Abstract
STUDY QUESTION
Is it safe to perform controlled ovarian stimulation (COS) for fertility preservation before starting anticancer therapies or ART after treatments in young breast cancer patients?
SUMMARY ANSWER
Performing COS before, or ART following anticancer treatment in young women with breast cancer does not seem to be associated with detrimental prognostic effect in terms of breast cancer recurrence, mortality or event-free survival (EFS).
WHAT IS KNOWN ALREADY
COS for oocyte/embryo cryopreservation before starting chemotherapy is standard of care for young women with breast cancer wishing to preserve fertility. However, some oncologists remain concerned on the safety of COS, particularly in patients with hormone-sensitive tumors, even when associated with aromatase inhibitors. Moreover, limited evidence exists on the safety of ART in breast cancer survivors for achieving pregnancy after the completion of anticancer treatments.
STUDY DESIGN, SIZE, DURATION
The present systematic review and meta-analysis was carried out by three blinded investigators using the keywords ‘breast cancer’ and ‘fertility preservation’; keywords were combined with Boolean operators. Eligible studies were identified by a systematic literature search of Medline, Web of Science, Embase and Cochrane library with no language or date restriction up to 30 June 2021.
PARTICIPANTS/MATERIALS, SETTING, METHODS
To be included in this meta-analysis, eligible studies had to be case-control or cohort studies comparing survival outcomes of women who underwent COS or ART before or after breast cancer treatments compared to breast cancer patients not exposed to these strategies. Survival outcomes of interest were cancer recurrence rate, relapse rate, overall survival and number of deaths. Adjusted relative risk (RR) and hazard ratio (HR) with 95% CI were extracted. When the number of events for each group were available but the above measures were not reported, HRs were estimated using the Watkins and Bennett method. We excluded case reports or case series with <10 patients and studies without a control group of breast cancer patients who did not pursue COS or ART. Quality of data and risk of bias were assessed using the Newcastle-Ottawa Assessment Scale.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 1835 records were retrieved. After excluding ineligible publications, 15 studies were finally included in the present meta-analysis (n = 4643). Among them, 11 reported the outcomes of breast cancer patients who underwent COS for fertility preservation before starting chemotherapy, and 4 the safety of ART following anticancer treatment completion. Compared to women who did not receive fertility preservation at diagnosis (n = 2386), those who underwent COS (n = 1594) had reduced risk of recurrence (RR 0.58, 95% CI 0.46–0.73) and mortality (RR 0.54, 95% CI 0.38–0.76). No detrimental effect of COS on EFS was observed (HR 0.76, 95% CI 0.55–1.06). A similar trend of better outcomes in terms of EFS was observed in women with hormone-receptor-positive disease who underwent COS (HR 0.36, 95% CI 0.20–0.65). A reduced risk of recurrence was also observed in patients undergoing COS before neoadjuvant chemotherapy (RR 0.22, 95% CI 0.06–0.80). Compared to women not exposed to ART following completion of anticancer treatments (n = 540), those exposed to ART (n = 123) showed a tendency for better outcomes in terms of recurrence ratio (RR 0.34, 95% CI 0.17–0.70) and EFS (HR 0.43, 95% CI 0.17–1.11).
LIMITATIONS, REASONS FOR CAUTION
This meta-analysis is based on abstracted data and most of the studies included are retrospective cohort studies. Not all studies had matching criteria between the study population and the controls, and these criteria often differed between the studies. Moreover, rate of recurrence is reported as a punctual event and it is not possible to establish when recurrences occurred and whether follow-up, which was shorter than 5 years in some of the included studies, is adequate to capture late recurrences.
WIDER IMPLICATIONS OF THE FINDINGS
Our results demonstrate that performing COS at diagnosis or ART following treatment completion does not seem to be associated with detrimental prognostic effect in young women with breast cancer, including among patients with hormone receptor-positive disease and those receiving neoadjuvant chemotherapy.
STUDY FUNDING/COMPETING INTEREST(S)
Partially supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC; grant number MFAG 2020 ID 24698) and the Italian Ministry of Health—5 × 1000 funds 2017 (no grant number). M.L. acted as consultant for Roche, Pfizer, Novartis, Lilly, AstraZeneca, MSD, Exact Sciences, Gilead, Seagen and received speaker honoraria from Roche, Pfizer, Novartis, Lilly, Ipsen, Takeda, Libbs, Knight, Sandoz outside the submitted work. F.S. acted as consultant for Novartis, MSD, Sun Pharma, Philogen and Pierre Fabre and received speaker honoraria from Roche, Novartis, BMS, MSD, Merck, Sun Pharma, Sanofi and Pierre Fabre outside the submitted work. I.D. has acted as a consultant for Roche, has received research grants from Roche and Ferring, has received reagents for academic clinical trial from Roche diagnostics, speaker’s fees from Novartis, and support for congresses from Theramex and Ferring outside the submitted work. L.D.M. reported honoraria from Roche, Novartis, Eli Lilly, MSD, Pfizer, Ipsen, Novartis and had an advisory role for Roche, Eli Lilly, Novartis, MSD, Genomic Health, Pierre Fabre, Daiichi Sankyo, Seagen, AstraZeneca, Eisai outside the submitted work. The other authors declare no conflict of interest. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
REGISTRATION NUMBER
N/A.
Keywords: controlled ovarian stimulation, assisted reproductive technologies, COS, ART, breast cancer, young women, fertility preservation
Introduction
Breast cancer is the most common malignancy and the leading cause of death in young women aged ≤40 years (Miller et al., 2020). Considering both the prognostic value of young age at diagnosis and the biology of tumors arising in these patients (Azim and Partridge, 2014; Partridge et al., 2016), premenopausal women with breast cancer are often candidate to receive multimodal treatments that include chemotherapy (Oktay et al., 2018; Lambertini et al., 2020). Long-term side effects of chemotherapy include premature ovarian insufficiency (POI) and subsequent impaired fertility (Lambertini et al., 2020; The ESHRE Guideline Group on Female Fertility Preservation et al., 2020). Many women have not completed their family planning at the time of breast cancer diagnosis. For this reason, gonadotoxicity of anticancer treatment, fertility preservation and family planning are crucial issues to be addressed during oncofertility counseling with all women of reproductive age with a newly diagnosed breast cancer (Lambertini et al., 2020; The ESHRE Guideline Group on Female Fertility Preservation et al., 2020).
Among the possible strategies to counteract the risk of chemotherapy-induced impaired fertility, controlled ovarian stimulation (COS) for oocyte/embryo cryopreservation before starting chemotherapy is standard of care for young women interested in fertility preservation (Lambertini et al., 2020; The ESHRE Guideline Group on Female Fertility Preservation et al., 2020).
Despite the recent availability of a growing number of studies demonstrating no increased risk of recurrence for patients undergoing COS, some oncologists still remain concerned about the safety of COS, particularly in patients who are candidates for neoadjuvant therapy and in the case of hormone-sensitive tumors (Lambertini et al., 2018). Nowadays, most breast cancer patients, especially those with hormone-receptor positive disease, undergoing oocyte or embryo cryopreservation are candidate to receive COS in combination with an aromatase inhibitor (AI) to reduce the levels of circulating estrogens or tamoxifen (Lambertini et al., 2020; The ESHRE Guideline Group on Female Fertility Preservation et al., 2020). Including an AI in the protocol for COS has proven to be effective in reducing circulating estrogen levels while it does not affect the efficacy of the protocol in terms of number of collected oocytes, their maturation and fertilization rates (Bonardi et al., 2020).
