Abstract
Background
Percutaneous left atrial appendage closure (LAAC) is an emerging alternative to oral anticoagulation for stroke prevention in atrial fibrillation (AF) in patients with AF, elevated stroke risk and contraindications to long-term anticoagulation treatment. Optimal pre-procedural planning is essential to ensure optimal procedural results.
Case summary
We report the case of a 62-year-old man with a history of right cerebellar haematoma referred for LAA closure. We describe the first use of FEops HEARTguide patient-specific computational simulation in the planning of LAAC with the Watchman Flex device (Boston Scientific, Marlborough, MA, USA) in an unusual ‘whale tail’-like LAA anatomy.
Discussion
Percutaneous left atrial appendage (LAA) closure is feasible in the majority of patients. However, certain LAA anatomies may pose substantial technical challenges. This case shows the crucial role of a pre-procedural assessment based on patient-specific computational simulations for LAA closure in difficult scenarios resulting in a more efficient procedure with the optimal result and good clinical outcomes.
Keywords: Left atrial appendage, Watchman FLX, Pre-procedural planning, FEops simulation software, Case report
Learning points.
Pre-procedural imaging based on computerized tomography imaging and transesophageal echo is crucial in the choice of a correct device in left atrial appendage closure procedure.
FEops HEARTguide simulation can add further information predicting the interaction of different devices with anatomical structures.
Introduction
Percutaneous left atrial appendage (LAA) closure is effective to prevent cardioembolic events and ischaemic stroke in case of non-valvular atrial fibrillation (AF) and is a recognized alternative in patients with AF contraindicated for oral anticoagulants due to relevant bleeding complications.1 Although LAA varies greatly in its morphology, there are 4four typical anatomies: windsock, cauliflower, cactus and chicken wing.2 However, some LAAs do not fall into any of these categories requiring deeper pre-procedural analysis to achieve a correct LAA closure device selection as well as an optimal implantation. This case-report illustrates the importance of using a three-dimensional simulation programme developed by FEops (FEops NV, Ghent, Belgium) and based on computed tomography (CT) angiography and image engineering to virtually test and select the working projection, the device type and size and its degree of compression.3
Timeline
| Baseline: Previous transurethral resection of bladder tumour. Recent pulmonary resection for lung adenocarcinoma. Cerebellar haematoma. Long term anticoagulation contraindication. |
| Day 0: FEops HEARTguide patient-specific computational simulation for Watchman Flex device in a whale tail like left atrial appendage (LAA). |
| Day 1: Watchman Flex 24 mm (Boston Scientific) device deployment according to FEops simulation |
| Day 2: Hospital discharge (Aspirin and Clopidogrel). |
| 45 days follow-up: Asymptomatic, optimal LAA closure, no residual leak at transesophageal echocardiography, aspirin alone. |
Case presentation
A 62-year-old male with permanent AF (CHA2DS2-VASc = 2) was referred for LAA closure due to right cerebellar haematoma on Warfarin in December 2020. He had a previous history of urothelial low grade adenocarcinoma treated with transurethral resection of bladder tumour and recently underwent pulmonary resection for lung adenocarcinoma.
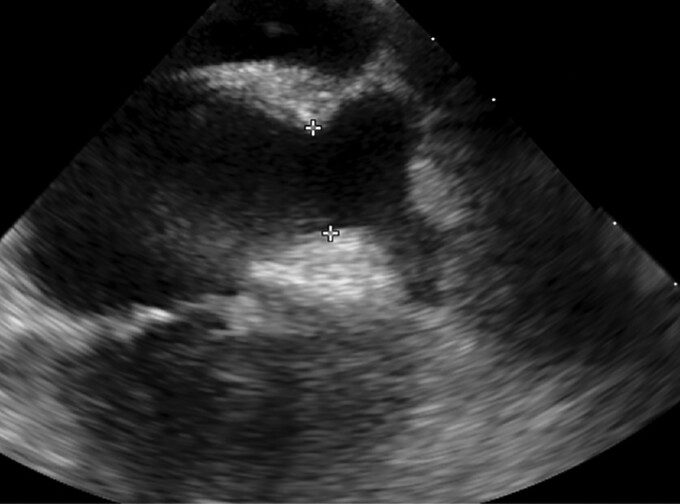
The pre-procedural CT showed a very unfavourable LAA anatomy with a short neck and two proximal symmetric lobes opposite one another. The landing zone measurements were 16 × 22 mm with an available depth of implant of 12 mm (Figure 1).
