Abstract
Study Objective
To evaluate whether the introduction of a 1‐hour high‐sensitivity cardiac troponin‐T (hs‐TnT) pathway for patients who present to the emergency department (ED) with suspected acute coronary syndrome (ACS) improves ED patient flow without changing the rate of “missed” major adverse cardiac events (MACE), compared to use of conventional cardiac troponin with an associated 3‐hour pathway.
Methods
This was a prospective, uncontrolled observational study conducted before and after implementation of a 1‐hour hs‐TnT pathway at a high‐volume urban ED. Patients undergoing evaluation for ACS in the ED were enrolled during their initial visit and clinical outcomes were assessed at 30 and 90 days. Throughput markers were extracted from the electronic medical record and compared. The primary outcome was provider‐to‐disposition decision time.
Results
A total of 1892 patients were enrolled, 1071 patients while using conventional troponin and 821 after introduction of hs‐TnT. With the new assay and pathway, median interval between troponin tests decreased from 4.7 hours (interquartile range [IQR] 3.9–5.7 hours) to 2.3 hours (IQR 1.5–3.4 hours) (P < 0.001). However, there was no difference in median provider‐to‐disposition decision time, which measured 4.7 hours (IQR 2.9–7.2) and 4.8 hours (IQR 3.1–7.1) (P = 0.428) respectively. Total 30‐day MACE rate in discharged patients was low in both groups, occurring in only 4/472 (0.85%) encounters in the first cohort and 4/381 (1.0%) encounters in the second.
Conclusion
Introduction of a 1‐hour hs‐TnT ACS evaluation pathway reduced the troponin collection interval but did not reduce provider to disposition time. There was no difference in rate of 30‐day MACE in patients discharged from the ED.
Keywords: acute coronary syndrome, high‐sensitivity cardiac troponin, length of stay, myocardial infarction, throughput, troponin
1. INTRODUCTION
1.1. Background
Evaluation for acute coronary syndrome (ACS) has long been a major consumer of emergency department resources. 1 , 2 ED visits for “chest pain” alone numbered more than 7 million during 2018 in the United States. 3 Over the years, a decreasing percentage of patients presenting to the ED with symptoms of possible ACS are ultimately found to have an acute cardiac event 4 and less than 5% of all chest pain visits are diagnosed with any emergent diagnosis, of which ACS is a subset. 5
The recent availability of high‐sensitivity cardiac troponin (hs‐Tn) in the United States changes the landscape of the evaluation of patients with possible ACS. These new assays have the potential to lower the rate of missed acute myocardial infarction (AMI) and improve care efficiency as their increased precision allows for both earlier detection and more rapid evaluation for ACS. 6 , 7 Whereas conventional troponin enzymes required serial measurements 3–6 hours apart to evaluate for ACS, an interval of 1‐hour between serial hs‐Tn is sufficient to guide ED discharge without increasing the rate of missed adverse events, 8 and that even a single troponin rule out strategy may be appropriate in some circumstances. 9
1.2. Importance
There have been concerns about the implications of the transition to hs‐Tn on ED patient flow given the broad use of troponin testing and possibility of undifferentiated troponin elevation. Studies have demonstrated the ability of hs‐Tn to rapidly exclude AMI and identify patients at low risk for ACS as well as reduce ED length of stay (LOS) compared to conventional troponin in clinical trial settings. 10 However, impact on patient flow in real‐world settings have been less frequently described and mostly in European and Australasian settings that may not translate to American EDs. 10 , 11 , 12 , 13 , 14 , 15 , 16
Understanding how the different test characteristic and diagnostic algorithms influence ED throughput is an important consideration in assessing the overall implications of hs‐Tn adoption.
The Bottom Line
Introduction of a high‐sensitivity troponin pathway has potential to reduce the rate of missed myocardial infarction while improving emergency department (ED) throughput. In this prospective, observational single‐center study of nearly 2000 patients with chest pain evaluated for acute coronary syndrome in an urban ED, the introduction of a high‐sensitivity troponin pathway reduced the time to repeat troponin testing by 2.4 hours compared to the conventional pathway but did not decrease the provider‐to‐disposition decision time. There were no differences in major adverse cardiac events at 30 days.
1.3. Goals of this investigation
We sought to measure the impact of the introduction of a high‐sensitivity cardiac troponin‐T assay in 1 ED across the full spectrum of patients with suspected ACS. We evaluated whether the introduction of a fifth‐generation (hs‐TnT) assay with an associated clinical pathway recommending troponin measurements 1 hour apart, compared to use of conventional fourth‐generation troponin assay with a pathway recommending measurements 3 hours apart, would reduce ED patient evaluation time without increasing the rate of missed major adverse cardiac events (MACE). We hypothesized that evaluation time, defined as the interval between initial physician evaluation and disposition decision, would be safely shortened.
