Abstract
STUDY QUESTION
What is the incidence of premature ovarian insufficiency (POI), has the incidence of POI changed over time, and what is the risk of POI among relatives of POI women?
SUMMARY ANSWER
The incidence of POI increased among females aged 15–19 years from 2007 onwards and decreased in older age groups, and among relatives of women with POI the risk of POI is significantly increased.
WHAT IS KNOWN ALREADY
So far, there has been no good quality, nationwide studies of the incidence of POI. Early menopause has been associated with the elevated risk of early menopause among relatives, but the knowledge of the familial risk of POI is scarce. Lower socioeconomic status has been associated with lower age at natural menopause.
STUDY DESIGN, SIZE, DURATION
Population-based study with 5011 women diagnosed with POI in 1988–2017. The data were collected from national registries and covers POI subjects in entire Finland.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women with hormone replacement therapy reimbursement for POI were identified from Social Insurance Institution (SII). We calculated POI incidence in different age groups and studied the changes in the incidence rate over time in 5-year segments. Four population-based controls were selected from the Digital and Population Data Services Agency (DVV) for each POI woman. Family members of the POI cases and controls were identified from the DVV and linked to SII reimbursement data to identify POI diagnoses among them. The familial risk of POI was estimated with a logistical regression model.
MAIN RESULTS AND THE ROLE OF CHANCE
The incidence was highest in the 35–39 age group, ranging from 73.8/100 000 women-years in 1993–1997 to 39.9/100 000 women-years in 2013–2017. From 2007, the incidence among 15- to 19-year-olds rose from 7.0 to 10.0/100 000 women-years in 2015–2017. Cumulative incidence of POI for women under 40 years in 1988–2017 was 478/100 000 women. The relative risk of POI among relatives of women with POI was 4.6 (95% CI 3.3–6.5) compared to relatives of women without POI. POI women tended to have slightly lower socioeconomic status and level of education compared to controls.
LIMITATIONS, REASONS FOR CAUTION
For some women with POI, diagnosis or reimbursement may be lacking. However, we presume that these women represent a minority due to the nature of the disease and the economic benefits of reimbursement. Some changes in the incidence of POI can reflect changes in clinical practice and changing treatments and reimbursement criteria.
WIDER IMPLICATIONS OF THE FINDINGS
The risk of developing POI is significantly higher in women who have first-degree relatives diagnosed with POI. Raising awareness of the increased risk might lead to earlier diagnosis and initiation of hormonal replacement therapy, possibly preventing adverse effects of low oestrogen levels, such as osteoporosis.
STUDY FUNDING/COMPETING INTEREST(S)
This work was financially supported by the Oulu University Hospital. H.S. received a grant from Finnish Menopause Society. S.M.S. received a grant from the Finnish Menopause Society, the Finnish Medical Foundation and the Juho Vainio Foundation. The authors do not have any competing interests to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: premature ovarian insufficiency, early menopause, genetic risk, hormone replacement therapy, familial risk, menopause
Introduction
Premature ovarian insufficiency (POI) is defined as the loss of ovarian function before the age of 40 (Nelson, 2009; Webber et al., 2016). The European Society of Human Reproduction and Embryology (ESHRE) defines POI as amenorrhoea for at least 4 months and a high, postmenopausal level of FSH (>25 U/l) in at least two samples at least 4 weeks apart (Webber et al., 2016). Loss of ovarian function causes infertility and menopausal symptoms and raises the risk for different diseases, such as osteoporosis and cardiovascular diseases (van Lennep et al., 2016; The ESHRE Guideline Group on POI et al., 2016; Anagnostis et al., 2019). According to the literature, POI affects ∼1% of women (Haller-Kikkatalo et al., 2015; Gruber et al., 2020).