Many women that did not have access to fertility preservation before starting treatment and that fail to conceive spontaneously may need to access the fertility units during oncology follow-up after completing anticancer therapies. More limited evidence exists on the safety of ART in breast cancer survivors after anticancer treatment completion with only few studies including a small number of patients that have addressed this issue (Goldrat et al., 2015; Rosenberg et al., 2019; Condorelli et al., 2021b), leaving an important unmet medical need in the oncofertility field (Lambertini et al., 2020).
Due to the limited available evidence, many physicians are still uncomfortable to deal with fertility treatments in breast cancer patients and survivors. Many women are still not properly informed about these important issues, reducing their chances of future conception after the end of treatment (Lambertini et al., 2019; Hershlag et al., 2020).
To provide up to date evidence on this important topic and to help physicians during the oncofertility counseling, we performed a systematic review and meta-analysis aiming to assess the safety of fertility treatments before or after anticancer treatments in young women with early breast cancer.
Materials and methods
This was a quantitative synthesis of studies evaluating the safety of COS before starting (neo)adjuvant chemotherapy and of studies assessing ART performed after completion of treatment in early breast cancer patients. Survival outcomes of patients exposed to COS or ART were compared to those of patients who did not undergo these techniques.
Search methods and study selection
Eligible studies were identified by a systematic literature search of Medline, Web of Science, Embase and Cochrane library with no language or date restriction up to 30 June 2021. The search strategy was carried out using the keywords ‘breast cancer’ and ‘fertility preservation’. Specific keywords were combined with Boolean operators. The systematic literature search was carried out independently by three authors (L.A., E.B. and M.M.L.) and any discrepancies were solved by discussion with a fourth author (M.L.). Cross-referencing from relevant studies was performed to confirm retrieval of all possible studies. Data on study design, study population characteristics, inclusion criteria, type of fertility preservation or ART and oncological outcomes were extracted. This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009).
Selection criteria and data extraction
To be included in this meta-analysis, eligible studies had to satisfy the following inclusion criteria: (i) retrospective or prospective case-control or cohort studies comparing survival outcomes of women who underwent COS before starting gonadotoxic treatments for breast cancer and/or ART at the end of treatments to a control group of breast cancer patients without access to these strategies; (ii) studies with available information on one or more survival endpoints (including cancer recurrence rate, event-free survival (EFS), overall survival, number of deaths); (iii) availability or possibility to estimate hazard ratio (HR) or relative risk (RR) and 95% CI. Adjusted RR and HR with 95% CI were extracted. When the number of events for each group were available but the above measures were not reported, HRs were estimated using the Watkins and Bennett method (Watkins and Bennett, 2018).
Exclusion criteria were: (i) absence of a control group of breast cancer patients who did not pursue COS or ART. (ii) Case reports and case series with <10 enrolled patients.
From all the included studies the name, year of publication, study design, number and median age of patients, follow-up time, type of fertility preservation techniques applied, survival status and type of events, and time between breast cancer diagnosis and start of oncological treatments were extracted. Quality assessment and risk of bias were performed using the Newcastle-Ottawa Assessment Scale (NOS) (Wells et al., 2014). The NOS assigns a maximum of 9 points according to three risks of bias domain for case-control or cohort study: selection of study participants, comparability, and exposure or outcomes bias. Studies were classified as follow: low risk of bias (9 points), moderate risk of bias (from 8 to 7 points) and high risk of bias (6 points or below) according to the different score obtained.
Study objectives
The main objectives of this analysis were to evaluate breast cancer recurrence rate, EFS and mortality ratio (MR) in patients who underwent COS or ART as compared to patients not exposed to these techniques. Subgroup analyses were performed to evaluate the prognostic impact of COS in patients with hormone receptor-positive disease and in those receiving neoadjuvant chemotherapy.
A potential delay in time to start chemotherapy in patients performing COS before starting oncological treatments was also evaluated.
Statistical analysis
Pooled RRs and HRs with their 95% CI were calculated with the method of DerSimonian and Laird using the random effects model (DerSimonian and Laird, 1986). Higgins I2 index was used to evaluate the quantitative measure of the degree of inconsistency in the results of the included studies (Higgins and Thompson, 2002). Egger’s asymmetry test was used to assess the probability of publication bias (Egger et al., 1997).
Pooled RRs and HRs were considered statistically significant with a P value of < 0.05 (two-sided). Sensitivity analyses were conducted to assess whether the pooled estimates were stable or depended on one single included study.
Differences in mean time from diagnosis to chemotherapy were analyzed using the random effects model. For studies reporting median values and ranges, mean values were estimated with the Hozo method (Hozo et al., 2005).
Results
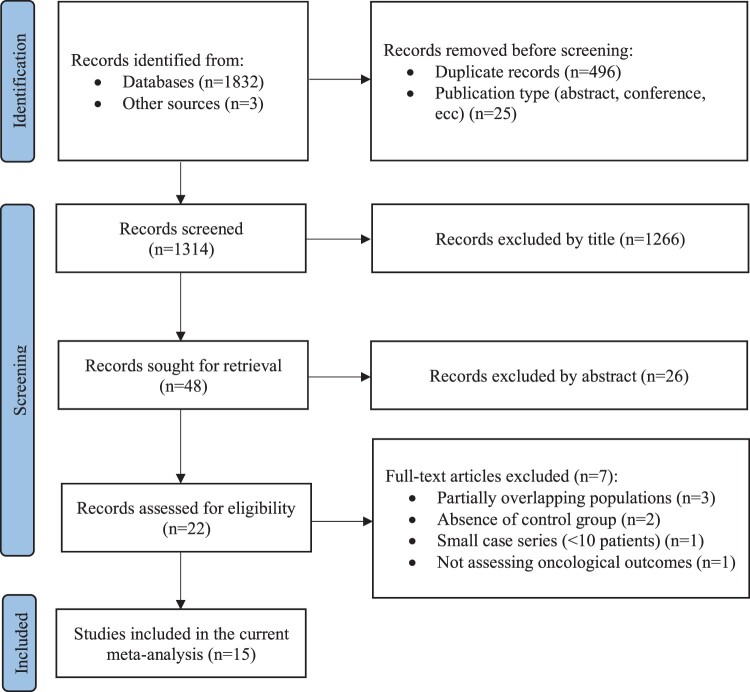
A total of 1835 records were retrieved. Among the 22 records assessed for eligibility, three were excluded because of overlapping population (Oktay et al., 2005; Moravek et al., 2018; Marklund et al., 2020), two because of the absence of a control group (Turan et al., 2013; Meirow et al., 2014), one because it did not assess oncological outcomes (Ben-Haroush et al., 2011) and another because it included a small case series of patients (Takuwa et al., 2018).
