ABSTRACT
Ulipristal acetate (UPA) is a medical treatment for uterine fibroids and was authorized for surgical pre-treatment in 2012 after the conduct of the PEARL I and II randomized controlled trials and for intermittent treatment after the observational PEARL III and IV trials. However, UPA came into disrepute due to its temporary suspension in 2017 and 2020 because of an apparent association with liver injury. This clinical opinion paper aims to review the process of marketing authorization and implementation of UPA, in order to provide all involved stakeholders with recommendations for the introduction of future drugs. Before marketing authorization, the European Medicines Agency (EMA) states that Phase III registration trials should evaluate relevant outcomes in a representative population, while comparing to gold-standard treatment. This review shows that the representativeness of the study populations in all PEARL trials was limited, surgical outcomes were not evaluated and intermittent treatment was assessed without comparative groups. Implementation into clinical practice was extensive, with 900 000 prescribed treatment cycles in 5 years in Europe and Canada combined. Extremely high costs are involved in developing and evaluating pre-marketing studies in new drugs, influencing trial design and relevance of chosen outcomes, thereby impeding clinical applicability. It is vitally important that the marketing implementation after authorization is regulated in such way that necessary evidence is generated before widespread prescription of a new drug. All stakeholders, from pharmaceutical companies to authorizing bodies, governmental funding bodies and medical professionals should be aware of their role and take responsibility for their part in this process.
Keywords: ulipristal acetate, leiomyoma, randomized controlled trials, clinical trials, Phase III, risk evaluation and mitigation
Introduction
Uterine fibroids are highly prevalent and cause symptoms that inversely influence quality of life (QoL) (Baird et al., 2003). Although often asymptomatic, about 25–30% of the women of reproductive age experience complaints depending on their number, volume and location in the uterus, varying from abnormal bleeding and pressure discomfort to fertility and pregnancy issues. Of the clinically apparent fibroids, about 25% causes symptoms so severe that they require treatment (Stewart et al., 2017; Herve et al., 2018). These symptoms can adversely influence women’s QoL (Downes et al., 2010). When conservative treatment fails or is not desired, uterine artery embolization (UAE), or surgery such as myomectomy or hysterectomy, can be offered. To facilitate surgery such as myomectomy or hysterectomy, pre-treatment with parenteral GnRH agonists (GnRHa) can decrease fibroid volume and stop menstrual bleeding (Stewart, 2001).
Another pharmacological treatment option for symptomatic fibroids is ulipristal acetate (UPA). UPA was authorized for pre-treatment of symptomatic fibroids in 2012 and for intermittent treatment in 2015. UPA is a selective progesterone receptor modulator which binds to the progesterone receptors in the myometrium, endometrium and fibroid tissue, and inhibits ovulation without affecting the anti-glucocorticoid activity and oestradiol levels. It also has a direct anti-proliferative and pro-apoptotic effects on fibroid cells through the progesterone receptor, enabling volume reduction (Donnez and Dolmans, 2016). Marketing authorization for UPA was granted in July 2012 and February 2013 in Europe and Canada, respectively, stating UPA to be indicated for pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age, with the treatment duration limited to 3 months (CHMP, 2011; Middelkoop et al., 2020). In 2015, extension of the indication for UPA to intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age was granted both by the European Medicines Agency (EMA) as well as by the Canadian Drug Expert Committee Recommendation (CADTH), leading to 900 000 prescribed treatment cycles in the 5 years (CHMP, 2015; CADTH, 2017; EMA, 2020a,b,c).
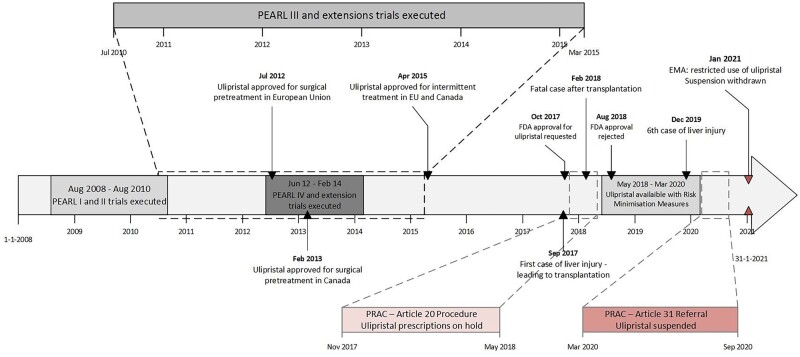
UPA’s popularity came to a sudden halt in September 2017 when UPA was thought to have caused a possible drug-induced liver injury (DILI), leading to liver transplantation in a woman using the first treatment course of UPA. This led to two subsequent investigations in 2018 and 2020 by the EMA’s safety committee: the Pharmacovigilance Risk Assessment Committee (PRAC) (Fig. 1) (EMA, 2018a,b,c, 2020a,b,c). Part of the PRAC report was an expert opinion report, that balanced the risks of surgery against the risk for DILI with UPA use (EMA, 2020a,b,c). Based on a 11:100 000 risk of DILI and a 0.6:100 000 risk on liver transplantation in severe cases (Middelkoop et al., 2020), the PRAC recommended revocation of the marketing authorization of UPA (EMA, 2020a,b,c). However, this recommendation was not supported by the Committee for Medicinal Products for Human Use (CHMP). They concluded that UPA has no clear advantage over existing pre-treatment with GnRHa and that risk of UPA-associated liver injury after intermittent use does not outweigh surgery-related risks (EMA, 2020a,b,c, 2021). Based on these conclusions, the EMA revoked the indication for pre-treatment with UPA, while maintaining the authorization of the indication for intermittent treatment, albeit with parameters for restricted use, especially regarding liver function. See Fig. 1 for a full authorization and implementation timeline (EMA, 2021).