Diverse aetiologic factors are associated with POI, including environmental toxins, genetic and chromosomal disorders, as well as iatrogenic and autoimmune causes (Nelson, 2009; De et al., 2010). X-chromosomal defects, such as Turner’s syndrome and Fragile X premutation, are the most commonly known genetic contributors (De et al., 2010), but specific autosomal mutations are increasingly reported (Qin et al., 2015). FSHR-gene mutations have been reported in the Finnish population, including a founder mutation (Aittomäki et al., 1995; Doherty et al., 2002). Being a child of a multiple pregnancy significantly increases the risk for POI compared to the general population (Gosden et al., 2007). Early menarche (before 11 years) and nulliparity are also identified as risk factors (Mishra et al., 2017). Smoking is an independent risk factor, as well as low BMI (Schoenaker et al., 2014; Tao et al., 2015; Mishra et al., 2019). The reasons for iatrogenic POI include surgery, chemotherapy and radiotherapy (Rebar, 2009). In a majority of cases, however, the aetiology remains unknown (Vujovic, 2009).
There are almost no findings reported about a relation of premature or early menopause and lower socioeconomic status. However, lower socioeconomic status has been associated with lower age at natural menopause (Costanian et al., 2018; Mishra et al., 2019).
Even less is known about the familial risk of POI. Almost 30 years ago, Cramer et al. (1995) reported that the risk of early menopause was 9-fold higher among women whose sister experienced early menopause and 12-fold higher among women who had multiple relatives who had experienced early menopause. Women with a family history of POI also had a 6-fold higher risk for early menopause (Cramer et al., 1995). We found no other studies about the familial risk of POI.
The incidence of POI varies in extant studies. The study’s primary outcome was the provision of new information about the incidence of POI and how it has changed over time. We also described the socioeconomic status and level of education among POI women as compared to population controls. The third aim was to identify the risk of POI in family members of POI women compared to family members of control women.
Materials and methods
Study population and design
We identified women with POI in Finland using reimbursement data for hormone replacement therapy (HRT) from the Social Insurance Institution of Finland (SII). Even though the reimbursement policy has changed over time (Table I), the criteria remain strict, and 100% reimbursement is reserved for patients who meet the international diagnostic criteria for POI. Obtaining reimbursement is a two-phase process. First, when the diagnosis of POI is made, a woman’s treating physician writes a doctor’s certificate. The certificate includes relevant information of the patient’s medical history and POI diagnosis, and it must show that the reimbursement and diagnostic criteria are fulfilled. Second, the certificate must be approved by an expert in the SII. Once the reimbursement has been approved, the woman receives full reimbursement for HRT medications until she reaches the age of 50 years. We identified all women who received the specific reimbursement code under 40 years old in 1970–2017 (n = 8846). We then excluded persons who had undergone gender reassignment (n = 235), as the same code was used for reimbursement of hormone therapies among individuals in the transgender process. We also excluded women with surgical POI by finding all patients who had undergone removal of both adnexa before POI diagnosis (n = 703). Because this information is available from 1986 onwards, we excluded all POI patients who received the diagnosis before 1988. This exclusion left us with 5011 POI patients.
Table I.
Changes in the reimbursement criteria in the Social Insurance Institution of Finland 1968–2020.
| Changes in the criteria for reimbursement of medicines for reproductive organ hormonal insufficiency | 1976 | 1981 | 1985 | 1994 | 1998 | 2004 | 2007 | 2015 |
|---|---|---|---|---|---|---|---|---|
| All reproductive organ hypofunctions/insufficiencies that require hormonal therapy | x | x | x | |||||
| Congenital ovarian development disorders, e.g. Turner’s syndrome | x | x | x | |||||
| Bilateral oophorectomy in fertile age | x | x | ||||||
| Bilateral oophorectomy before the age of 45 | x | x | x | x | x | x | ||
| Substitution of medical expenses until the age of 50 | x | x | x | x | x | x | ||
| Severe primary and secondary gonadal insufficiencies | x | x | x | x | x | |||
| Early menopause: minimum 8 months amenorrhoea, high FSH and signs of oestrogen deficiency before the age of 40 | x | x | x | x | x | |||
| Not granted for preventing or treating osteoporosis | x | x | x | x | x | |||
| After allogenic stem cell transplant if pre-transplant treatments cause gonal insufficiency | x | x | x | |||||
| Gender reassignment, when the change is formalized and new personal identity number with new sex is granted | x | x | ||||||
| Gender reassignment, when set medical criteria are fulfilled | x |
Data collection
After identifying the women with POI, we combined data from other national registries by using unique personal identity codes (PICs), which are given to all Finnish citizens and permanent residents. The use of PICs as a linkage key allows a reliable combination of data from different registries. In this study, we used data from Statistics Finland, the SII, Finnish Institute for Health and Welfare (THL) and the Digital and Population Data Services Agency (DVV).