Finally, 15 studies were included in the present meta-analysis (Azim et al., 2008; Goldrat et al., 2015; Kim et al., 2016; Chien et al., 2017; Rodriguez-Wallberg et al., 2018; Muñoz et al., 2019; Rosenberg et al., 2019; Letourneau et al., 2020; Vriens et al., 2020; Fredriksson et al., 2021; Greer et al., 2021; Marklund et al., 2021; Moravek et al., 2021; Condorelli et al., 2021a,b), of which 11 reporting on the outcomes of patients who underwent COS for fertility preservation before starting chemotherapy (Azim et al., 2008; Kim et al., 2016; Chien et al., 2017; Rodriguez-Wallberg et al., 2018; Muñoz et al., 2019; Letourneau et al., 2020; Vriens et al., 2020; Fredriksson et al., 2021; Greer et al., 2021; Marklund et al., 2021; Moravek et al., 2021) and 4 studies reporting the outcomes of survivors who underwent ART following anticancer treatment completion (Goldrat et al., 2015; Rosenberg et al., 2019; Condorelli et al., 2021a,b). Among the latter group of studies, one (Condorelli et al., 2021a) has been published after the literature search was performed on 30 June 2021, but it has been included because data were propriety of two of the authors (M.C. and M.L.) (Fig. 1).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram of study selection process.
Main characteristics of the included studies are reported in Table I. Among them, 2 were prospective nonrandomized controlled studies (Azim et al., 2008; Kim et al., 2016), 1 was a prospective cohort study (Vriens et al., 2020), 1 was ambispective (Muñoz et al., 2019) and 10 were retrospective cohort studies (Goldrat et al., 2015; Chien et al., 2017; Rodriguez-Wallberg et al., 2018; Rosenberg et al., 2019; Letourneau et al., 2020; Fredriksson et al., 2021; Greer et al., 2021; Marklund et al., 2021; Moravek et al., 2021; Condorelli et al., 2021b).
Table I.
Main characteristics of the studies included in the present meta-analysis.
| Reference | Country | Years | Study design | Patients exposed to COS or ART, n | Patients not exposed to COS or ART, n | Timing of ovarian stimulation n, (%) |
FP strategy and concomitant medications | Mean/median follow-up time in FP vs no FP cohort |
Matching criteria for choosing controls/ controlling factors |
Baseline differences between study populations and controls | Outcomes | Risk of bias* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azim et al. (2008) 1 | USA | 2002–2007 | Prospective nonrandomized controlled study | 79 | 136 | Before adjuvant chemotherapy | COS + AI (100%) | FP: 23.4 months (7.5–63.6) No FP: 33.05 months (4.5–63.6) |
N.R. | No statistically significant differences between populations | Delay to treatments, recurrence rate, RFS | Moderate |
|
| ||||||||||||
| Kim et al. (2016) 1 | USA | 2002–2014 | Prospective nonrandomized controlled study | 120 | 217 | 14 (11.7) Before surgery 106 (88.3) After surgery |
COS + AI (100%) | FP: 5 years (±2.1) No FP: 6.9 years (±3.6) |
N.R. | FP patients had lower node involvement (p=.02) than controls | Recurrence rate, RFS | Moderate |
|
| ||||||||||||
| Chien et al. (2017) 2 | USA | 2010 –2017 | Retrospective study | 34 | 48 | Before neoadjuvant chemotherapy | N.R. | FP: 79 months No FP: 79 months |
N.R. | No statistically significant differences between populations | Delay to treatments, recurrence rate, RFS | Moderate |
|
| ||||||||||||
| Rodriguez‐Wallberg et al. (2018) | Sweden | 1999–2013 | Retrospective cohort study | 148 | 378 | 23 (15.5) Before neoadjuvant chemotherapy 123 (83.1) Before adjuvant chemotherapy 2 (1.3) N.R. |
COS ± AI (46%) | FP: 5.8 years (0.3–17.9) No FP: 5.8 years (0.3–17.9) |
Age at diagnosis; adjustments for confounders: tumor size, node involvement, estrogen receptor status and chemotherapy administered | No statistically significant differences between populations | Recurrence rate | Low |
|
| ||||||||||||
| Muñoz et al. (2019) | Spain | 2008–2016 | Ambispective cohort study | 148 | 111 | 20 (13.5) Before neoadjuvant chemotherapy 128 (86.5) Before adjuvant chemotherapy |
COS + AI (100%) |
FP: 5 years No FP: 5 years |
Patients with same characteristics but who did not want to preserve their fertility | No statistically significant differences between populations | DFS | Moderate |
|
| ||||||||||||
| Letourneau et al. (2020) 2 | USA | 2007–2017 | Retrospective cohort study | 207 | 122 | 85 (41.1) Before neoadjuvant chemotherapy 122 (58.9) Before adjuvant chemotherapy |
COS + AI or TAM in hormone-receptor positive breast cancer |
FP: 42 months (2–114) No FP: 46 months (10–132) |
Demographic, reproductive health, cancer treatments and tumor characteristics considered as potential confounders and included in the multivariable survival analysis in case of P < 0.2 | No statistically significant differences between populations | Recurrence rate, DFS | High |
|
| ||||||||||||
| Vriens et al. (2020) | The Netherlands | 2008–2015 | Prospective cohort study | 34 | 84 | Before adjuvant chemotherapy | COS + AI (100%) |
FP: 52 moths (48–58) No FP: 51 months (42–63) |
N.R. | FP patients had lower tumor size at diagnosis (P = 0.04) | Recurrence rate, DFS | Moderate |
| Marklund et al. (2021) | Sweden | 1994–2017 | Retrospective cohort study | 425 | 850 | 105 (24.7) Before neoadjuvant chemotherapy 320 (75.3) Before adjuvant chemotherapy |
N.R. | FP: 5.8 years (±4.2) No FP: 5.2 years (±4) |
Age at diagnosis (5-year periods), time of diagnosis (3-year periods) and health care region | FP patients were younger (P < 0.001), estrogen receptor positive (P = 0.034), and received more chemotherapy (P = 0.002) | MR | Moderate |
|
| ||||||||||||
| Moravek et al. (2021) | USA | 2005–2018 | Retrospective cohort study | 150 | 165 | 39 (26.0) Before neoadjuvant chemotherapy 111 (74.0) Before adjuvant chemotherapy |
COS without any AI or TAM | FP: 4 years (0.48–12.40) No FP: 6.19 years (0.72–11.12) |
N.R. | No statistically significant differences between populations | Delay to treatments, recurrence rate, MR | High |
|
| ||||||||||||
| Fredriksson et al. (2021) | Sweden | 2005–2014 | Retrospective cohort study | 126 | 126 | Before adjuvant chemotherapy | COS ± AI | FP: N.R. No FP: N.R. |
Age at breast cancer diagnosis ±5 years, tumor size and node involvement | No statistically significant differences between populations | Recurrence rate, RFS | Moderate |
|
| ||||||||||||
| Greer et al. (2021) | USA | 2007–2017 | Retrospective cohort study | 123 | 149 | 37 (30.1) before neoadjuvant 86 (69.9) before adjuvant chemotherapy |
COS ± AI (60.2%) or TAM (3.3%) | FP: 50 months (32–71) No FP: 49 months (31–75) |
N.R. | More FP patients were BRCA mutation carriers (P = 0.021) | Delay to treatments, DFS, OS | Moderate |
|
| ||||||||||||
| Goldrat et al. (2015) | Belgium, Italy, Spain and Denmark | 2000–2009 | Retrospective cohort study | 25 | 173 | After adjuvant chemotherapy | ART | FP: 102 months (81–131) No FP: 107 months (85–123) |
N.R. | Patients not exposed to ART were younger (P = 0.009) and higher grade 3 tumors (P = 0.033) | Recurrence rate | Moderate |
|
| ||||||||||||
| Rosenberg et al. (2019) | Sweden | 1982–2014 | Retrospective cohort study | 37 | 148 | After adjuvant chemotherapy | ART | FP: 10.3 years (±4.2) No FP: 10.7 years (±4.4) |
Breast cancer stage and year of diagnosis (±5 years) |
No statistically significant differences between populations | Recurrence rate | Low |
|
| ||||||||||||
| Condorelli et al. (2021b) | Belgium | 2006–2016 | Retrospective cohort study | 39 | 73 | After adjuvant chemotherapy | ART | FP: 9.4 years (4.5–22.2) No FP: 12.1 years (6.8–19.8) |
BRCA status, breast cancer stage, anticancer treatments and age | ART patients were younger than controls (P < 0.001) | Recurrence rate, RFS | Low |
| Condorelli et al. (2021a) | Europe, USA and Israel | 2000–2012 | Retrospective cohort study | 22 | 146 | After adjuvant chemotherapy | ART | FP: 7.5 years (3.0–16.0) No FP: 8.8 years (2.5–18.4) |
N.R. | ART patients were older (P = 0.004), had lower grade (P = 0.008) and higher hormone-receptor positive (P = 0.016) tumors | DFS, OS | High |
AI, aromatase inhibitors; COS, controlled ovarian stimulation; DFS, disease-free survival; FP, fertility preservation; MR, mortality rate; N.R., not reported; OS, overall survival; RFS, relapse-free survival; TAM, tamoxifen.
