Abstract
Background
Pregnancy is a risk factor for progression from latent tuberculosis infection to symptomatic tuberculosis. However, how pregnancy influences T-cell responses to Mycobacterium tuberculosis is unknown.
Methods
We measured M. tuberculosis-specific cytokines, T-cell memory markers, and overall CD4+ and CD8+ T-cell activation by flow cytometry from 49 women (18 with and 31 without HIV) who became pregnant while enrolled in a randomized controlled trial of preexposure prophylaxis for HIV. We analyzed data using COMPASS, an established statistical method for evaluating overall antigen-specific T-cell responses.
Results
Pregnant women with latent tuberculosis infection demonstrated significantly diminished M. tuberculosis-specific CD4+ cytokine responses in the third trimester (COMPASS polyfunctional score [PFS], 0.07) compared before (PFS, 0.15), during (PFS, 0.13 and 0.16), and after pregnancy (PFS, 0.14; P = .0084, Kruskal-Wallis test). Paradoxically, M. tuberculosis-specific CD8+ cytokines and nonspecifically activated T-cells increased during late pregnancy. Nonspecific T-cell activation, a validated biomarker for progression from latent tuberculosis infection to tuberculosis disease, increased in latent tuberculosis infection-positive women postpartum, compared with latent tuberculosis infection-negative women.
Conclusions
Pregnancy-related functional T-cell changes were most pronounced during late pregnancy. Both M. tuberculosis-specific T-cell changes during pregnancy and increases in immune activation postpartum may contribute to increased risk for tuberculosis progression.
Clinical Trials Registration
NCT0557245.
Keywords: M. tuberculosis, pregnancy, T-cell activation, T-cell memory, T cells
Pregnancy is a risk factor for tuberculosis disease. The influence of pregnancy on Mycobacterium tuberculosis-specific T-cell responses is unknown. M. tuberculosis-specific CD4+T-cell responses were selectively diminished and simplified in the third trimester of pregnancy.
Tuberculosis in pregnancy and postpartum is associated with poor maternal and infant outcomes [1, 2]. Recent large cohort studies demonstrate that pregnancy is associated with increased rates of tuberculosis disease up to 6 months postpartum [3, 4]. However, the immune mechanisms underpinning this observation are not clear. Successful pregnancy requires alteration of the local immune environment [5–7]. These adaptations to fetal development are accompanied by cell-specific systemic immune alterations [8]. Pathogen-specific responses may be influenced by these immune changes, which can alter disease risk [9–11]. Understanding pathogen-specific changes to the immune response during pregnancy may improve efforts to prevent and control disease.
T-cell responses are a critical aspect of Mycobacterium tuberculosis host defense. Multiple lines of evidence, including studies in mouse and nonhuman primate studies of CD4+ T-cell knockout or depletion, human genetic studies, and observational studies, demonstrate that CD4+ T cells are essential for M. tuberculosis control [12–15]. Interferon-γ (IFN-γ) responses in CD4+ T cells are also essential for M. tuberculosis protection [16]. The frequency of CD4+ T cells simultaneously producing interleukin 2 (IL-2), tumor necrosis factor (TNF), and IFN-γ (eg, that are polyfunctional) is correlated with M. tuberculosis protection in mice [17]. Other T-cell phenotypes, such as the frequency of antigen-specific central memory (Tcm) and CD8+ T cells, contribute to M. tuberculosis host defense [18–21]. Conversely, nonspecifically activated HLA-DR+CD4+ T cells are a correlate of tuberculosis progression [22]. Because studies of pregnancy commonly begin enrollment during the first or second trimester of pregnancy, they are often unable to capture immune variation prior to and earlier in pregnancy. We leveraged a unique biorepository of samples and data collected longitudinally before, during, and after pregnancy from women in sub-Saharan Africa enrolled in a randomized controlled trial of preexposure prophylaxis for human immunodeficiency virus (HIV) prevention to evaluate the effects of various stages of pregnancy on M. tuberculosis-specific T-cell responses.