To compare POI women to those of the general population, four female population controls were selected by the DVV for each POI woman (n = 20 031). The controls were matched by month and year of birth and municipality of residence. The controls had to be living in Finland on the date of POI diagnosis of the respective case. For eight POI women, the DVV provided less than four population controls because there were not enough women who fulfilled the eligibility criteria. PICs of mothers, daughters and sisters of the cases and controls to our POI diagnosis data of 5011 patients were also extracted by DVV.
Education and the socioeconomic status of the cases and controls were taken from the census data compiled by Statistics Finland. The level of education was divided into basic, secondary and tertiary education. Basic education includes persons whose highest degree is upper secondary education or lower (up to 9 years). Secondary education includes vocational upper secondary education and training, post-secondary non-tertiary qualifications, polytechnic bachelor’s degrees and bachelor’s degrees (10–15 years). Tertiary education involves master’s or equivalent-level degrees and doctoral degrees (16 years or more). Information of the adnexal surgery was found from Hospital Discharge Register (Finnish Institute for Health and Welfare) by using operative codes from Finnish Medical Association and since 1997 NOMESCO (Nordic Medico-Statistical Committee) classification of surgical procedures. We excluded women with two separate unilateral adnex removals or both adnexa removed at the same time to exclude surgical POI cases. Detailed list of operative codes can be found from Supplementary Table SI.
Study approvals
We obtained approval for this study from the Finnish Institute for Health and Welfare (THL/1973/5.05.00/2019), the Social Insurance Institution (135/522/2018) and the Digital and Population Data Services Agency (VRK 4304-2019-2). Anonymization was done before analyses, and hence we had no access to identifiable personal data. Due to the register-based nature of the study, ethics committee approval was not required. The data were analysed and reported in accordance with the STROBE statement.
Data analysis
To calculate the incidence rate of POI in different age groups, we divided the number of new patients by the total number of women-years in the entire Finnish population in that specific period and 5-year age category. We also calculated cumulative incidence from age 10 to age 40 years, based on the age-group specific incidence rates.
Multiple data points were available for both socioeconomic status and education. We used the data points in the same year or closest available before the date of receiving the reimbursement code. A Chi-square significance test was performed by comparing the group of the highest socioeconomical class and education to other groups (upper white collar vs all other socioeconomic levels and tertiary education vs basic or secondary).
We used the data of first-degree relatives to find out whether the person has a relative or relatives with POI in data. We used the same data to generate the family key to illustrate the family relations. To calculate the risk of POI among relatives of our POI cases compared to the risk of POI among the relatives of control women, we used a binary logistical regression analysis. The response variable was having POI (yes/no) and as an independent variable we used the POI diagnosis of a relative (yes/no). The model was carried out using the SAS glimmix procedure, and family key was noted in the model as a random effect.
The data were processed with RStudio, SAS Enterprise guide 7.1 and IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA). Statistical analyses were performed using IBM SPSS Statistics 26.0. and SAS Enterprise Guide 7.1.
Results
We conducted a historical review of the reimbursement policies concerning POI in Finland (Table I). Although changes in these policies have occurred over time, they have mostly pertained to making the criteria stricter and more precise. In 1994, a criteria reform ruled out reimbursement for patients who received HRT exclusively for treating osteoporosis.
