Abstract
Background
Reagent strip to detect microhematuria as a proxy for Schistosoma haematobium infections has been considered an alternative to urine filtration for individual diagnosis and community-based estimates of treatment needs for preventive chemotherapy. However, the diagnostic accuracy of reagent strip needs further investigation, particularly at low infection intensity levels.
Methods
We used existing data from a study conducted in Tanzania that employed urine filtration and reagent strip testing for S. haematobium in two villages, including a baseline and six follow-up surveys after praziquantel treatment representing a wide range of infection prevalence. We developed a Bayesian model linking individual S. haematobium egg count data based on urine filtration to reagent strip binary test results available on multiple days and estimated the relation between infection intensity and sensitivity of reagent strip. Furthermore, we simulated data from 3,000 hypothetical populations with varying mean infection intensity to infer on the relation between prevalence observed by urine filtration and the interpretation of reagent strip readings.
Principal findings
Reagent strip showed excellent sensitivity even for single measurement reaching 100% at around 15 eggs of S. haematobium per 10 ml of urine when traces on reagent strip were considered positive. The corresponding specificity was 97%. When traces were considered negative, the diagnostic accuracy of the reagent strip was equivalent to urine filtration data obtained on a single day. A 10% and 50% urine filtration prevalence based on a single day sampling corresponds to 11.2% and 48.6% prevalence by reagent strip, respectively, when traces were considered negative, and 17.6% and 57.7%, respectively, when traces were considered positive.
Conclusions/Significance
Trace results should be included in reagent strip readings when high sensitivity is required, but excluded when high specificity is needed. The observed prevalence of reagent strip results, when traces are considered negative, is a good proxy for prevalence estimates of S. haematobium infection by urine filtration on a single day.
Author summary
Control of schistosomiasis, a parasitic worm infection affecting more than 200 million people worldwide, relies mainly on mass drug administration of praziquantel to school-age children as well as adults in areas where the disease is particularly rampant. The World Health Organization has set thresholds of observed prevalence that require intervention to reach the goal of eliminating schistosomiasis as a public health problem by 2025. Intervention thresholds are defined based on parasitologic methods, which is urine filtration for Schistosoma haematobium. There are alternative diagnostic methods to detect S. haematobium, such as the detection of blood in urine that is a common symptom of urogenital schistosomiasis. We determined the diagnostic sensitivity and specificity of a reagent strip to detect microhematuria using data from two villages in Tanzania at multiple time points (once before and several times after treatment with the deworming drug praziquantel) and translate the urine filtration intervention thresholds to reagent strip equivalents. We show that the reagent strip including trace results is almost perfectly sensitive for infections above 15 eggs of S. haematobium per 10 ml of urine and that a 10% observed prevalence by urine filtration corresponds to 17.6% observed prevalence by reagent strip including traces.
Introduction
Schistosoma haematobium is the most prevalent of the schistosome species parasitizing humans, causing urogenital schistosomiasis if left untreated [1, 2]. More than 200 million individuals are infected with any species of Schistosoma that, collectively, cause a global burden of 2.1 million disability-adjusted life years (DALYs) [3–5]. While mortality is relatively low, there is considerable morbidity that might be manifested as anemia, growth stunting, impaired cognition, increased risk for cancer of the bladder, and HIV infections [6, 7].
There has been a surge in investment in the control of schistosomiasis as part of the Millennium Development Goals (MDGs) after 2000 and the Sustainable Development Goals (SDGs) from 2015 onwards [8, 9]. Reduction of disease burden is primarily achieved through preventive chemotherapy with the antischistosomal drug praziquantel [2, 10]. Treatment needs are commonly assessed with parasitologic methods [11–13]. In 2012, the World Health Organization (WHO) put forward a roadmap for elimination of schistosomiasis as a public health problem (i.e., prevalence of heavy infections under a threshold of 1%) and interruption of transmission in suitable settings by 2025 [14].
The WHO recommended diagnostic method for S. haematobium is urine filtration. A midday urine specimen is collected and, after vigorous shaking, 10 ml of urine are filtered and examined under a light microscope by experienced laboratory technicians [11, 15]. Alternative diagnostic techniques exist, which are often used in parallel on the same individual to increase diagnostic sensitivity. Blood in urine is a common symptom of urogenital schistosomiasis, although it is not fully specific to the disease [16]. As blood in urine is relatively easier to detect than S. haematobium eggs, three blood-based diagnostic tests are available; namely (i) a simple questionnaire regarding recent history of blood in the urine; (ii) inspection of a urine sample for visible blood; and (iii) a reagent strip for detection of microhematuria (detects visible as well as non-visible blood in urine) [17, 18]. Reagent strip for microhematuria allow for a semi-quantitative assessment of infection-intensity with four grades distinguishing the severity of an infection. Other diagnostic approaches include the detection of a specific antigen in urine and polymerase chain reaction (PCR)-based methods to detect genetic material in urine [19, 20].
In this study, we determined the infection intensity-dependent diagnostic accuracy of reagent strip to detect microhematuria and urine filtration for S. haematobium eggs. We considered repeated measurements obtained over consecutive days. Previous studies assessed the sensitivity and specificity of the aforementioned methods, but there is no study that models S. haematobium egg counts directly and takes into account day-to-day variation of infection intensity [15, 17, 21–24]. We extended our egg count model for individual-level data, previously developed for the analysis of Kato-Katz thick smears and a point-of-care circulating cathodic antigen (POC-CCA) urine cassette test for the diagnosis of S. mansoni. We developed a model to estimate ‘true’ prevalence and infection intensity-dependent sensitivity for urine filtration and reagent strip testing and determined the specificity of the latter diagnostic test [25, 26]. Additionaly, we employed a simulation to translate current WHO urine filtration intervention thresholds to microhematuria analogues based on reagent strip test results.
Methods
Ethics statement
All data included in this study have been published elsewhere [27]. Ethics approval and informed consent procedures are given in the aforementioned study from which the data have been extracted.