Quality assessment and risk of bias performed using the Newcastle-Ottawa Assessment Scale.
Partially overlapping population.
Partially overlapping population.
COS was performed in association with concomitant AI or tamoxifen in all but one study (Moravek et al., 2021), although in some studies only a portion of the included women were treated with concomitant AI or tamoxifen during COS (Rodriguez-Wallberg et al., 2018; Letourneau et al., 2020; Fredriksson et al., 2021; Greer et al., 2021) (Table I).
The timing of COS differed between the included studies, being before or after surgery in one (Kim et al., 2016), before neoadjuvant chemotherapy in one (Chien et al., 2017), before adjuvant therapy in three (Azim et al., 2008; Vriens et al., 2020; Fredriksson et al., 2021) and both before neoadjuvant or adjuvant chemotherapy in the remaining six studies (Rodriguez-Wallberg et al., 2018; Muñoz et al., 2019; Letourneau et al., 2020; Greer et al., 2021; Marklund et al., 2021; Moravek et al., 2021) (Supplementary Fig. S1).
Follow-up time ranged from 23.4 months (Azim et al., 2008) to 79 months (Chien et al., 2017) in studies evaluating COS and between 7.5 and 10.3 years (Rosenberg et al., 2019) in studies evaluating ART treatments in breast cancer survivors.
A total of 4643 breast cancer patients were included in these studies, of whom 1594 underwent COS for fertility preservation before starting anticancer treatments and 2386 did not undergo fertility preservations strategies at time of breast cancer diagnosis, 123 breast cancer survivors were exposed to ART after the end of anticancer treatments and 540 breast cancer survivors were not exposed to ART.
Safety of COS in breast cancer patients before starting (neo)adjuvant treatments
Among the 11 studies included in the analysis, 10 reported recurrence rate (Azim et al., 2008; Kim et al., 2016; Chien et al., 2017; Rodriguez-Wallberg et al., 2018; Muñoz et al., 2019; Letourneau et al., 2020; Vriens et al., 2020; Fredriksson et al., 2021; Greer et al., 2021; Moravek et al., 2021), 7 EFS (4 as relapse-free survival (Azim et al., 2008; Kim et al., 2016; Chien et al., 2017; Fredriksson et al., 2021), 3 disease-free survival (Letourneau et al., 2020; Vriens et al., 2020; Greer et al., 2021)) and 4 mortality rate (Muñoz et al., 2019; Marklund et al., 2020; Greer et al., 2021; Moravek et al., 2021). Sensitivity analyses of all outcomes are available as Supplementary Tables SI, SII, SIII.
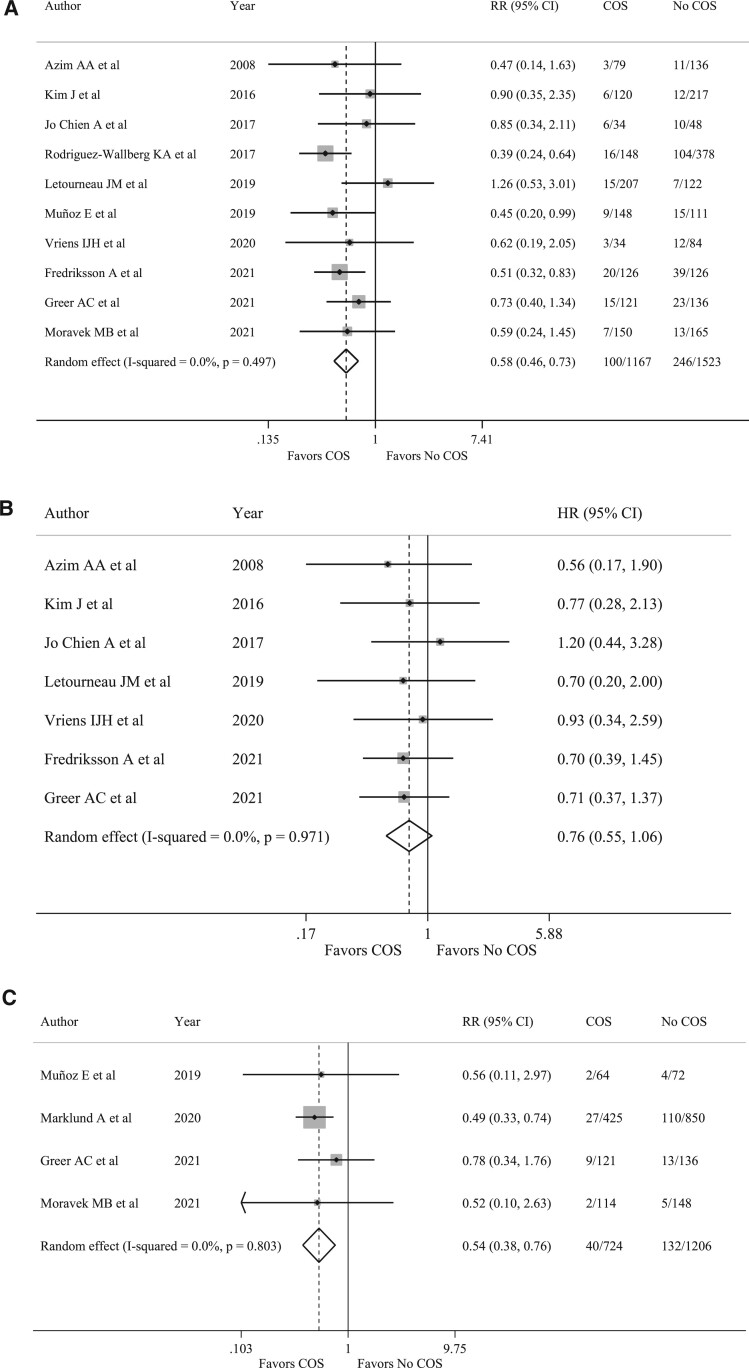
Overall, 100 recurrences (8.6%) occurred among the 1167 women who underwent COS at the time of breast cancer diagnosis whereas 246 (16.2%) occurred among the 1523 patients who did not undergo COS. Compared to women who did not receive fertility preservation at diagnosis, those who underwent COS had a reduced risk of recurrence (RR 0.58, 95% CI 0.46–0.73, P < 0.001) (Fig. 2A). No heterogeneity (I2 = 0.0%, P = 0.497) nor publication bias (P = 0.109) were observed (Supplementary Table SI).