Figure 1.
Timeline of ulipristal acetate (UPA) implementation in relation to the PEARL trials and European Medicines Agency (EMA) highlights.
In this article, we evaluate the marketing authorization and implementation of ulipristal and reflect upon lessons learned, commencing with an overview of the general authorization process and how this was executed in the case of ulipristal. We will identify the involved stakeholders and make recommendations in order to increase the chance of successful and sustainable implementation of future and innovative drugs in gynaecology and other medical specialties.
Pre-marketing registration process of new drugs
Before authorization of a new drug, regulatory bodies such as the EMA (for Europe) and the Food and Drug Administration (FDA, for the USA) require information on its safety and efficacy. This information can be provided by performing preclinical studies (i.e. laboratory trials and animal testing) followed by Phases I–III clinical trials in humans. Each phase focuses on a different part of drug safety and efficacy and consequently has different clinical endpoints or outcomes (see Table I). The EMA subsequently checks whether the trials have been conducted well and whether the chosen outcomes were met, and thereafter an indication label is applied to the medicine. An important aspect in the assessment for marketing authorization are the clinical objectives that registration trials need to investigate to obtain regulatory approval (FDA USFaDA, 2018; EMA, 2019), which are defined as: (i) demonstrate treatment benefit (i.e. are the relevant outcomes studied?); (ii) study the intended patient population (i.e. is the study population representative?); (iii) compare with placebo or the current gold-standard treatment; and (iv) collect longer-term safety data to reveal chronic or rare side effects.
Table I.
Clinical phases of drug development (FDA USFaDA, 2018; EMA, 2019).
| PHASE | PURPOSE | CLINICAL OBJECTIVES | STUDY PARTICIPANTS | LENGTH OF STUDY | |
|---|---|---|---|---|---|
| PRE- CLINICAL PHASE | 0 | Laboratory trials and animal testing | |||
|
| |||||
| PRE- MARKETING PHASE | I | Safety and dosage |
|
20–100 healthy volunteers or patients | Several months |
| II | Efficacy and side effects |
|
Up to several hundred patients | Several months to 2 years | |
| III | Efficacy and monitoring of adverse reactions (Pivotal studies or registration trials) |
|
Several hundred to thousands of patients | 1–4 years | |
|
| |||||
| POST- MARKETING PHASE | IV | Safety and efficacy |
|
Several thousand patients | Not predefined |
EMA, European Medicines Agency; FDA, Food and Drug Administration.
For UPA, an overview of the registration trials on which the application for marketing authorization was based is shown in Table II (Donnez et al., 2012a,b, 2014, 2015, 2016; Fauser et al., 2017). In the following paragraphs, we evaluate how the four stated requirements for clinical objectives, Criterias 1–4, were met in the registration trials, the PEARL I–IV trials (Donnez et al., 2012a,b, 2014, 2015, 2016; Fauser et al., 2017).
Table II.
PEARL trial characteristics with patient baseline characteristics and outcomes.
| PEARL I: Ulipristal vs placebo for 3 months (Donnez et al., 2012a) | PEARL II: Ulipristal vs GnRHa* for 3 months (Donnez et al., 2012b) | PEARL III-Extensions: Ulipristal 10 mg 4 repeated courses (Donnez et al., 2014) | PEARL III-2nd Extension: Ulipristal 10 mg 8 repeated courses (Fauser et al., 2017) | PEARL IV: Ulipristal 5 vs 10 mg 2 repeated courses (Donnez et al., 2015) | PEARL IV-Extension: Ulipristal 5 vs 10 mg 4 repeated courses (Donnez et al., 2016) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Design | Randomized, parallel-group, double-blind, placebo-controlled | Randomized, parallel-group, double-blind, double-dummy, active-comparator–controlled | Repeated intermittent ulipristal courses, followed by randomized double-blind NETA† or placebo | Optional, long-term, open-label extension, available to PEARL III first extension participants | Randomized, double-blind controlled trial | Randomized, double- blind controlled trial | |||||||||
| Patients | 85% Caucasian | 85% Caucasian | 86% Caucasian | 94% Caucasian |
|
|
|||||||||
| Baseline fibroid characteristics: | Total fibroids: | 3 Largest fibroids: | 3 Largest fibroids: | BL fibroid characteristics: not mentioned in publication | BL fibroid characteristics for PEARL IV and its extension are the same, as this is the same BL population | ||||||||||
| 5 mg ulipristal | 5 mg ulipristal | GnRHa | 1 Course | 4 Courses | 3 Largest fibroids: | 5 mg | 10 mg | ||||||||
| Volume (cm3) | 100.7 | 79.6 | 59.2 | 53.9 | 49.8 | 42.6 | 43.6 | ||||||||
| Diameter (cm) | ∼5.8 | ∼5.3 | ∼4.8 | ∼4.7 | ∼4.6 | ∼4.3 | ∼4.4 | ||||||||
| Complaints based on Questionnaires‡ | Mild: | Severe: | Mild: | Severe: | Mild: | Severe: | Mild: | Severe: | |||||||
|
PBAC >200 |
|
PBAC >200 |
|
PBAC >200 | – | – | – | PBAC > 200 | ||||||
| VAS | 39.5 | 43.0 | |||||||||||||
| SSS | 50.0 | 50.0 | |||||||||||||
| HRQOL | 56.9 | 55.2 | |||||||||||||
| Intervention |
|
|
Ulipristal 10 mg + 10 days NETA 10 mg | Ulipristal 10 mg | Ulipristal 5 mg: 228 patients | ||||||||||
| Comparison | Placebo: 48 patients | GnRHa: 101 patients | Ulipristal 10 mg + 10 days placebo | – | Ulipristal 10 mg: 223 patients | ||||||||||
| Outcomes | Ulipristal showed a significant effect compared to placebo in terms of:
Adverse events (AE) monitored (PAECs•) for up to 6 months after stopping treatment Surgical parameters were not evaluated |
Surgical parameters were not evaluated |
Fibroid volume reduction increased slightly with repeated courses, after course 4:
|
|
|
Stable outcomes in both treatment groups and over repeated courses regarding:
|
|||||||||
GnRHa: GnRH agonist.