Data
The analysis was carried out using a readily available dataset from a study conducted in two villages in Tanzania in 1993. The study involved 533 school-age children (7–18 years) and included a baseline and six follow-up surveys (at 2, 4, 6, 12, 18, and 24 months) after an initial treatment with praziquantel just after the baseline survey. At each survey, urine samples were collected and subjected to urine filtration and reagent strip testing (Boehringer Mannheim; Mannheim, Germany). Urine filtration was performed with samples of 10 ml of urine using Nucleopore membranes with 12 μl pore filters. Readings of the reagent strip were done semi-quantitatively. The results of the reagent strip used here consist of 0 (negative), trace T (<5 red blood cells (RBC)/μl of urine), 1+ (5–10 RBC/μl of urine), 2+ (∼50 RBC/μl of urine), and 3+ (∼250 RBC/μl of urine) [27]. For each survey, efforts were made to collect urine specimens over five consecutive days (between 10:00 and 14:00 hours). The aim of the study was to characterize the evolution of S. haematobium pathology after a single dose of praziquantel (40 mg/kg). However, 52 children were re-treated after 18 months (i.e., 6 months before the last follow-up) because they had heavy infections (≥ 50 eggs/10 ml urine), macrohematuria, or major lesions of the urinary tract. Summary measures of the data, stratified by village at the seven time points, are presented in Table 1 and a comparison of prevalence based on test results with reagent strip versus urine filtration for each day at the different time points is given in Fig 1.
Table 1. Observed prevalence and mean infection intensity by urine filtration and prevalence by microhematuria using reagent strip based on the first sample for all individuals and based on individuals with complete data, stratified by village (Tanzania survey, 1993 [27]).
| Village | Survey | Urine filtration | Reagent strip | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 day all | 1 day complete | 1 day all | 1 day complete | ||||||||||
| N | Positive (%) | μ+ | N | Positive (%) | μ+ | N | Positive T+1 (%) | Positive T-2 (%) | N | Positive T+1 (%) | Positive T-2 (%) | ||
| A | Baseline | 310 | 56.5 | 203.4 | 152 | 56.6 | 187.5 | 310 | 59.4 | 53.2 | 152 | 59.2 | 53.3 |
| 2 months | 288 | 15.3 | 9.5 | 17 | 17.6 | 2.3 | 288 | 24.0 | 19.4 | 17 | 23.5 | 17.6 | |
| 4 months | 204 | 12.3 | 37.8 | 20 | 0 | 0 | 204 | 23.0 | 20.1 | 20 | 20.0 | 20.0 | |
| 6 months | 241 | 40.7 | 13.6 | 14 | 28.6 | 8.0 | 241 | 33.6 | 25.7 | 14 | 21.4 | 14.3 | |
| 12 months | 219 | 37.9 | 31.1 | 91 | 35.2 | 24.6 | 219 | 47.5 | 32.4 | 91 | 49.5 | 31.9 | |
| 18 months | 164 | 70.1 | 41.0 | 1 | 100 | 5.0 | 164 | 100 | 76.8 | 1 | 100 | 0 | |
| 24 months | 122 | 57.4 | 23.5 | 0 | - | - | 122 | 100 | 75.4 | 0 | - | - | |
| B | Baseline | 178 | 82.6 | 189.2 | 138 | 86.2 | 179.4 | 178 | 87.6 | 59.0 | 138 | 91.3 | 62.3 |
| 2 months | 163 | 15.3 | 14.4 | 13 | 7.7 | 1.0 | 163 | 42.3 | 23.3 | 13 | 15.4 | 7.7 | |
| 4 months | 129 | 10.9 | 6.1 | 10 | 0 | 0 | 129 | 17.1 | 9.3 | 10 | 10.0 | 0 | |
| 6 months | 172 | 23.8 | 10.4 | 14 | 0 | 0 | 172 | 25.0 | 16.3 | 14 | 0 | 0 | |
| 12 months | 26 | 46.2 | 38.7 | 0 | - | - | 26 | 100 | 61.5 | 0 | - | - | |
| 18 months | 103 | 79.6 | 153.2 | 1 | 100 | 6.0 | 103 | 100 | 79.6 | 1 | 100 | 100 | |
| 24 months | 69 | 69.6 | 60.3 | 0 | - | - | 69 | 100 | 71.0 | 0 | - | - | |
N is the number of individuals tested; μ+ is the mean number of eggs per 10 ml of urine in the positive individuals.
1 Trace results are regarded as positive.
2 Trace results are regarded as negative.
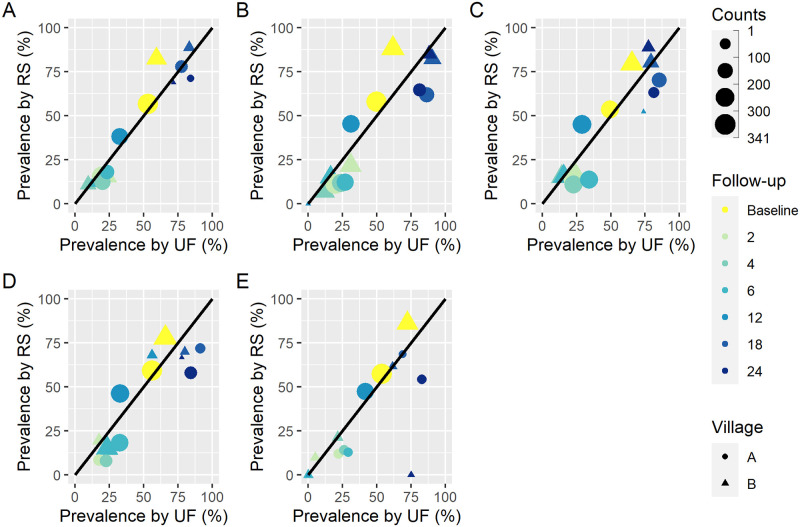
Fig 1. Comparison of prevalence by reagent strip (with trace negative) and urine filtration computed from raw data.
The dots and triangles represent the prevalence at baseline (before treatment) and follow-up (at 2, 4, 6, 12, 18, and 24 months post-treatment) for each study site and ‘Counts’ show the number of individuals tested. A shows the prevalence computed from individuals with complete data at day 1 regarding reagent strip and urine filtration tests. B, C, D, and E show the same for days 2, 3, 4, and 5, respectively.