Incidence
We identified 5011 women diagnosed with POI in 1988–2017. Changes in the age-specific incidence rates of POI during that period can be seen in Fig. 1. There are similar types of slight incidence changes in all age groups until 2007. After that, the incidence has remained relatively stable, apart from the age group of 15- to 19-year-olds, in which the incidence rate rose from 7.0 to 10.0/100 000 women-years in 2013–2017. As expected, the incidence was highest in the 35–39 age group, ranging from 73.8/100 000 women-years in 1993–1997 to 39.9/100 000 women-years in 2013–2017. Cumulative incidence of POI up to the age of 40 years in 1988–2017 was 0.48%. The mean age at the POI diagnosis was highest in 1993–1997 (33.5 years) and lowest in 2013–2017 (31.4 years).
Figure 1.
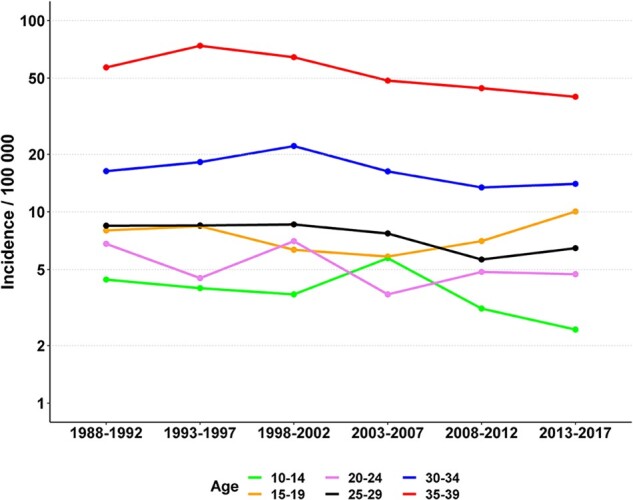
Changes in the incidence of premature ovarian insufficiency (POI) among the Finnish female population 1988–2017.
Socioeconomic status and level of education
Socioeconomic status and level of education were available from the registries for practically all POI women and their controls (missing for 0.04% of POI patients and 0.02% of controls). The results can be seen in Table II. Compared to matched controls, the proportion of upper white collar workers was lower among POI women (P < 0.001). Also, the level of education of POI women is slightly lower than the level of education of population controls (P < 0.001).
Table II.
Socioeconomic status and level of education among women with premature ovarian insufficiency (POI) and controls.
| POI patients (n = 5011) |
Controls (n = 20031) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Socioeconomic status | ||||
| Upper white collar worker | 612 | 12.2 | 3126 | 15.6 |
| Lower white collar worker | 1884 | 37.6 | 7217 | 36.0 |
| Blue collar worker | 907 | 18.1 | 3503 | 17.5 |
| Others (pensioners, students, entrepreneurs) | 1606 | 32.1 | 6182 | 30.9 |
| Unknown | 2 | 3 | ||
| Level of education | ||||
| Basic | 681 | 13.6 | 2413 | 12.0 |
| Secondary | 3885 | 77.5 | 15 275 | 76.3 |
| Tertiary | 445 | 8.9 | 2342 | 11.7 |
| Unknown | 0 | 1 | ||
Relatives
Of the women with POI, 2.6% (n = 129) had at least one first-degree relative (mother, sister or daughter) diagnosed with POI at the age of under 40 years in 1988–2017. We identified 63 POI families with at least two members with POI. Sixteen families consisted of mother and daughter, 44 families had 2 sisters with POI and 3 families had 3 sisters with POI. The odds ratio for POI was 4.6 (95% CI 3.3–6.5, P < 0.001) for first-degree relatives of POI cases compared to the first-degree relatives of the population controls.
The ages at POI diagnosis were similar for POI women with relatives with POI (31.6 years) and POI women without relatives with POI (32.6 years).
Discussion
Our study shows that the incidence of POI has slightly decreased between 1988 and 2017 except in the group of women aged 15–19 years. In another study, the prevalence of POI seems not to have changed over time (Golezar et al., 2019). We believe the increase in the incidence in younger age groups can reflect a change towards a more active approach to primary amenorrhoea among adolescent women. Public health nurses at the schools systematically screen adolescent girls with primary amenorrhoea at the age of 16, and further examinations will be performed in secondary and tertiary care clinics.