NETA: norethisterone acetate.
Questionnaires: SF-MGPQ: Short-Form McGill Pain Questionnaire (range 0–45 points, with higher scores indicating more severe pain); VAS: Visual Analogue Scale (range 0–100 points, with higher scores indicating more severe pain); Discomfort: measurement of discomfort questionnaire (range 0–28 points, with higher scores indicating greater discomfort); PBAC: pictorial blood-loss assessment chart. Higher scores indicate more blood loss with cut-off for HMB was set on 100 points in the PEARL trials. SSS: Symptom Severity Score (range 0–100, higher scores indicating increased severity); HRQOL: health-related quality of life score (range 0–100, higher scores indicating a better quality of life).
PAEC: PRM-Associated-Endometrial Changes.
Laboratory parameters: Hb: haemoglobin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TB: total bilirubin.
Demonstratation of treatment benefit (are the relevant outcomes studied?)
The benefits of pre-treatment with UPA can be: (i) pre-surgical improvement in general health such as increased haemoglobin levels or enhanced QoL; (ii) surgical facilitation and reduction of blood loss and (iii) post-surgical reduction of hospital stay and faster recovery (Lethaby et al., 2017). For intermittent use of UPA, clinical outcomes such as QoL, amount of blood loss including amenorrhoea, pain and bulk pressure symptoms can be assessed. Symptoms can be quantified with, for example the Uterine Fibroid Symptom and Quality of Life questionnaire (UFS-QOL, consisting of a symptom severity and a QoL domain) (Spies et al., 2010). Tables II and III describe relevant outcomes indicating treatment benefit and in which trial they were evaluated. The PEARL I–IV trials demonstrated improvement of bleeding symptoms and QoL, but pre- and postoperative outcomes were not evaluated. As fibroid volume can be related to fibroid complaints, volume reduction is a potential treatment benefit. The PEARL II data show similar effects on fibroid volume reduction, with a −36% (−58% to −11%) versus −53% (−69% to −36%) change from baseline in the UPA and GnRHa groups, respectively. Uterine volume was significantly more reduced in the GnRHa group than in the UPA group, −47% (−57% to −35%) versus −20% (−40% to +3%) (Donnez et al., 2012b).
Table III.
Effectiveness and safety outcomes of ulipristal, studied in registration trials.
| PRETREATMENT | ||
|---|---|---|
| Enhancing preoperative parameters (Lethaby et al., 2017) | ||
| Outcome | Studied?* | Effect/commentary |
| Amenorrhoea rates/preoperative bleeding | Yes (+) | Majority reached amenorrhoea within 7–10 days after start treatment (Donnez et al., 2012a) |
| Increases preoperative haemoglobin (Hb) levels | Yes (±) | Improvement, but could be related to additional daily iron supplementation only (Donnez et al., 2012a) |
| Reduces fibroid volume | Yes (±) | Significant effect compared to placebo (Donnez et al., 2012a), and similar effect compared to GnRHa (Donnez et al., 2012b) |
| Reduces uterine volume | Yes (±) | Significant effect compared to placebo (Donnez et al., 2012a), but inferior to GnRHa (Donnez et al., 2012b) |
| Quality of life (symptom reduction by validated questionnaires/scales) | Yes (+) | Less pain (Donnez et al., 2012a) and similar effect of pain and quality of life (Donnez et al., 2012b) |
|
| ||
|
Enhancing per- and postoperative parameters (Lethaby et al., 2017) | ||
|
No | Trials focused on preoperative treatment but were not designed to evaluate possible treatment-related differences in surgical outcomes (Donnez et al., 2012a,b) |
|
| ||
|
Safety† | ||
| Endometrial changes | Yes (±) | Higher incidence than with placebo/GnRHa (Donnez et al., 2012a,b) |
| Laboratory values (e.g. Hb, serum hormone levels, lipids, glucose) | Yes (+) | Laboratory parameters did not change significantly during repeated courses (Donnez et al., 2012a,b) |
| Adverse effects | Yes (+) | Less hot flushes than GnRHa (Donnez et al., 2012b) |
|
| ||
|
INTERMITTENT TREATMENT | ||
|
Sustained effect (also in therapy free interval)‡ | ||
| Amenorrhoea rates/controlled bleeding | Yes (±) | Sustained effect with repeated courses (Donnez et al., 2014, 2015, 2016) |
| Fibroid volume | Yes (±) | Sustained effect with repeated courses (Donnez et al., 2014, 2015, 2016) |
| Uterine volume | Yes (±) | Sustained effect with repeated courses (Donnez et al., 2014, 2015) |
| Quality of life (symptom reduction by validated questionnaires/scales) | Yes (±) | Sustained effect with repeated courses (Donnez et al., 2015, 2016) Not all fibroids symptoms were assessed, e.g. pressure symptoms, abdominal distension |
| Fibroid recurrence | Yes (±) | No regrowth recurrence at follow-up 3 months after cessation of therapy (Donnez et al., 2016) |
|
| ||
|
Safety† | ||
| Endometrial changes | Yes (+) | Changes apparent, but no concerns regarding endometrial histology (Donnez et al., 2016; Fauser et al., 2017) |
| Adverse effects | Yes (+) | No concerns regarding laboratory safety (such as Hb, liver enzymes) (Donnez et al., 2016; Fauser et al., 2017) |
Colour meanings; Green: studied in specific trials; Yellow: partly studied or studied in a non-representative patient population; Red: not studied in specific trials.