S. haematobium egg count model
We assumed that the S. haematobium infection status and intensity of each individual was independent across follow-ups. For each individual, reagent strip (RS) data results were converted into 3 categorical variables with values T when the RS result was at least T (i.e., T/1+/2+/3+), 1 when the RS result was at least 1 (i.e., 1+/2+/3+), and 2 when the RS result was at least 2 (i.e., 2+/3+). The data consist of measurements over 5 consecutive days, and hence, each of the aforementioned variables were split into 5 binary variables corresponding to the z first days, z = 1 (i.e., first day of testing), 2 (i.e., first and second day), 3 (i.e., first 3 days), …, 5 (i.e., all 5 days) leading to 15 binary variables. Data from each cross-sectional time point and village were assumed to arise from a separate, independent population. As we have 7 cross-sectional time surveys corresponding to baseline and 6 follow-up surveys and 2 villages included in the study, we considered 14 independent populations. A joint Bayesian model was fitted separately for each binary RS variable with the corresponding urine filtration (UF) egg count. No diagnostic ‘gold’ standard was asummed. RS sensitivity for T,1,2 at z was related to infection intensity.
For each individual i in population j, j = 1, …, 14, let be the observed egg counts from urine filtration on day d, d = 1, 2, …, 5. The results from a semi-quantitative reagent strip for microhematuria were coded into 15 binary variables: describes all individuals with at least result trace, with at least result 1+, and 2+, respectively. The models were fitted separately for each of the 15 binary results , x = T, 1, 2, combined with all , where all individuals with both test results at a cross-sectional survey were included, with exception of a few individuals with multiple entries. We inferred on the sensitivity of repeated microhematuria measurements without having to model the correlation structure explicitly.
We assumed that each population consists of a proportion of infected individuals πj, where each individual had a disease status Dji and infection intensity λji. Infected individuals with Dji = 1 measurements on consecutive days were modeled as a negative binomial distribution with mean equal to the infection intensity λji and dispersion parameter depending on it. The infection intesities were assumed to be distributed as a gamma distribution as follows:
| (1) |
kji is the dispersion parameter of individual i in population j that depends on parameters k0 and k1, is the mean infection intensity of a positive individual in population j, and αj is the aggregation parameter that describes the aggregation of infection intensities in population j. Individuals with infection status Dji = 0 have observations , which is equivalent to 100% specificity. The sensitivity of urine filtration sjin for individual i in population j after n days was calculated using the probability of repeated zero measurements under a negative binomial model, that is , where .
Microhematuria measurements were modeled by a Bernoulli distribution,
| (2) |
A parametric model was used to relate infection intensity with diagnostic sensitivity, where sRS,x,z is the infection intensity-dependent sensitivity defined by four parameters. sets the sensitivity for infections approaching to zero intensity, describes the rate of increase with infection intensity, changes the shape of the curve, and limits the maximum sensitivity for very severe infections. cRS,x,z is the specificity of the microhematuria reagent strip, that is the probability of no positive result for an uninfected individual where positive is defined as having at least once a value of x after z observations.
Models were also run with sensitivity and specificity parameters of the reagent strip stratified by sex. The model was formulated using a Bayesian paradigm. We chose a uniform prior for πj, a gamma distribution with mean 100 and standard deviation (SD) 100 for the mean infection intensity , a gamma distribution with mean 1 and SD 1 for the population variation αj, a beta distribution with parameters 10 and 1 for cRS, and normal distributions with mean 0 and SD 2 for k0 and k1. For a0 a normal distribution with mean -1 and SD 1.5 was chosen, a gamma distribution with shape and scale parameters 5 and 30 for a1, a normal distribution with mean 10 and SD 10 for a2, and a beta distribution with parameters 10 and 1 for a3 to ensure a non-informative distribution of sensitivity curves in the relevant range of infection intensities. Priors for the sensitivity of the reagent strip were chosen using prior predictive checks to ensure non-informativity [28]. Other parameters have semi informative prior distributions over sensible parameter values. Inference was done using Markov chain Monte Carlo (MCMC) simulation. Model fit was carried out in Stan version 1.2.1335 (Stan Development Team; mc-stan.org), running 20 chains for 2,000 iterations of which the first 500 were discarded [29]. There were no divergent transitions, and convergence was assessed using the Gelman-Rubin diagnostics as well as visual inspection of chains [30].
Simulation
To assess the relation between prevalence observed by microhematuria and urine filtration for one, two, three, four, and five days, we run extensive simulations of hypothetical populations in diverse transmission settings. We assumed that worms are negative binomially distributed in the population with worm aggregation parameter wagg and that a proportion of 30% of the worms are female [31, 32]. The mean number of eggs per 10 ml of urine per female worm was selected from a publication of Truscott et al. who estimated it to be 5.2 [33]. For the parameters a0, a1, a2, a3, k0, and k1, 100 draws were taken directly from the posterior distribution and thereby correlation between parameters was incorporated in the simulation. The mean number of worms per individual in a population was varied in 40 equal steps on the log-scale from 1 to 200. We used wagg according to a normal distribution with a mean of 0.2 and a SD of 0.03. For each hypothetical population, worm load for 6,000 individuals and corresponding urine filtration and reagent strip results were simulated for all five days.
Results
Descriptive data analysis
Prevalence by a single urine filtration ranged from 10.9% to 82.6%, while the cumulative prevalence over 5 consecutive days ranged from 20.9% to 98.7%. The mean infection intensity in the study population at the different time points ranged from 0.4 to 107 eggs/10 ml of urine, while the corresponding range in positive individuals was 1.8 to 116.6 eggs/10 ml. Reagent strip prevalence ranged from 17.1% to 100% when traces were considered positive and from 9.3% to 84.2% when traces were considered negative based on observations from a single day. Use of reagent strip over 5 consecutive days increased the observed prevalence from 33.9% to 100% when traces were considered positive and from 22.8% to 89.3% when traces were considered negative.
