpensive, and convenient measures of muscle mass could improve the evidence base available to examine the relation between dietary patterns and sarcopenia. Finally, limitations of observational nutrition studies on foods and dietary patterns have been recognized elsewhere and include the following: unclear contribution of individual foods to observed dietary pattern associations, lack of standardized food grouping, lack of generalizability across populations, varying scoring systems for the same named dietary patterns, long-term variability of intake, unknown correlation between food intake and exploratory substitutions, measurement error, and the semi-quantitative nature of dietary data (7, 66).
Our study also has several strengths. We relied on the most recent consensus (13) regarding the definition of sarcopenia to identify intermediate markers for meta-analysis. It has been noted that research findings in the field of sarcopenia over the last decade have raised many questions and use of a clear definition and diagnostic criteria are needed (13). By focusing on accepted intermediate markers, results from the current analysis may help guide future research design regarding sarcopenia and dietary patterns. In addition, by using the systematic review protocol designed by NESR experts and completing a meta-analysis, our current review complements and expands upon that used to inform the 2020–2025 DGA and may provide insights for designing a protocol to investigate sarcopenia risk and dietary patterns for the 2025–2030 DGA cycle.
In conclusion, while our analysis finds that adherence to healthy dietary patterns may preserve gait speed in older adults, the evidence base is limited by serious risk of bias. More research is needed to understand the association between healthy dietary patterns and reduced risk of sarcopenia.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kristy Hancock of W.K. Kellogg Health Sciences Library, Dalhousie University, for her assistance in implementing the search strategy for this review. The authors’ responsibilities were as follows—MEVE, LT, CSL, and CJS: designed and conducted research, analyzed data, and wrote the manuscript; MEVE: had primary responsibility for the main manuscript content; LT: had primary responsibility for supplementary material content; and all authors: read and approved the final manuscript.
Notes
Supported by the Beef Checkoff.
Author disclosures: CSL is currently employed by the National Cattlemen's Beef Association (NCBA), a contractor to the Beef Checkoff, as the Senior Director of Human Nutrition Research. MEVE, CJS, and LT are consultants and have been paid by NCBA, a contractor to the Beef Checkoff, for work related to this manuscript. The sponsor had no role in the writing or imposed any restrictions regarding the submission of this report for publication.
Supplemental Tables 1–15, Supplemental Files 1 and 2, and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AMDR, Acceptable Macronutrient Distribution Range; BSD, Baltic Sea Diet; DGA, Dietary Guidelines for Americans; DGAC, Dietary Guidelines Advisory Committee; DII, Dietary Inflammatory Index; MA, Meta-Analysis; NESR, Nutrition Evidence Systematic Review; PC, Prospective Cohort; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; RoB-Nobs, Risk of Bias for Nutrition Observational Studies tool; SPPB, Short Physical Performance Battery.
Contributor Information
Mary E Van Elswyk, Email: mveconsulting@q.com, Van Elswyk Consulting, Inc., Clark, CO, USA.
Lynn Teo, Teo Research Consulting, Portland, ME, USA.
Clara S Lau, National Cattlemen's Beef Association, a contractor to the Beef Checkoff, Washington, DC, USA.
Christopher J Shanahan, Unleash Creatives, LLC, Reynoldsburg, OH, USA.
Data Availability
All data described in the manuscript will be made available upon request. Statistical code is available from Mr. Shanahan (e-mail: shanahan.christopher@gmail.com); data sets (study characteristics and risk-of-bias assessment) are available from Dr. Van Elswyk (e-mail: mveconsulting@q.com).
