Abstract
Background:
While Tacrolimus/Sirolimus (T/S)-based graft-versus-host disease (GvHD) prophylaxis has been effective in preventing acute GvHD post hematopoietic cell transplantation (HCT), its efficacy and long-term outcome in matched (MUD) and mismatched unrelated donor (mMUD) setting is not well defined.
Methods:
Herein, we evaluated a consecutive case-series of 482 patients who underwent unrelated donor (URD) HCT (2005 – 2013) with T/S-based GvHD prophylaxis.
Results:
With a median follow-up of 6.2 years (range=2.4–11.3), the 5-year overall survival (OS) and relapse/progression-free survival were 47.5% (95%CI: 43.0–52.0) and 43.6% (95%CI: 39.1–48.1), respectively; and the 5-year cumulative incidence of non relapse mortality (NRM) and relapse were 24.9%, and 31.5%, respectively. In this cohort, mMUD was associated with worse OS (39.0% vs. 50.7% at 5 years, p=0.034), primarily due to greater risk of NRM (33.5% vs. 21.7%, p=0.038). While rates of relapse, acute (II-IV or III-IV) or chronic GvHD (limited or extensive) were not different, death caused by chronic GvHD (20.8% vs. 12.8%, p=0.022) and infection (33.0% vs. 18.1%, p<0.01) were significantly greater in mMUD. In multivariable analysis, high-risk disease (HR= 2.21, 95%CI: 1.16–4.23; p<0.01) and mMUD (HR=1.55, 95%CI:.1.15–2.08; p=0.004) were independent predictive factors for OS.
Conclusions:
T/S-based GvHD prophylaxis is an effective and acceptable GvHD prophylactic regimen. However, survival after mMUD remained poor, possibly related to the severity of chronic GvHD.
INTRODUCTION
Despite the increasing number of volunteer donors available through the registry, around 30–75% of patients do not have a well-matched (8/8 HLA-A, -B, -C, -DRB1) unrelated donor (MUD).1 While in most cases a 7/8 single HLA-mismatched donor is available for such patients, based on previously reported registry studies,2–4 mismatched unrelated donor (mMUD) allogeneic hematopoietic stem cell transplantation (alloHCT) is associated with inferior transplant outcomes regardless of the intensity of preparative regimen (myeloablative [MAC] or reduced intensity [RIC]) or graft source (bone marrow5 or peripheral blood stem cell [PBSC]).2,6,7 Lower overall survival (OS) rate in these recipients is largely due to the increased risk of graft-versus-host disease (GvHD) and non relapse mortality (NRM). Consequently, there is no currently established standard GvHD prophylaxis for mMUD.
We and others have evaluated a combination of tacrolimus and sirolimus (T/S), and demonstrated that this GvHD prophylactic regimen is associated with reduced incidence/severity of acute GvHD and NRM.8–15 In a randomized phase III trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN), no significant differences were seen in grades II-IV acute GvHD-free survival (GFS), disease-free survival (DFS), or overall survival (OS) when T/S-regimen was compared with tacrolimus/methotrexate (T/MTX) in the context of matched related donor (MRD) transplantation with total body irradiation (TBI)–based conditioning regimen. However, patients treated with T/S were found to have a significantly faster time to engraftment and lower incidence of oral mucositis.8 Similar results were achieved in a smaller single-center phase II randomized study of both MRD and MUD recipients, comparing T/S regimen with T/MTX.14
Currently, and in the absence of large multicenter randomized clinical trials, it is not clear whether T/S-based GvHD prophylaxis could improve the outcomes after MUD or mMUD HCT. In a recent study, Parody and colleagues reported their case series of 159 patients who received alloHCT from 8/8- (n=139) or 7/8-matched donors (n=20) with T/S as GvHD prophylactic regimen and demonstrated similar 3-year OS between the two groups despite the increased rate of grade II-IV acute GvHD in 7/8. The authors concluded that T/S-based regimen is associated with favorable outcomes after alloHCT, with OS of 55–70%, and nonsignificant differences in the overall outcomes irrespective of the presence of any mismatches at obligatory loci.16 However, this study was limited by its small sample size and inclusion of both MRD and MUD recipients.
In this study, we sought to assess the efficacy of T/S-based GvHD prophylactic regimen after alloHCT in the unrelated donor setting, and evaluated clinical variables associated with transplant outcome. We also explored the impact of individual HLA locus mismatch on transplant outcomes. To our knowledge this is the largest single-center analyses describing the outcome of patients undergoing HCT from MUD/mMUD with T/S-based GvHD prophylaxis with a long follow-up duration.
METHODS
Study Population
This study includes patients who underwent their first URD HCT at the City of Hope National Medical Center between 2005 and 2013 for malignant hematological disease. Myeloablative conditioning regimen (MAC) was defined as a single dose total body irradiation (TBI) > 500 cGy or > 800 cGy in fractionated doses, busulfan with dose of > 9 mg/kg, or melphalan with dose of > 150 mg/m2. Graft source was PBSC for all patients. Diseases included acute myeloid or lymphoid leukemia (AML or ALL), chronic myeloid leukemia (CML), other leukemia (chronic myelomonocytic leukemia, acute leukemia bi-phenotypic and undifferentiated, and polymorphocytic leukemia), myeloproliferative neoplasm (MPN), myelodysplastic syndrome (MDS), multiple myeloma (MM), Hodgkin’s (HL) and non-Hodgkin’s lymphoma (NHL). Disease Risk Index (DRI) was based on what has been described by Armand et al.17 Research was approved and conducted under the supervision and approval of Institutional Review Board (IRB) at the City of Hope.
GvHD Prophylaxis and Supportive Care
GvHD prophylaxis was administered according to previously published reports.9,12,13,15 Briefly, 12 mg (loading dose) of sirolimus was administered by mouth, 3 days prior to alloHCT (day −3), followed by 4 mg daily, with subsequent dose adjustments to maintain blood levels between 3 and 10 ng/mL. Tacrolimus was initially administered intravenously (IV) at the dose of 0.02 mg/kg per day, starting on day −3, then switched to an equivalent oral dose when oral intake was adequate after engraftment and before hospital discharge (approximately 3 to 4 weeks post HCT), to maintain target blood levels of 5 to 10 ng/mL. T/S were given for at least 6 months after transplantation unless there was a toxicity warranting discontinuation. Additional MTX was administered at a dose of 5 mg/m2 on days +1, +3, and +6 when the risk of GvHD was considered high (i.e. <10/10 MUD). The study cohort also included patients who received antithymocyte globulin (ATG) on a previously reported clinical trial13 or off-protocol at a dose of 0.5 mg/kg on day −3, 1.5 mg/kg on day −2, and 2.5 mg/kg on day −1. Other supportive care including infection prophylaxis were provided according to the City of Hope HCT Standard of Procedures (COH SOPs).