Sensitivity of urine filtration
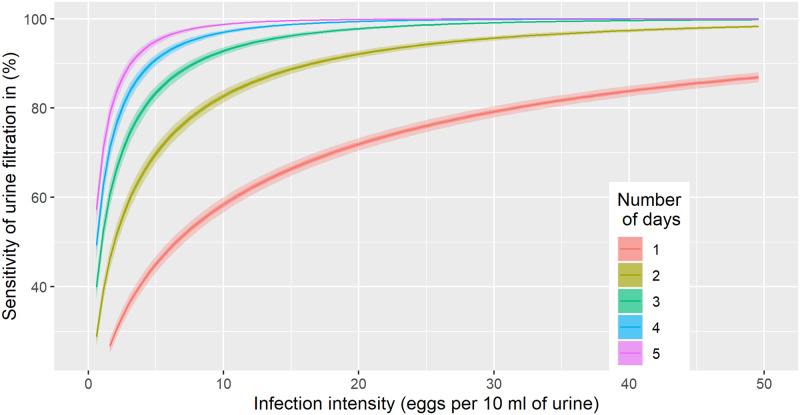
Using the egg count model, we estimated the sensitivity of the urine filtration from a single sample to a total of five samples over consecutive days. The posterior medians for parameters k0 and k1, which define the relation between the sensitivity and mean infection intensity and were estimated at -2.3 and 0.4, respectively. The influence of the choice of RS, x, z on estimates of k0 and k1 was small (see S1 Text). The aggregation parameter of the negative binomial distribution increased from 0.15 to 0.48 for infection intensity increasing from 0 to 50 eggs/10 ml of urine, which corresponds to a reduction in overdispersion when the infection intensity increases. This is equivalent to a SD of 20 at a density of 10 eggs/10 ml of urine, 73 at a density of 50 eggs/10 ml of urine, and 3.3 at a density of 1 egg/10 ml of urine. The posterior distributions of k0 and k1 with the probability for repeated zeros under the negative binomial model enabled us to estimate the sensitivity of urine filtration. In Fig 2, we present the estimated sensitivity of urine filtration cumulatively for one to five days (i.e., single versus multiple tests) as a function of infection intensity.
Fig 2. Sensitivity estimates of urine filtration (10 ml of urine) from a single to a total of five samples collected over consecutive days.
Curves indicate posterior medians, while dark shaded areas provide a 50% and light shaded areas a 95% Bayesian credible interval.
A single urine filtration had a sensitivity of above 85% for heavy infections (≥50 eggs/10 ml of urine), while the sensitivity was below 50% at around 7 eggs/10 ml of urine. The sensitivity showed a considerable increase as a function of repeated urine filtration. For example, the sensitivity increased from 50% to 75% when comparing a single with a duplicate urine filtration at a low infection intensity of 7 eggs/10 ml of urine. After five days of urine filtration, an average infection with 1 egg/10 ml of urine showed a probability of around 60% to be detected.
Sensitivity of reagent strip
The model was run for each interpretation of the reagent strip RS, x, z separately, where x is either T for trace, 1, or 2, and z the total number of samples from 1 to 5 (i.e., the test is positive when at least on one of the first z days a value of at least x in the semi-quantitative interpretation of the reagent strip is reported). The parameters a0, a1, a2, a3, and c fully define the diagnostic accuracy of each RS, x, z. The specificity c for each interpretation is summarized in Table 2. The specificity is estimated to basically 100% even after multiple days when only 2+ and 3+ test readings were considered positive while, when trace results were considered positive, the specificity of even a single sample is 97% and only 87% after five days.
Table 2. Specificity of each interpretation of the reagent strip RS, x, z defined as a result of at least x (x being T for trace, 1, or 2), on the first z days.
Values are given between 0 and 1, where 1 corresponds to zero probability of a false-positive, values in brackets represent the 95% Bayesian credible interval.
| Reading | RS,x,1 | RS,x,2 | RS,x,3 | RS,x,4 | RS,x,5 |
|---|---|---|---|---|---|
| x = T | 0.97 (0.92–1.00) | 0.93 (0.86–0.99) | 0.92 (0.85–0.99) | 0.89 (0.82–0.98) | 0.87 (0.80–0.96) |
| x = 1 | 0.99 (0.96–1.00) | 0.99 (0.97–1.00) | 0.99 (0.96–1.00) | 0.98 (0.94–1.00) | 0.97 (0.92–1.00) |
| x = 2 | 1.00 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.97–1.00) | 0.99 (0.97–1.00) | 0.99 (0.96–1.00) |
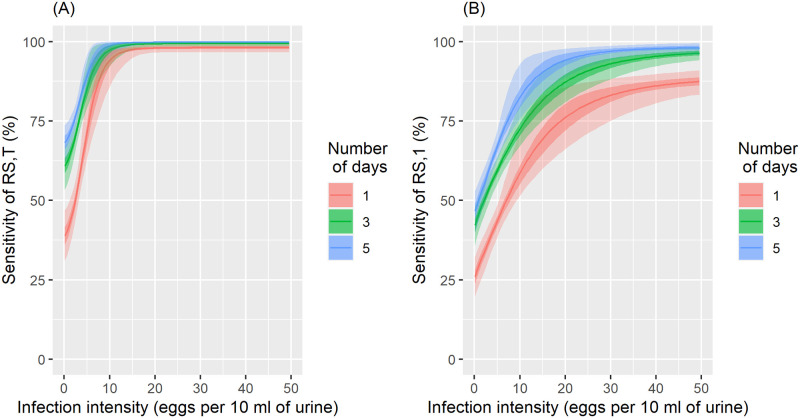
The parameters a0, a1, a2, and a3 are reported for all 15 interpretations (S1 Text). Combined with Eq 2, these parameters estimate the infection intensity-dependent sensitivity of the reagent strip for microhematuria to diagnose S. haematobium. Fig 3A and 3B show the sensitivity of the reagent strip when traces were considered positive or negative, for cumulative results from one, three, and five days. When traces were considered positive, a single reagent strip detected basically all infections with an intensity above 15 eggs/10 ml of urine, and still 40% of infections with only 1 egg/10 ml of urine. Repeating the test over consecutive days increased the sensitivity, for example from 40% to 70% for very light infections after 5 consecutive days, but this increase was modest when compared to urine filtration.