References
- 1. US Department of Agriculture; US Department of Health and Human Services . Dietary guidelines for Americans, 2020–2025. [Internet]. 9th ed.December 2020. [Accessed 2022 Feb 8]. Available from: https://www.dietaryguidelines.gov/. [Google Scholar]
- 2. Du K, Goates S, Arensberg MB, Pereira S, Gaillard T. Prevalence of sarcopenia and sarcopenic obesity vary with race/ethnicity and advancing age. Divers Equal Health Care. 2018;15(4):175–83. [Google Scholar]
- 3. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta Det al. . Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 4. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KSet al. . Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paddon-Jones D, Campbell WW, Jacques PF, Kritchevsky SB, Moore LL, Rodriguez NR, van Loon LJ. Protein and healthy aging. Am J Clin Nutr. 2015;101(6):1339S–45S. [DOI] [PubMed] [Google Scholar]
- 6. Phillips SM, Chevalier S, Leidy HJ. Protein “requirements” beyond the RDA: implications for optimizing health. Appl Physiol Nutr Metab. 2016;41(5):565–72. [DOI] [PubMed] [Google Scholar]
- 7. Schulze MB, Martinez-Gonzalez MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. [Internet]. 2020. [Accessed 2021 Dec 4]. Available from: https://www.dietaryguidelines.gov/2020-advisory-committee-report. [Google Scholar]
- 9. 2020 Dietary Guidelines Advisory Committee . Meeting 2. Day 1: July 10, 2019. Dietary Patterns Subcommittee Presentation. [Internet]. [Accessed 2022 Feb 8]. Available from: https://www.youtube.com/watch?v=iqitbzrmiTM. [Timestamp: 6:29]. [Google Scholar]
- 10. 2020 Dietary Guidelines Advisory Committee . Meeting 3. Day 1: October 24, 2019. Dietary Patterns Subcommittee Presentation. [Internet]. [Accessed 2022 Feb 8]. Available from: https://www.youtube.com/watch?v=CRpwBugSBHs. [Timestamp: 2:47, 10:49]. [Google Scholar]
- 11. 2020 Dietary Guidelines Advisory Committee . Meeting 4. Day 2: January 24, 2020, Morning Session. Dietary Patterns Subcommittee Presentation. [Internet]. [Accessed 2022 Feb 8]. Available from: https://www.youtube.com/watch?v=2RnX37Xoz18. [Timestamp: 35:56]. [Google Scholar]
- 12. 2020 Dietary Guidelines Advisory Committee and Nutrition Evidence Systematic Review Team . Dietary patterns and sarcopenia: a systematic review. [Internet]. 2020. [Accessed 2021 Apr 5]. Available from: https://nesr.usda.gov/2020-dietary-guidelines-advisory-committee-systematic-reviews/dietary-patterns-subcommittee/dietary-patterns-sarcopenia. [PubMed] [Google Scholar]
- 13. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AAet al. . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Sterne JA, Savovic J, Page MJ, Hrobjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, Clarke M, McKenzie J, Boutron I, Welch Veditors. Cochrane methods. Cochrane Database Syst Rev. 2016;10(Suppl 1):29–31. doi: 10.1002/14651858.CD201601. [DOI] [Google Scholar]
- 16. Higgins JPT, Savović J, Page M, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). [Internet]. [Accessed 2022 Feb 8]. 2019. Available from: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view. [Google Scholar]
- 17. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 19. Laclaustra M, Rodriguez-Artalejo F, Guallar-Castillon P, Banegas JR, Graciani A, Garcia-Esquinas E, Lopez-Garcia E. The inflammatory potential of diet is related to incident frailty and slow walking in older adults. Clin Nutr. 2020;39(1):185–91. [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agarwal P, Wang Y, Buchman AS, Bennett DA, Morris MC. Dietary patterns and self-reported incident disability in older adults. J Gerontol A Biol Sci Med Sci. 2019;74(8):1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birnie K, Ben-Shlomo Y, Gunnell D, Ebrahim S, Bayer A, Gallacher J, Holly JM, Martin RM. Childhood milk consumption is associated with better physical performance in old age. Age Ageing. 2012;41(6):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bishop NJ, Zuniga KE, Lucht AL. Latent profiles of macronutrient density and their association with mobility limitations in an observational longitudinal study of older U.S. adults. J Nutr Health Aging. 2018;22(6):645–54. [DOI] [PubMed] [Google Scholar]
- 24. Cervo MM, Shivappa N, Hebert JR, Oddy WH, Winzenberg T, Balogun S, Wu F, Ebeling P, Aitken D, Jones Get al. . Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin Nutr. 2020;39(2):516–23. [DOI] [PubMed] [Google Scholar]
- 25. Chan R, Leung J, Woo J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J Am Med Dir Assoc. 2016;17(4):336–42. [DOI] [PubMed] [Google Scholar]
- 26. Germain L, Latarche C, Kesse-Guyot E, Galan P, Hercberg S, Briançon S. Does compliance with nutrition guidelines lead to healthy aging? A quality-of-life approach. J Acad Nutr Diet. 2013;113(2):228–40.e2. [DOI] [PubMed] [Google Scholar]