METHODS
Study Population
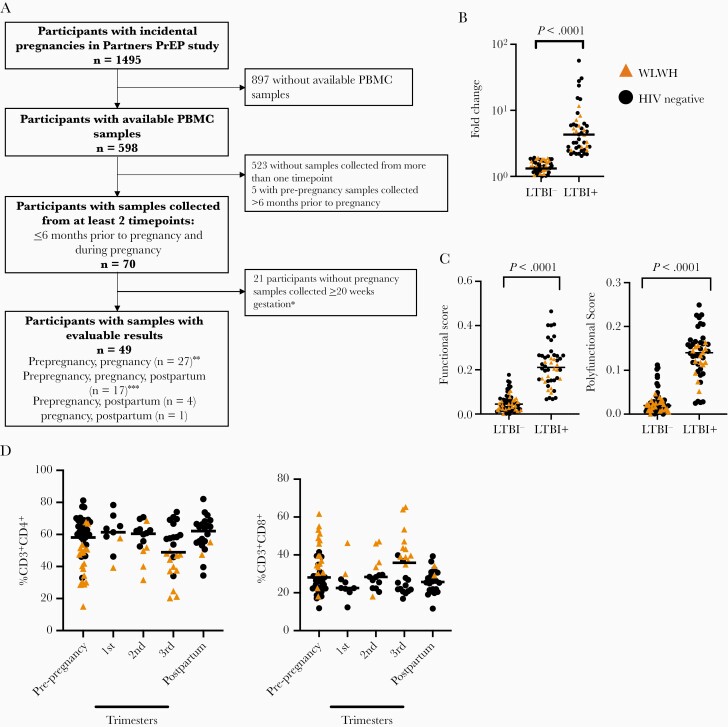
The protocol for this study was approved by the University of Washington Human Subjects Review Committee and ethics review committee at each study site. All participants provided written informed consent. The Partners PrEP Study (NCT0557245) was a randomized clinical trial of antiretroviral preexposure prophylaxis (PrEP) in HIV serodiscordant couples [23]. In total, 4758 participants were enrolled from 9 sites in Kenya and Uganda between 2007 and 2012. Partners with HIV and CD4 cell count > 250, without history of AIDS-defining diagnosis or current use of antiretroviral therapy (ART) were eligible. Participants without HIV underwent monthly HIV testing and had urine pregnancy testing at enrollment and monthly thereafter. Women living with HIV (WLWH) not already on ART who became pregnant were referred for prevention of maternal to child transmission services. Participants were eligible for this analysis if they had at least 2 samples available within 6 months prior to and during incident pregnancy. If available, additional samples were tested from within 6 months postpartum (Figure 1A). For participants with samples available from only before and during pregnancy, paired testing was done on participants with pregnancy samples from > 20 weeks’ gestation. WLWH were provided ART for prevention of maternal to child transmission of HIV.
Figure 1.
Baseline immune characteristics are stable across pregnancy. A, Flowchart of stored sample selection of participants with incidental pregnancies in the Partners PrEP study. B, Fold change of the proportion of CD4+ T cells expressing IFN-γ after incubation with ESAT-6 and CFP-10 pooled peptides, compared to vehicle control. C, COMPASS functional score and polyfunctional score stratified by latent tuberculosis infection status. D, Proportion of CD4+ T cells and CD8+ T cells, stratified by pregnancy trimester and HIV status. Prepregnancy sample, n = 49; 1st trimester, n = 9; 2nd trimester, n = 14; 3rd trimester, n = 24; postpartum, n = 22. Circles indicate HIV-negative study participants, while triangles indicate WLWH. Bars indicate median values. ∗Among participants with pre-pregnancy and pregnancy samples only. ∗∗Includes one participant with a sample collected during pregnancy < 20 weeks of gestation without an evaluable postpartum sample. ∗∗∗Includes two participants with two samples collected during pregnancy. Abbreviations: HIV, human immunodeficiency virus; IFN-γ, interferon-γ; LTBI, latent tuberculosis infection; PBMC, peripheral blood mononuclear cell; PrEP, preexposure prophylaxis; WLWH, women living with HIV.
Flow Cytometry
Cryopreserved peripheral blood mononuclear cells were thawed, washed, and rested overnight. Cells were stimulated and captured on a BD LSRFortessa flow cytometer (Supplementary Table 1) using established protocols (Supplementary Materials).
Identification of Latent Tuberculosis Infection-Positive Participants
Because real-time latent tuberculosis diagnostic tests were not available to us at the time of the parent study, we used a flow cytometry-based assay that demonstrated strong concordance with interferon-γ release assay (IGRA) results in pilot studies and in the published literature. We chose this approach due to its capacity to identify individuals with latent tuberculosis and to identify M. tuberculosis-specific immune response that are missed by traditional IGRA, including polyfunctionality, CD8+ T-cell responses, and IFN-γ–independent T-cell responses [24, 25]. We identified latent tuberculosis infection-positive individuals if the frequency of IFN-γ+CD4+ T cells doubled after ESAT-6 and CFP-10 pooled peptide stimulation compared to dimethyl sulfoxide with a frequency above 0.002% [24, 25].
Statistical Analysis
We used the R package COMPASS to analyze flow cytometry responses. COMPASS uses a Bayesian hierarchical framework to model all observed cell subsets and select those most likely to have antigen-specific responses [26, 27]. COMPASS provides a functional score (FS), which is the proportion of antigen specific subsets detected among all possible ones, and polyfunctional score (PFS), which weighs subsets to favor those producing multiple cytokines. Our primary outcome of interest was the COMPASS PFS from CD4+ and CD8+ T cells of latent tuberculosis infection-positive women, restimulated with ESAT-6 and CFP10 pooled peptides. Differences in median values were compared among time points of interest using Kruskal-Wallis tests followed by Dunn test to compare individual groups. Comparisons combined women with and without HIV as 1 group except where indicated, to optimize sample size. For studies of T-cell memory and activation markers in M. tuberculosis-specific, cytokine-producing cells, a minimum of 100 cytokine-producing cells were required for inclusion.
