Glioblastoma (GBM) is the most common and deadly primary malignant brain tumor in adults and is characterized by rapid growth and diffuse infiltration. Even with current treatment strategies consisting of aggressive surgical resection followed by chemotherapy and radiation, GBM evades treatment and recurs due to remaining therapy-resistant cell populations, including infiltrative self-renewing neural stem cell-like GBM stem cells (GSCs). Thus, there is a pressing need to identify new treatment strategies for GBM. Surmounting this challenge depends on exploiting our understanding of the biological drivers of tumor formation and progression for therapeutic purposes.
GBMs have been subject to extensive molecular characterization, which show that frequent alterations in tumor cells include overexpression, amplification, and/or mutation of receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR), in IDH1 wild-type tumors. A recent study from Tome-Garcia et al showed that the TEAD transcription factors are important regulators of cell migration in EGFR-expressing infiltrative GSCs.1 TEAD family transcription factors directly bind to DNA and stimulate expression of transcriptional target genes in response to binding by the paralogous transcriptional co-activators YAP and TAZ, which are inactivated by the Hippo pathway and activated by RTK pathways.2 Several recent studies have reconstructed transcriptional regulatory networks associated with GSC identity and functionality, and these studies have identified YAP and TAZ as master transcriptional determinants that define GSC populations induced downstream of well-established oncogenic drivers of GBM,3–6 such as RTKs including EGFR. Together with Tome-Garcia et al, these studies establish that TEAD-dependent YAP/TAZ transcriptional targets in GSCs include gene expression programs that govern epithelial-to-mesenchymal transition, migration, and neural differentiation, and stem/progenitor cell self-renewal and proliferation, including EGFR itself.1,4,5 In nervous system development, YAP and TAZ are expressed in radial glia and cortical and ependymal neural stem/progenitor cells, where they are required for TEAD-dependent proliferation, expansion, and structural organization of these cell types during neurogenesis.7 Thus, YAP/TAZ-TEAD recapitulate their neurodevelopmental roles in GBM, and drive an EGFR-mediated feedforward loop that promotes the pathogenic properties of GSCs.
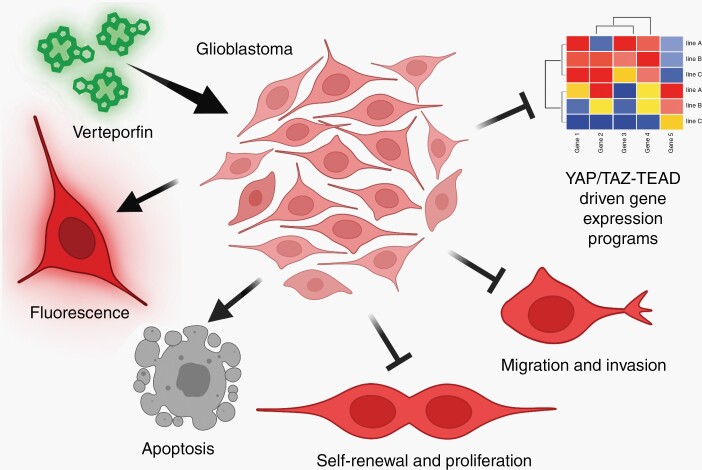
In this issue of Neuro-Oncology, Barrette et al build upon this work to explore the therapeutic efficacy of verteporfin, an inhibitor of YAP/TAZ-TEAD (Figure 1).8 Verteporfin acts as a dual-targeting irreversible inhibitor of YAP/TAZ in that verteporfin binds to conserved TEAD interaction domains in YAP and TAZ to block TEAD binding and, in GSC models, provokes YAP/TAZ and TEAD protein degradation, thereby reducing nuclear localization and preventing transcriptional transactivation.4,8,9 Barrette et al found that verteporfin, which downregulated YAP/TAZ-TEAD-dependent gene expression programs, reduced proliferation and viability of multiple GSC lines and standard GBM cell lines that together contain cells that represent the three different predominant transcriptional states of GBM cells, proneural, mesenchymal, and classical. Barrette et al extend on previous reports of verteporfin chemotoxicity in GBM, including Vigneswaran et al, which found that verteporfin showed no/low toxicity toward normal human neural progenitor cells but decreased YAP/TAZ-TEAD-dependent gene expression and induced apoptosis in EGFR-mutant or amplified GSCs, and reduced tumor growth in xenograft and mouse genetic models of EGFR-mutant GBM.4 Verteporfin is an intrinsically fluorescent porphyrin derivative similar to protoporphyrin IX,8 which is used for fluorescence-assisted intraoperative tumor mapping for GBM. Similarly, fluorescence can be used to visualize verteporfin uptake into GBM tumors using several techniques, including IVIS imaging.4,9 Barrette et al confirmed verteporfin absorption in xenografts, and showed that verteporfin treatment decreased tumor cell migration in vitro and reduced infiltration and conferred survival benefit in vivo in a GSC xenograft model of primary GBM. Verteporfin combined with radiation and temozolomide chemotherapy also had transient pro-survival effects in a xenograft model of recurrent GBM and significantly increased tumor necrosis in bulk tumor mass compared to radiation and temozolomide treatment alone.9 Together with previous studies, Barrette et al support the prospect that anti-YAP/TAZ-TEAD therapy with verteporfin may result in apoptosis and conversion of GSCs into less infiltrative and more differentiated tumor cell types.