Safety outcomes discussed in Section D: Longer-term safety data collected to show long-term or rare side effects.
As described in Section B: Intended patient population is studied: the study population involved relatively small fibroids and mild fibroid symptoms.
Study of the intended patient population (is the study population representative?)
UPA is indicated for women of reproductive age with moderate to severe symptoms of uterine fibroids (Richter, 2018). A randomized controlled trial (RCT) to evaluate fibroid therapy should study a typical, affected patient population, making the study findings relevant and generalizable. This includes an ethnically diverse population, as women with an African-American background have a higher incidence of uterine fibroids, and their natural history and response to treatment may differ. Furthermore, it needs to include a population across the reproductive age range, experiencing the gamut of severe symptoms caused by significant uterine fibroids including a variety of sizes, locations and number (Eltoukhi et al., 2014; Stewart, 2020).
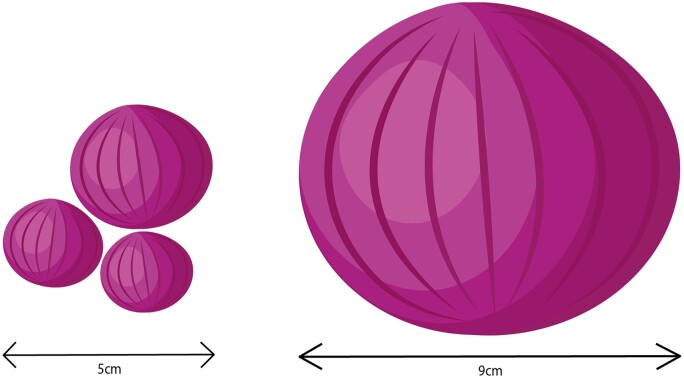
Table IV compares some of the baseline characteristics of the PEARL trials with other trials that have evaluated fibroid treatment outcomes (Spies et al., 2010; Donnez et al., 2012b; Manyonda et al., 2020). Baseline fibroid complaints differ, and as Fig. 2 illustrates, the combined fibroid diameter of the largest three fibroids ranged from 4.3 to 5.8 cm in the PEARL I and II trials (Donnez et al., 2012a,b) compared to the 9.5 cm diameter of the single largest fibroid in the FEMME trial, which compared UAE and myomectomy (Manyonda et al., 2020).
Table IV.
Comparison of baseline fibroid characteristics of the PEARL-II trial, the trial from Spies et al. and the FEMME trial.
| PEARL-II trial (Donnez et al., 2012b) | Spies et al. 2010 (Spies et al., 2010) | FEMME trial (Manyonda et al., 2020) | |
|---|---|---|---|
| Intervention group (n) |
|
|
|
| Ethnicity |
|
|
|
| Baseline fibroid volume (cm3) |
|
– |
|
| Baseline fibroid diameter (cm) * |
|
|
|
| Baseline uterine volume (cm3) |
|
|
|
| Baseline UFS-QOL SSS c |
|
|
|
| Baseline UFS-QOL HRQL d |
|
|
|
Spies et al. (2010) assessed the severity of fibroid related symptoms before and after surgical treatment. The FEMME trial compared uterine artery embolization (UAE) with myomectomy.
GnRHa: GnRH agonist.
UAE: Uterine Artery Embolization.
UFS-QOL SSS: Uterine Fibroid Symptom and Quality of Life Symptom Severity Score (higher score denotes increased severity).
HRQL: health-related quality of life score (lower score denotes poorer quality of life).
When diameters were not given in the original trials, this was calculated based on the formula: V = 4/3 × π × r³, V: volume and r: radius.
Figure 2.
Baseline fibroid size comparison between PEARL-II (left) and FEMME trial (right). Fibroid sizes are in proportion with scale 1:1.
Comparison with placebo or the current gold-standard treatment
UPA was compared as pre-treatment with both placebo and the existing gold-standard treatment (GnRHa) for the outcomes: reduction in fibroid and uterus volume, bleeding control and adverse events (AE). No surgical outcomes or post-surgical complications, hospitalization or recovery were evaluated in these trials (Donnez et al., 2012a,b). For intermittent treatment, marketing authorization was granted without performance of comparative studies of UPA with gold-standard treatment. Two publicly funded Phase IV RCTs comparing intermittent UPA treatment with (i) a medical gold-standard (levonorgestrel-releasing intrauterinesystem (LNG-IUS)) and (ii) a surgical gold-standard (hysterectomy, myomectomy and UAE) were still recruiting, while intermittent UPA treatment was widely implemented in clinical practice. These trials were the UCON trial (EudraCT number 2014-003408-65), comparing intermittent UPA with LNG-IUS for conventional management of heavy menstrual bleeding (Euctr, 2014) and the MYOMEX-2 trial (EudraCT number 2017-005120-16; NTR6860) comparing intermittent UPA with surgery in women with symptomatic uterine fibroids (Middelkoop et al., 2020) (Supplementary Table SI). The UCON trial was funded on 25 June 2014, and started to recruit in April 2015, finishing recruitment of in total 236 women in October 2020. The MYOMEX-2 trial was funded on 1 June 2017, and started to recruit in November 2018, with currently 38 women recruited of the intended 179 women.