RESULTS
Cohort Characteristics and Timing of Sample Collection
We identified 70 participants with incident pregnancy. Of these, 49 of these met criteria for this study, with paired samples collected during pregnancy and another collected less than 6 months prior to the pregnancy; 17 participants had samples collected from 3 time points (pre-, during, and postpregnancy); and 32 participants had samples collected before and during pregnancy (Figure 1A). A total of 117 samples with evaluable results were analyzed: 48 prepregnancy (median 18.7 weeks; interquartile range [IQR], 12.4–22.2), 47 during pregnancy (median 26.9 weeks’ gestation; IQR, 12.7–30.9); and 22 postpartum (median 23.1 weeks; IQR, 18.4–39.6). The median age of study participants was 26.4 years (IQR, 23.2–31.1 years); 36.7% (18/49) were WLWH with median CD4 766 cells/mm3 (IQR, 507–1070 cells/mm3) (Table 1).
Table 1.
Baseline Characteristics of Participants and Timing of Samples With Evaluable Results
| Characteristic | Overall | Latent Tuberculosis Infection Positivea | Latent Tuberculosis Infection Negative | RR (95% CI) | P |
|---|---|---|---|---|---|
| Baseline | |||||
| n = 49b | n = 19 | n = 30 | |||
| Age, y, median (IQR) | 26.4 (23.2–31.3) | 26.8 (23.2–31.1) | 26.2 (22.7–31.5) | 1.02 (.94–1.10) | .624 |
| WLWH | 18 (36.7) | 6 (31.6) | 12 (40.0) | 0.79 (.36–1.74) | .565 |
| CD4, cells/mm3, median (IQR) | 765.5 (507–1070) | 868.5 (588–1272) | 730.5 (476.5–1042) | 1.00 (1.00–1.00) | .258 |
| Samples | |||||
| n = 117 | n = 48 | n = 69 | |||
| Pregnancy stage | 1.10 (.83–1.47) | .496 | |||
| Prepregnancyd | 48 (41.0) | 18 (37.5) | 30 (43.5) | ||
| Pregnancy | 47 (40.2) | 20 (41.7) | 27 (39.1) | 0.77 (.52–1.14) | .184 |
| 1st trimester (0 to < 14 wk) | 9 (19.2) | 5 (25.0) | 4 (14.8) | ||
| 2nd trimester (14 to < 28 wk) | 14 (29.8) | 7 (35.0) | 7 (25.9) | ||
| 3rd trimester (≥28 wk) | 24 (51.1) | 8 (40.0) | 16 (59.3) | ||
| Postpregnancyc | 22 (18.8) | 10 (20.8) | 12 (17.4) | ||
| Timing of sample collection, wk | |||||
| Prepregnancy, median (IQR) | −18.7 (−22.2 to −12.4) | −18.3 (−22.3 to −11.7) | −18.8 (−22.1 to −13.1) | 1.00 (.96–1.03) | .836 |
| During pregnancy, median (IQR) | 26.9 (12.7–30.9) | 18.9 (11.7–28.1) | 28.7 (22.4–32.0) | 0.97 (.94–1.00) | .077 |
| Postpregnancy, median (IQR) | 23.1 (18.4–39.6) | 19.5 (17.3–45.6) | 30.5 (21.4–38.7) | 1.00 (.97–1.03) | .980 |
Data are No. (%) except where indicated.
Abbreviations: CI, confidence interval; IFN-γ, interferon-γ; IQR, interquartile range; RR, relative risk; WLWH, women living with HIV.
Latent tuberculosis infection diagnosis determined by 2 × increase of frequency of IFN-γ+CD4+ cells after stimulation with Mycobacterium tuberculosis antigens over control.
Includes 1 participant whose prepregnancy sample was unevaluable.
Up to 6 months after delivery.
Samples collected up to 6 months before date of conception.
Identification of Latent Tuberculosis Infection-Positive Participants
We defined individuals as latent tuberculosis infection positive by flow cytometry if the frequency of ESAT-6 and CFP-10–specific IFN-γ+CD4+ T cells was double baseline, and above 0.002% frequency. This measurement correlates well with commercial IGRA tests [24, 25, 28, 29]. Twenty-two of 49 (45%) individuals were latent tuberculosis infection positive (Figure 1B). Increasing the threshold for positivity to 3-fold above background reduced the proportion of latent tuberculosis infection-positive individuals slightly, from 22 (44%) to 19 (41%). These observations are similar in frequency to other studies of women of childbearing age in regions with high M. tuberculosis burden [1]. We used the more inclusive cutoff to ensure we identified all individuals with preexisting immune responses to M. tuberculosis. We identified 3 individuals who were latent tuberculosis infection negative at baseline but latent tuberculosis infection positive by their pregnancy visit. In these situations, we included these individuals as latent tuberculosis infection positive from their first positive result onward. COMPASS FS and PFS of CD4+ T cells from all samples strongly correlated with our latent tuberculosis infection measurements (Figure 1C).