- 27. Gopinath B, Russell J, Flood VM, Burlutsky G, Mitchell P. Adherence to dietary guidelines positively affects quality of life and functional status of older adults. J Acad Nutr Diet. 2014;114(2):220–9. [DOI] [PubMed] [Google Scholar]
- 28. Granic A, Jagger C, Davies K, Adamson A, Kirkwood T, Hill TR, Siervo M, Mathers JC, Sayer AA. Effect of dietary patterns on muscle strength and physical performance in the very old: findings from the Newcastle 85+ study. PLoS One. 2016;11(3):e0149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Siervo M, Mathers JC, Jagger C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: the Newcastle 85+ study. Clin Nutr. 2020; 39(1):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hagan KA, Chiuve SE, Stampfer MJ, Katz JN, Grodstein F. Greater adherence to the alternative healthy eating index is associated with lower incidence of physical function impairment in the Nurses' Health Study. J Nutr. 2016;146(7):1341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagan KA, Grodstein F. The Alternative Healthy Eating Index and physical function impairment in men. J Nutr Health Aging. 2019;23(5):459–65. [DOI] [PubMed] [Google Scholar]
- 32. Isanejad M, Sirola J, Mursu J, Rikkonen T, Kröger H, Tuppurainen M, Erkkilä AT. Association of the Baltic Sea and Mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur J Nutr. 2018;57(4):1435–48. [DOI] [PubMed] [Google Scholar]
- 33. Karlsson M, Becker W, Michaëlsson K, Cederholm T, Sjögren P. Associations between dietary patterns at age 71 and the prevalence of sarcopenia 16 years later. Clin Nutr. 2020;39(4):1077–84. [DOI] [PubMed] [Google Scholar]
- 34. León-Muñoz LM, García-Esquinas E, López-García E, Banegas JR, Rodríguez-Artalejo F. Major dietary patterns and risk of frailty in older adults: a prospective cohort study. BMC Med. 2015;13(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. León-Muñoz LM, Guallar-Castillón P, López-García E, Rodríguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc. 2014;15(12):899–903. [DOI] [PubMed] [Google Scholar]
- 36. Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Dietary protein is associated with musculoskeletal health independently of dietary pattern: the Framingham Third Generation Study. Am J Clin Nutr. 2017;105(3):714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meng X, Zhu K, Devine A, Kerr DA, Binns CW, Prince RL. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women. J Bone Miner Res. 2009;24(11):1827–34. [DOI] [PubMed] [Google Scholar]
- 38. Milaneschi Y, Bandinelli S, Corsi AM, Lauretani F, Paolisso G, Dominguez LJ, Semba RD, Tanaka T, Abbatecola AM, Talegawkar SAet al. . Mediterranean diet and mobility decline in older persons. Exp Gerontol. 2011;46(4):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mulla UZ, Cooper R, Mishra GD, Kuh D, Stephen AM. Adult macronutrient intake and physical capability in the MRC National Survey of Health and Development. Age Ageing. 2013;42(1):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parsons TJ, Papachristou E, Atkins JL, Papacosta O, Ash S, Lennon LT, Whincup PH, Ramsay SE, Wannamethee SG. Healthier diet quality and dietary patterns are associated with lower risk of mobility limitation in older men. Eur J Nutr. 2019;58(6):2335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perälä MM, Von Bonsdorff M, Männistö S, Salonen MK, Simonen M, Kanerva N, Pohjolainen P, Kajantie E, Rantanen T, Eriksson JG. A healthy Nordic diet and physical performance in old age: findings from the longitudinal Helsinki Birth Cohort Study. Br J Nutr. 2016;115(5):878–86. [DOI] [PubMed] [Google Scholar]
- 42. Perälä MM, Von Bonsdorff MB, Männistö S, Salonen MK, Simonen M, Kanerva N, Rantanen T, Pohjolainen P, Eriksson JG. The healthy Nordic diet predicts muscle strength 10 years later in old women, but not old men. Age Ageing. 2017;46(4):588–94. [DOI] [PubMed] [Google Scholar]
- 43. Pérez-Tasigchana RF, León-Muñoz LM, López-García E, Banegas JR, Rodríguez-Artalejo F, Guallar-Castillón P. Mediterranean diet and health-related quality of life in two cohorts of community-dwelling older adults. PLoS One. 2016;11(3):e0151596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pilis K, Pilis A, Stec K, Pilis W, Langfort J, Letkiewicz S, Michalski C, Czuba M, Zych M, Chalimoniuk M. Three-year chronic consumption of low-carbohydrate diet impairs exercise performance and has a small unfavorable effect on lipid profile in middle-aged men. Nutrients. 2018;10(12):1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pilleron S, Pérès K, Jutand MA, Helmer C, Dartigues JF, Samieri C, Féart C. Dietary patterns and risk of self-reported activity limitation in older adults from the Three-City Bordeaux Study. Br J Nutr. 2018;120(5):549–56. [DOI] [PubMed] [Google Scholar]
- 46. Rahi B, Ajana S, Tabue-Teguo M, Dartigues JF, Peres K, Feart C. High adherence to a Mediterranean diet and lower risk of frailty among French older adults community-dwellers: results from the Three-City-Bordeaux Study. Clin Nutr. 2018;37(4):1293–8. [DOI] [PubMed] [Google Scholar]