2. METHODS
2.1. Study design and setting
This was a prospective, uncontrolled, single‐center before and after implementation study on the impact of a new evaluation pathway using hs‐TnT. Approval for the study was obtained from the institutional review board at our institution. This study was registered with clinicaltrials.gov (NCT03590535). We followed the Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) guidelines for cohort studies.
The study was performed in the adult ED at a single large academic medical center in New York City, which averages over 100,000 annual visits and serves a diverse patient population. The hospital has neither an observation unit nor dedicated ACS unit. Patients were enrolled in the study from October 2018 until January 2020 and follow‐up was continued through March 2020.
2.2. Selection of participants
We included adult patients, age 19 years and older, who presented with symptoms that the treating ED clinician considered to be potentially caused by an ACS and who were receiving troponin testing as part of their evaluation. To accurately represent the spectrum of presentations in which ACS is considered in the ED, no particular symptoms were required for inclusion. We excluded patients without capacity to consent, acute ST‐segment–elevation myocardial infarction, pre‐heart transplant, left ventricular assist device, who were presenting after a cardiac arrest, lacked fluency in either English or Spanish, or were otherwise unable to participate in telephone follow‐up.
Patients were recruited by trained research associates and clinical study staff. The research associates were present in the ED from 8 am until midnight for 7 days a week during most of the study period, with occasional overnight coverage as well. Research associates obtained verbal consent for participation and contact information. Clinicians and patients were blinded to the study hypotheses.
2.3. Troponin testing pathways
Before July 2019, patients who presented with possible ACS were tested with either a point of care troponin I assay (Abbott i‐STAT Troponin‐I) or with a fourth‐generation cardiac troponin T assay performed in the core laboratory (Roche fourth‐generation Elecsys TnT, Roche Diagnostics GmbH). The existing ED pathway recommended repeat troponin testing at 3 hours for all patients and shared decision‐making regarding admission for patients with negative troponins based on HEART score risk stratification. 17 (Figure 1, Conventional Pathway, Supplemental Material).
FIGURE 1.
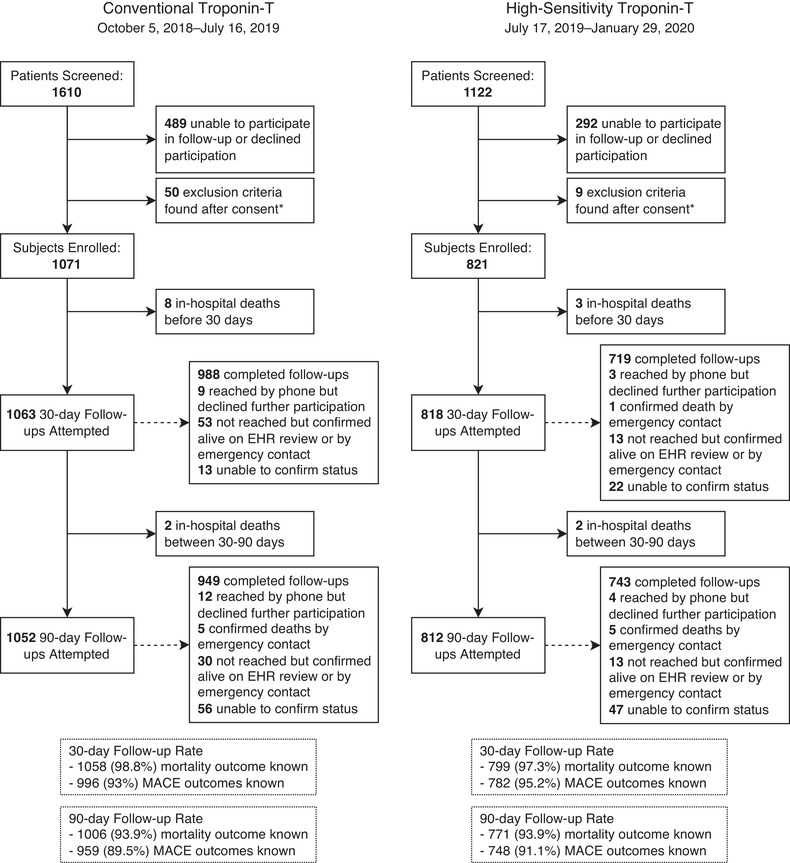
Flow diagram of patient enrollment. * These were mostly encounters where troponin testing was ultimately not performed in the ED, either because of misplaced specimens or change in the planned clinical workup. Other reasons for exclusion were 4 patients with heart transplant or left‐ventricular assist device, 2 patients who did not speak English or Spanish, 1 patient with STEMI, and 1 patient who had psychiatric decompensation noted during the ED and was likely lacked capacity to consent.” Abbreviations: ED, emergency department; EHR, electronic health record; MACE, major adverse cardiac events; STEMI, ST‐segment–elevation myocardial infarction