CD4+ and CD8+ T-Cell Frequency Is Consistent Before, During, and After Pregnancy
We identified the frequency of CD4+ and CD8+ T cells from samples collected before, during, and after pregnancy (gating shown in Supplementary Figure 1A). The frequency of CD4+ and CD8+ T cells was similar across this study (Figure 1D). We observed diminished frequency of CD4+ T cells in WLWH, irrespective of pregnancy status (Supplementary Figure 1B), and decreased CD8+ T-cell frequency (Supplementary Figure 1C).
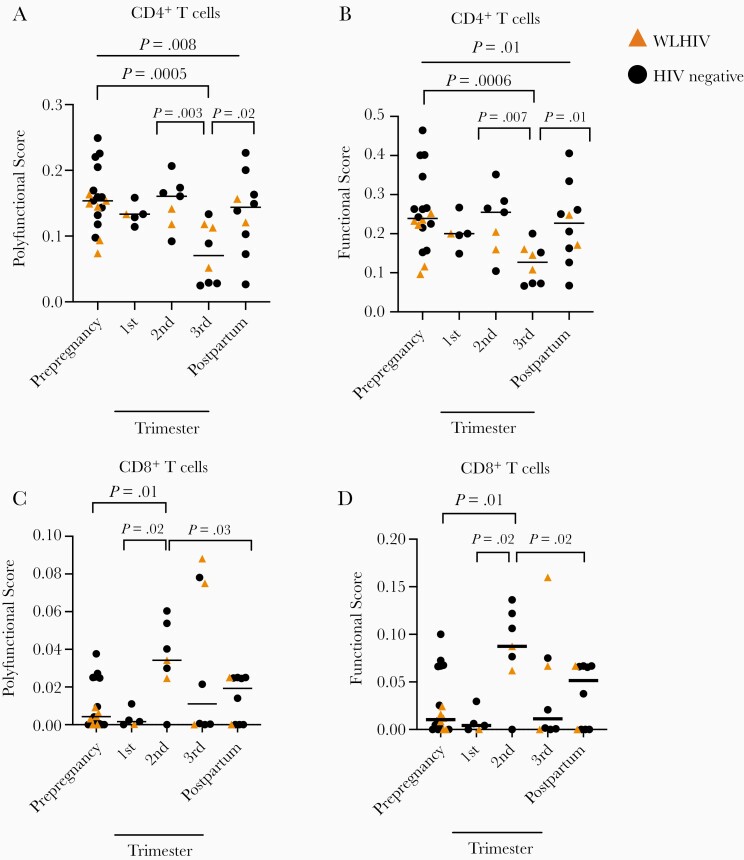
M. tuberculosis-Specific CD4+ T Cells Polyfunctional Responses Decreased During the Third Trimester of Pregnancy
M. tuberculosis induces a polyfunctional T-cell response that may act independently of IFN-γ, and these cells are critical for M. tuberculosis control [30]. In M. tuberculosis-specific CD4+ T cells, PFS was significantly different before, during, and after pregnancy (Figure 2A, gating in Supplementary Figure 2; P = .0084, Kruskal-Wallis test). PFS was significantly decreased in the third trimester compared with prepregnancy (P = .0005), second trimester (P = .0034), and postpregnancy (P < .017), but not during the first trimester (P = .099; Figure 2A). We also evaluated the changes of T-cell responses during pregnancy over time. Using gestational age as a continuous variable, we performed simple linear regression of PFS as a function of gestational time from the second to the third trimester. We found that PFS decreased during this time (P = .0036, R2 = 0.22, regression coefficient = −0.0037). FS results were similarly distributed (Figure 2B). We detected fewer M. tuberculosis-specific cytokine-producing subsets during third trimester, and very few subsets producing multiple cytokines simultaneously (Supplementary Figure 3A). We noted diminished single cytokine responses during the third trimester in IL-2+CD4+ cells compared with the second trimester (P = .026; Supplementary Figure 3B) which trended lower compared with prepregnancy (P = .13), first trimester (P = .12), and postpregnancy (P = .09). CD4+TNF+ or CD4+IFN-γ+ responses were similar across pregnancy (Supplementary Figure 3C and D). Therefore, using COMPASS, we were able to detect changes in M. tuberculosis-specific T-cell responses that may be clinically important but not immediately detectable by analyzing single cytokines separately.
Figure 2.
Mycobacterium tuberculosis-specific CD4+ T-cell responses are diminished during the third trimester of pregnancy. A-B, PFS (A) and FS (B) generated from COMPASS analysis of CD4+ T cell responses, stratified by pregnancy trimester. C-D, PFS (C) and FS (D) generated from COMPASS analysis of CD8+ T cell responses, stratified by pregnancy trimester. Circles indicate an HIV-negative study participant, while triangles indicate a WLWH. Bars indicate median values, Kruskal-Wallis test. Column-to-column comparisons are made with Dunn test. Abbreviations: FS, functional score; HIV, human immunodeficiency virus; PFS, polyfunctional score; WLWH, women living with HIV.