Collection of longer-term safety data to show long-term or rare side effects
As shown in Tables II and III, the PEARL trials studied general adverse effects (AEs) and a specific AE described as (reversible) endometrial changes, termed ‘Progesterone receptor modulator-Associated-Endometrial Changes’ (PAECs). The extensions studies of PEARL III and IV showed that repeated treatment up to eight intermittent courses were not associated with higher incidences of PAECs. Also, in cases where PAEC occurred, no (pre)malignancies were found and endometrium recovered back to normal after the treatment course.
Liver function was assessed in the PEARL III and IV trials at baseline and after repeated courses, with laboratory values including alanine transaminase (ALT), aspartate aminotransferase (AST) and total bilirubin (TB) staying within normal ranges (Table II) (Donnez et al., 2014, 2015, 2016; Fauser et al., 2017). The second extension of PEARL III, included 64 patients and was an open-label cohort and follow-up study of eight repeated courses (Fauser et al., 2017). The PEARL IV and its extension compared UPA 5 mg and 10 mg and showed that laboratory values (including ALT, AST and TB) and PAECs remained stable and benign and reversible, respectively (Donnez et al., 2015, 2016).
Implementation
Phase IV trials post-marketing authorization
The EMA demands that post-marketing safety should be constantly monitored through AE reports by patients and healthcare professionals, in clinical studies or publications. Also, a new medicine needs to be regularly assessed through reports by the pharmaceutical company and evaluated through post-authorization safety studies (EMA, 2019). Phase IV trials can be performed in larger populations, for a longer period of time, in order to identify more infrequent AEs and to study the medicine in heterogenic patient populations, who are less likely to be included in earlier phase trials (see Table I) (EMA, 2019). For example, the PEARL I, II and IV trials included patients of age up to 50 years old and the PEARL III trial included patients up to 48 years old (Donnez et al., 2012a,b, 2014, 2015, 2016; Fauser et al., 2017). Looking at the PRAC reports discussing the severe DILI cases, they showed that four out of the seven patients were ≥54 years old (EMA, 2018a,b,c, 2020a,b,c). So severe DILI occurred mostly (57%) in patients that would not have been included in the PEARL trials. In addition, a 11:100 000 risk on DILI is so rare that this could only have been picked up in large post-marketing studies or databases.
Clinical trial registration databases (EMA, Medicine, Netherlands) mention three registered observational trials on Clinicaltrials.gov: the PGL 14-001 PREMIUM-study (NCT02748460; for long-term safety), a Canadian study (NCT02580578; registration of different fibroid treatments and their effect on fibroid characteristics and complaints) (Bedaiwy et al., 2018) and an Italian study (NCT03972917; fibroid complaints and endometrial safety) (Medicine). At this moment, none of them have published results.
As for the patients mentioned in the PRAC reports, we could not identify whether they had been included in and identified through the observational PREMIUM-study in the EU, or whether they were identified by a different database or information source. The PRAC report of 2020 mentions 91 identified cases with serious AEs within the hepatic disorder spectrum. The majority of these cases do not provide sufficient information to identify UPA as the main cause of hepatic impairment. Seven cases provided sufficient information to assess causality and in five of these cases a causative role of UPA was thought to be possible (EMA, 2018a,b,c, 2020a,b,c).
Discussion
By evaluating the process leading to marketing authorization of UPA, we observed that the registration trials missed essential outcomes and studied a non-representative population, limiting the value of the randomized comparison for the indication ‘pre-treatment’ of fibroids. For ‘intermittent treatment’ of heavy menstrual bleeding associated with fibroids, no comparison was available at the time that extension of the marketing authorization was granted. Randomized trials comparing intermittent treatment with placebo or gold-standard medications, conducted by independent researchers, were only started several years after marketing authorization. Indeed, over 900 000 cycles had been prescribed before temporary revocation of the drug in 2020 occurred due to a rare complication of liver failure. Some publications in esteemed journals even suggested prescribing UPA for most fibroids, without a solid scientific basis (Singh et al., 2017; Middelkoop and Huirne, 2018), before the outcomes of any post-marketing studies or independent trial data were reported.
How could this situation have arisen and why was this drug implemented in routine clinical practice despite the shortcomings, identified in this article, of the research assessing the safety and effectiveness of UPA? To answer this question, we need to understand the process for implementing a new pharmacological agent and the stakeholders involved. From pre-marketing studies to marketing authorization and subsequent introduction of a new drug, stakeholders influencing decision-making include: (i) the pharmaceutical company; (ii) the (inter)national authorizing bodies such as the EMA and FDA; (iii) individual medical professionals and their (national) societies such as the American College of Obstetricians and Gynecologists (ACOG), the British Royal College of Obstetricians and Gynaecologists (RCOG) and the Dutch Society for Obstetricians and Gynaecologists (Nederlandse Vereniging voor Obstetrie en Gynaecologie, NVOG); (iv) fibroid-researchers; and (v) (inter)national bodies involved in research and funding. All stakeholders have their own responsibilities and as a result may be liable to potential pitfalls during the drug approval process and the following clinical implementation. We evaluate the process by addressing all stakeholders.
Firstly, the pharmaceutical company (i) is considered. Developing new medicines and executing clinical trials are vastly expensive processes. A recent cross-sectional study of the approval of 101 pharmacological agents by the FDA from 2015 to 2017, showed median costs per approved agent of $48 million (interquartile range: $20–102 million). For UPA, this was not different with an investment in the patent holding firm PregLem of US$70 million (PregLem). The need for return on investment is likely to influence the chosen primary outcomes and included study population of the PEARL trials. Since no core outcome sets (COS) are available for uterine fibroids, the manufacturer could choose outcomes with little risk of negative results.