HLA Typing and Analysis
Patient and donor HLA high resolution typing (allele at 4 digits) included a combination of the following testing methods: sequence-based typing (SBT), sequence-specific primers (SSP) and sequence –specific oligonucleotide (SSO). HLA allele or antigen mismatch included both graft-versus-host and host-versus-graft directions. Alleles from HLA “G” groups were considered identical for this paper.
Outcome Definitions
OS was defined as time from HCT to death from any cause, or censored on the last known to be alive. Engraftment was defined as achieving an absolute neutrophil count of 500/mL for 3 consecutive days. NRM was defined as death from causes not related to disease relapse/progression, and relapse/progression was considered as a competing risk. NRM was censored at time of last follow-up if patients were alive and remained relapse/progression free. DFS and relapse were defined per CIBMTR criteria,7 NRM was considered a competing risk event for relapse. DFS and relapse were censored at time of last follow-up when they remained alive and free of relapse/progression. Grades II-IV and III-IV acute GvHD were defined by the Glucksberg scale,18 and chronic GvHD was defined as limited or extensive chronic GvHD according to the Seattle criteria.19 Relapse and NRM were considered as competing risk events for engraftment and GVHD. GvHD-free and relapse-free survival was defined as survival without disease relapse, acute GvHD grades III-IV and extensive chronic GvHD.
Statistical Analysis
The differences in the baseline patient and donor characteristics, conditioning regimen intensity, and GvHD prophylaxis regimen by HLA match type were showed in contingency tables and tested using two-sample Wilcoxon and chi-square tests whenever appropriate. OS and DFS by HLA match type were examined using Kaplan-Meier curves and log-rank test in the univariate analysis and Cox proportional hazards model in the multivariable analyses. NRM, relapse, engraftment, acute and chronic GvHD by HLA match type were assessed using cumulative incidence curves and Gray test in the univariate analysis and the proportional sub-distribution hazards model (Fine and Gray) for competing risks in the multivariable analysis. The stepwise backward selection procedure was used with variables having a univariable P value <0.20 being considered first. Forward selection was then applied for other variables in the multivariable regression models. The final multivariable regression models were constructed by keeping variables having a multivariable P value <0.10. The assumptions of proportionality for both Cox regression and Fine and Gray models were checked by corresponding tests and plots of the scaled Schoenfeld residuals or the cumulative sums of residuals whenever appropriate.20 No violations were found.
All tests were 2-sided at a significance level of 0.05. SAS 9.4 (SAS Institute, Cary, NC) was used to perform the analyses.
RESULTS
Patient Characteristics
In this retrospective study, approved by City of Hope Institutional Review Board, we evaluated a consecutive case series of 482 patients who underwent alloHCT using PBSC as graft source from 2005 to 2013. Of these, 131 patients were transplanted with a mMUD (mismatch in HLA allele or antigen) and the remaining 351 patients received transplantation from an 8/8 MUD (HLA-A, -B, -C, -DRB1). Patient and transplant characteristics are shown in Table 1. In summary, MUD recipients were older (median age of 54 vs. 45; p<0.001), more likely to be conditioned with RIC regimen (61.3% vs. 45.8%; p=0.002), had poorer performance status (24.8% vs. 13.7% with KPS of 70–80%; p=0.027), and more likely to have a CMV seronegative donor (59.5% vs. 45.0%, p=0.026). All patients in both groups received PBSC as graft source and T/S-based regimen for GvHD prophylaxis. Of the MUD HCT recipients, 20.8% received additional GvHD prophylaxis (i.e. MTX, ATG or both) compared to 73.3% in the mMUD group (p<0.001).
Table 1:
Patient, Disease and Transplant Characteristics
| 8/8 Match or HLA-DQ Mismatch MUD (N=351) | ≤7/8 at A, B, C, DR or Multiple Mismatch MMUD (N=131) | Total (N=482) | P value | |