M. tuberculosis-Specific CD8+ T Cells Increase in Activity During the Second and Third Trimester of Pregnancy
CD8+ T cells contribute to M. tuberculosis host defense and are measured in latent tuberculosis infection diagnostic tests [31, 32], but little is known about how pregnancy impacts M. tuberculosis-specific CD8+ T-cell responses. We found low but detectable M. tuberculosis-specific CD8+ T-cell cytokine responses in most samples from latent tuberculosis infection-positive participants (gating strategy in Supplementary Figure 2). The PFS and FS in CD8+ T cells changed significantly over pregnancy (P = .0033 and P = .038, respectively, Kruskal-Wallis test; Figure 2C and 2D). Median PFS and FS in CD8+ T cells was nearly undetectable before pregnancy and significantly increased in the second trimester from prepregnancy (P = .015), the first trimester (P = .023), and postpregnancy (P = .034). The complexity of the CD8+ T-cell response increased during the second and third trimester, and we detected subsets producing multiple cytokines simultaneously (Supplementary Figure 4A). We did not detect changes in IL-2, TNF, and IFN-γ+ CD8+ T-cell responses when evaluated singly, but we found a trend toward increased responses during the second and third trimester (Supplementary Figure 4B–4D). Taken together, M. tuberculosis-specific CD8+ T-cell responses increased during the second and third trimester but M. tuberculosis-specific CD4+ T-cell responses diminished.
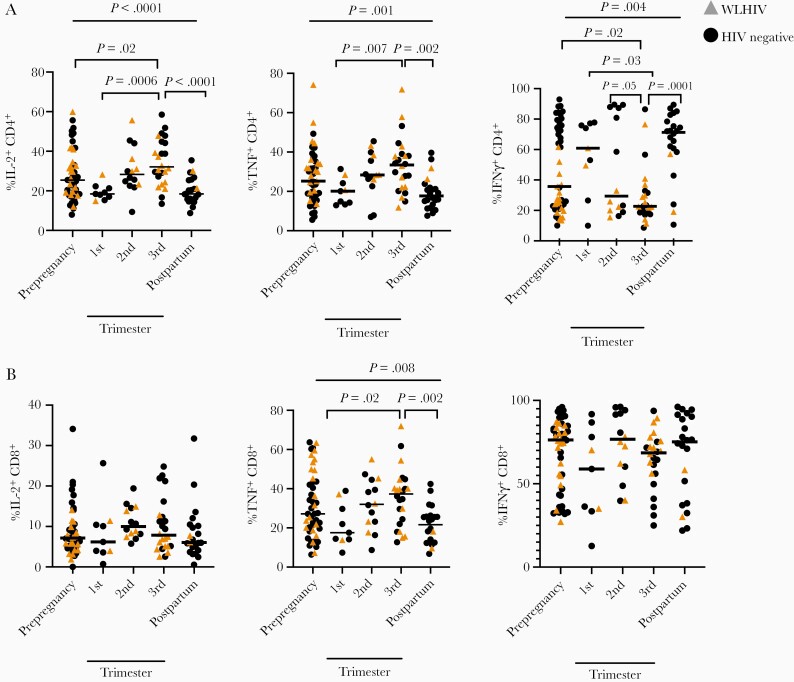
CD4+ T-Cell Responsiveness to a Mitogen Is Altered During the Third Trimester
Clinical laboratory tests for latent tuberculosis infection include mitogen controls to assess overall T-cell reactivity; impacts to mitogen-induced CD4+ and CD8+ T-cell responses during pregnancy directly alter latent tuberculosis infection diagnostic test performance [33]. We compared T-cell responses after stimulation with phorbol 12-myristate 13-acetate and ionomycin (PMA/ionomycin) over pregnancy in all study participants. We evaluated these T-cell responses using traditional flow cytometry analysis, not COMPASS, as COMPASS is designed to detect antigen-specific responses observed at low frequency. The frequency of IL-2+CD4+ T cells responding to PMA/ionomycin were significantly changed over the duration of pregnancy (P < .0001, Kruskal-Wallis test; Figure 3A). IL-2+CD4+ responses in the third trimester were increased compared to prepregnancy (P = .022), first trimester (P = .0006), and postpregnancy (P < .0001). TNF+CD4+ responses were also significantly increased during the third trimester compared to first trimester (P = .0073) and postpregnancy (P = .0002; P = .0011 overall; Figure 3A). IFN-γ+CD4+ cells decreased across pregnancy (P = .0036). The third trimester response was significantly diminished compared to prepregnancy (P = .016), first trimester (P = .026), second trimester (P = .049), and postpregnancy (P = .0001; Figure 3A). IL-2+, TNF+, and IFN-γ+ CD4+ T-cell frequencies were each significantly different before and after pregnancy (P = .006, P = .008, and P = .04, respectively). These data demonstrate distinct patterns of CD4+ T-cell activation across pregnancy as compared to M. tuberculosis-specific T cells.
Figure 3.
PMA/ionomycin-stimulated CD4+ T-cell cytokine responses are dynamic across pregnancy. A, Proportion of IL-2+CD4+, TNF+CD4+, or IFN-γ+CD4+ T cells stratified by pregnancy trimester. B, Proportion of IL-2+CD8+, TNF+CD8+, and IFN-γ+CD8+ T cells stratified by pregnancy trimester. Black dots indicate an HIV-negative study participant, while gold triangles indicate an HIV-positive study participant. Bars indicate median values. P values calculated by Kruskal-Wallis test. Column-to-column comparisons are made with Dunn test. Abbreviations: HIV, human immunodeficiency virus; IFN-γ, interferon-γ; IL-2, interleukin 2; TNF, tumor necrosis factor.