- 47. Robinson SM, Westbury LD, Cooper R, Kuh D, Ward K, Syddall HE, Sayer AA, Cooper C. Adult lifetime diet quality and physical performance in older age: findings from a British birth cohort. J Gerontol A Biol Sci Med Sci. 2018;73(11):1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shahar DR, Houston DK, Hue TF, Lee JS, Sahyoun NR, Tylavsky FA, Geva D, Vardi H, Harris TB. Adherence to mediterranean diet and decline in walking speed over 8 years in community-dwelling older adults. J Am Geriatr Soc. 2012;60(10):1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stefler D, Hu Y, Malyutina S, Pajak A, Kubinova R, Peasey A, Pikhart H, Rodriguez-Artalejo F, Bobak M. Mediterranean diet and physical functioning trajectories in Eastern Europe: findings from the HAPIEE study. PLoS One. 2018;13(7):e0200460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Struijk EA, Guallar-Castillón P, Rodríguez-Artalejo F, López-García E. Mediterranean dietary patterns and impaired physical function in older adults. J Gerontol A Biol Sci Med Sci. 2018;73(3):333–9. [DOI] [PubMed] [Google Scholar]
- 51. Talegawkar SA, Bandinelli S, Bandeen-Roche K, Chen P, Milaneschi Y, Tanaka T, Semba RD, Guralnik JM, Ferrucci L. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr. 2012;142(12):2161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yokoyama Y, Nishi M, Murayama H, Amano H, Taniguchi Y, Nofuji Y, Narita M, Matsuo E, Seino S, Kawano Yet al. . Dietary variety and decline in lean mass and physical performance in community-dwelling older Japanese: a 4-year follow-up study. J Nutr Health Aging. 2017;21(1):11–6. [DOI] [PubMed] [Google Scholar]
- 53. Zhu J, Xiang YB, Cai H, Li H, Gao YT, Zheng W, Shu XO. A prospective investigation of dietary intake and functional impairments among the elderly. Am J Epidemiol. 2018;187(11):2372–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baker ME, DeCesare KN, Johnson A, Kress KS, Inman CL, Weiss EP. Short-term Mediterranean diet improves endurance exercise performance: a randomized-sequence crossover trial. J Am Coll Nutr. 2019;38(7):597–605. [DOI] [PubMed] [Google Scholar]
- 55. Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. 1995;62(1):30–9. [DOI] [PubMed] [Google Scholar]
- 56. Dipla K, Makri M, Zafeiridis A, Soulas D, Tsalouhidou S, Mougios V, Kellis S. An isoenergetic high-protein, moderate-fat diet does not compromise strength and fatigue during resistance exercise in women. Br J Nutr. 2008;100(2):283–6. [DOI] [PubMed] [Google Scholar]
- 57. Van Zant RS, Conway JM, Seale JL. A moderate carbohydrate and fat diet does not impair strength performance in moderately trained males. J Sports Med Phys Fitness. 2002;42(1):31–7. [PubMed] [Google Scholar]
- 58. Waijers PM, Feskens EJ, Ocke MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97(2):219–31. [DOI] [PubMed] [Google Scholar]
- 59. Zaragoza-Marti A, Cabanero-Martinez MJ, Hurtado-Sanchez JA, Laguna-Perez A, Ferrer-Cascales R. Evaluation of Mediterranean diet adherence scores: a systematic review. BMJ Open. 2018;8(2):e019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2020;120(12):1998–2031.e15. [DOI] [PubMed] [Google Scholar]
- 61. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VAeditors. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane;. 2021. [Internet]. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 62. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32(2):2–5. [PubMed] [Google Scholar]
- 63. Coelho-Junior HJ, Milano-Teixeira L, Rodrigues B, Bacurau R, Marzetti E, Uchida M. Relative protein intake and physical function in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10(9):1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rogers-Soeder TS, Peters KE, Lane NE, Shikany JM, Judd S, Langsetmo L, Hoffman AR, Evans WJ, Cawthon PM. Dietary intake, D3Cr muscle mass, and appendicular lean mass in a cohort of older men. J Gerontol A Biol Sci Med Sci. 2020;75(7):1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, Stefanick ML, Shikany JM, Strotmeyer ES, Glynn NWet al. . Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74(6):844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tapsell LC, Neale EP, Probst Y. Dietary patterns and cardiovascular disease: insights and challenges for considering food groups and nutrient sources. Curr Atheroscler Rep. 2019;21(3):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 68. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17(12):2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the Meddiet score. Prev Med. 2007;44(4):335–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data described in the manuscript will be made available upon request. Statistical code is available from Mr. Shanahan (e-mail: shanahan.christopher@gmail.com); data sets (study characteristics and risk-of-bias assessment) are available from Dr. Van Elswyk (e-mail: mveconsulting@q.com).