Figure 2.
Forest plots describing breast cancer recurrence, event-free survival and mortality in patients undergoing controlled ovarian stimulation before starting oncological treatments. Solid vertical line: Significance line (line of no effect). Gray squares: represents the weighted mean (point estimate) of each study. Horizontal bars: 95% CI line. Diamond: represents the mean of effect sizes obtained by the meta-analysis. Its width corresponds to its 95% CI. (A) Recurrence rate in patients undergoing COS. (B) Event-free survival in patients undergoing COS. (C) Mortality rate in patients undergoing COS. COS, controlled ovarian stimulation; HR, hazard ratio; RR, relative risk. I-squared is Higgins I2 index (Higgins and Thompson, 2002).
No detrimental effect of COS was observed in terms of EFS (HR 0.76, 95% CI 0.55–1.06, P = 0.112; I2 =0.0%, P = 0.971) (Fig. 2B) (Supplementary Table SII).
Patients exposed to COS had a reduced risk of dying, with 40 deaths (5.5%) observed among the 724 patients that pursued COS and 132 (10.9%) among the 1206 patients not exposed to COS (RR 0.54, 95% CI 0.38–0.76, P < 0.001; I2 = 0.0%, P = 0.803) (Fig. 2C) (Supplementary Table SIII).
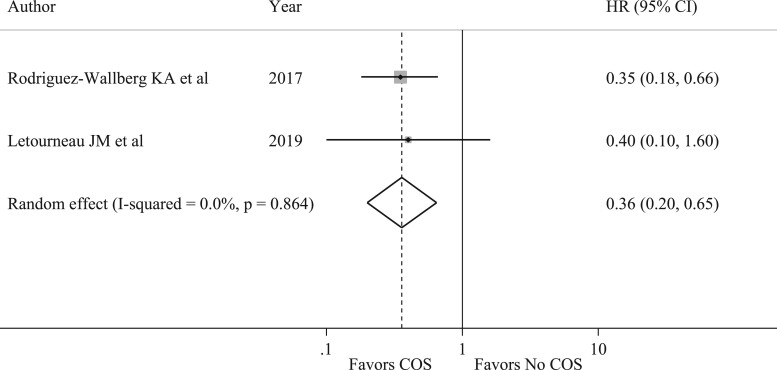
Two studies (Rodriguez-Wallberg et al., 2018; Letourneau et al., 2020) reported a subgroup analysis of EFS in patients with hormone-receptor positive breast cancer. Similarly to the results of the main analysis, a tendency for a better outcome in terms of EFS was observed in patients with hormone-receptor positive breast cancer who underwent COS as compared to those not exposed to fertility preservation procedures (HR 0.36, 95% CI 0.20–0.65) (Fig. 3).
Figure 3.
Forest plot describing analysis of event-free survival in hormone-receptor positive disease in patients undergoing controlled ovarian stimulation. COS, controlled ovarian stimulation; HR, hazard ratio. I-squared is Higgins I2 index (Higgins and Thompson, 2002).
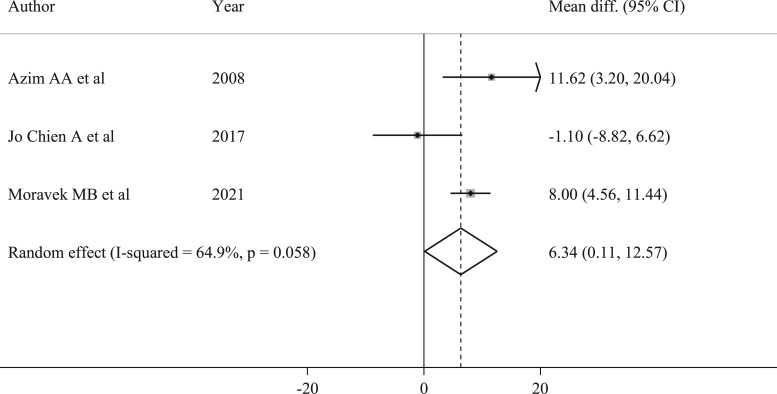
Among the included studies, three evaluated differences in time to therapy initiation between patients undergoing or not COS (Azim et al., 2008; Chien et al., 2017; Moravek et al., 2021). Different definitions of time to therapy were used: Azim et al. (2008) computed the time between surgery and start of adjuvant chemotherapy, Chien et al. (2017) the time between the date of invasive breast cancer diagnosis by biopsy to the first dose of neoadjuvant chemotherapy, and Moravek et al. (2021) the time between the first contact with reproductive team to cancer treatment start. Overall, patients that underwent COS had a 6-day longer time to chemotherapy compared to patients not exposed to COS (mean difference 6.34, 95% CI 0.11–12.57, P = 0.046) (Fig. 4).
Figure 4.
Forest plot describing analysis of delay-time to start of treatments in patients undergoing controlled ovarian stimulation. I-squared is Higgins I2 index (Higgins and Thompson, 2002).
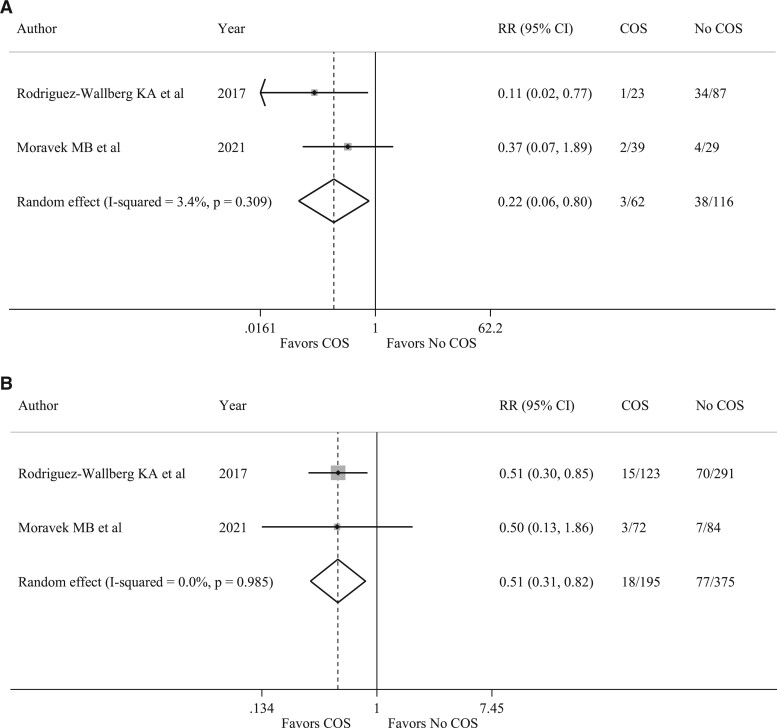
Two studies (Rodriguez-Wallberg et al., 2018; Moravek et al., 2021) reported data on breast cancer recurrences in patients who received COS before neoadjuvant chemotherapy as compared to controls and in those who received COS before adjuvant chemotherapy as compared to controls. A reduced risk of recurrence was observed among patients receiving COS before starting neoadjuvant chemotherapy (RR 0.22, 95% CI 0.06–0.80, P = 0.021) (Fig. 5A) and in those receiving COS before initiation of adjuvant therapy (RR 0.51, 95% CI 0.31–0.82, P = 0.005) (Fig. 5B).