|---|---|---|---|---|
|
| ||||
| Age at HSCT, years | <0.001 | |||
| Median | 54 | 45 | 52 | |
| Interquartile range | 43, 61 | 32, 57 | 39, 61 | |
| Range | (18–73) | (18–71) | (18–73) | |
| <50 | 131 (37.3%) | 76 (58%) | 207 (42.9%) | <0.001 |
| 50–59 | 107 (30.5%) | 29 (22.1%) | 136 (28.2%) | |
| 60+ | 113 (32.2%) | 26 (19.8%) | 139 (28.8%) | |
| Recipient sex | 0.063 | |||
| Male | 181 (51.6%) | 80 (61.1%) | 261 (54.1%) | |
| Female | 170 (48.4%) | 51 (38.9%) | 221 (45.9%) | |
| Year of HCT | 0.19 | |||
| 2005 | 5 (1.4%) | 2 (1.5%) | 7 (1.5%) | |
| 2006 | 23 (6.6%) | 8 (6.1%) | 31 (6.4%) | |
| 2007 | 35 (10%) | 14 (10.7%) | 49 (10.2%) | |
| 2008 | 40 (11.4%) | 17 (13%) | 57 (11.8%) | |
| 2009 | 53 (15.1%) | 23 (17.6%) | 76 (15.8%) | |
| 2010 | 43 (12.3%) | 23 (17.6%) | 66 (13.7%) | |
| 2011 | 61 (17.4%) | 10 (7.6%) | 71 (14.7%) | |
| 2012 | 57 (16.2%) | 16 (12.2%) | 73 (15.1%) | |
| 2013 | 34 (9.7%) | 18 (13.7%) | 52 (10.8%) | |
| Female donor to male recipient | 0.068 | |||
| Yes | 44 (12.5%) | 25 (19.1%) | 69 (14.3%) | |
| No | 307 (87.5%) | 106 (80.9%) | 413 (85.7%) | |
| Diagnosis | 0.82 | |||
| Acute Myeloid Leukemia | 164 (46.7%) | 53 (40.5%) | 217 (45%) | |
| Acute Lymphocytic Leukemia | 50 (14.2%) | 24 (18.3%) | 74 (15.4%) | |
| Chronic Myeloid Leukemia | 11 (3.1%) | 5 (3.8%) | 16 (3.3%) | |
| Chronic Lymphocytic Leukemia | 9 (2.6%) | 3 (2.3%) | 12 (2.5%) | |
| Leukemia, Other | 12 (3.4%) | 5 (3.8%) | 17 (3.5%) | |
| Myelodysplastic Syndrome | 38 (10.8%) | 10 (7.6%) | 48 (10%) | |
| Myeloproliferative Disorder | 16 (4.6%) | 5 (3.8%) | 21 (4.4%) | |
| Non-Hodgkin Lymphoma | 45 (12.8%) | 23 (17.6%) | 68 (14.1%) | |
| Hodgkin Lymphoma | 3 (0.9%) | 1 (0.8%) | 4 (0.8%) | |
| Multiple Myeloma | 3 (0.9%) | 2 (1.5%) | 5 (1%) | |
| Disease risk index | 0.91 | |||
| Low Risk | 16 (4.6%) | 6 (4.6%) | 22 (4.6%) | |
| Intermediate Risk | 191 (54.4%) | 75 (57.3%) | 266 (55.2%) | |
| High Risk | 122 (34.8%) | 41 (31.3%) | 163 (33.8%) | |
| Very High | 22 (6.3%) | 9 (6.9%) | 31 (6.4%) | |
| Conditioning intensity | 0.002 | |||
| Reduced Intensity | 215 (61.3%) | 60 (45.8%) | 275 (57.1%) | |
| Myeloablative | 136 (38.7%) | 71 (54.2%) | 207 (42.9%) | |
| GVHD prophylaxis | <0.001 | |||
| Tacrolimus, Sirolimus | 278 (79.2%) | 35 (26.7%) | 313 (64.9%) | |
| Tacrolimus, Sirolimus, +MTX | 49 (14%) | 77 (58.8%) | 126 (26.1%) | |
| Tacrolimus, Sirolimus, +ATG | 24 (6.8%) | 19 (14.5%) | 43 (8.9%) | |
| Stem cell source | - | |||
| Peripheral blood stem cells | 351 (100%) | 131 (100%) | 482 (100%) | |
| ABO blood group compatibility | 0.96 | |||
| ABO compatible | 162 (46.2%) | 62 (47.3%) | 224 (46.5%) | |
| Minor mismatch (donor is O) | 71 (20.2%) | 27 (20.6%) | 98 (20.3%) | |
| Major mismatch (recipient is O) | 80 (22.8%) | 27 (20.6%) | 107 (22.2%) | |
| Bidirectional (none are O) | 38 (10.8%) | 15 (11.5%) | 53 (11%) | |
| Donor/Recipient CMV serostatus | 0.026 | |||
| D−/R− | 44 (12.5%) | 10 (7.6%) | 54 (11.2%) | |
| D−/R+ | 165 (47%) | 49 (37.4%) | 214 (44.4%) | |
| D+/R− | 30 (8.5%) | 12 (9.2%) | 42 (8.7%) | |
| D+/R+ | 112 (31.9%) | 60 (45.8%) | 172 (35.7%) | |
| DQB1 | 0.10 | |||
| 1 | 22 (6.3%) | 14 (10.7%) | 36 (7.5%) | |
| 2 | 329 (93.7%) | 117 (89.3%) | 446 (92.5%) | |
| Karnofsky performance status % | 0.027 | |||
| 90–100 | 198 (56.4%) | 88 (67.2%) | 286 (59.3%) | |
| 70–80 | 87 (24.8%) | 18 (13.7%) | 105 (21.8%) | |
| Unknown | 66 (18.8%) | 25 (19.1%) | 91 (18.9%) | |
| HCT comorbidity index | 0.94 | |||
| 0 | 157 (44.7%) | 56 (42.7%) | 213 (44.2%) | |
| 1–2 | 79 (22.5%) | 30 (22.9%) | 109 (22.6%) | |
| >2 | 49 (14%) | 21 (16%) | 70 (14.5%) | |
| Unknown | 66 (18.8%) | 24 (18.3%) | 90 (18.7%) | |
Overall outcomes
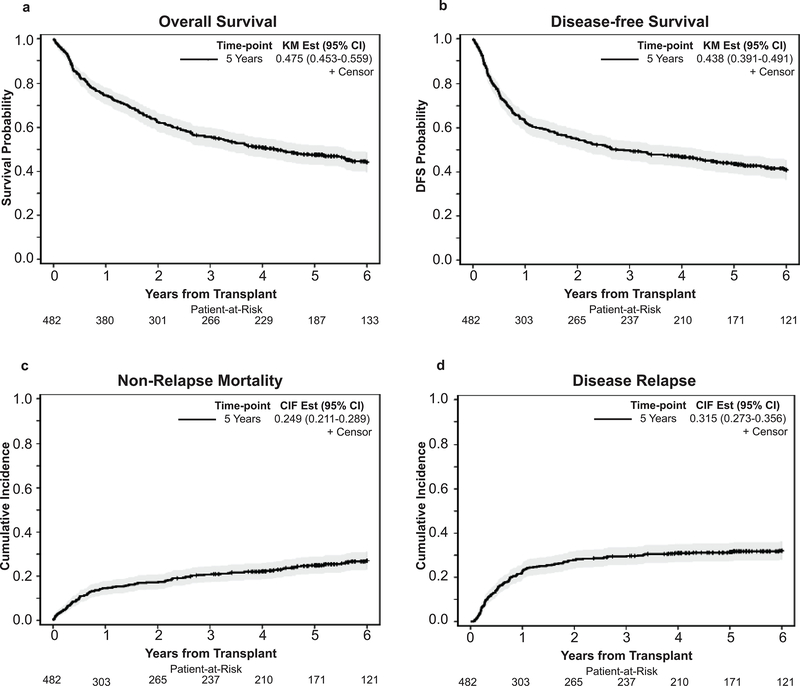
With a median follow up of 6.2 years (range: 2.4–11.3) for survivors, the probability of 5-year OS and DFS were 47.5%, (95%CI: 43.0–52.0) and 43.6% (95%CI: 39.1–48), respectively. The cumulative incidence of NRM was 24.9% (95%CI: 21.1–28.9) and disease relapse was 31.5% (95%CI: 27.3–35.6) at 5 years. (Figure 1)
Figure 1.