On July 17, 2019, the existing laboratory troponin assay was replaced with the Elecsys Troponin T Gen 5 assay (Roche Diagnostics). In conjunction with the rollout of this hs‐TnT assay, a new diagnostic pathway was also introduced. At the time of the hs‐TnT pathway design there was a wide range of workflows and reference limit values described in the literature. 18 A multidisciplinary group from emergency medicine, laboratory medicine, and cardiology, as well as nursing and administrative leadership, designed a local pathway consistent with expert recommendations developed to guide implementation in the United States. 19 It drew from the accelerated diagnostic protocol recommended in the 2015 European Society of Cardiology guidelines based on 0/1 hour hs‐Tn testing. 20 We adapted the European Society of Cardiology (ESC) guideline to use the US Food and Drug Administration (FDA)‐approved sex‐specific 99th percentile values of 14 ng/L in women and 22 ng/L in men, and the FDA cutoff limit of detection of 6 ng/L; we also kept the framework of encouraging shared decision‐making based on HEART score risk stratification in patients without troponin elevation. 21 Change in troponin concentration over time was also addressed. 22 Because of uncertainty regarding the ability to reliably repeat specimen collection at any fixed interval, rather than using a set delta troponin threshold we used a novel, not yet validated approach we termed a troponin “velocity,” which was calculated by dividing the change in troponin measurement by the actual time interval between specimen collections. Patients who were below the 99th percentile values on 0‐ and 1‐hour testing and who did not have troponin velocity above the threshold (2.5 ng/L/h) were considered to have AMI excluded as a diagnosis. (Figure 2, hs‐TnT Pathway, Supplemental Material).
FIGURE 2.
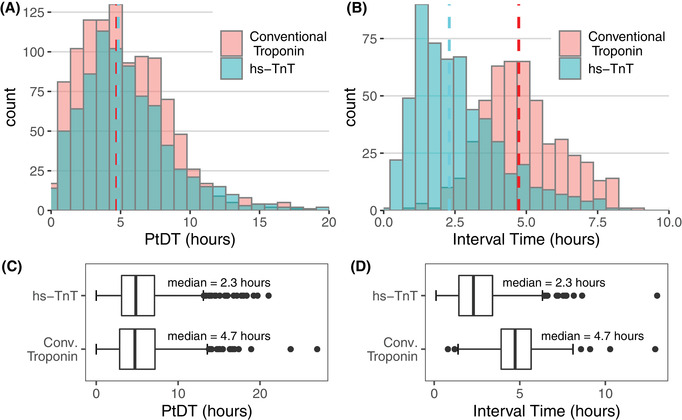
Provider‐to‐decision time (PtDT) and serial troponin draw intervals before and after rapid algorithm and hs‐TnT implementation. Boxes A and B show histograms of PtDT and troponin interval time, respectively. The pink and teal bars represent the count of encounters in each bin for conventional and hs‐TnT cohorts, respectively; the shading is darker where the heights of the bars overlap. The dashed lines denote the medians. Note the axes are scaled differently between these 2 diagrams. Boxes C and D show the same data as box plots. The dark vertical line represents the median, and the limits of the box denote the interquartile range (IQR). The whiskers are set at 1.5*IQR above and below the 75th and 25th percentiles, respectively. Data beyond the end of the whiskers are plotted individually. The median PtDT was not significantly different between groups (P = 0.428); however, the median troponin draw interval did decrease significantly (P < 0.001).” Abbreviation: hs‐TnT, high‐sensitivity troponin T
2.4. Measurements
We collected baseline clinical characteristics to identify imbalance between cohorts. Race and ethnicity were collected from patient self‐report at the time of consent. Treating clinicians were asked to report the ECG findings to the research coordinator; other clinical data elements such as age, sex, comorbidities, vital signs, examination findings, and laboratory values were abstracted from the electronic medical records (EMR) by trained members of the study team. Abstraction was performed following an explicit protocol with pre‐set variables on a standardized instrument hosted and managed on a secure REDCap electronic data capture platform. 23 , 24 A sample of 10% of each abstractor's charts were independently reviewed by the principal investigator to measure interrater agreement, and we obtained a Cohen's kappa of 0.94, indicating excellent agreement.