Figure 5.
Forest plots describing analysis of recurrence rate among patients undergoing controlled ovarian stimulation before chemotherapy. (A) Recurrence rate among patients undergoing COS before neoadjuvant chemotherapy compared to patients who did not receive fertility preservation techniques. (B) Recurrence rate among patients undergoing COS before adjuvant chemotherapy compared to patients who did not receive fertility preservation techniques. RR, relative risk; COS, controlled ovarian stimulation. I-squared is Higgins I2 index (Higgins and Thompson, 2002).
Safety of ART in breast cancer survivors
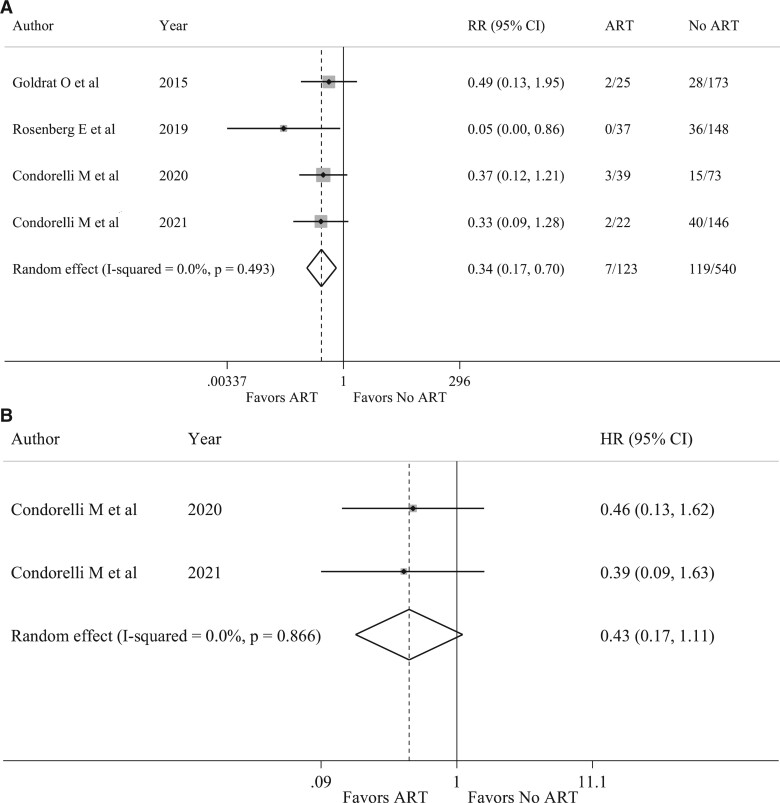
Among the four included studies, recurrence rates were reported in all studies (Goldrat et al., 2015; Rosenberg et al., 2019; Condorelli et al., 2021a,b), and EFS in two (Condorelli et al., 2021a,b). Fewer recurrences were observed in patients receiving ART with 7 (5.7%) among the 123 patients who underwent ART and 119 (22.0%) among the 540 patients in the control group (RR 0.34, CI 95% 0.17–0.70, P = 0.003; I2 = 0.0%, P = 0.493) (Fig. 6A) (Supplementary Table SIV).
Figure 6.
Forest plots describing breast cancer recurrence rate and event-free survival in breast cancer survivors undergoing assisted reproductive technologies. (A) Recurrence rate in survivors that performed assisted reproductive technologies. (B) Event-free survival in survivors that performed ARTs. HR, hazard ratio; RR, relative risk. I-squared is Higgins I2 index (Higgins and Thompson, 2002).
Moreover, no detrimental effect in terms of EFS was observed (HR 0.43, 95% CI 0.17–1.11, P = 0.081; I2 = 0.0%, P = 0.866) (Fig. 6B).
Discussion
To our knowledge, this is the first systematic review and meta-analysis assessing the safety of hormonal stimulation in young women with breast cancer before starting anticancer treatments and in survivors who underwent ART after anticancer treatment completion. Among women who were exposed to COS before starting chemotherapy, we observed fewer recurrences and deaths as compared to patients not exposed to fertility preservation strategies, with no difference in EFS. A 6-day delay in the time to chemotherapy start was found in patients who had access to COS for fertility preservation. Similarly, we observed no apparent detrimental prognostic effect of ART in breast cancer survivors with a tendency for lower recurrences in patients exposed to ART as compared to those not exposed to ART, with no difference in EFS.
Nowadays, it is imperative to ensure that all young patients are given the chance to potentially complete their family planning after cancer diagnosis and treatment (Perachino et al., 2020). Therefore, oncofertility counseling should be offered to all young women at the time of breast cancer diagnosis to discuss the risk of chemotherapy-induced POI and subsequent impaired fertility and the available strategies for counteracting these side effects, including ovarian function and fertility preservation (Lambertini et al., 2020; The ESHRE Guideline Group on Female Fertility Preservation et al., 2020).
However, being breast cancer a hormonally driven tumor, both young patients and their oncologists are still concerned about the safety of fertility treatments that include ovarian stimulation. This is mainly due to the limited safety evidence available regarding these techniques as well as the limitations of the studies addressing this important issue (e.g. short-term follow-up, different inclusion criteria, timing of hormonal manipulations and type of COS performed). Our results provide more robust evidence on the safety of COS in breast cancer patients.
We included studies that were mainly retrospective, enrolled patients from different countries around the world (particularly from Europe and America) and used different fertility preservation techniques (i.e. random vs. conventional start, COS associated or not with concomitant AI or tamoxifen). Despite the limitations of the included studies, overall, we observed clearly no detrimental prognostic effect of COS with a similar trend of no alarming signals in all the studies.
As reported in a recent meta-analysis (Bonardi et al., 2020), concomitant use of letrozole reduces circulating estrogen levels and it does not impair the efficacy of COS. Therefore, the inclusion of letrozole in the protocol for COS could be considered as alternative to standard stimulations in breast cancer patients. Notably, only one study included in the present meta-analysis did not use AI or tamoxifen as part of the protocol for COS (Moravek et al., 2021).
Many oncologists are still concerned about whether patients with hormone-receptor positive tumors should undergo COS. Although only two studies reported survival outcomes according to hormone receptor status of the tumor, our results indicate no detrimental effect of COS in this setting.
A recent analysis of the Pregnancy and Fertility (PREFER) study (Blondeaux et al., 2021) has shown that 11.7% of young breast cancer patients refuse to preserve their fertility mainly because of the concerns about a possible delay in cancer treatment. In our meta-analysis, we observed a 6-day longer time to chemotherapy start. In the breast cancer setting, differently from aggressive hematologic diseases, such short additional time should not be considered a contraindication to the procedure for the majority of patients. In fact, adjuvant chemotherapy is equally effective up to 12 weeks after definitive surgery, although this time should be kept within a maximum of 60 days for patients with more aggressive malignancies at higher risk of relapse (i.e. those with triple-negative or human epidermal growth factor receptor 2 positive disease) (Lohrisch et al., 2006; de Melo Gagliato et al., 2014). Similar considerations apply for neoadjuvant chemotherapy but taking into account that shortening the interval to treatment initiation would be particularly relevant in this setting (de Melo Gagliato et al., 2020).