Overall outcomes at 5 years post-HCT. (a) Overall Survival, (b) Disease-free survival, (c) Non relapse mortality, and (d) Relapse
Outcomes after MUD and mMUD HCT
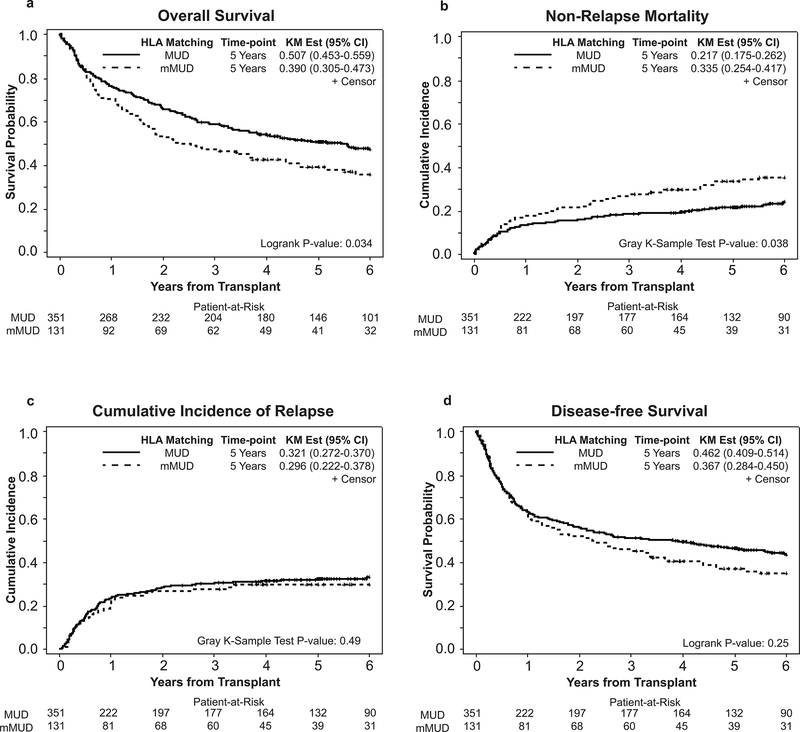
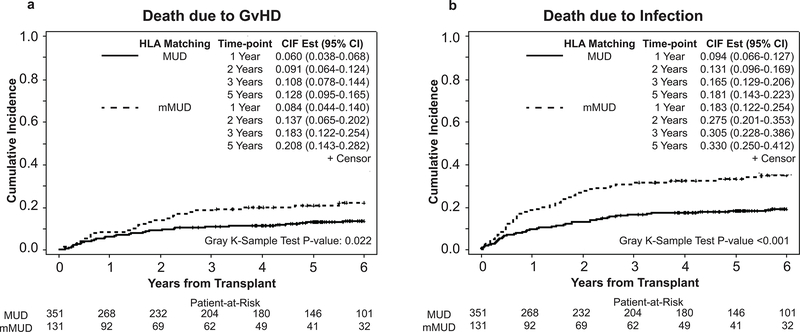
In our cohort, the probability of 5-year OS was significantly worse in mMUD recipients when compared to MUD (39.0 % vs. 50.7%, p=0.034) (Figure 2a), primarily due to the increased NRM in the mMUD group (at 1 year: 17.6% vs. 13.4%, at 5 years 33.5% vs. 21.7%; p=0.038) (Figure 2b). The cumulative incidence of disease relapse at 5 years was similar between MUD and mMUD groups (29.8% vs. 32.1%; p=0.49) (Figure 2c), and the 5-year DFS was 46.2% in MUD compared with 36.7% in mMUD (p=0.25). (Figure 2d) Interestingly, with T/S-based GvHD prophylaxis, patients receiving transplant from mMUD and MUD had similar rates of grades II-IV (52.7% in mMUD vs. 53.0% in MUD; p=0.91), (Figure S1a) grades III-IV acute GvHD at 100 days (19.1% in mMUD vs. 20.3% in MUD, p=0.81), (Figure S1b), and chronic GvHD at 3 years post alloHCT (68.7% in mMUD vs. 69.5% in MUD, p=0.95). (Figure S1c) However, at 5 years post alloHCT, we observed increased rates of death due to GvHD (20.8% in mMUD vs. 12.8% in MUD, p=0.022), (Figure 3a) and infections (33.0% in mMUD vs. 18.1% in MUD, p<0.001), (Figure 3b) in mMUD group when compared with MUD patients. Lastly, at 1-year post alloHCT, similar rates of GRFS was observed among patients receiving transplant form a matched or mismatched unrelated donor (15% percent in mMUD and 19% in MUD, P= 0.74), data not shown.
Figure 2.
Transplant outcomes after MUD and mMUD transplant. (a) Overall survival, (b) Non relapse mortality, (c) Relapse, and (d) Disease-free survival.
Figure 3.
Mortality rates post transplantation in MUD and mMUD transplants. (a) Death due to GvHD, and (b) Death due to infection
To investigate toxicities of this regimen among MUD and mMUD recipients, we also collected selected toxicity endpoints including ICU transfers, need for dialysis, thrombotic microangiopathy requiring plasma exchange, veno occlusive disease treated with defibrotide and did not find any differences between these toxicities among the two cohorts (Table S1).
Univariate and multivariable analyses for factors associated with outcomes.
Tables 2a and 2b summarize the univariate and multivariable analyses of factors for HCT outcomes. Among all factors studied in the univariate analysis, patients with higher disease risk (HR= 2.26, 95%CI: 1.18–4.31; p<0.001) or patients who received transplant from a mMUD (HR= 1.31, 95%CI: 1.02–1.69; p=0.034) had higher risk of mortality. Disease risk was significantly associated with OS in the multivariable Cox regression model (HR=2.21, 95%CI: 1.16–4.23; p<0.001. In the multivariable Cox regression models, mMUD was found to be associated with higher risk of death (HR=1.55, 95%CI: 1.15–2.08, p =0.004) after adjusting for disease risk and GVHD prophylaxis, with mMUD being significantly associated with increased NRM (HR= 2.01, 95%CI: 1.31–3.10, p=0.002) after adjusting for age, female donor to male recipient, and GVHD prophylaxis. GVHD prophylaxis (HR=0.46, 95%CI: 0.24–0.89; p= 0.048), and patient’s age at the time of transplant (HR= 2.04, 95%CI: 1.37–3.03; p=0.001) were also independent factors affecting NRM on multivariable analysis. Age at HCT was found to affect incidence of post transplant relapse in our analysis (Gray test p=0.021), where older patients who were ≥60 years old were less likely to relapse compared to their younger counterparts who were <50 years old (HR=0.54, 95%CI: 0.35–0.82; p=0.014).
Table 2a.