2.5. Outcomes
The prespecified primary outcome of interest was the time interval between first clinician provider evaluation to disposition decision time (provider‐to‐disposition time, PtDT). We chose PtDT rather than the ED length of stay, as LOS would have been confounded by prolonged time intervals between arrival to initial provider evaluation (nearly 1 hour on average) and admission decision to departure from the ED (over 9 hours on average). Measurements were obtained from patient movement timestamps present in the EMR. The provider contact timestamp generates when a clinician assigns themselves to a patient's care on the EMR and the disposition timestamp when an order is placed to discharge the patient from the ED (including discharge against medical advice) or when a bed is requested for hospital admission. We also evaluated as a secondary clinical outcome the prevalence of MACE diagnosed within 30 and 90 days of the initial ED visit. We were particularly interested in MACE occurring within 30 days of the initial evaluation when the clinician discharged the patient from the ED. We defined MACE to include the diagnosis of type I AMI, coronary revascularization procedures, ventricular arrhythmia, high degree atrioventricular block requiring intervention, cardiogenic shock requiring mechanical support, cardiac arrest with return of spontaneous circulation, and death. When data were obtained from hospital records, the diagnosis of type I myocardial infarction followed the third universal definition of myocardial infarction and was adjudicated by a cardiologist on the team; type II AMI was not categorized as a MACE. 25 We accepted the diagnosis as reported by the patient when the outcomes were identified only on telephone follow‐up.
Telephone follow‐up was performed by trained research personnel fluent in English and Spanish. An explicit protocol was used that involved structured interview questions prepared in both languages, and data were collected on a standardized instrument. At 30 days and 90 days after the initial visit, the EMR was reviewed to identify medical encounters and diagnoses, both at our hospital and in the regional health care network. To ensure accuracy, all charts that were identified by the abstraction team as containing a MACE were independently reviewed by a cardiologist. The previous protocols remained in place from the commencement of the study until the end, and there was no difference in method of assessment between cohorts.
2.6. Analysis
Given a large amount of variability in provider to disposition time in general practice, we estimated based on a 2‐sided 2‐sample equal‐variance t test that for a significance level (alpha) of 0.05 we would require 770 subjects per group to detect a minimum difference of 30 minutes at 80% power if SD was 210 minutes in each.
Statistical analyses were performed with R (version 3.6.0; R Core Team, Vienna, Austria). For the primary hypothesis, we explored the difference in the distribution of PtDT between cohorts using a nonparametric Wilcoxon rank‐sum test. Differences in baseline clinical factors between groups were summarized with Χ2 for categorical variables and either t test for normally distributed or Mann‐Whitney U test for non‐normally distributed continuous variables. Clinical outcomes such as rates of ED disposition, AMI, or MACE were summarized with Χ2 and Fisher exact tests and 95% confidence intervals (CI) around differences in proportions were derived using Wilson's method.
3. RESULTS
3.1. Characteristics of study subjects
We enrolled 1071 patients in the first cohort and 821 in the second. Slight delay in the date of hs‐TnT deployment in the laboratory caused the imbalance in group size. Baseline characteristics of study participants within each cohort are reported in Table 1. There were minimal differences in conventional cardiac risk factors between cohorts except for body mass index (BMI). BMI was infrequently reported in the earlier cohort, with height and weight data temporally close to the encounter being available only 25.6% of the time, whereas in the latter cohort it was documented in 48.7% of encounters. The rate of successful follow‐up was similar between groups; we achieved 93% and 89.5% follow‐up in the first cohort at 30 and 90 days, and 95.2% and 91.1% at 30 and 90 days in the second. In some cases, we were able to ascertain whether the patient was alive or deceased but were not able to assess for all MACE outcomes. Data on follow‐up success by outcome, time‐period, and cohort are included in Figure 1.
TABLE 1.
Patient baseline characteristics
| Conventional troponin | hs‐TnT | P value | |
|---|---|---|---|
| Patients (n) | 1071 | 821 | |
| Age in years (mean, SD) | 60.3 (15.8) | 60.4 (15.9) | 0.816 |
| Female (n, %) | 538 (50.2) | 447 (54.4) | 0.077 |
| Race and ethnicity (n, %): | |||
| Hispanic | 587 (56.7) | 472 (60.4) | 0.117 |
| American Indian/Alaska Native | 12 (1.1) | 6 (0.7) | 0.531 |
| Asian | 23 (2.1) | 21 (2.6) | 0.665 |
| Native Hawaiian or OPI | 3 (0.3) | 3 (0.4) | 1 |
| Black or African American | 298 (27.8) | 209 (25.5) | 0.271 |
| White or Caucasian | 187 (17.5) | 110 (13.4) | 0.019 |
| Other | 502 (46.9) | 441 (53.7) | .004 |
| Do not wish to disclose | 57 (5.3) | 43 (5.2) | 1 |
| Systolic blood pressure (mean, SD) | 142.9 (27.1) | 140.3 (27.4) | 0.204 |
| Heart rate (median [IQR]) | 82 (70,95) | 81 (70,94) | 0.075 |
| Comorbidity (n, %): | |||
| Hypertension | 765 (71.4) | 563 (68.6) | 0.196 |
| Hyperlipidemia | 436 (40.7) | 367 (44.7) | 0.09 |
| Diabetes | 375 (35.0) | 297 (36.2) | 0.635 |
| Tobacco use | 136 (13.2) | 85 (11.2) | 0.225 |
| Family historya | 100 (14.8) | 80 (15.4) | 0.837 |
| Obesityb | 159 (57.8) | 165 (41.2) | <0.001 |
| Previous CAD diagnosis | 288 (26.9) | 213 (25.9) | 0.682 |
| Any atherosclerotic disease | 347 (32.4) | 270 (32.9) | 0.861 |
| Congestive heart failure | 199 (18.6) | 148 (18.0) | 0.806 |
| Chronic kidney disease | 164 (15.3) | 131 (16.0) | 0.742 |
| On hemodialysis | 36 (3.4) | 27 (3.3) | 1 |
No other past medical history and risk factors category had a significant proportion of missing data, with the next highest category being tobacco history at 5.3% missing.