According to the literature, POI affects ∼1% of women (Haller-Kikkatalo et al., 2015; Gruber et al., 2020). Our result expressed as cumulative incidence by the age of 40 years is roughly comparable with prevalence rate at the age of 40 years. Our rate of about 0.5% is lower than what is reported in previous studies of POI prevalence rates (1.9–3.7%) (Mishra et al., 2017; Lagergren et al., 2018; Golezar et al., 2019). The lower rate in our study could be explained with more complex, two-step process with strict criteria for reimbursement, while other studies have relied partly on indirect sources, such as drug prescription register (Lagergren et al., 2018) or self-reported age at menopause (Mishra et al., 2017).
We found slight differences in socioeconomic status and level of education between POI women and population controls. Earlier, a weak association between childhood socioeconomic status and age of menopause was found (Hardy and Kuh, 2002). This association could be explained by comorbidities and environmental factors. For example, smoking is more common in lower socioeconomic groups (Laaksonen et al., 2005; Hiscock et al., 2011), and it is also associated with the menopausal transition at an earlier age (Whitcomb et al., 2018). Also, environmental factors like air pollution have been suspected to play a role in the aetiology of POI (Vabre et al., 2017). We did not have information on environmental or lifestyle-based potential confounding factors due to the register-based nature of our study.
One of the significant findings of the current study was that first-degree relatives of POI women have a 4- to 5-fold excess risk of having POI themselves. A few earlier studies have reported even higher prevalences of the family history of POI in POI patients (Vegetti et al., 1998, Bachelot et al., 2009). However, in the study by Vegetti et al. (1998) also more distant than first-degree relatives were reported. In the study of Bachelot et al. (2009), the information about relativés menopausal ages were reported by the POI patients and not checked from the medical records. Since POI women are at increased risk of infertility (Piedade et al., 2021), the POI women tend to have fewer children, which would cause bias to our data. This would further mean that the real excess risk among POI relatives might be slightly higher if the number of children would have been taken into account in the analyses.
Our study has several strengths. To our knowledge, our study is the most extensive population-based study of POI women from a 30-year observation period. The diagnoses of POI in our data are reliable because they were based on two independent physicians’ evaluation and nationally defined criteria for reimbursement since 1970. Finnish health registries are highly reliable data sources (Heino et al., 2017), and PICs represent a strong linkage key. We had no issues with missing data, and none of our data was based on self-reporting. To identify women with real ovarian insufficiency, surgical POI cases were excluded by using codes for surgical procedures.
This study has some limitations, however. There is a chance that some POI patients do not apply for reimbursement, and therefore these patients would not have been included in our data set. That said, we consider this risk to be relatively low since the economic gain from full reimbursement is significant. Also, we cannot exclude the possibility that some women with POI remain undiagnosed, as these women may not have visited health care facilities, or healthcare professionals have not recognized their POI. However, due to the nature of the disease and the reasonable availability of affordable public health care services in Finland, we suggest that these women comprise a small minority. Other limitation in our study is that during the study period, there has been some minor changes in the reimbursement criteria. During the first years of the study, reimbursement for HRT was also granted for the treatment of osteoporosis. However, in our cohort, none of the women with POI had received reimbursement for HRT with the indication of pure osteoporosis. During the study period, the awareness of the potential risks of HRT has increased, which was strongly influenced by the initial results of Women’s Health Initiative (WHI), causing a deep decline in HRT use (Crawford et al., 2018). In our study, the diagnosis of POI is based on the reimbursement right for HRT, which is applied by the treating physician and granted by default as the diagnosis of POI is made, unaffected by HRT purchaces. In addition, in Finland, the sales drop in HRT medications after WHI was smallest of all Nordic countries from 1995 to 2005 (Hemminki et al., 2008), and significantly lower than the sales drop in the USA (Crawford et al., 2018). Furthermore, we have not separated the different aetiological backgrounds of POI, for example history with potentially gonadotoxic therapies or autoimmune diseases. Surgical POI is an immediate consequence of bilateral oophorectomy, and therefore we excluded those patients. The causality of the gonadotoxic therapies or autoimmune conditions is not as straightforward. The aim of this study was to investigate the incidence of POI in the population in general, not only primary POI, and we plan to dive deeper to the aetiologic factors in further studies.