Mitogen-Induced TNF+CD8+ T-Cell Responses Increase During Pregnancy
We measured PMA/ionomycin-stimulated CD8+ T-cell responses over the course of pregnancy in all participants. The frequency of IL-2+CD8+ cells was unchanged over pregnancy (P = .14, Kruskal-Wallis test; Figure 3B). TNF+CD8+ T-cell frequency changed over the course of pregnancy (P = .0084; Figure 3B), increasing in the third trimester compared with the first trimester (P = .015) and postpregnancy (P = .0018). IFN-γ+CD8+ T-cell frequencies were similar (Figure 3B). These responses were distinct from the changes observed in M. tuberculosis-specific CD8+ T cells, where multiple subsets increased in frequency during the second and third trimester.
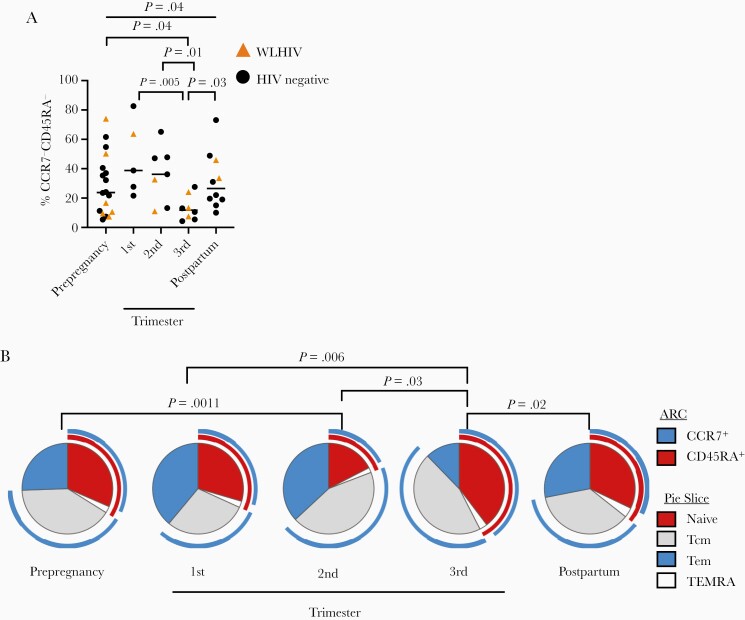
M. tuberculosis-Specific Effector Memory T-Cell Populations Diminish During the Third Trimester
T cells display cell surface markers CD45RA and CCR7, which define the T-cell memory phenotype [34]. Naive (CCR7+CD45RA+), central memory (Tcm; CCR7+CD45RA−), and effector memory (Tem; CCR7−CD45RA−) T cells display distinct functional profiles and tissue homing sites, and Tcm are important for M. tuberculosis control [18, 35, 36]. The frequency of total CD4+ and CD8+ T cells expressing CD45RA and CCR7 did not change over pregnancy (Supplementary Figure 5A–5C). We evaluated CD45RA and CCR7 expression in M. tuberculosis-specific CD4+ T cells, which were ESAT-6 and CFP-10 peptide-incubated, cytokine-producing, CD4+ T cells from latent tuberculosis infection-positive individuals. The proportion of M. tuberculosis-specific naive and Tcm CD4+ T cells remained stable throughout pregnancy (data not shown), but the frequency of M. tuberculosis-specific Tem decreased during the third trimester (P = .037, Kruskal-Wallis test; Figure 4A) compared to prepregnancy (P = .049), first trimester (P = .005), second trimester (P = .014), and postpartum (P = .031). Viewed proportionally, naive CD4+ cells increased while Tem decreased during the third trimester of pregnancy compared to the first trimester, second trimester, and postpartum, without substantial changes to Tcm frequency (Figure 4B). We did not detect adequate M. tuberculosis-specific CD8+ T cells to evaluate T-cell phenotypes in this population. In summary, Tem and IFN-γ+CD4+ cells were reduced during the third trimester, but without increases in the Tcm population, which is important for M. tuberculosis host defense and may predict worsened M. tuberculosis control.
Figure 4.
The proportion of Mycobacterium tuberculosis-specific naive effector memory T cells decreases during the third trimester of pregnancy. A, Overall proportion of Tem (CCR7−CD45RA−) among all cytokine-producing CD4+ T cells after restimulation with ESAT-6 and CFP-10 antigens, stratified by pregnancy status. Black dots indicate an HIV-negative study participant, while gold triangles indicate a sample from WLWH study participant. P values calculated by Kruskal-Wallis test. Column-to-column comparisons are made with Dunn test. B, Pie chart of Boolean-gated T helper memory phenotype frequencies, comparing the third trimester with other time points, connected by overhead bar. Arc: blue, CCR7+ cells; red, CD45RA+ cells. Pie slice: red, naive CD4+ T cells; blue, Tem CD4+ T cells; gray, Tcm; white, TEMRA cell. Signifcance determined by permutation test of 10 000 iterations. Abbreviations: HIV, human immunodeficiency virus; Tcm, central memory T cell; Tem, effector memory T cell; WLWH, women living with HIV.