P values were calculated using Χ2 with continuity correction for categorical variables and t test or Mann‐Whitney U test for continuous variables.
Abbreviations: CAD, coronary artery disease; hs‐TnT, high‐sensitivity troponin T; IQR, interquartile range; OPI, Other Pacific Islander
aFamily history of coronary artery disease was available in only 64.1% of encounters.
bDiagnosis of obesity or measurement of body mass index was available in only 25.6% of encounters in the first cohort and 48.7% of encounters in the second.
3.2. Main results
The primary outcome was PtDT. There was no significant difference in median PtDT between cohorts, which was 4.7 hours (IQR, 2.9–7.2) in the conventional troponin group and 4.8 hours (IQR, 3.1–7.1) in the hs‐TnT group (P = 0.428). There was also no difference in the overall ED LOS between the cohorts, which was median 11.3 hours (IQR, 8.1–20.2) in first group and 11.5 hours (IQR 7.6–22.9) in the second (P = 0.962). Given these findings, we conducted an analysis of the time intervals between serial troponin tests. These, in contrast, decreased significantly between the 2 groups, from a median of 4.7 hours (IQR 3.92–5.67) in the conventional troponin cohort to 2.3 hours (IQR 1.45–3.42) in the hs‐TnT cohort (P < 0.001). A significantly higher percentage of patients received serial troponin testing in the hs‐TnT cohort, 560/821 (68.2%) versus 484/1071 (45.2%) (difference = 23%; 95% CI = 18.5%, 27.5%; P < 0.001). A graphic of the distribution of PtDT and troponin interval between groups is provided in Figure 2.
Secondary outcomes are summarized in Table 2. Difference in disposition from the ED between groups did not reach statistical significance. The proportion of patients discharged from the ED in the hs‐TnT cohort compared to the conventional troponin cohort was 48.8% versus 45.8%, (difference = 3%; 95% CI = ‐1.6%, 4.5%, P = 0.212); the combined proportion of patients who left against medical advice or eloped from the ED before completing evaluation was 3.0% versus 2.6% (difference 0.4%; 95% CI ‐2.1%, 1.2%, P = 0.673).
TABLE 2.
Disposition and clinical outcomes
| Conventional troponin | hs‐TnT | ||||||
|---|---|---|---|---|---|---|---|
| Number | N | % | Number | N | % | P value | |
| First troponin > 99th percentile | 156 | 1071 | 14.6 | 260 | 821 | 31.7 | <0.001 |
| Disposition from the ED | 1071 | 821 | 0.519 | ||||
| Discharged | 491 | 45.8 | 401 | 48.8 | |||
| Admitted | 551 | 51.4 | 395 | 48.1 | |||
| Eloped | 15 | 1.4 | 15 | 1.8 | |||
| Left against medical advice | 13 | 1.2 | 10 | 1.2 | |||
| Transferred | 1 | 0.1 | 0 | 0 | |||
| Outcomesa | |||||||
| AMI within 30 daysb | 21 | 996 | 2.1 | 20 | 782 | 2.6 | 0.640 |
| MACE within 30 daysc | 69 | 996 | 6.9 | 45 | 782 | 5.8 | 0.366 |
| MACE between 30–90 days | 31 | 959 | 3.2 | 22 | 748 | 2.9 | 0.839 |
| MACE during hospital admission after initial ED visitd | 57 | 551 | 10 | 35 | 395 | 8.9 | 0.517 |
| MACE within 30 days when discharged after initial ED visit | 4 | 472 | 0.85 | 4 | 381 | 1.0 | 1 |
| MACE within 90 days when discharged after initial ED visite | 9 | 432 | 2.0 | 6 | 362 | 1.7 | 0.796 |
aOutcome assessments reflect completed follow‐ups, see Figure 1 for details.
bPrevalence of AMI in each cohort rather than the incident number of AMIs.
cPrevalence of MACE in each cohort, inclusive of AMI which are also separately reported above.
dAll patients who had a MACE during hospitalization had one occur within 30 days of ED visit.
ePrevalence of MACE within 90 days, inclusive of 30‐day MACE outcomes.