Fig 3. Sensitivity of a reagent strip (RS) in relation to infection intensity for measurements from a single to a total of 3 and 5 cumulative tests.
Trace results were included in the positives (A) and trace results were included in the negatives (B). Dark shaded areas are the 50% and light shaded areas the 95% Bayesian credible interval.
Considering traces as negative resulted in higher specificity of 99% for a single reagent strip and still 97% after five samples. When traces were considered positive, the specificity after five samples was reduced to 87%, however, the sensitivity was higher compared to traces considered negative. A single reagent strip only has a 60% chance to detect an infection of 10 eggs/10 ml of urine and still less than 90% for heavy infections (≥50 eggs/10 ml of urine). Repeated sampling over 5 consecutive days increased the sensitivity up to 80% at 10 eggs/10 ml of urine compared to a sensitivity of almost 100% at the same intensity when traces were considered positive. The sensitivity of a single reagent strip when traces were considered negative is similar to a single urine filtration regardless of the level of infection intensity. Stratification by sex did not show any difference in parameter estimates that would indicate an important interaction, for example with menstruation.
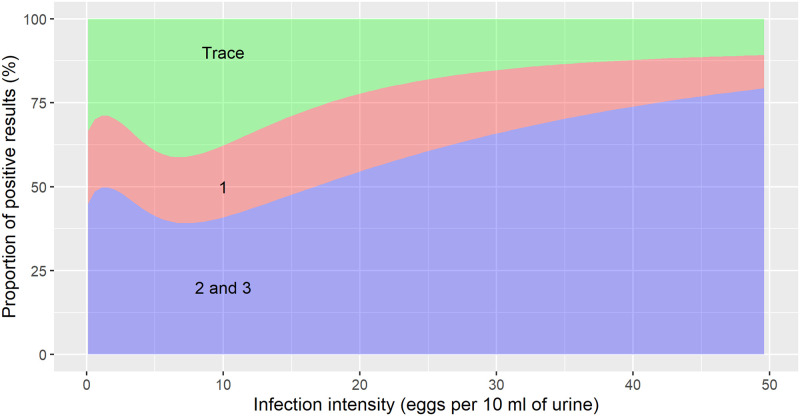
The semi-quantitative results of the reagent strip were closely correlated with the infection intensity of an individual. The proportion of trace, 1+, and >1+ results for infection intensities up to 50 eggs/10 ml of urine are shown in Fig 4. The non-monotonic behavior close to an infection intensity of zero is due to increased uncertainty in the sensitivity estimates (not shown in the plot, but visible in Fig 3A and 3B). At very low infection intensities, there was a considerable probability (∼40%) for readings of 2+ or 3+, while at 50 eggs/10 ml of urine almost 80% of tests showed a 2+ or 3+. Trace results, on the other hand, decreased from a proportion of about 30% to around 10% at 50 eggs/10 ml of urine, while the proportion of samples with reading 1+ remained relatively constant.
Fig 4. Proportion of semi-quantitative results of reagent strip for microhematuria in relation to S. haematobium infection intensity.
The 2+ and 3+ readings were grouped together.
Relation between the observed prevalence by urine filtration and reagent strip
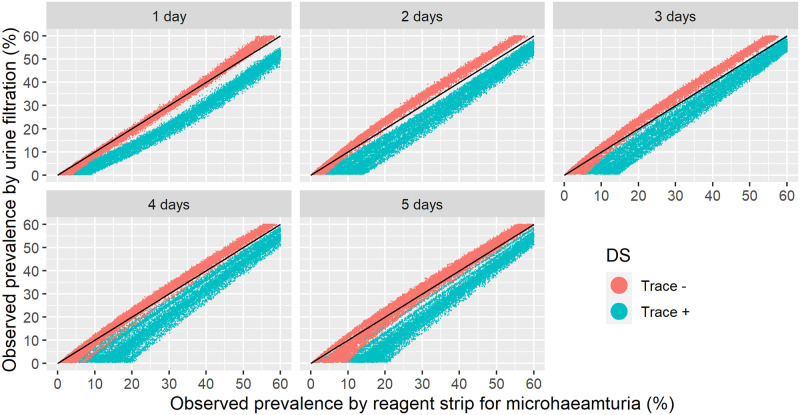
We conducted extensive simulations of 3,000 hypothetical populations with 6,000 individuals each and generated observations of the two diagnostic tests over 5 consecutive days based on the individual-level results from the egg count model detailed above. Fig 5 depicts the relation between the observed prevalences by reagent strip and urine filtration for up to five samples collected over consecutive days. Traces were treated either as positive or negative. Measurements based on a single reagent strip reading showed equal or higher observed prevalence than urine filtration. When all five samples were considered, the prevalence was higher by urine filtration compared to reagent strip when traces were considered negative. For the sampling scheme recommended by WHO, i.e., single day sample, and reagent strip without trace results, the observed prevalence by both tests are similar, independent of the setting.
Fig 5. Simulated relation between observed prevalence of S. haematobium by urine filtration and by reagent strip based on one to five urine samples collected over consecutive days with traces considered either positive or negative.
The black line indicates equivalence between urine filtration and reagent strip results.
We translated WHO prevalence thresholds from urine filtration into microhematuria by taking all simulated populations with observed prevalence by urine filtration within a narrow interval of ±0.5% around the threshold and calculating the mean of the observed prevalence by reagent strip and the corresponding Bayesian credible intervals. Table 3 shows results for three thresholds of urine filtration, 10%, 25%, and 50%, and the reagent strip when traces were considered positive or negative, for sampling schemes based on a single up to five urine samples over consecutive days. Most relevant for evaluating treatment needs are results for sampling on a single day. When traces were considered negative, the prevalence thresholds for microhematuria were very close to urine filtration thresholds. Including traces in the positive required upwards adjustment of the thresholds, for example a 10% urine filtration corresponds to about 20% prevalence by reagent strip.
Table 3. Translation of prevalence thresholds from urine filtration (UF) into reagent strip (RS) for the diagnosis of S. haematobium for sampling schemes varying from one to five urine samples over consecutive days when traces were considered either positive or negative.