Nonspecific T-Cell Activation Increases During Pregnancy
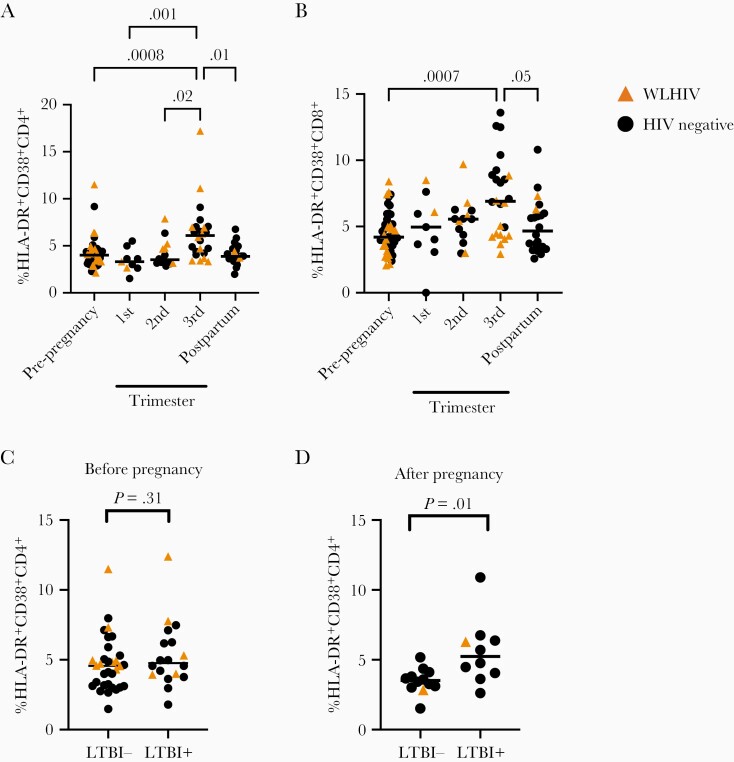
Increased nonspecific CD4+ T-cell activation is a correlate of risk for developing M. tuberculosis disease [22, 37]. We measured the frequency of HLA-DR+CD38+CD4+ and CD8+ T cells across pregnancy, using fluorescence minus 1 controls (Supplementary Figure 6). The frequency of HLA-DR+CD38+CD4+ T cells significantly changed over pregnancy (P = .0001, Kruskal-Wallis test; Figure 5A), highest in the third trimester compared to prepregnancy (P = .008), first trimester (P = .001), second trimester (P = .02), or postpartum (P = .01). HLA-DR+CD38+CD8+ T-cell frequency also changed similarly to CD4+ T cells (P = .002, Kruskal-Wallis test; Figure 5B).
Figure 5.
Nonspecific T-cell activation increases during pregnancy and latent tuberculosis infection-positive women demonstrate increased T-cell activation postpartum. A and B, Proportion of (A) HLA-DR+CD38+CD4+ T cells and (B) HLA-DR+CD38+CD8+ T cells stratified by pregnancy status. C and D, Proportion of (C) HLA-DR+CD38+CD4+ T cells from samples collected prepregnancy and (D) proportion of HLA-DR+CD38+CD4+ T cells from samples collected postpartum stratified by latent tuberculosis infection status of the participant. Circles indicate an HIV-negative study participant, while triangles indicate sample from WLWH. Bars indicate median values. P values calculated by Kruskal-Wallis test. Column-to-column comparisons are made with Dunn test in multiple group comparisons or Mann-Whitney U test for tests between 2 groups. Abbreviations: HIV, human immunodeficiency virus; LTBI, latent tuberculosis infection; WLWH, women living with HIV.
We leveraged the observation that CD4+ T-cell activation is a biomarker for tuberculosis risk to evaluate if pregnancy might increase the risk for tuberculosis progression from asymptomatic to symptomatic disease. We hypothesized that selective increases in nonspecific T-cell activation would be present in latent tuberculosis infection-positive women after pregnancy due to their increased risk for postpartum M. tuberculosis progression but not for latent tuberculosis infection-negative women. Prepregnancy, the proportion of HLADR+CD38+CD4+ T cells was equivalent between latent tuberculosis infection-negative and latent tuberculosis infection-positive individuals (Figure 5C). Postpartum, latent tuberculosis infection-positive individuals demonstrated significantly higher HLA-DR+CD38+CD4+ T cells as compared to latent tuberculosis infection-negative individuals (P = .008, Mann-Whitney test; Figure 5D). Immune changes of pregnancy in latent tuberculosis infection-positive individuals may substantially contribute to overall T-cell activation in the postpartum period and predict increased risk for tuberculosis progression.