Subsequently, the authorizing body (ii) (EMA) monitors the quality of the trials upon which the authorization is being requested. They do not evaluate the choice of primary outcomes, nor the specific population characteristics, but only look at the methodological quality of the executed trials. After publication of the PEARL trials, UPA was granted marketing authorization and the label stated that UPA was indicated ‘for moderate to severe symptoms of uterine fibroids in adult women of reproductive age’. The initial authorization label in 2012 mentioned ‘pre-treatment’ only, which was extended to ‘intermittent treatment’ in 2015, without comparative research with gold-standard treatments. This labelling lacked specificity, being applicable to any patient with fibroids, regardless of their size, location, number or severity of associated symptoms. Moreover, ethnic distribution within the licensing trials was non-representative and fibroid volume and symptoms in the studied population, were minor in comparison to other trials evaluating patients with moderate to severe complaints of fibroids (Spies et al., 2010; Manyonda et al., 2020; de Milliano et al., 2020a,b). Therefore, the EMA should have considered narrowing the label, ensuring it corresponded with the characteristics of the population UPA was evaluated on, such as restricting indications to a total fibroid volume up to 100 cm3 (diameter 5.8 cm) and Caucasian patients up to 50 years of age. Aduhelm is drug for Alzheimer’s disease and a recent example of narrowing the label after approval. Initially, this drug was approved by the FDA for anyone with Alzheimer’s disease although the registration trials of Aduhelm, tested only patients with mild dementia and cognitive impairment. After protest from physicians and patients advocates, the FDA narrowed the label to patient groups in alignment with the initial studied population (Higgins-Dun, 2021). Registration for general use of UPA was based upon inadequate outcomes and a limited patient representativeness in the registration trials. If the EMA had involved independent experts in the registration process, this labelling could have been narrowed.
The next stakeholders are the medical professionals and their national societies (iii). After marketing authorization UPA was implemented in daily practice as proven by the 900 000 prescribed cycles between 2012 and 2018 (CHMP, 2015; CADTH, 2017; EMA, 2020a,b,c). This occurred despite the aforementioned research design flaws and the important Phase IV RCTs, comparing intermittent treatment with gold-standard treatment, was yet to be completed (Euctr, 2014; Middelkoop et al., 2021). Without evidence from comparative RCTs in real-life practice, healthcare professionals should refrain from prescribing the new treatment outside of a research setting. Moreover, medical professionals should work together with fibroid researchers (iv) on COS and categorizing symptom severity through quantification. Since fibroids and their associated symptoms are currently not categorized according to levels of severity as the UFS-QOL only gives symptom severity scores and QOL scores, a positive treatment effect leads to marketing authorization with a ‘broad’ label. A COS could provide relevant primary outcomes for registration trials, making fibroid therapy research more reproducible and valid and enabling justifiable direct implementation after marketing authorization.
Finally, the (inter)national bodies (v) involved in research and funding are considered. Despite several attempts to acquire governmental funding, the necessary randomized trials were only granted sponsorship in 2014 (UCON-trial (Euctr, 2014)) and 2018 (MYOMEX-2 trial (Middelkoop et al., 2021), with results to be expected many years after grant approval. When a potentially valuable drug is available, the procedures for grant acquisition should be dramatically shortened. Individual professionals and their national societies (ACOG/RCOG/NVOG) should advocate the need for further (comparative) research trials before of supporting implementation. Such direction from influential sources independent from industry could also stimulate governmental grant allocation and help to shorten trial execution time and thereby trial costs, as patient recruitment could be done faster if the new therapy is only available within a research setting. A great step forward is the implementation of the new clinical trials regulation from the European Commission, that among other points, supports the execution of multinational trials and facilitates specific Phase IV trials, identified as so-called low intervention trials, to economize trial costs (European Commission, 2021).
Despite the deficiencies highlighted in the evaluation of UPA, we believe that the extremely rare complication of liver transplantation associated with the drugs usage (risk 1:180 000), would not have been picked up by Phase IV trials. In addition, the incidence of DILI related to UPA is comparable or lower than several drugs that are not subject to additional liver tests such as diclofenac or several antibiotics (Middelkoop et al., 2020). An alternative way of post-marketing surveillance could be compulsory registration of all AE by all prescribing physicians providing a fast way to accumulate safety data. In Europe, this can be done in the EudraVigilance database for suspected adverse drug reactions for authorized medicines, as has been done extensively with the introduction of COVID-19 vaccines, e.g. shown by 108 500 reported AE for >61 million Spikevax vaccines (EMA, EMA). In addition, a special awareness symbol exists, the so-called black inverted triangle on medical packaging for newly introduced medicines, indicating that the medicine is under additional monitoring by the EMA. This should stimulate both health care professionals and patients to report AE for these specific new drugs (EMA).
Conclusion
Extremely high costs involved in developing and evaluating pre-marketing studies in new drugs may influence trial design and the relevance of chosen outcomes, in turn influencing clinical applicability. In the absence of a fibroid COS and quantification of symptom severity, UPA was labelled ‘for moderate to severe symptoms of uterine fibroids’ after investigation in a non-diverse population with small fibroids and relatively mild symptoms. Authorizing bodies should involve independent researchers in evaluating registration trials for marketing authorization. Also, the granted label should be narrowed to the investigated population. It is vitally important that drug authorization is regulated in such a way that the necessary evidence is generated before widespread implementation of a new drug. All stakeholders, from pharmaceutical companies to authorizing bodies, governmental funding bodies and medical professionals should be aware of their role and responsibilities when scrutinizing and implementing new pharmacological drugs.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgements
The authors thank Maurits F.J.M. Vissers for his valuable input regarding clinical trial execution and drug authorization and Anna L. Tomson, for her help in the visualization of Fig. 2.