All numerical values are percentages and the brackets contain 95% percentiles from the simulation.
| RS | UF (%) | 1 day | 2 days | 3 days | 4 days | 5 days |
|---|---|---|---|---|---|---|
| Traces + |
10 | 17.6 (13.4–21.8) | 16.5 (10.7–22.3) | 19.2 (12.6–25.8) | 19.4 (15.9–22.9) | 21.4 (14.4–28.4) |
| 25 | 35.0 (31.6–38.5) | 31.3 (25.7–36.8) | 32.1 (27.8–36.5) | 32.9 (29.2–36.6) | 34.2 (28.1–40.3) | |
| 50 | 57.7 (55.2–60.3) | 54.8 (51.4–58.3) | 54.2 (50.5–57.9) | 55.6 (53.3–57.9) | 56.2 (51.8–60.5) | |
| Traces - |
10 | 11.2 (9.0–13.3) | 8.5 (7.2–9.8) | 8.9 (6.9–11.0) | 9.1 (6.9–11.3) | 11.7 (7.7–15.6) |
| 25 | 25.7 (23.1–28.4) | 21.5 (19.7–23.3) | 21.4 (19.1–23.3) | 22.7 (20.1–25.4) | 24.0 (19.7–28.3) | |
| 50 | 48.6 (46.6–50.6) | 46.0 (44.4–47.6) | 45.8 (449–47.6) | 46.9 (44.5–49.3) | 48.4 (45.5–51.3) |
Discussion
We determined the diagnostic accuracy of a reagent strip used for detection of microhematuria (a proxy for S. haematobium infection) from survey data where participants were subjected simultaneously to urine filtration and reagent strip testing, using a Bayesian egg count model. The study was conducted in the frame of a treatment intervention with a 24-month follow-up post-praziquantel intervention, thus characterized by different infection intensity. The semi-quantitative results of the reagent strip were incorporated into the modeling by determining the sensitivity and specificity profiles, when trace results of the reagent strip were considered either positive or negative, and when only the 2+ and 3+ readings were considered as positive. As the reagent strip results can be influenced by menstruation in females, stratification by sex was also done. Furthermore, we related the observed prevalence by the two tests and translated the urine filtration treatment prevalence thresholds recommended by WHO into thresholds by reagent strip. We analyzed the data cross-sectionally because the longitudinal nature captures the variation in the infection intensity, which is taken into account in the modeling by assuming that the sensitivity parameters of the two tests depend on the infection intensity.
For individual-level diagnosis, the reagent strip showed almost perfect sensitivity when infection intensity was above 15 eggs/10 ml of urine, while specificity was high (97%) for a single urine sample when traces were included in the positives. When traces were excluded, a much more similar profile to urine filtration was created across all infection intensities. A key difference between microhematuria and urine filtration is that results are more correlated across samples for microhematuria. This means that for urine filtration, an infection might be missed if tested only once, but captured by an additional urine filtration, whereas for microhematuria repeated tests will more likely reveal the same result leading to less improvement in sensitivity by repeating measurements. It is conceivable that the presence of blood in urine is less variable than the egg excretion by parasitic worms in the case where few worm-pairs are present as the eggs also may remain trapped in the walls of the urinary tract. The reduction in the overdispersion of the negative binomial distribution of egg outputs with increasing infection intensity supports arguments that in the presence of a larger number of worms, the day-to-day variations are low due to averaging. We did not observe a sex difference, which might be explained by the young age of the participants (7–18 years, 58% between 7 and 12 years) although a proportion of the females must have experienced menarche. Yet, the menstruation would have to have occurred at the same time as urine samples were collected and subjected to reagent strip testing to have an influence.
Point estimates of urine filtration and reagent strip sensitivity as well as specificity from other studies are difficult to compare to the results presented here because previous studies did not take into account the relation to infection intensity and they are averaged over the population. Hence, findings are setting specific. Urine filtration is considered the ‘gold’ standard for S. haematobium diagnosis [34]. However, results depend on the sampling scheme, as collection of multiple urine sample increases sensitivity. For example, a study conducted by Kosinski et al. (2011) reported a sensitivity of reagent strip using a single urine sample of about 50%, while the corresponding sensitivity for duplicate or triplicate reagent strip were 60% and 70%, respectively. For urine filtration, these estimates were 50%, 80%, and 100%, respectively [23]. Especially in low-endemic settings, a single urine filtration can lead to low sensitivity. Specificity of reagent strip testing decreases from 93% for single, to 88% for two, and 83% for three urine samples. These results are generally in agreement with our estimates, especially considering that those values over-estimate the diagnostic error because of the definition of urine filtration as ‘gold’ standard. Day-to-day variation in egg output is considerable, rendering a stable classification of infection intensity into severity classes difficult. Our study revealed that the semi-quantitative results derived from reagent strip testing are correlated with infection intensity, as determined by urine filtration. Variation in microhematuria seems to be lower, indicating that classification based on a reagent strip might be more stable and more insightful.
Our results were derived from the analysis of a study conducted in two villages in Tanzania in 1993 using a reagent strip produced by Boehringer Mannheim [27], a company that was bought and incorporated into Roche in 1997. It is unclear how representative the results are for other reagent strip tests currently on the market. For example, a study carried out in Kenya utilized a reagent strip called Hemastix (Ames, Bie and Bernsten; Copenhagen, Denmark). Mafe (1997) and Kahama et al. (1999), in studies carried out in Nigeria, also used Hemastix [17, 35]. More recently Kosinski et al. (2011) evaluated a semi-quantitative reagent strip called U-11 Urinalysis Reagent Strip (Mindray Co. Ltd.; Shenzhen, China). However, this test seems to have been on the market before; indeed, it has been evaluated by Mott et al. in 1985 [23, 36]. More data should be incorporated to study potential differences between reagent strips from different companies or from the same company over time. Some of the most recent data have been collected in the frame of large-scale preventive chemotherapy programs, which influence microhematuria following repeated praziquantel administration. Hence, this is an important confounder that should be included into future modeling studies.