Among the included studies, the time from diagnosis to anticancer treatments initiation ranged from a median of 35 days (Moravek et al., 2021) to 45 days (Azim et al., 2008). Despite being statistically significant, the short delay of 6 days that we observed in our analysis does not appear to be clinically relevant and would not lead to exceeding the 60-day cutoff.
These results are also indirectly confirmed by a further analysis showing no increased risk of breast cancer recurrence in patients receiving COS before undergoing neoadjuvant or adjuvant chemotherapy as compared to controls. Though evidence remains limited, these data are encouraging in showing that performing COS before starting neoadjuvant chemotherapy does not seem to worsen the prognosis of breast cancer patients. Even if this strategy is not allowed in the neoadjuvant setting in some countries, our data support the current recommendation that COS for fertility preservation is not contraindicated (Lambertini et al., 2020).
In this work, the safety of ART in breast cancer survivors following anticancer treatment completion was also assessed. This is a very controversial area where limited data are available. Indeed, only four studies fulfilled the inclusion criteria and thus could be included in this analysis (Goldrat et al., 2015; Rosenberg et al., 2019; Condorelli et al., 2021a,b). Despite the small number of women included in these studies (n = 663), reduced recurrences in women undergoing ART and no differences in EFS were found suggesting again no apparent detrimental prognostic effect. Among the four included studies, only one demonstrated a statistically significant reduction in recurrences in breast cancer patients exposed to ART (Rosenberg et al., 2019), while the other three did not reach statistical significance (Goldrat et al., 2015; Condorelli et al., 2021a,b). It should be noted that these studies were retrospective and included a low number of patients, particularly those deemed at low risk of recurrence, such as patients with luminal-like disease, generally with small tumor size and low nodal involvement. This may be due to the fact that ART in breast cancer survivors is not considered standard of care and many physicians are uncomfortable to suggest it after the end of anticancer therapies.
Although these data demonstrate that performing COS at diagnosis or ART following treatment completion do not seem to worsen the prognosis of young breast cancer patients, it should be noted that there are several limitations in our work. First of all, our meta-analysis is based on abstracted data and the majority of the studies included were retrospective cohort studies, with different matching criteria between the study population and controls. Among all the studies included in our analysis, less than half of them reported a trend towards less aggressive baseline breast cancer characteristics (i.e. smaller tumor size, less nodal involvement and grading, higher frequency of hormone receptor-positive disease) in patients undergoing fertility preservation techniques as compared to controls (Goldrat et al., 2015; Kim et al., 2016; Vriens et al., 2020; Greer et al., 2021; Marklund et al., 2021; Condorelli et al., 2021a,b).
Moreover, most of the studies reported just the rate of cancer recurrence without reporting ‘time-to-event’ endpoints and follow-up is relatively short for many of the included studies. Even if breast cancer recurrences are higher during the first 5 years from diagnosis, some patients like those with hormone receptor-positive disease are also at high risk of late recurrence (Pan et al., 2017). Median follow-up was shorter than 5 years in six of the included studies, thus results are to be considered immature to capture long-term differences in this setting and longer follow-up is needed. The risk of bias in the selection of patients with favorable prognostic characteristics and the relatively short follow-up of some of the studies included in this meta-analysis are the two main factors that were considered in the risk of bias assessment. The results of risk of bias assessment and potential confounding factors in the included studies must be considered in the overall interpretation of our results. Although our results seem to indicate a better prognosis of patients undergoing fertility preservation strategies, considering these limitations, we conclude that COS and ART are unlikely to increase the risk of breast cancer recurrence more than having a protective effect. However, despite these limitations, our meta-analysis provides important updated evidence that accessing COS at diagnosis or ART following breast cancer treatment completion does not appear to be associated with any detrimental prognostic effect in young women, including patients with hormone receptor-positive disease or those receiving neoadjuvant chemotherapy, while it is associated with a short and non-clinically relevant 6-day delay in starting anticancer treatments. These results are important to reassure patients and oncologists on the safety of these procedures in order to increase patients’ chances of future conception. However, future well-designed prospective studies with long-term follow-up are needed to further strengthen these findings.
Data availability
The data underlying this article are available on Medline, Web of Science, Embase and Cochrane library.
Authors’ roles
L.A., E.B. and M.L. conceived and designed the work. L.A., E.B., M.M.L., A.B., C.M., M.G.R., D.F., S.S., M.C., C.M., O.G., L.D.M., I.D. and M.L. acquired the data and revised the manuscript for important intellectual content. M.B., M.C. and E.B. performed the statistical analysis. All authors interpreted the data. L.A., E.B. and M.L. drafted the work and all other authors revised it critically and gave final approval for publication.
Funding
This study was partially supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC; grant number MFAG 2020 ID 24698) and the Italian Ministry of Health—5 × 1000 funds 2017 (no grant number).
Conflict of interest
M.L. acted as consultant for Roche, Pfizer, Novartis, Lilly, AstraZeneca, MSD, Exact Sciences, Gilead, Seagen and received speaker honoraria from Roche, Pfizer, Novartis, Lilly, Ipsen, Takeda, Libbs, Knight, Sandoz outside the submitted work. F.S. acted as consultant for Novartis, MSD, Sun Pharma, Philogen and Pierre Fabre, and received speaker honoraria from Roche, Novartis, BMS, MSD, Merck, Sun Pharma, Sanofi, and Pierre Fabre outside the submitted work. I.D. has acted as a consultant for Roche, has received research grants from Roche and Ferring, has received reagents for academic clinical trial from Roche diagnostics, speaker’s fees from Novartis, and support for congresses from Theramex and Ferring outside the submitted work. L.D.M. reported honoraria from Roche, Novartis, Eli Lilly, MSD, Pfizer, Ipsen, Novartis and had an advisory role for Roche, Eli Lilly, Novartis, MSD, Genomic Health, Pierre Fabre, Daiichi Sankyo, Seagen, AstraZeneca, Eisai outside the submitted work. The other authors declare no conflict of interest. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Supplementary Material
References
- Azim AA, Costantini-Ferrando M, Oktay K.. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol 2008;26:2630–2635. [DOI] [PubMed] [Google Scholar]
- Azim HA, Partridge AH.. Biology of breast cancer in young women. Breast Cancer Res 2014;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haroush A, Farhi J, Ben-Aharon I, Sapir O, Pinkas H, Fisch B.. High yield of oocytes without an increase in circulating estradiol levels in breast cancer patients treated with follicle-stimulating hormone and aromatase inhibitor in standard gonadotropin-releasing hormone analogue protocols. Isr Med Assoc J 2011;13:753–756. [PubMed] [Google Scholar]
- Blondeaux E, Massarotti C, Fontana V, Poggio F, Arecco L, Fregatti P, Bighin C, Giannubilo I, Ruelle T, Razeti MG. et al. The PREgnancy and FERtility (PREFER) Study Investigating the need for ovarian function and/or fertility preservation strategies in premenopausal women with early breast cancer. Front Oncol 2021;11:690320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi B, Massarotti C, Bruzzone M, Goldrat O, Mangili G, Anserini P, Spinaci S, Arecco L, Del Mastro L, Ceppi M. et al. Efficacy and safety of controlled ovarian stimulation with or without letrozole co-administration for fertility preservation: a systematic review and meta-analysis. Front Oncol 2020;10:574669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Chambers J, Mcauley F, Kaplan T, Letourneau J, Hwang J, Kim M-O, Melisko ME, Rugo HS, Esserman LJ. et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II–III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat 2017;165:151–159. [DOI] [PubMed] [Google Scholar]