Abbreviations: AMI, acute myocardial infarction; ED, emergency department; hs‐TnT, high‐sensitivity troponin T; MACE, major adverse cardiac event.
The percentage of patient encounters that had any troponin measurement above the 99th percentile threshold rose markedly from 14.6% to 31.7% between the 2 groups (difference = 17.1%; 95% CI = 13.2%, 21.0%; P < 0.001. However, the rate of AMI and other MACE in the entire study population over the study period was low and largely similar between cohorts (see Table 2). The number of patients reporting a MACE after being discharged from the ED during their initial visit was acceptably low in both groups at both 30 and 90 days.
4. LIMITATIONS
This study has several limitations. Baseline characteristics between the 2 groups may have differed with respect to obesity, which we are unable to determine owing to a large amount of missing data; obesity data availability was greater in the postintervention period due to the introduction of an automated body mass index calculation in the EMR.
The study design permits entry of other potential confounding factors as well. Although no major operational changes happened during the study period other than the implementation of hs‐TnT, we were not able to undertake an analysis of competing secular trends that might disguise the effect of the new algorithm and assay. Tracking movement data from the EMR conforms to standard reporting of operational metrics but is only a proxy for the actual clinician's disposition decision time. 26 Most subjects were approached between 8 am and midnight, which could bias toward enrolling patients with longer lengths of stay as patients seen and discharged overnight would have been missed.
A significant number of patients in our study were not evaluated according to the recommended serial‐troponin pathway. A total 55% of patients in the preimplementation cohort and 32% of patients in the postimplementation cohort had only a single troponin sent before disposition, a finding that has the potential to have foreshortened disposition time in the preintervention compared with the postintervention groups and therefore led to a convergence of results with respect to the primary outcome. Deviation from local pathways is reflective of actual clinical practice and should be considered in that context when weighing its importance as a potential limitation. Repeat troponin enzyme testing in our cohorts may also have been affected by hemolysis affecting interpretation of hs‐TnT, which would require repeat testing to resolve. 27
The use of PtDT as our primary outcome measure limits direct comparison to the more commonly reported outcome of ED LOS. Further, our pathway used a not yet validated troponin “velocity” approach rather than a fixed delta interval to set the threshold for abnormal rise, which may also have negative implications for the generalizability of our results.
Finally, there was imbalance in group size (enrollment ratio of approximately 0.77) and significant skew in the distribution of the primary outcome requiring a nonparametric test. However, this did not significantly affect our power to detect a difference in our primary outcome as the enrolled sample size was still larger than the required one to detect a 30‐minute difference at 80% power with at that ratio.
5. DISCUSSION
Introduction of a 1‐hour hs‐TnT based pathway instead of a 3‐hour conventional troponin pathway did not reduce median provider to decision time in patients despite an over 2‐hour reduction in the interval between serial troponin collections.
Inconsistent impact on ED throughput despite reduction in troponin testing intervals has been observed in previous studies as well. In the RAPID‐TnT trial, median ED LOS decreased by only 1 hour despite a 2‐hour reduction in the time interval between troponin testing. 10 , 28 Similarly, the APACE investigators reported a median decrease in time to discharge of only 79 minutes after adopting a protocol that reduced interval time by 3 hours. 11 In the multisite TRAPID‐AMI study, investigators found 2 sites actually showed a LOS increase after implementation of a 1‐hour hs‐TnT protocol compared to existing local standard care. 6 We suggest there are common factors driving these findings.
Underlying ED processes inefficiencies can clearly counteract improvement in throughput. This was demonstrated in studies such as Rapid‐CPU, where transition from the ESC 0/3‐hour protocol to the 0/1‐hour algorithm resulted in a 2 hour improvement in LOS, except during periods of ED crowding where the improvement disappeared. 12 , 13 Although we had chosen PtDT as our primary outcome to attenuate the confounding impact of crowding on throughput measurement, baseline operating conditions still erode the effect of more rapid troponin protocols. This is evinced by the gap between the recommended and actual troponin collection intervals in both the conventional and hs‐TnT cohorts.