On the population level, we see a clear relation between the observed prevalence by urine filtration and reagent strip. The size of the 3,000 simulated populations was fixed at 6,000 to be able to observe the influence of uncertainty in diagnostic accuracy, while limiting the influence of sampling error. Important assumptions in the simulation model were primarily the negative binomial distribution of worms in the population and the aggregation parameter that was considered to be independent of infection intensity. The latter may not hold because it has been assessed for hookworm infections, showing that the aggregation parameter increases with infection intensity [37]. In accordance with recommendations put forward by WHO and the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) for estimation of S. haematobium prevalence from a single day [14, 38], we recommend translating urine filtration thresholds of 10%, 25%, and 50% into 10%, 24%, and 48% when a single reagent strip is employed, considering traces as negative. Trace positive individuals should, however, also be treated with praziquantel, as it might indicate a very light infection with S. haematobium that might cause subtle morbidity [39, 40]. The reagent strip with traces considered negative serves as a convenient proxy to estimate prevalence almost equivalent to single-day urine filtration. If national control programs would make use of reagent strip instead of urine filtration, the costs could be reduced substantially and proceedings could be accelerated allowing more children to get tested.
Our study has several limitations that are offered for consideration. First, the data stem from a single type of reagent strip from a survey carried out in two villages of Tanzania almost 30 years ago with an age of participants ranging between 7 and 18 years. Importantly though, urine samples were obtained at multiple time points over the course of 24 months after a single oral dose of praziquantel. It is imperative to validate the results with additional data, for example using the Hemastix and U-11 reagent strips, particularly in settings where S. haematobium is close to elimination. Second, the simulation depends on the assumption of negative binomial distribution of worms in the population with a constant aggregation parameter, which is likely a good approximation at higher mean worm counts, but cannot be extrapolated to low prevalences below 10% observed urine filtration prevalence. This is reflected in our results making no recommendations for translation of lower thresholds. Third, it was not uncommon to have individuals where only one diagnostic test was performed on a specific sampling time point, and hence, observed prevalence by reagent strip and urine filtration at the same time point and village are not directly comparable.
Regarding the ethics of the analyzed study, the principal investigator emphasized that the children were treated when they were positive upon enrollment and then followed up for 24 months without treatment unless there were important symptoms. All infected children were treated at the end of the study, knowing that their potential or proven morbidity could be cleared with it. Based on previous experience, the study conductors could assume that within the 24 months, no serious morbidity that could not be reversed by treatment will occur, suggesting that such a study is ethically justifiable. The analyzed study was pivotal in defining the intervals possible for treatment and retreatment schemes that otherwise could not have been established for future actions.
Conclusion
Reagent strip testing for microhematuria might be appropiate to test for S. haematobium infection, as it showed results with a high sensitivity and a high specificity. Indeed, at infection intensities above 15 eggs/10 ml of urine, sensitivity is close to 100%, while still maintaining a high specificity above 97%. When higher specificity is required, traces can be considered as negative to exclude the majority of false-positives, which lead to a sensitivity almost equal to a single sample urine filtration, therefore enabling direct translation of observed prevalence in the population. Further research is still warranted with additional data, particularly in settings where the prevalence of S. haematobium is low and infection intensity below 50 eggs/10 ml of urine.
Supporting information
(PDF)
(PDF)
(DOCX)
(CSV)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This study received financial support from the European Research Council, ERC-2012-AdG-323180 (PV) and the Schistosomiasis Consortium for Operational Research and Evaluation, SCORE (JU). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018;4:13. doi: 10.1038/s41572-018-0013-8 [DOI] [PubMed] [Google Scholar]
- 3. Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, et al. The Global Burden of Disease Study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865. doi: 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdulamir AS, Hafidh RR, Kadhim HS, Abubakar F. Tumor markers of bladder cancer: the schistosomiasis bladder tumors. J Exp Clin Cancer Res. 2009;28:27. doi: 10.1186/1756-9966-28-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kjetland EF, Hegertun IE, Baay MF, Onsrud M, Ndhlovu PD, Taylor M. Genital schistosomiasis and its unacknowledged role on HIV transmission in the STD intervention studies. Int J STD AIDS. 2014;25:1060–1064. doi: 10.1177/0956462414523743 [DOI] [PubMed] [Google Scholar]
- 8. Molyneux D. “Neglected” diseases but unrecognised successes—challenges and opportunities for infectious disease control. Lancet. 2004;364:380–383. doi: 10.1016/S0140-6736(04)16728-7 [DOI] [PubMed] [Google Scholar]
- 9. Molyneux D, Savioli L, Engels D. Neglected tropical diseases: progress towards addressing the chronic pandemic. Lancet. 2017;389:312–325. doi: 10.1016/S0140-6736(16)30171-4 [DOI] [PubMed] [Google Scholar]
- 10. WHO. Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2018. Wkly Epidemiol Rec. 2019;94:601–612. [Google Scholar]
- 11. Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization; 1998. [Google Scholar]
- 12.WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organ Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 13. Lai YS, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 2015;15:927–940. doi: 10.1016/S1473-3099(15)00066-3 [DOI] [PubMed] [Google Scholar]
- 14.WHO. Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. Geneva: World Health Organization; 2013.