- Condorelli M, Bruzzone M, Ceppi M, Ferrari A, Grinshpun A, Hamy AS, de Azambuja E, Carrasco E, Peccatori FA, Di Meglio A. et al. Safety of assisted reproductive techniques in young women harboring germline pathogenic variants in BRCA1/2 with a pregnancy after prior history of breast cancer. ESMO Open 2021a;6:100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli M, De Vos M, Lie Fong S, Autin C, Delvigne A, Vanden Meerschaut F, Wyns C, Imbert R, Cheruy C, Bouziotis J. et al. Impact of ARTs on oncological outcomes in young breast cancer survivors. Hum Reprod 2021b;36:381–389. [DOI] [PubMed] [Google Scholar]
- de Melo Gagliato D, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, Hortobagyi GN, Chavez-Macgregor M.. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014;32:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo Gagliato D, Lei X, Giordano SH, Valero V, Barcenas CH, Hortobagyi GN, Chavez-MacGregor M.. Impact of delayed neoadjuvant systemic chemotherapy on overall survival among patients with breast cancer. Oncologist 2020;25:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Rosenberg E, Einbeigi Z, Bergh C, Strandell A.. Gonadotrophin stimulation and risk of relapse in breast cancer. Hum Reprod Open 2021;2021:hoaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrat O, Kroman N, Peccatori FA, Cordoba O, Pistilli B, Lidegaard O, Demeestere I, Azim HA.. Pregnancy following breast cancer using assisted reproduction and its effect on long-term outcome. Eur J Cancer 2015;51:1490–1496. [DOI] [PubMed] [Google Scholar]
- Greer AC, Lanes A, Poorvu PD, Kennedy P, Thomas AM, Partridge AH, Ginsburg ES.. The impact of fertility preservation on the timing of breast cancer treatment, recurrence, and survival. Cancer 2021;127:3872–3880. [DOI] [PubMed] [Google Scholar]
- Hershlag A, Mullin C, Bristow SL.. Is fertility preservation feasible and safe with neoadjuvant therapy for breast cancer? JCO Glob Oncol 2020;356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Turan V, Oktay K.. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab 2016;101:1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, Paluch-Shimon S, Loibl S, Partridge AH, Demeestere I. et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast 2018;42:41–49. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Di Maio M, Poggio F, Pagani O, Curigliano G, Mastro LD, Paluch-Shimon S, Loibl S, Partridge AH, Azim HA. et al. Knowledge, attitudes and practice of physicians towards fertility and pregnancy-related issues in youngBRCA-mutated breast cancer patients. Reprod Biomed Online 2019;38:835–844. [DOI] [PubMed] [Google Scholar]
- Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg J-B, Paluch-Shimon S, Halaska MJ, Uzan C, Meissner J. et al. ; ESMO Guidelines Committee. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2020;31:1664–1678. [DOI] [PubMed] [Google Scholar]
- Letourneau JM, Wald K, Sinha N, Juarez-Hernandez F, Harris E, Cedars MI, McCulloch CE, Dolezal M, Chien AJ, Rosen MP.. Fertility preservation before breast cancer treatment appears unlikely to affect disease-free survival at a median follow-up of 43 months after fertility-preservation consultation. Cancer 2020;126:487–495. [DOI] [PubMed] [Google Scholar]
- Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, Olivotto IA.. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 2006;24:4888–4894. [DOI] [PubMed] [Google Scholar]
- Marklund A, Eloranta S, Wikander I, Kitlinski ML, Lood M, Nedstrand E, Thurin-Kjellberg A, Zhang P, Bergh J, Rodriguez-Wallberg KA.. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer—a prospective nationwide Swedish multicenter study. Hum Reprod 2020;35:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund A, Lundberg FE, Eloranta S, Hedayati E, Pettersson K, Rodriguez-Wallberg KA.. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol 2021;7:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Raanani H, Maman E, Paluch-Shimon S, Shapira M, Cohen Y, Kuchuk I, Hourvitz A, Levron J, Mozer-Mendel M. et al. Tamoxifen co-administration during controlled ovarian hyperstimulation for in vitro fertilization in breast cancer patients increases the safety of fertility-preservation treatment strategies. Fertil Steril 2014;102:488–495.e3. [DOI] [PubMed] [Google Scholar]
- Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL.. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin 2020;70:443–459. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravek MB, Confino R, Lawson AK, Smith KN, Kazer RR, Klock SC, Gradishar WJ, Jeruss JS, Pavone ME.. Predictors and outcomes in breast cancer patients who did or did not pursue fertility preservation. Breast Cancer Res Treat 2021;186:429–437. [DOI] [PubMed] [Google Scholar]
- Moravek MB, Confino R, Smith KN, Kazer RR, Klock SC, Lawson AK, Gradishar WJ, Pavone ME.. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil Steril 2018;109:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E, Domingo J, De Castro G, Lorenzo I, García-Velasco JA, Bellver J, Pellicer A, Garrido N.. Ovarian stimulation for oocyte vitrification does not modify disease-free survival and overall survival rates in patients with early breast cancer. Reprod Biomed Online 2019;39:860–867. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z.. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005;23:4347–4353. [DOI] [PubMed] [Google Scholar]
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW.. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, et al. ; EBCTCG. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017;377:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong Y-N, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP. et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016;34:3308–3314. [DOI] [PubMed] [Google Scholar]
- Perachino M, Massarotti C, Razeti MG, Parisi F, Arecco L, Damassi A, Fregatti P, Solinas C, Lambertini M.. specific aspects related to type of fertility preservation strategies and access to fertility care. ESMO Open 2020;5:e000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Eloranta S, Krawiec K, Lissmats A, Bergh J, Liljegren A.. Safety of fertility preservation in breast cancer patients in a register-based matched cohort study. Breast Cancer Res Treat 2018;167:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E, Fredriksson A, Einbeigi Z, Bergh C, Strandell A.. No increased risk of relapse of breast cancer for women who give birth after assisted conception. Hum Reprod Open 2019;2019:hoz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa H, Hagiwara R, Takahara S, Yamauchi A.. Assisted reproductive technology is effective for but does not affect the prognosis of young patients with breast cancer: experience in a single institution. Breast J 2018;24:1001–1005. [DOI] [PubMed] [Google Scholar]
- The ESHRE Guideline Group on Female Fertility Preservation; Anderson RA, Amant F, Braat D, D’Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020;2020:hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan V, Bedoschi G, Moy F, Oktay K.. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril 2013;100:1681–1685.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens IJH, Welle-Butalid EM, ter Boer M, de Die-Smulders CEM, de Derhaag JG, Geurts SME, Hellemond IEG, van Luiten EJT, Dercksen MW, Lemaire BMD. et al. Preserving fertility in young women undergoing chemotherapy for early breast cancer; the Maastricht experience. Breast Cancer Res Treat 2020;181:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins C, Bennett I.. A simple method for combining binomial counts or proportions with hazard ratios for evidence synthesis of time-to-event data. Res Synth Methods 2018;9:352–360. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P.. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses, 2014. Available to download from Ottawa Hospital Research Institute’s website: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (November 2021, last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available on Medline, Web of Science, Embase and Cochrane library.