The high rate of single‐troponin evaluations is a major driver of our findings as well. Despite the conventional troponin pathway recommending repeating testing at 3 hours on all patients who had a negative initial troponin, half of the patients in the first cohort received just a single test. A comparable experience was reported by a large UK trial evaluating implementation of a single‐troponin hs‐Tn protocol at multiple sites with existing local 2‐troponin pathways. There, investigators found no improvement in the rate of patients being discharged within 4 hours postimplementation. Though only 9% of patients should have met criteria for early discharge under existing pathways, in fact 37% of patients were already being discharged early. 29
We further note that the introduction of the hs‐Tn assays seems to alter the way clinicians use the tests in clinical practice. The adoption of fifth‐generation hs‐TnT has previously been associated with decreasing the proportion of all ED patients who are evaluated with troponin enzymes. 30 We found increased use of serial troponin testing after introduction of hs‐TnT. This is consistent with data from Australia reporting the rate of single troponin evaluation encounters fell from 46.7% to 37.6% postimplementation of hs‐Tn. 14 There are likely physician, nurse, and patient factors underlying these trends. Physicians, being unfamiliar with the characteristics of the new enzyme, may have been less comfortable with “off‐protocol” practice and more likely to complete the serial testing. Although there was no difference in the method of troponin sample collection, nursing staff received dedicated training on hs‐TnT given the suggested 1‐hour interval; this could have driven increased attention to completing phlebotomy orders on these patients. Patients and clinicians may have simply been more willing to wait for a second troponin test with the knowledge that they were being asked to add only 1 hour to their stay, as opposed to 3 as was the case with conventional troponins. As clinical familiarity builds and environments of care change, we anticipate that practice patterns will continue to evolve.
Although the introduction of a 1‐hour pathway did not improve PtDT, the rate of MACE in patients discharged after pathway implementation remained acceptably low without increase in the admission rate. These findings suggest that deploying a rapid high‐sensitivity troponin T protocol provides an acceptable level of safety even in clinical practice environments with significant operational challenges. However, our results also reinforce previous literature that indicates underlying operational inefficiencies cannot always be overcome by more rapid protocols alone, and improvement in patient flow requires attention to the specific factors that driving delay and missed time targets. Finally, we highlight the complex nature of expert clinical practice and the difficulties that poses for forecasting the consequences of innovations. Our study found that emergency physicians were already rapidly identifying patients at low risk for MACE using conventional troponin enzymes and introduction of hs‐TnT with associated rapid 1‐hour protocol did not improve either throughput outcomes or clinical safety. We anticipate as clinicians continue to adapt the new test to their practice, further research on real world use of hs‐TnT may yield important insights and future improvements in care.
CONFLICTS OF INTEREST
A.J.E.: received speaker's fee from Ionetix, consulting fees from W.L. Gore & Associates; and has grants/grants pending from Canon Medical Systems, GE Healthcare, and W.L. Gore & Associates. All of the other authors report no other conflicts of interest.
AUTHOR CONTRIBUTIONS
Edward Hyun Suh, Andrew J. Einstein, Aleksandr M. Tichter, Andrew Amaranto, Betty C. Chang, Alexander Kratz, and LeRoy E. Rabbani were responsible for the conception and design of the study and obtained research funding. Edward Hyun Suh, Lauren S. Ranard, Rebekah Jihae Lee, Phong Anh Huynh, Betty C. Chang, and Dana Sacco were involved in acquisition and/or interpretation of study data. Edward Hyun Suh, Andrew J. Einstein, and Aleksandr M. Tichter led the data analysis. Edward Hyun Suh drafted the manuscript and all authors contributed substantially to its revision.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
REDCap tool was hosted by Mailman School of Public Health Clinical and Translational Science Award Hub. The authors thank Dr. Marc Probst for help with production of the manuscript. This study was funded by an investigator‐initiated industry grant from Roche Diagnostics, the producers of the assay used. The study conception, design, execution, and analysis were the sole responsibility of the investigator team with the exception that Roche requested that the study follow patients for 90 days instead of an initially planned 30‐day period. Summarized statistical findings were shared with Roche prior to publication, but they did not have access to the source data, nor did they give input into the writing of the manuscript or restrict submission in any way. This study was funded by an investigator‐initiated grant from Roche Diagnostics, RD003699.
Biography
Edward H. Suh, MD, is an Assistant Professor of Emergency Medicine at Columbia University Irving Medical Center in New York, NY.

Suh EH , Tichter AM, Ranard LS, et al. Impact of a rapid high‐sensitivity troponin pathway on patient flow in an urban emergency department. JACEP Open. 2022;3:e12739. 10.1002/emp2.12739
Supervising Editors: Nichole Bosson, MD, MPH; Nicholas Johnson, MD.