Figure 1.
(A) Baseline computed tomography scans: whale tail anatomy with a very short neck ending in two symmetric lobes. (B) Landing zone measurements taken at level of the circumflex artery (the narrowest portion of the neck): minimum diameter of 16 mm, maximum of 22 mm, left atrial appendage depth of 12 mm.
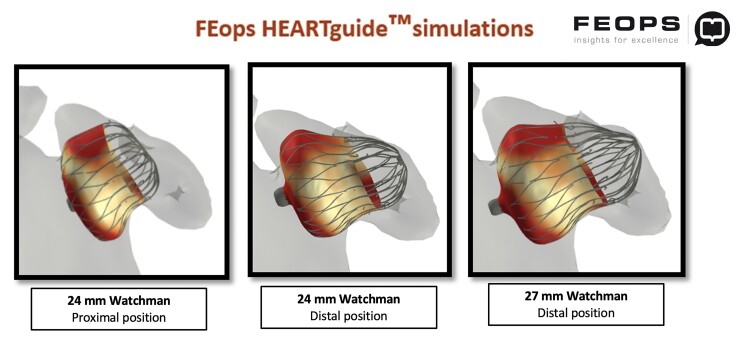
Considering this rare and challenging anatomy, also known as ‘Whale Tail’ LAA, we obtained a prediction of implantation with a Watchman FLX device with the simulation support of FEops HEARTguide. According to Watchman sizing chart, this appendage was fit for a 24 mm FLX device, aiming at a very proximal deployment. The main question we asked to the software was if the 24 mm size was suitable for this anatomy, achieving a good closure with no final significant leaks or excessive protrusion into the left atrium (LA). FEops analysis confirmed the suitability of the 24 mm device suggesting a proximal implant to avoid any leaks, with a predicted final compression of ∼10%, good apposition degree and not excessive buldge into the LA. We also tried a simulation with a 27 mm FLX device with a deeper deployment but it was expected to extremely protrude into the LA (Figure 2).
Figure 2.
FEops Heartguide simulations with Watchman Flex devices 24 mm (with proximal or distal deployment) and 27 mm.
The procedure was performed with the patient under general anaesthesia, transesophageal echocardiography (TEE) and fluoroscopy guidance. Adequate anticoagulation with heparin throughout the procedure was maintained by testing the activated clotting time every 20–30 min to gain a level of 200 to 300 s.
Intraprocedural TEE confirmed the bilobated whale tail-like LAA with landing zone diameters of 19 × 15 mm and a maximum depth of 12 mm. No thrombi were found (Figure 3).
Figure 3.
Transesophageal echocardiography of the left atrial appendage: whale tail left atrial appendage with 19 × 15 mm diameters at lending zone, maximum depth of 12 mm and no thrombi.
By using a 14-F delivery system, the 24 mm Watchman Flex device (Boston Scientific, Marlborough, MA, USA) was definitely selected and implanted, as intended, in the proximal LAA.