- 15. Knopp S, Ame SM, Hattendorf J, Ali SM, Khamis IS, Bakar F, et al. Urogenital schistosomiasis elimination in Zanzibar: accuracy of urine filtration and haematuria reagent strips for diagnosing light intensity Schistosoma haematobium infections. Parasit Vectors. 2018;11:552. doi: 10.1186/s13071-018-3136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krauth SJ, Greter H, Stete K, Coulibaly JT, Traoré SI, Ngandolo BNR, et al. All that is blood is not schistosomiasis: experiences with reagent strip testing for urogenital schistosomiasis with special consideration to very-low prevalence settings. Parasit Vectors. 2015;8:584. doi: 10.1186/s13071-015-1165-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mafe MA. The diagnostic potential of three indirect tests for urinary schistosomiasis in Nigeria. Acta Trop. 1997;68:277–284. doi: 10.1016/S0001-706X(97)00102-2 [DOI] [PubMed] [Google Scholar]
- 18. Lengeler C, Makwala J, Ngimbi D, Utzinger J. Simple school questionnaire can map both Schistosoma mansoni and Schistosoma haematobium in the Democratic Republic of Congo. Acta Trop. 2000;74:77–87. doi: 10.1016/S0001-706X(99)00046-7 [DOI] [PubMed] [Google Scholar]
- 19. Knopp S, Corstjens PLAM, Koukounari A, Cercamondi CI, Ame SM, Ali SM, et al. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis. 2015;9:e0003752. doi: 10.1371/journal.pntd.0003752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiff C. Accurate diagnostics for schistosomiasis: a new role for PCR. Rep Parasitol. 2015;4:23–29. doi: 10.2147/RIP.S74319 [DOI] [Google Scholar]
- 21. Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–633. doi: 10.1179/136485908X337490 [DOI] [PubMed] [Google Scholar]
- 22. Midzi N, Butterworth AE, Mduluza T, Munyati SM, Deelder AM, van Dam G. Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis. Trans R Soc Trop Med Hyg. 2009;103:45–51. doi: 10.1016/j.trstmh.2008.08.018 [DOI] [PubMed] [Google Scholar]
- 23. Kosinski KC, Bosompem K, Stadecker MJ, Wagner AD, Plummer J, Durant JL, et al. Diagnostic accuracy of urine filtration and dipstick tests for Schistosoma haematobium infection in a lightly infected population of Ghanaian schoolchildren. Acta Trop. 2011;118:123–127. doi: 10.1016/j.actatropica.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 24. Stete K, Krauth SJ, Coulibaly JT, Knopp S, Hattendorf J, Müller I, et al. Dynamics of Schistosoma haematobium egg output and associated infection parameters following treatment with praziquantel in school-aged children. Parasit Vectors. 2012;5:298. doi: 10.1186/1756-3305-5-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bärenbold O, Raso G, Coulibaly JT, N’Goran EK, Utzinger J, Vounatsou P. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl Trop Dis. 2017;11:e0005953. doi: 10.1371/journal.pntd.0005953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bärenbold O, Garba A, Colley DG, Fleming FM, Haggag AA, Ramzy RMR, et al. Translating preventive chemotherapy prevalence thresholds for Schistosoma mansoni from the Kato-Katz technique into the point-of-care circulating cathodic antigen diagnostic test. PLoS Negl Trop Dis. 2018;12:e0006941. doi: 10.1371/journal.pntd.0006941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hatz C, Vennervald BJ, Nkulila T, Vounatsou P, Kombe Y, Mayombana C, et al. Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg. 1998;59:775–781. doi: 10.4269/ajtmh.1998.59.775 [DOI] [PubMed] [Google Scholar]
- 28. Grinsztajn L, Semenova E, Margossian CC, Riou J. Bayesian workflow for disease transmission modeling in Stan. Stat Med. 2021;40:6209–6234. doi: 10.1002/sim.9164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carpenter B, Gelman A, Hoffman M, Lee D, Goodrich B, Betancourt M, et al. Stan: a probabilistic programming language. J Stat Softw. 2017;76:1–32. doi: 10.18637/jss.v076.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–511. doi: 10.1214/ss/1177011136 [DOI] [Google Scholar]
- 31. Anderson RM. The population dynamics and epidemiology of intestinal nematode infections. Trans R Soc Trop Med Hyg. 1986;80:686–696. doi: 10.1016/0035-9203(86)90367-6 [DOI] [PubMed] [Google Scholar]
- 32. May RM, Woolhouse MEJ. Biased sex ratios and parasite mating probabilities. Parasitology. 1993;107:287–295. doi: 10.1017/S0031182000079269 [DOI] [PubMed] [Google Scholar]
- 33. Truscott JE, Gurarie D, Alsallaq R, Toor J, Yoon N, Farrell SH, et al. A comparison of two mathematical models of the impact of mass drug administration on the transmission and control of schistosomiasis. Epidemics. 2017;18:29–37. doi: 10.1016/j.epidem.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stothard JR, Stanton MC, Bustinduy AL, Sousa-Figueiredo JC, van Dam GJ, Betson M, et al. Diagnostics for schistosomiasis in Africa and Arabia: a review of present options in control and future needs for elimination. Parasitology. 2014;141:1947–1961. doi: 10.1017/S0031182014001152 [DOI] [PubMed] [Google Scholar]
- 35. Kahama AI, Odek AE, Kihara RW, Vennervald BJ, Kombe Y, Nkulila T, et al. Urine circulating soluble egg antigen in relation to egg counts, hematuria, and urinary tract pathology before and after treatment in children infected with Schistosoma haematobium in Kenya. Am J Trop Med Hyg. 1999;61:215–219. doi: 10.4269/ajtmh.1999.61.215 [DOI] [PubMed] [Google Scholar]
- 36. Mott KE, Dixon H, Osei-Tutu E, England EC, Ekue K, Tekle A. Evaluation of reagent strips in urine tests for detection of Schistosoma haematobium infection: a comparative study in Ghana and Zambia. Bull World Health Organ. 1985;63:125–133. [PMC free article] [PubMed] [Google Scholar]
- 37. Truscott J, Ower A, Werkman M, Halliday K, Oswald WE, Gichuki PM, et al. Heterogeneity in transmission parameters of hookworm infection within the baseline data from the TUMIKIA study in Kenya. Parasit Vectors. 2019;12:442. doi: 10.1186/s13071-019-3686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King CH, Bertsch D, Andrade GN, Burnim M, Ezeamama AE, Binder S, et al. The Schistosomiasis Consortium for Operational Research and Evaluation rapid answers project: systematic reviews and meta-analysis to provide policy recommendations based on available evidence. Am J Trop Med Hyg. 2020;103(1 Suppl):92–96. doi: 10.4269/ajtmh.19-0806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis—why it matters now. Trends Parasitol. 2006;22:575–582. doi: 10.1016/j.pt.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 40. King CH, Bertsch D. Meta-analysis of urine heme dipstick diagnosis of Schistosoma haematobium infection, including low-prevalence and previously-treated populations. PLoS Negl Trop Dis. 2013;7:e2431. doi: 10.1371/journal.pntd.0002431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(DOCX)
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.