The association of patient and transplant characteristics with clinical outcomes in the univariate and multivariate analyses of PFS and OS
| PFS | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 5 Yr (95%CI) | HR (95%CI) | P value* | Adjusted HR (95%CI)† | P value† | 5 Yr (95%CI) | HR (95%CI) | P value* | Adjusted HR (95%CI)† | P value† | |
|
| |||||||||||
| Age at HSCT, yrs | |||||||||||
| <50 | 207 | 0.423(0.354,0.490) | Reference | 0.38 | Reference | 0.25 | 0.461(0.391,0.529) | Reference | 0.54 | Reference | 0.28 |
| 50–59 | 136 | 0.403(0.320,0.484) | 1.16(0.89,1.53) | 1.24(0.94,1.64) | 0.453(0.368,0.535) | 1.15(0.87,1.52) | 1.26(0.94,1.68) | ||||
| 60+ | 139 | 0.488(0.401,0.569) | 0.96(0.73,1.26) | 1.01(0.76,1.34) | 0.516(0.428,0.597) | 1.00(0.75,1.33) | 1.07(0.80,1.43) | ||||
| Recipient sex | |||||||||||
| Male | 261 | 0.414(0.353,0.474) | Reference | 0.21 | Reference | 0.32 | 0.459(0.396,0.519) | Reference | 0.28 | Reference | 0.44 |
| Female | 221 | 0.462(0.395,0.527) | 0.86(0.69,1.09) | 0.89(0.71,1.12) | 0.494(0.426,0.559) | 0.88(0.69,1.11) | 0.91 (0.72,1.15) | ||||
| Female donor to male recipient | 0.35 | ||||||||||
| Yes | 413 | 0.440(0.391,0.488) | Reference | Reference | 0.479(0.429,0.526) | Reference | Reference | ||||
| No | 69 | 0.414(0.296,0.528) | 1.10(0.81,1.50) | 0.53 | 1.14(0.84,1.56) | 0.40 | 0.456(0.334,0.570) | 1.15(0.84,1.57) | 0.39 | 1.16(0.85,1.60) | |
| Disease Risk Index | |||||||||||
| Low Risk | 22 | 0.633(0.398,0.797) | Reference | <0.001 | Reference | <0.001 | 0.682(0.446,0.834) | Reference | <0.001 | Reference | <0.001 |
| Intermediate Risk | 266 | 0.509(0.447,0.568) | 1.45(0.76,2.75) | 1.41(0.74,2.69) | 0.554(0.491,0.612) | 1.26(0.66,2.39) | 1.24(0.65,2.36) | ||||
| High Risk | 163 | 0.315(0.245,0.388) | 2.54(1.33,4.84) | 2.47(1.29,4.71) | 0.345(0.273,0.419) | 2.26(1.18,4.31) | 2.21(1.16,4.23) | ||||
| Very High | 31 | 0.323(0.169,0.486) | 2.11(1.00,4.45) | 2.08(0.98,4.39) | 0.352(0.191,0.517) | 1.83(0.86,3.88) | 1.80(0.85,3.83) | ||||
| Conditioning Intensity | |||||||||||
| RIC | 275 | 0.453(0.393,0.511) | Reference | 0.64 | Reference | 0.95 | 0.497(0.436,0.555) | Reference | 0.51 | Reference | 0.94 |
| MAC | 207 | 0.415(0.346,0.482) | 1.06(0.84,1.33) | 1.01(0.79,1.28) | 0.447(0.378,0.514) | 1.08(0.85,1.37) | 1.01 (0.79,1.29) | ||||
| GVHD prophylaxis | |||||||||||
| Tac/Siro | 313 | 0.431(0.375,0.486) | Reference | 0.10 | Reference | 0.078 | 0.468(0.411,0.523) | Reference | 0.21 | Reference | 0.057 |
| Tac/Siro/+MTX | 126 | 0.408(0.321,0.493) | 0.98(0.76,1.27) | 0.85(0.63,1.15) | 0.455(0.366,0.540) | 0.94(0.72,1.23) | 0.77(0.56,1.04) | ||||
| Tac/Siro/+ATG | 43 | 0.558(0.398,0.691) | 0.63(0.41,0.98) | 0.60(0.38,0.94) | 0.581(0.421,0.712) | 0.68(0.44,1.06) | 0.61 (0.39,0.97) | ||||
| ABO blood group compatibility | |||||||||||
| Compatible | 224 | 0.453(0.386,0.518) | Reference | 0.82 | Reference | 0.57 | 0.498(0.430,0.562) | Reference | 0.78 | Reference | 0.65 |
| Minor | 98 | 0.407(0.307,0.504) | 1.03(0.76,1.40) | 1.04(0.77,1.42) | 0.457(0.354,0.554) | 0.99(0.72,1.36) | 1.00(0.73,1.37) | ||||
| Major | 107 | 0.401(0.308,0.492) | 1.15(0.86,1.54) | 1.23(0.92,1.65) | 0.438(0.342,0.529) | 1.13(0.84,1.52) | 1.20(0.89,1.62) | ||||
| Bidirectional | 53 | 0.486(0.346,0.613) | 1.07(0.73,1.57) | 1.03(0.70,1.52) | 0.485(0.345,0.612) | 1.14(0.78,1.68) | 1.10(0.75,1.63) | ||||
| Donor/Recipient CMV serostatus | |||||||||||
| D−/R+ | 54 | 0.386(0.257,0.513) | Reference | 0.53 | Reference | 0.42 | 0.461(0.324,0.587) | Reference | 0.82 | Reference | 0.52 |
| D−/R+ | 214 | 0.426(0.358,0.492) | 0.86(0.60,1.25) | 0.87(0.60,1.26) | 0.459(0.390,0.525) | 0.96(0.65,1.40) | 0.95(0.65,1.39) | ||||
| D+/R− | 42 | 0.493(0.334,0.634) | 0.71(0.43,1.20) | 0.74(0.44,1.25) | 0.493(0.334,0.634) | 0.87(0.51,1.48) | 0.89(0.52,1.51) | ||||
| D+/R+ | 172 | 0.452(0.376,0.524) | 0.79(0.54,1.16) | 0.74(0.51,1.10) | 0.497(0.420,0.570) | 0.86(0.58,1.28) | 0.79(0.52,1.18) | ||||
| DQB1_status | |||||||||||
| 2 | 446 | 0.436(0.389,0.482) | Reference | 0.86 | Reference | 0.74 | 0.479(0.431,0.525) | Reference | 0.87 | Reference | 0.92 |
| 1 | 36 | 0.437(0.272,0.592) | 0.96(0.62,1.49) | 0.93(0.60,1.44) | 0.437(0.272,0.592) | 1.04(0.67,1.60) | 0.98(0.63,1.52) | ||||
| Karnofsky performance status % | |||||||||||
| 90–100 | 286 | 0.475(0.415,0.532) | Reference | 0.20 | Reference | 0.55 | 0.502(0.443,0.559) | Reference | 0.34 | Reference | 0.63 |
| 70–80 | 105 | 0.387(0.292,0.481) | 1.21(0.91,1.61) | 1.10(0.81,1.48) | 0.413(0.316,0.508) | 1.15(0.86,1.55) | 1.08(0.80,1.46) | ||||
| HCTCI | |||||||||||
| 0 | 213 | 0.485(0.416,0.551) | Reference | 0.31 | Reference | 0.49 | 0.527(0.457,0.592) | Reference | 0.10 | Reference | 0.16 |
| 1–2 | 109 | 0.432(0.336,0.524) | 1.20(0.88,1.62) | 1.15(0.85,1.56) | 0.449(0.352,0.541) | 1.25(0.92,1.70) | 1.23(0.90,1.67) | ||||
| >2 | 70 | 0.385(0.269,0.500) | 1.25(0.89,1.76) | 1.19(0.85,1.69) | 0.383(0.266,0.498) | 1.40(1.00,1.98) | 1.36(0.96,1.93) | ||||
| HLA Match | |||||||||||
| MUD | 351 | 0.462(0.409,0.514) | Reference | 0.25 | Reference | 0.078 | 0.507(0.453,0.559) | Reference | 0.034 | Reference | 0.004 |
| mMUD | 131 | 0.367(0.284,0.450) | 1.16(0.90,1.49) | 1.30(0.97,1.74) | 0.390(0.305,0.473) | 1.31(1.02,1.69) | 1.55(1.15,2.08) | ||||
Based on log-rank test.