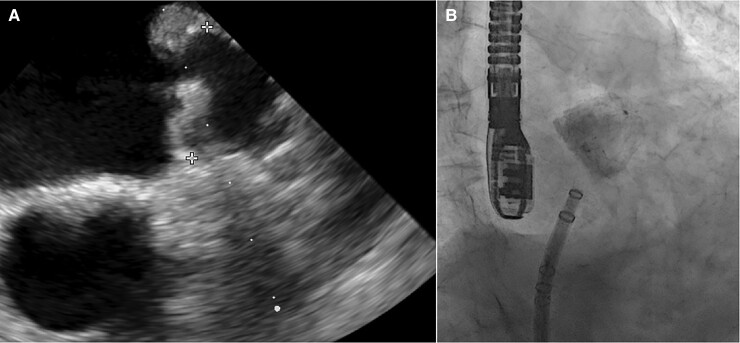
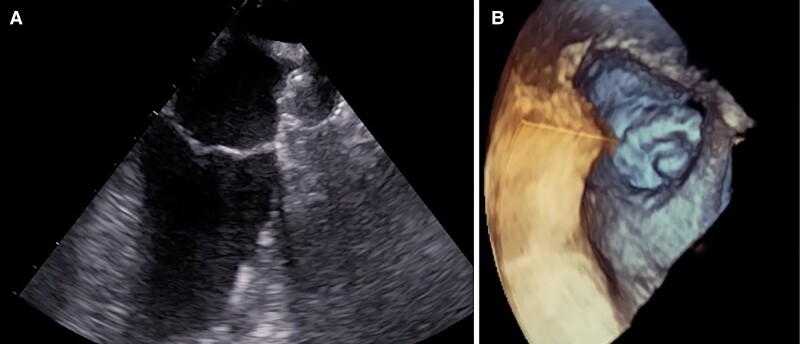
After two attempts of proximal deployment, the device was sitting tilted towards the inferior lobe, leaving a wide leak around the opposite one. Then we pulled it into the LAA ostial part with gentle retraction, obtaining a perfect sealing with adequate compression and stability at push and pull test. The device final position and deformation confirmed both at TEE and angiography resulted quite similar to FEops prediction (Figures 2–4). The patient was discharged at home on postprocedural day 2, the in-hospital course was uneventful. His home therapy included only aspirin after three months of dual antiplatelet therapy without oral anticoagulants, according to his high bleeding risk profile. At 45-day follow-up TEE control, the device was in its correct position with no residual leak and no evidence of embolization or thrombi (Figure 5).
Figure 4.
(A) Intraprocedural transesophageal echocardiography shows optimal device delivery, with good apposition and compression (maximum device diameter 21.4 mm with 13% of compression). (B) Angiographic right anterior oblique view: optimal deployment without peri-device leaks at contrast media injection.
Figure 5.
Follow up transesophageal echocardiography at 45 days (A) showing stationary results also at three-dimensional reconstruction (B).
Discussion
In common practice, LAA closure device selection is still based on two-dimensional TEE measurements. The use of CT scan is becoming the standard of care in pre-procedural planning, due to the non-invasiveness of the exam and to the amount of information it provides.4 To achieve an even more complete analysis, especially when the risk of procedural failure is higher, a CT-based simulation technology, FEops HEARTguide, has been validated for LAA closure and also for transcatheter aortic valve replacement.5 This patient-specific computational simulation provides a deeper insight into the behaviour of the LAA closure device before the procedure, reproducing its mechanical interaction with patient’s anatomy. Our case shows the advantages of resorting to this simulation software in one of the most complex LAA morphologies, the so called whale tail LAA, with only 12 mm of available implantation depth. A device with a lobe and a disc, such as the Amplatzer Amulet, has been already used with success,6 but in this particular scenario we felt it might not have enough depth to fit LAA body with a kind of ‘sandwich technique’, resulting in device prolapse. On the other hand, a ball-shape device, such as the Watchman FLX, tends to leave a leak towards one of the two lobes and, once deployed, has to be accurately sized to the ostium of the appendage, with a risk of under compression and instability too. Therefore, a step forward in planning came from FEops simulation, suggesting not only the best device and size, but also the right placement just at the ostium of the appendage to avoid leakages (Figure 2). This kind of unfavourable LAA anatomy carries a greater risk of long and complex procedures, even testing multiple devices to find the correct one. Therefore, a detailed pre-procedural assessment, enhanced by a patient-specific computational simulation software, should be considered even by experienced operators. Another help could come from three-dimensional-printed custom moulds, designed from volume-rendered CT scans, to enable personalized LAA device implants.7 To the best of our knowledge, this is the first described case of a whale tail anatomy successfully managed with a ball-shape device. Even if at the moment there are no trials on FEops HEARTguide technology applied to Watchman Flex devices, we show the first use of this software to choose a Watchman FLX in such very uncommon anatomy. This case offers new insights on possible application of this new simulation programme, even considering cost implication and availability, to difficult LAA morphology, avoiding mismatches or procedural mistakes and obtaining optimal clinical outcomes.8
Conclusion
LAA closure is a safe and effective procedure, very well codified in the most common settings. However, some challenging anatomic variations may increase procedure complexity. In these scenarios, an accurate pre-procedure planning becomes paramount, especially with the additional simulation of device implantation through FEops platform. Thanks to this advanced analysis, our case of whale tail LAA was treated successfully with a fast uncomplicated procedure and optimal outcomes at follow up.