Fig. 1.
Schematic showing the effects of the drug verteporfin on glioblastoma. Created with Biorender.com.
Verteporfin is FDA-approved in a liposomal formulation (brand name Visudyne), which has a long history of safe clinical use to treat macular degeneration. To evaluate its therapeutic potential, Vigneswaran et al performed a phase 0 clinical trial in which liposomal verteporfin was administered to GBM patients prior to surgery. Using fluorescence and mass spectrometry, this phase 0 trial found that GBM cells in human patients can readily absorb verteporfin, which showed no evidence of toxicity. Moreover, tumor tissue from verteporfin recipients showed low YAP/TAZ protein levels compared with representative untreated control tissues, which suggests that sufficient verteporfin may be absorbed to disrupt YAP/TAZ protein expression in humans. In mouse models, Barrette et al observed verteporfin efficacy in vivo in the absence of systemic toxicity, with up to 10 months of daily treatment. Therefore, adjuvant therapy using verteporfin in GBM patients has the potential to be more effective than current radio-chemo-therapeutic regimens, and an ongoing phase I/2 clinical trial of liposomal verteporfin in recurrent GBM is underway in support of repurposing this drug (NCT04590664).
Yet, many questions remain regarding verteporfin’s mechanism of action. For example, verteporfin has YAP/TAZ-TEAD-independent effects on autophagy and oxidative phosphorylation that enhance its anti-neoplastic activity and augment immunotherapies in brain tumors.4,10 Furthermore, the mechanisms whereby verteporfin affects chemosensitivity and radioresistance are not clear, although Barrette et al show that verteporfin treatment increases MGMT methylation, which is a clinically relevant biomarker for temozolomide response in GBM patients. Finally, to treat macular degeneration, verteporfin has been used as a photosensitizer in photodynamic therapy (PDT), where it is activated by far-red laser light to focally generate reactive oxygen species (ROS) to eliminate abnormal tissue. Emerging efforts to adapt PDT for neurosurgical treatment of GBM using protoporphyrin IX and other fluorescent drugs could be directed toward verteporfin, which would leverage combined YAP/TAZ-TEAD inhibition with ROS generation in novel ways. Additionally, efforts are warranted to reformulate verteporfin to improve delivery and potency for brain tumors and to test other investigational YAP/TAZ-TEAD inhibitors for GBM.
Acknowledgments
This text is the sole product of the author and no third party had input or gave support for its writing.
Conflict of interest statement. The author reports research support from Bausch Health.
References
- 1. Tome-Garcia J, Erfani P, Nudelman G, et al. Analysis of chromatin accessibility uncovers TEAD1 as a regulator of migration in human glioblastoma. Nat Commun. 2018;9(1):4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng Y, Pan D. The hippo signaling pathway in development and disease. Dev Cell. 2019;50(3):264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castellan M, Guarnieri A, Fujimura A, et al. Single-cell analyses reveal YAP/TAZ as regulators of stemness and cell plasticity in glioblastoma. Nat Cancer. 2021;2(2):174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vigneswaran K, Boyd NH, Oh SY, et al. YAP/TAZ transcriptional co-activators create therapeutic vulnerability to verteporfin in EGFR mutant glioblastoma. Clin Cancer Res. 2021;27(5):1553–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao M, Y F, Zhou W, et al. EGFR activates a TAZ-driven oncogenic program in glioblastoma. Cancer Res. 2021;81(13):3580–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhat KP, Salazar KL, Balasubramaniyan V, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25(24):2594–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lavado A, Gangwar R, Pare J, Wan S, Fan Y, Cao X. YAP/TAZ maintain the proliferative capacity and structural organization of radial glial cells during brain development. Dev Biol. 2021;480:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrette AM, Ronk H, Joshi T, et al. Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models. Neuro Oncol. 2022;24(5):694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang J, Wang L, Wang C, et al. Verteporfin inhibits PD-L1 through autophagy and the STAT1-IRF1-TRIM28 signaling axis, exerting antitumor efficacy. Cancer Immunol Res. 2020;8(7):952–965. [DOI] [PMC free article] [PubMed] [Google Scholar]