Based on the Cox proportional hazards regression model adjusting for disease risk index, GVHD prophylaxis, and HLA match.
Table 2b.
The association of patient and transplant characteristics with clinical outcomes in the univariate and multivariate analyses of relapse and NRM
| Relapse | NRM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cumulative Incidence at 1 Yr (95%CI) | P value* | HR (95%CI)† | P value† | Cumulative Incidence at 5 Yrs (95%CI) | P value* | HR (95%CI)† | P value† | |
|
| |||||||||
| Age at HSCT, yrs | |||||||||
| <50 | 207 | 0.295(0.234,0.358) | 0.021 | Reference | 0.014 | 0.213(0.159,0.272) | 0.013 | Reference | 0.001 |
| 50–59 | 136 | 0.191(0.130,0.262) | 0.90(0.62,1.30) | 0.258(0.188,0.335) | 1.72(1.13,2.62) | ||||
| 60+ | 139 | 0.158(0.103,0.224) | 0.54(0.35,0.82) | 0.295(0.220,0.374) | 2.04(1.37,3.03) | ||||
| Recipient sex | |||||||||
| Male | 261 | 0.222(0.174,0.274) | 0.51 | Reference | 0.28 | 0.252(0.200,0.307) | 0.33 | Reference | 0.61 |
| Female | 221 | 0.231(0.178,0.288) | 0.84(0.61,1.16) | 0.247(0.191,0.306) | 1.10(0.75,1.62) | ||||
| Female donor to male recipient | |||||||||
| Yes | 413 | 0.237(0.197,0.279) | 0.30 | Reference | 0.37 | 0.239(0.198,0.281) | 0.043 | Reference | 0.071 |
| No | 69 | 0.159(0.084,0.256) | 0.80(0.50,1.30) | 0.311(0.203,0.424) | 1.44(0.97,2.15) | ||||
| Disease Risk Index | |||||||||
| Low Risk | 22 | 0.0 (no events) | 0.002 | Reference | 0.002 | 0.273(0.108,0.469) | 0.56 | Reference | 0.66 |
| Intermediate Risk | 266 | 0.177(0.133,0.225) | 3.57(0.93,13.71) | 0.223(0.174,0.276) | 0.82(0.39,1.70) | ||||
| High Risk | 163 | 0.331(0.260,0.404) | 5.99(1.56,23.09) | 0.279(0.212,0.350) | 1.01 (0.48,2.15) | ||||
| Very High | 31 | 0.258(0.120,0.421) | 5.24(1.23,22.26) | 0.290(0.141,0.458) | 0.97(0.36,2.57) | ||||
| Conditioning Intensity | |||||||||
| RIC | 275 | 0.185(0.142,0.234) | 0.063 | Reference | 0.58 | 0.265(0.214,0.319) | 0.12 | Reference | 0.95 |
| MAC | 207 | 0.280(0.221,0.343) | 1.11(0.76,1.62) | 0.228(0.172,0.288) | 0.98(0.63,1.53) | ||||
| GVHD prophylaxis | |||||||||
| Tac/Siro | 313 | 0.236(0.191,0.285) | 0.87 | Reference | 0.69 | 0.254(0.206,0.304) | 0.18 | Reference | 0.048 |
| Tac/Siro/–ATG | 126 | 0.206(0.140,0.281) | 1.19(0.79,1.79) | 0.266(0.191,0.347) | 0.72(0.46,1.12) | ||||
| Tac/Siro/–ATG | 43 | 0.209(0.102,0.342) | 1.10(0.60,2.01) | 0.163(0.070,0.289) | 0.46(0.24,0.89) | ||||
| ABO blood group compatibility | |||||||||
| Compatible | 224 | 0.214(0.163,0.270) | 0.37 | Reference | 0.28 | 0.246(0.191,0.305) | 0.65 | Reference | 0.68 |
| Minor | 98 | 0.235(0.156,0.323) | 1.11(0.72,1.70) | 0.267(0.181,0.360) | 0.84(0.54,1.31) | ||||
| Major | 107 | 0.280(0.199,0.368) | 1.37(0.92,2.04) | 0.235(0.159,0.319) | 0.87(0.56,1.35) | ||||
| Bidirectional | 53 | 0.151(0.070,0.261) | 0.79(0.44,1.42) | 0.265(0.154,0.390) | 1.17(0.68,1.99) | ||||
| Donor/Recipient CMV serostatus | |||||||||
| D−/R− | 54 | 0.241(0.136,0.362) | 0.86 | Reference | 0.67 | 0.298(0.181,0.424) | 0.84 | Reference | 0.69 |
| D−/R+ | 214 | 0.243(0.188,0.302) | 1.09(0.64,1.85) | 0.246(0.189,0.306) | 0.75(0.44,1.27) | ||||
| D+/R− | 42 | 0.214(0.105,0.349) | 1.07(0.51,2.24) | 0.216(0.105,0.353) | 0.66(0.30,1.46) | ||||
| D+/R+ | 172 | 0.203(0.147,0.267) | 0.87(0.50,1.51) | 0.246(0.184,0.313) | 0.75(0.43,1.30) | ||||
| DQB1_status | |||||||||
| 2 | 446 | 0.222(0.185,0.262) | 0.71 | Reference | 0.69 | 0.250(0.211,0.292) | 0.51 | Reference | 0.33 |
| 1 | 36 | 0.278(0.143,0.431) | 1.13(0.61,2.12) | 0.229(0.105,0.382) | 0.73(0.39,1.37) | ||||
| Karnofsky performance status | |||||||||
| 90–100 | 286 | 0.199(0.155,0.248) | 0.053 | Reference | 0.21 | 0.261 (0.211,0.314) | 0.47 | Reference | 0.46 |
| 70–80 | 105 | 0.286(0.202,0.374) | 1.30(0.86,1.95) | 0.250(0.169,0.339) | 0.84(0.53,1.33) | ||||
| HCT comorbidity index | |||||||||
| 0 | 213 | 0.216(0.163,0.274) | 0.67 | Reference | 0.84 | 0.242(0.186,0.301) | 0.26 | Reference | 0.78 |
| 1–2 | 109 | 0.257(0.179,0.342) | 1.14(0.74,1.75) | 0.247(0.168,0.334) | 1.04(0.66,1.62) | ||||
| >2 | 70 | 0.186(0.104,0.285) | 1.05(0.64,1.74) | 0.325(0.216,0.440) | 1.19(0.73,1.93) | ||||
| HLA Match | |||||||||
| MUD | 351 | 0.234(0.191,0.279) | 0.49 | Reference | 0.31 | 0.217(0.175,0.262) | 0.038 | Reference | 0.002 |
| mMUD | 131 | 0.206(0.141,0.279) | 0.82(0.57,1.20) | 0.335(0.254,0.417) | 2.01(1.31,3.10) | ||||