Authors’ roles
M.-A.M., W.J.K.H., M.E.d.L. and J.A.F.H. were responsible for the conceptualization of this manuscript. T.J.C., B.W.J.M., P.M.B. and J.A.F.H. were major and substantial contributors in writing the manuscript. M.-A.M., M.E.d.L. and W.J.K.H. were responsible for the original draft, but this was extensively reviewed and critically revised by all other authors to clarify all sides of this review. All authors approved the final version of this manuscript and agreed that they are accountable for all aspects of the work.
Funding
The authors received no funding for this opinion paper.
Conflict of interest
Authors M.E.d.L. and P.M.B. declare no conflicts of interest, financial or otherwise. W.J.K.H., J.A.F.H. and M.-A.M. declare they have been involved in a Dutch investigator initiated trial (NCT 02288130, sponsored by Gedeon Richter PregLem, manufacturer of ulipristal), investigating UPA versus leuproreline prior to laparoscopic myomectomy. PregLem did not influence the outcome of this trial, nor did any statistical analyses and this trial was allowed to be published, irrespective of the outcome (de Milliano et al., 2020a,b). They also initiated a Dutch clinical trial (NTR6860, funded by NWO, a Dutch Research Council), evaluating UPA versus surgical treatment for symptomatic uterine fibroids (Middelkoop et al., 2020). T.J.C. declares being a faculty member of an international educational programme in benign gynaecological disorders, funded by Gedeon Richter PregLem, and a co-applicant on an United Kingdom clinical trial, funded by the National Institute for Health Research (Efficacy and Mechanism Evaluation Programme: Award ID: 12/206/52; October 2014–October 2021). This UCON trial evaluates UPA versus conventional management of HMB (Euctr, 2014). B.W.J.M. reports grants from NHMRC and personal fees from Guerbet. He is a former advisory board member and stock holder for ObsEva and he previously received research funding from Merck KGaA.
References
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM.. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Janiszewski P, Singh SS; CAPTURE Steering Committee. A patient registry for the management of uterine fibroids in Canada: protocol for a multicenter, prospective, noninterventional study. JMIR Res Protoc 2018;7:e10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADTH. CADTH Canadian Drug Expert Committee Recommendation: Ulipristal Acetate (Fibristal—Allergan Inc): Indication: Uterine Fibroids. Ottawa (ON): CADTH, 2017. [PubMed]
- CHMP. Assessment Report Esmya. 2011. https://www.ema.europa.eu/en/documents/assessment-report/esmya-epar-public-assessment-report_en.pdf (17 December 2021, date last accessed).
- CHMP. Assessment Report Esmya. 2015. https://www.ema.europa.eu/en/documents/variation-report/esmya-h-c-2041-ii-0028-epar-assessment-report-variation_en.pdf (17 December 2021, date last accessed).
- de Milliano I, Huirne JAF, Thurkow AL, Radder C, Bongers MY, van Vliet H, van de Lande J, van de Ven PM, Hehenkamp WJK.. Ulipristal acetate vs gonadotropin-releasing hormone agonists prior to laparoscopic myomectomy (MYOMEX trial): short-term results of a double-blind randomized controlled trial. Acta Obstet Gynecol Scand 2020a;99:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Milliano I, Middelkoop MA, Huirne JAF, Kwee J, Geomini P, Schoot BC, Van Baal M, Bosmans JE, Hehenkamp WJK.. Ulipristal acetate versus gonadotropin-releasing hormone agonists prior to laparoscopic myomectomy (MYOMEX trial): long term results of a double-blind randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2020b;252:256–264. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans M-M.. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016;22:665–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Donnez O, Matule D, Ahrendt H-J, Hudecek R, Zatik J, Kasilovskiene Z, Dumitrascu MC, Fernandez H, Barlow DH. et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016;105:165–173.e4. [DOI] [PubMed] [Google Scholar]
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt H-J, Zatik J, Kasilovskiene Z, Dumitrascu MC, Fernandez H, Barlow DH. et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2015;103:519–527.e3. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E. et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012a;366:409–420. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E. et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012b;366:421–432. [DOI] [PubMed] [Google Scholar]
- Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BCJM, Barlow DH, Palacios S, Donnez O, Bestel E. et al. ; PEARL III and PEARL III Extension Study Group. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565–1573.e18. [DOI] [PubMed] [Google Scholar]
- Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, Dodd SL, Maroulis C, Subramanian D.. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol 2010;152:96–102. [DOI] [PubMed] [Google Scholar]
- Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA.. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol 2014;210:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA. Annex IV—Scientific Conclusions Esmya 5 mg. 2018a. ema.europa.eu/en/documents/referral/esmya-article-20-procedure-scientific-conclusions_en.pdf (17 December 2021, date last accessed).
- EMA. Assessment Report on Provisional Measures. 2018b. https://www.ema.europa.eu/en/documents/referral/esmya-article-20-procedure-assessment-report-provisional-measures_en.pdf (1 April 2018, date last accessed).
- EMA. Assessment Report on Temporary Measures. 2020a. https://www.ema.europa.eu/en/documents/referral/ulipristal-acetate-5mg-medicinal-products-article-31-referral-assessment-report-temporary-measures_en.pdf (26 July 2020, date last accessed).
- EMA. CHMP Scientific Conclusions and PRAC Assessment Report of the Review under Article 31 of Directive 2001/83/EC Resulting from Pharmacovigilance Data. 2020b. https://www.ema.europa.eu/en/documents/referral/ulipristal-acetate-5mg-medicinal-products-article-31-referral-chmp-scientific-conclusions-prac_en.pdf (17 December 2021, date last accessed).
- EMA. EU Clinical Trials Register. The EU Clinical Trials Register contains information on interventional clinical trials on medicines conducted in the European Union (EU), or the European Economic Area (EEA) which started after 1 May 2004. https://www.clinicaltrialsregister.eu/ (23 January 2021, date last accessed).