Clinical Trials Registration: NCT03590535
REFERENCES
- 1. Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91(2):229–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 3. Cairns C, Kang K, Santo L. National hospital ambulatory medical care survey: 2018 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2018_ed_web_tables‐508.pdf
- 4. Bhuiya Farida A., Pitts Stephen R., McCaig Linda F.. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS Data Brief: National Center for Health Statistics; 2010(43):1–8. [PubMed] [Google Scholar]
- 5. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life‐threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176(7):1029–1032. [DOI] [PubMed] [Google Scholar]
- 6. Ambavane A, Lindahl B, Giannitsis E, et al. Economic evaluation of the one‐hour rule‐out and rule‐in algorithm for acute myocardial infarction using the high‐sensitivity cardiac troponin T assay in the emergency department. PLoS One. 2017;12(11):e0187662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCord J. Will high‐sensitivity troponin assays lead to improved outcomes in patients with acute coronary syndrome? Coron Artery Dis. 2013;24(8):713–715. [DOI] [PubMed] [Google Scholar]
- 8. Ljung L, Lindahl B, Eggers KM, et al. A rule‐out strategy based on high‐sensitivity troponin and HEART score reduces hospital admissions. Ann Emerg Med. 2019;73(5):491–499. [DOI] [PubMed] [Google Scholar]
- 9. Musey PI, Jr. , Bellolio F, Upadhye S, et al. Guidelines for reasonable and appropriate care in the emergency department (GRACE): recurrent, low‐risk chest pain in the emergency department. Acad Emerg Med. 2021;28(7):718–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chew DP, Lambrakis K, Blyth A, et al. A randomized trial of a 1‐hour troponin T protocol in suspected acute coronary syndromes: the rapid assessment of possible acute coronary syndrome in the emergency department with high‐sensitivity troponin T study (RAPID‐TnT). Circulation. 2019;140(19):1543–1556. [DOI] [PubMed] [Google Scholar]
- 11. Twerenbold R, Jaeger C, Rubini Gimenez M, et al. Impact of high‐sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J. 2016;37(44):3324–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stoyanov KM, Hund H, Biener M, et al. RAPID‐CPU: a prospective study on implementation of the ESC 0/1‐hour algorithm and safety of discharge after rule‐out of myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2020;9(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoyanov KM, Biener M, Hund H, et al. Effects of crowding in the emergency department on the diagnosis and management of suspected acute coronary syndrome using rapid algorithms: an observational study. BMJ Open. 2020;10(10):e041757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenslade JH, Parsonage W, Foran L, et al. Widespread introduction of a high‐sensitivity troponin assay: assessing the impact on patients and health services. J Clin Med. 2020;9(6):1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vasile VC, Jaffe AS. High‐sensitivity cardiac troponin for the diagnosis of patients with acute coronary syndromes. Curr Cardiol Rep. 2017;19(10):92. [DOI] [PubMed] [Google Scholar]
- 16. Vigen R, Diercks DB, Hashim IA, et al. Association of a novel protocol for rapid exclusion of myocardial infarction with resource use in a US Safety Net Hospital. JAMA Netw Open. 2020;3(4):e203359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16(6):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howell SJ, Amsterdam EA, Mumma BE, López JE, Tran NK. Implementation of high‐sensitivity cardiac troponin: challenges from the international experience. Crit Pathw Cardiol. 2018;17(4):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Januzzi JL, Jr. , Mahler SA, Christenson RH, et al. Recommendations for institutions transitioning to high‐sensitivity troponin testing: JACC scientific expert panel. J Am Coll Cardiol. 2019;73(9):1059–1077. [DOI] [PubMed] [Google Scholar]
- 20. Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. [DOI] [PubMed] [Google Scholar]
- 21. Mahler SA, Stopyra JP, Apple FS, et al. Use of the HEART pathway with high sensitivity cardiac troponins: a secondary analysis. Clin Biochem. 2017;50(7–8):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4–5):201–203. [DOI] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581–1598. [DOI] [PubMed] [Google Scholar]
- 26. Welch SJ, Stone‐Griffith S, Asplin B, Davidson SJ, Augustine J, Schuur JD. Emergency department operations dictionary: results of the second performance measures and benchmarking summit. Acad Emerg Med. 2011;18(5):539–544. [DOI] [PubMed] [Google Scholar]
- 27. Li A, Brattsand G. Stability of serum samples and hemolysis interference on the high sensitivity troponin T assay. Clin Chem Lab Med. 2011;49(2):335–336. [DOI] [PubMed] [Google Scholar]
- 28. Papendick C, Blyth A, Seshadri A, et al. A randomized trial of a 1‐hour troponin T protocol in suspected acute coronary syndromes: design of the rapid assessment of possible ACS in the emergency department with high sensitivity troponin T (RAPID‐TnT) study. Am Heart J. 2017;190:25–33. [DOI] [PubMed] [Google Scholar]
- 29. Carlton EW, Ingram J, Taylor H, et al. Limit of detection of troponin discharge strategy versus usual care: randomised controlled trial. Heart. 2020;106(20):1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandoval Y, 3rd Askew JW, , Newman JS, et al. Implementing high‐sensitivity cardiac troponin T in a US Regional Healthcare System. Circulation. 2020;141(23):1937–1939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