Based on Gray’s test.
Based on the proportional subdistribution hazards model for competing risks adjusting for age, disease risk index, and HLA match for relapse; for age, female donor to male recipient, GVHD prophylaxis, and HLA match.
Impact of ATG and additional GvHD agent (methotrexate)
Given the heterogeneity of the 3rd agent combined with T/S in some of our patients, we next examined the impact of MUD vs. mMUD in subgroups of patients who received T/S only (n=313), T/S with additional agent (MTX) without ATG (n=126) and T/S with ATG (n=43). (Table 3) Univariate comparison in the subgroups of T/S only, T/S with MTX, and T/S with ATG showed 5-yr OS of 49% vs. 31% (p=0.017), 53% vs. 40% (p=0.23), and 67% vs. 47% (p=0.080), respectively. Thus patients with mMUD remained to have lower OS compared to MUD in each group. Additionally we did not detect any statistically significant interactions from these three different subgroups of T/S-based GVHD prophylaxis on the impact of MUD vs. mMUD regarding OS or other HCT outcomes (PFS, relapse and NRM) (P value for interaction>0.5). (Table 3)
Table 3.
Impact of HLA match (MUD vs MMUD) on overall survival in different type of GVHD prophylaxis
| Event/Total | 5-Yr (95% CI) | Hazard Ratio (95% CI)* | P-value* | |
|---|---|---|---|---|
|
| ||||
| TS only | ||||
| HLA Match | ||||
| MUD | 156/278 | 0.488 (0.427–0.546) | Reference | 0.017 |
| MMUD | 25/35 | 0.314 (0.171–0.468) | 1.66 (1.09–2.54) | |
| Adding MTX | ||||
| HLA Match | ||||
| MUD | 29/49 | 0.531 (0.383–0.658) | Reference | 0.23 |
| MMUD | 49/77 | 0.404 (0.292–0.513) | 1.33 (0.84–2.11) | |
| Adding ATG | ||||
| HLA Match | 0.080 | |||
| MUD | 11/24 | 0.667 (0.443–0.817) | Reference | |
| MMUD | 12/19 | 0.474 (0.244–0.673) | 2.09 (0.90–4.86) | |
Based on univariate analysis and log-rank test
Individual Locus Mismatch and Patient Outcomes
We next explored whether mismatching at a specific HLA locus impacted URD transplant outcomes. While the number of patients in each HLA locus mismatch group was relatively small (Table 4), mismatches at HLA-B and -C were more common than HLA-A or -DRB1, and HLA DRB1 mismatch was only at the allele level and not the antigen. In multivariable Cox regression analysis, and relative to patients receiving MUD transplant, inferior OS was associated with single allele/antigen mismatch at A and C (HR= 1.76, 95%CI: 1.14–2.73; p=0.011) and (HR= 1.64, 95%CI: 1.09–2.45; p=0.017), and a trend was observed in single allele mismatch at B (HR=1.50, 95% CI: 0.96–2.33; p=0.074).
Table 4.
Individual locus mismatch and overall survival
| MMUD (N=131) | MUD (N=351) | Adjusted HR (95%CI) † | P value † | |
|---|---|---|---|---|
|
| ||||
| A Mismatch | 36 (27.5%) | Ref | 1.76 (1.14– 2.73) | 0.011 |
| B Mismatch | 44 (33.6%) | Ref | 1.50 (0.96–2.33) | 0.074 |
| C Mismatch | 50 (38.2%) | Ref | 1.64 (1.09–2.45) | 0.017 |
| DRB1 Mismatch | 22 (16.8%) | Ref | 0.95 (0.47–1.91) | 0.89 |
Based on the Cox proportional hazards regression model adjusting for disease risk index and GVHD prophylaxis.
DISCUSSION
Only 25–30% of patients with the indication of HCT have an available HLA-matched sibling donor (MSD).1,21 For the remainder of patients, unrelated donor (URD) alloHCT has become the standard treatment. Availability of a matched unrelated donor (MUD), depending on patients’ racial and ethnic background varies between 20–70% with only 25–30% availability for underrepresented minority (i.e., black Americans, Hispanics, Asians, etc.).1 In such cases, alternative donors such as HLA- mMUD can be an option. Historically, outcome of transplant using mMUD has been less than optimal, mostly due to the increased incidence of GvHD and the subsequent NRM,11,22 T/S-based GvHD prophylaxis regimens has been shown to be at least equally effective to “standard” methotrexate and CNI combination in GvHD prevention, with further beneficial effects including faster time to engraftment and reduced oral mucositis in the setting of both MRD and MUD transplant.8,12 However, to date, there have been no studies specifically evaluating the role of T/S-based GvHD prophylaxis in mMUD HCT. Thus we retrospectively studied consecutive set of 482 patients who underwent URD transplant at our center in the last 10 years during which the T/S-based regimen were implemented as our institutional standard GvHD prophylaxis.