Contributor Information
Francesca Maria Di Muro, Structural Interventional Cardiology, Department of Clinical and Experimental Medicine, Careggi University Hospital, Clinica Medica, Room 124, Largo Brambilla 3, 50134 Florence, Italy.
Miroslava Stolcova, Structural Interventional Cardiology, Department of Clinical and Experimental Medicine, Careggi University Hospital, Clinica Medica, Room 124, Largo Brambilla 3, 50134 Florence, Italy.
Carlo Di Mario, Structural Interventional Cardiology, Department of Clinical and Experimental Medicine, Careggi University Hospital, Clinica Medica, Room 124, Largo Brambilla 3, 50134 Florence, Italy.
Francesco Meucci, Structural Interventional Cardiology, Department of Clinical and Experimental Medicine, Careggi University Hospital, Clinica Medica, Room 124, Largo Brambilla 3, 50134 Florence, Italy.
Lead author biography

Born in 1994, she graduated with top marks and honours at Catholic University of the Sacred Heart, Rome. She is a Cardiology resident with a special interest in Interventional Cardiology and Structural Heart Disease at Careggi University Hospital of Florence, where she is attending the Catheterization Laboratory directed by Professor Carlo Di Mario.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated, text has been obtained from the patient in line with COPE guidance.
Conflict of interest: C.D.M. is the recipient of institutional research grants from AMGEN, Abbott Vascular, Behring, Boston, Scientific, Chiesi Pharmaceuticals, Daiichi-Sankyo, Edwards Lifescience, Medtronic, Shockwave Medical, Volcano Philips. F.M. reports receiving speaker and consultation fees from Medtronic, Edwards and Boston Sientific. The other authors have no conflicts of interest to report.
Funding: None declared.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging 2014;7:1251–1265. [DOI] [PubMed] [Google Scholar]
- 3. Garot P, Iriart X, Aminian A, Kefer J, Freixa X, Cruz-Gonzalez I, Berti S, Rosseel L, Ibrahim R, Korsholm K, Odenstedt J, Nielsen-Kudsk JE, Saw J, Sondergaard L, De Backer O. Value of FEops HEARTguide patient-specific computational simulations in the planning of left atrial appendage closure with the Amplatzer Amulet closure device: rationale and design of the PREDICT-LAA study. Open Heart 2020;7:e001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaafarani M, Saw J, Daniels M, Song T, Rollet M, Kesinovic S, Lamorgese T, Kubiak K, Qi Z, Pantelic M, O’Neill W, Wang DD. Role of CT imaging in left atrial appendage occlusion for the WATCHMANTM device. Cardiovasc Diagn Ther 2020;10:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bavo AM, Wilkins BT, Garot P, De Bock S, Saw J, Søndergaard L, De Backer O, Iannaccone F. Validation of a computational model aiming to optimize preprocedural planning in percutaneous left atrial appendage closure. J Cardiovasc Comput Tomogr 2020;14:149–154. [DOI] [PubMed] [Google Scholar]
- 6. Freixa X, Panaro A, Carballo J. Percutaneous closure of a “whale tail” left atrial appendage. Rev Esp Cardiol (Engl Ed) 2017;70:770. [DOI] [PubMed] [Google Scholar]
- 7. Robinson SS, Alaie S, Sidoti H, Auge J, Baskaran L, Avilés-Fernández K, Hollenberg SD, Shepherd RF, Min JK, Dunham SN, Mosadegh B. Patient-specific design of a soft occluder for the left atrial appendage. Nat Biomed Eng 2018;2:8–16. [DOI] [PubMed] [Google Scholar]
- 8. Korsholm K, Berti S, Iriart X, Saw J, Wang DD, Cochet H, Chow D, Clemente A, De Backer O, Møller Jensen J, Nielsen-Kudsk JE. Expert recommendations on cardiac computed tomography for planning transcatheter left atrial appendage occlusion. JACC Cardiovasc Interv 2020;13:277–292. [DOI] [PubMed] [Google Scholar]