Women with POI are at increased risk of infertility and adverse health outcomes during their lifespan (van Lennep et al., 2016). Awareness of the increased risk of POI among first-degree relatives of women with POI would improve the likelihood of diagnosing POI earlier, thereby preventing unfavourable health outcomes and providing timely and invaluable counsel to women with an increased risk of POI on fertility preservation possibilities before ovarian capacity is lost.
Conclusion
According to our study’s findings, having a relative with POI is a remarkable risk factor for POI. If the likelihood of getting diagnosed with POI under the age of 40 in the female population is about 0.5%, the likelihood among the relatives of POI patients is more than 2%. The incidence of POI slightly decreased during the follow-up except in the age group 15–19 years. We believe this reflects the improved recognition of primary amenorrhoea among adolescent girls. As fertility preservation methods are developing and becoming increasingly available, it is crucial to raise awareness of the risks of POI, especially among relatives of the POI women.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
All authors participated in planning the study design. P.P. and H.S. performed the data analyses, while the drafting of the article was accomplished by H.S., S.M.S., P.P. and M.N. All authors were involved in critical discussions and comments on the manuscript.
Funding
This work was financially supported by the Oulu University Hospital. H.S. received a grant from Finnish Menopause Society. S.M.S. received a grant from the Finnish Menopause Society, the Finnish Medical Foundation and the Juho Vainio Foundation.
Conflict of interest
None of the authors have conflict of interest to declare.
Supplementary Material
References
- Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehväslaiho H, Engel AR. et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 1995;82:959–968. [DOI] [PubMed] [Google Scholar]
- Anagnostis P, Christou K, Artzouchaltzi A-M, Gkekas NK, Kosmidou N, Siolos P, Paschou SA, Potoupnis M, Kenanidis E, Tsiridis E. et al. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol 2019;180:41–50. [DOI] [PubMed] [Google Scholar]
- Bachelot A, Rouxel A, Massin N, Dulon J, Courtillot C, Matuchansky C, Badachi Y, Fortin A, Paniel B, Lecuru F. et al. ; OF-GIS Study Group. Phenotyping and genetic studies of 357 consecutive patients presenting with premature ovarian failure. Eur J Endocrinol 2009;161:179–187. [DOI] [PubMed] [Google Scholar]
- Costanian C, McCague H, Tamim H.. Age at natural menopause and its associated factors in Canada: cross-sectional analyses from the Canadian Longitudinal Study on Aging. Menopause 2018;25:265–272. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Xu H, Harlow BL.. Family history as a predictor of early menopause. Fertil Steril 1995;64:740–745. [DOI] [PubMed] [Google Scholar]
- Crawford SL, Crandall CJ, Derby CA, El Khoudary SR, Waetjen LE, Fischer M, Joffe H.. Menopausal hormone therapy trends before versus after 2002: impact of the Women’s Health Initiative Study Results. Menopause N Y N 2018;26:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M, Vos MD, Devroey P, Fauser BCJM.. Primary ovarian insufficiency. Lancet 2010;376:911–932. [DOI] [PubMed] [Google Scholar]
- Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, Aittomäki K.. A novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab 2002;87:1151–1155. [DOI] [PubMed] [Google Scholar]
- Golezar S, Tehrani FR, Khazaei S, Ebadi A, Keshavarz Z.. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric 2019;22:403–411. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Treloar SA, Martin NG, Cherkas LF, Spector TD, Faddy MJ, Silber SJ.. Prevalence of premature ovarian failure in monozygotic and dizygotic twins. Hum Reprod 2007;22:610–615. [DOI] [PubMed] [Google Scholar]