Our results show that the long-term outcome of URD HCT using T/S-based GvHD prophylaxis was overall favorable. However, HLA mismatch remains a significant factor for poor outcome after URD HCT using T/S-based GvHD prophylaxis. In a multivariable model, mMUD was significantly associated with worse OS and increased NRM after adjusting for other clinical variables. Interestingly, there was no significant difference in the incidence or severity of acute or chronic GvHD between MUD and mMUD. Further analysis of mortality causes revealed that inferior survival in mMUD was associated with increased GvHD- and infection-related death. Since our study database used the old grading for chronic GvHD (limited vs. extensive), it is possible that the severity of chronic GvHD was not as well captured as would have been if graded by the 2014 NIH criteria.23
Besides HLA-mismatch, HCT-CI and disease risk were independent risk factors for OS. Older patients with lower performance status and high-risk disease had more relapses, and older patients, with female-to-male donor who had higher risk disease had higher NRM.
Despite the small number of patients in each HLA mismatch group, there was a trend towards worse survival associated with HLA-A or -C mismatch (p= 0.06 and 0.08, respectively); these results were in accordance with what has been reported by the CIBMTR study.3 However, in our study, mismatch at DRB1 did not affect patients’ survival; most likely due to the small number of patients with mismatch at the allele (not at the antigen level) at this locus, as it is our practice to avoid using donors with DRB1 antigen mismatch. Lastly, consistent with published data2,3,24 single DQB1 mismatch did not affect OS in this study.
To our knowledge, our study is the largest single-center analyses describing the outcome of patients undergoing HCT from URD with T/S-based GvHD prophylaxis with a long follow-up duration of over 5 years. Despite of the inherent limitations due to the heterogeneity and the retrospective nature of the study, we demonstrate that the survival outcome after mMUD HCT using T/S-based GVHD prophylaxis is suboptimal even with the intensified immunosuppression with additional MTX or ATG. The lower OS was not directly due to the incidence of acute/chronic GvHD but likely because severity of GvHD was not well captured in the current acute GvHD grading or previous chronic GvHD grading without limited/extensive designation.
In conclusion, HLA-mismatch remains to be a major barrier for successful URD transplant with T/S-based GvHD prophylaxis likely due to chronic GvHD and infection-related late mortality. Novel GvHD prophylaxis agents and immune reconstitution strategies are needed to improve outcomes of mMUD HCT. In addition, our data also provide an important piece of information to the existing literature, which can facilitate further discussions about optimizing the highly complex donor selection processes between mMUD/cord blood/haploidentical HCT for patients without available matched related or unrelated donors.
Supplementary Material
ACKNOWLEDGMENTS
Authors thank City of Hope staff and nurses, as well as the patients and their families, without whom this work would not be possible. This study was partially supported by NIH P30 CA033572 (Biostatistics Core).
Footnotes
Conflict of Interest: Authors declare no conflict of interest.
Reference List
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. [DOI] [PubMed] [Google Scholar]
- 3.Verneris MR, Lee SJ, Ahn KW, et al. HLA Mismatch Is Associated with Worse Outcomes after Unrelated Donor Reduced-Intensity Conditioning Hematopoietic Cell Transplantation: An Analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(10):1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersdorf EW, Gooley T, Malkki M, et al. Clinical significance of donor-recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69 Suppl 1:25–30. [DOI] [PubMed] [Google Scholar]
- 5.Center for International Blood and Marrow Transplant Research. CIBMTR Sample Type and Inventory Summary. Available at https://www.cibmtr.org/Samples/Inventory/Pages/index.aspx. Published 2017.
- 6.Woolfrey A, Lee SJ, Gooley TA, et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: role of cell source and disease risk category. Biol Blood Marrow Transplant. 2010;16(10):1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. [DOI] [PubMed] [Google Scholar]
- 8.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez R, Nakamura R, Palmer JM, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(5):1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601–1605. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura R, Palmer JM, O’Donnell MR, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leuk Res. 2012;36(9):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaled SK, Palmer J, Stiller T, et al. A phase II study of sirolimus, tacrolimus and rabbit anti-thymocyte globulin as graft-versus-host prophylaxis after unrelated-donor peripheral blood stem cell transplant. Bone Marrow Transplant. 2013;48(2):278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pidala J, Kim J, Jim H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97(12):1882–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder DS, Palmer J, Gaal K, et al. Improved outcomes using tacrolimus/sirolimus for graft-versus-host disease prophylaxis with a reduced-intensity conditioning regimen for allogeneic hematopoietic cell transplant as treatment of myelofibrosis. Biol Blood Marrow Transplant. 2010;16(2):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parody R, Lopez-Corral L, Godino OL, et al. GVHD prophylaxis with sirolimus-tacrolimus may overcome the deleterious effect on survival of HLA mismatch after reduced-intensity conditioning allo-SCT. Bone Marrow Transplant. 2015;50(1):121–126. [DOI] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 19.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. [DOI] [PubMed] [Google Scholar]
- 20.Gambacorti-Passerini CB, Donadoni C, Parmiani A, et al. Recurrent ETNK1 mutations in atypical chronic myeloid leukemia. Blood. 2015;125(3):499–503. [DOI] [PubMed] [Google Scholar]
- 21.Saber W, Opie S, Rizzo JD, et al. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119(17):3908–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Homsi AS, Cole K, Muilenburg M, et al. Calcineurin and mTOR Inhibitor-Free Post-Transplantation Cyclophosphamide and Bortezomib Combination for Graft-versus-Host Disease Prevention after Peripheral Blood Allogeneic Hematopoietic Stem Cell Transplantation: A Phase I/II Study. Biol Blood Marrow Transplant. 2017;23(10):1651–1657. [DOI] [PubMed] [Google Scholar]
- 23.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersdorf EW, Longton GM, Anasetti C, et al. Definition of HLA-DQ as a transplantation antigen. Proc Natl Acad Sci U S A. 1996;93(26):15358–15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.