- EMA. EudraVigilance—European Database of Suspected Adverse Drug Reactions, for Authorised Medicines. European Medicines Agency. Amsterdam, the Netherlands
- EMA. From laboratory to patient: the journey of a centrally authorised medicine. In: European Medicines Agency (ed). Amsterdam: European Medicines Agency, 2019. Online access From laboratory to patient - the journey of a medicine assessed by EMA. https://www.ema.europa.eu/en/documents/other/laboratory-patient-journey-centrally-authorised-medicine_en.pdf (25 January 2022, date last accessed).
- EMA. List of Medicines Under Additional Monitoring. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medicines-under-additional-monitoring/list-medicines-under-additional-monitoring (17 December 2021, date last accessed).
- EMA. PRAC Recommends Revoking Marketing Authorisation of Ulipristal Acetate for Uterine Fibroids. 2020c. https://www.ema.europa.eu/en/news/prac-recommends-revoking-marketing-authorisation-ulipristal-acetate-uterine-fibroids (17 December 2021, date last accessed).
- EMA. Procedure under Article 20 of Regulation (EC) No 726/2004 Resulting from Pharmacovigilance Data. 2018c. https://www.ema.europa.eu/en/documents/referral/esmya-article-20-procedure-assessment-report-provisional-measures_en.pdf (17 December 2021, date last accessed).
- EMA. Safety of COVID-19 Vaccines. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/safety-covid-19-vaccines (17 December 2021, date last accessed).
- EMA. Ulipristal Acetate for Uterine Fibroids: EMA Recommends Restricted Use. 2021. https://www.ema.europa.eu/en/documents/referral/ulipristal-acetate-5mg-medicinal-products-article-31-referral-ulipristal-acetate-uterine-fibroids_en.pdf (17 December 2021, date last accessed).
- Euctr GB. Comparison of Drug Ulipristal Acetate with Existing Treatment of Levonorgestrel-Releasing Intra-Uterine System. 2014. https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-003408-65/GB (25 January 2022, date last accessed).
- European Commission. Clinical trials—Regulation EU No 536/2014. 2021. https://ec.europa.eu/health/human-use/clinical-trials/regulation_en; https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-1/reg_2014_536/reg_2014_536_en.pdf (17 December 2021, date last accessed).
- Fauser BC, Donnez J, Bouchard P, Barlow DH, Vazquez F, Arriagada P, Skouby SO, Palacios S, Tomaszewski J, Lemieszczuk B. et al. Safety after extended repeated use of ulipristal acetate for uterine fibroids. PLoS One 2017;12:e0173523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA USFaDA. Step 3: Clinical Research. 2018. https://www.fda.gov/patients/drug-development-process/step-3-clinical-research (15 October 2020, date last accessed).
- Herve F, Katty A, Isabelle Q, Celine S.. Impact of uterine fibroids on quality of life: a national cross-sectional survey. Eur J Obstet Gynecol Reprod Biol 2018;229:32–37. [DOI] [PubMed] [Google Scholar]
- Higgins-Dun N. Biogen, FDA walk back controversial Aduhelm label after weeks of fierce criticism. In: FiercePharma (ed). 2021. FiercePharma. https://www.fiercepharma.com/pharma/facing-pushback-biogen-and-fda-agree-to-narrow-aduhelm-s-broad-label (25 January 2022, date last accessed).
- Lethaby A, Puscasiu L, Vollenhoven B.. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database Syst Rev 2017;11:CD000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyonda I, Belli AM, Lumsden MA, Moss J, McKinnon W, Middleton LJ, Cheed V, Wu O, Sirkeci F, Daniels JP. et al. Uterine-artery embolization or myomectomy for uterine fibroids. N Engl J Med 2020;383:440–451. [DOI] [PubMed] [Google Scholar]
- Medicine USNLo. ClinicalTrials.Gov.https://clinicaltrials.gov/ (23 January 2021, date last accessed).
- Middelkoop MA, Bet PM, Drenth JPH, Huirne JAF, Hehenkamp WJK.. Risk-efficacy balance of ulipristal acetate compared to surgical alternatives. Br J Clin Pharmacol 2020;87:2685–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelkoop MA, Huirne JAF, van der Weide MCJ, Bosmans JE, Hehenkamp WJK; MYOMEX-2 TRIAL GROUP. A multi-centre, randomized, non-inferiority trial to compare ulipristal with standard surgical treatment in women with symptomatic uterine fibroids: Protocol of the MYOMEX-2 trial. Eur J Obstet Gynecol Reprod Biol 2021;256:63–69. [DOI] [PubMed] [Google Scholar]
- Middelkoop MA, Huirne JAF.. Re: The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am J Obstet Gynecol 2018;219:424–425. [DOI] [PubMed] [Google Scholar]
- Netherlands C. Netherlands Trial Register. https://www.trialregister.nl/ (23 January 2021, date last accessed).
- PregLem. https://www.preglem.com/ (17 December 2021, date last accessed).
- Richter G. Summary of Product Characteristics of Esmya. 2018. Budapest, Hungary
- Singh SS, Belland L, Leyland N, von Riedemann S, Murji A.. The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am J Obstet Gynecol 2017;218:563–572. [DOI] [PubMed] [Google Scholar]
- Spies JB, Bradley LD, Guido R, Maxwell GL, Levine BA, Coyne K.. Outcomes from leiomyoma therapies: comparison with normal controls. Obstet Gynecol 2010;116:641–652. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R.. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501–1512. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Comparing apples to apples for fibroids. N Engl J Med 2020;383:489–490. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet 2001;357:293–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.