- Gruber N, Kugler S, de Vries L, Brener A, Zung A, Eyal O, Rachmiel M, Koren I, Tenenbaum-Rakover Y, Hershkovitz E. et al. Primary ovarian insufficiency nationwide incidence rate and etiology among Israeli adolescents. J Adolesc Health 2020;66:603–609. [DOI] [PubMed] [Google Scholar]
- Haller-Kikkatalo K, Uibo R, Kurg A, Salumets A.. The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: a general population registry-based study. Hum Reprod 2015;30:1229–1238. [DOI] [PubMed] [Google Scholar]
- Hardy R, Kuh D.. Does early growth influence timing of the menopause? Evidence from a British birth cohort. Hum Reprod 2002;17:2474–2479. [DOI] [PubMed] [Google Scholar]
- Heino A, Niinimäki M, Mentula M, Gissler M.. How reliable are health registers? Registration of induced abortions and sterilizations in Finland. Inform Health Soc Care 2017;43:1–10. [DOI] [PubMed] [Google Scholar]
- Hemminki E, Kyyrönen P, Pukkala E.. Postmenopausal hormone drugs and breast and colon cancer: Nordic countries 1995–2005. Maturitas 2008;61:299–304. [DOI] [PubMed] [Google Scholar]
- Hiscock R, Bauld L, Amos A, Fidler J, Munafò M.. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2011;1248:107–123. [DOI] [PubMed] [Google Scholar]
- Laaksonen M, Rahkonen O, Karvonen S, Lahelma E.. Socioeconomic status and smoking: analysing inequalities with multiple indicators. Eur J Public Health 2005;15:262–269. [DOI] [PubMed] [Google Scholar]
- Lagergren K, Hammar M, Nedstrand E, Bladh M, Sydsjö G.. The prevalence of primary ovarian insufficiency in Sweden; a national register study. BMC Womens Health 2018;18:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GD, Chung HF, Cano A, Chedraui P, Goulis DG, Lopes P, Mueck A, Rees M, Senturk LM, Simoncini T. et al. EMAS position statement: predictors of premature and early natural menopause. Maturitas 2019;123:82–88. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Pandeya N, Dobson AJ, Chung H-F, Anderson D, Kuh D, Sandin S, Giles GG, Bruinsma F, Hayashi K. et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod 2017;32:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LM. Primary ovarian insufficiency. N Engl J Med 2009;360:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedade KC, Spencer H, Persani L, Nelson LM.. Optimizing fertility in primary ovarian insufficiency: case report and literature review. Front Genet 2021;12:676262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Simpson JL, Chen ZJ.. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update 2015;21:787–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar RW. Premature Ovarian Failure. Obstet Gynecol2009;113:1355–1363. [DOI] [PubMed] [Google Scholar]
- Schoenaker D, Jackson C, Rowlands J, Mishra G.. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014;43:1542–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Jiang A, Yin L, Li Y, Tao F, Hu H.. Body mass index and age at natural menopause. Menopause 2015;22:469–474. [DOI] [PubMed] [Google Scholar]
- Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, Leandri RD.. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health 2017;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lennep J, Heida KY, Bots ML, Hoek A; on behalf of the orators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:178–186. [DOI] [PubMed] [Google Scholar]
- Vegetti W, Grazia Tibiletti M, Testa G, de Lauretis Y, Alagna F, Castoldi E, Taborelli M, Motta T, Bolis PF, Dalpra L. et al. Inheritance in idiopathic premature ovarian failure: analysis of 71 cases. Hum Reprod 1998;13:1796–1800. [DOI] [PubMed] [Google Scholar]
- Vujovic S. Aetiology of premature ovarian failure. Menopause Int2009;15:72–75. [DOI] [PubMed] [Google Scholar]
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E. et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Whitcomb BW, Purdue-Smithe AC, Szegda KL, Boutot ME, Hankinson SE, Manson JE, Rosner B, Willett WC, Eliassen AH, Bertone-Johnson ER.. Cigarette smoking and risk of early natural menopause. Am J Epidemiol 2018;187:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