DISCUSSION
In this study, we performed a comprehensive analysis of pregnancy stage on M. tuberculosis-specific T-cell phenotypes in sub-Saharan African women, including WLWH. This is, to our knowledge, the first longitudinal assessment of systemic T-cell responses to M. tuberculosis in pregnancy that includes samples collected prior to pregnancy. Late pregnancy is associated with multiple changes to M. tuberculosis-specific T-cell function: (1) overall M. tuberculosis-specific CD4+ T-cell responses diminished and simplified; (2) M. tuberculosis-specific CD4+ Tem, cells that strongly produce IFN-γ, decreased [34]; (3) M. tuberculosis-specific CD8+ T-cell responses increased; and (4) nonspecific T-cell activation increased. Nonspecific T-cell activation increased in latent tuberculosis infection-positive individuals compared to latent tuberculosis infection-negative individuals postpartum, despite being equivalent prepregnancy. Increased nonspecific T-cell activation, a correlate of risk for M. tuberculosis disease in latent tuberculosis infection-positive individuals, supports the observation that tuberculosis risk increases in the early postpartum period [22]. Together, late pregnancy induces complex changes in the known T-cell response to M. tuberculosis associated with tuberculosis progression, and latent tuberculosis infection-positive women develop increased correlates of risk for tuberculosis in the immediate postpartum period.
We observed distinct changes between M. tuberculosis-specific and more generalized mitogen (PMA/ionomycin)-induced T-cell cytokine responses (Table 2). These results have direct implications for the performance of latent tuberculosis infection diagnostics in pregnant women. M. tuberculosis-specific CD4+ T-cell responses decreased during the third trimester but increased in the second trimester, and M. tuberculosis-specific CD8+ T-cell responses were low but increased during the second and third trimester. By contrast, overall mitogen responses in CD4+ T cells, especially IL-2 responses, increased during late pregnancy. In other cohorts, discordance between the 2 commonest latent tuberculosis infection tests, tuberculin skin test (TST) and IGRA, has been noted, especially among women in the late second and early third trimester, which may be partly due to the changes in the immune responses of pregnancy [33, 38]. Part of the difference may be due to the absence of mitogen testing in most TSTs. Understanding this discordance in pregnancy may have implications for tuberculosis progression. Among those with positive IGRA but negative TST, M. tuberculosis-specific IL-2 responses associated with postpartum tuberculosis progression from asymptomatic infection to symptomatic disease [39]. In combination, discordance between M. tuberculosis-specific and nonspecific immune responses may contribute to increased false-negative latent tuberculosis infection testing during the third trimester and may contribute to M. tuberculosis progression.
Table 2.
Model Summary
| T Cells | Prepregnancy | 1st Trimester | 2nd Trimester | 3rd Trimester | Postpartem |
|---|---|---|---|---|---|
| Mycobacterium tuberculosis-specific T cells | |||||
| CD4+ | ↔ | ↔ | ↓ | ↓↓↓ | ↔ |
| CD8+ | ↓↓↓ | ↓↓ | ↑↑ | ↑ | ↓↓ |
| Mitogen-stimulate T cells | |||||
| CD4+ | ↔ | ↔ | ↔ | ↑↓ | ↑↓ |
| CD8+ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Nonspecific T-cell activation | |||||
| HLA-DR+CD38+CD4+ | ↔ | ↓ | ↑↑ | ↑↑↑ | ↑ |
We observed distinct changes in Mycobacterium tuberculosis-specific T-cell responses as compared to mitogen-stimulated T cells. Arrows indicate the overall frequency of the responses measured. A downward arrow followed by an upward arrow indicates diminished IL-2/TNF responses with simultaneous increases in IFN-γ+ cell frequency, and an upward arrow followed by a downward arrow indicates increases in IL-2/TNF+ T-cell frequency with decreases in IFN-γ+ responses.
Abbreviations: IFN-γ, interferon-γ; IL-2, interleukin 2; TNF, tumor necrosis factor.
M. tuberculosis-specific CD8+ T-cell responses increased during late pregnancy, when CD4+ T-cell responses were diminished. The role of CD8+ T cells in tuberculosis pathogenesis is uncertain. In children, M. tuberculosis-specific CD8+ T-cell frequencies are correlated with M. tuberculosis disease [21]. In animals, CD8+ T-cell responses correlate strongly with M. tuberculosis bacterial burden in the lung [40, 41]. CD8+ T cells recognize antigens presented on major histocompatibility complex I, which presents intracellular antigens. CD8+ T cells preferentially recognize cells with increased bacterial burden [42]. During active intracellular infection, these responses may increase proportionally. It is unclear if some of these responses reflect emerging tuberculosis disease or if there are general postpartum factors that enhance CD8+ responses. CD8+ T-cell responses may increase to compensate for diminished CD4+ T-cell responses but may be unable to fully reproduce CD4+ T-cell immune control.
The strengths of this study include its longitudinal nature, including samples obtained before, during, and after pregnancy. Most studies of the effects of pregnancy enroll women during pregnancy and often focus on the second and third trimester, which do not capture prepregnancy and first trimester. Several T-cell phenotypes change between prepregnancy and the first trimester, and other phenotypes differed between prepregnancy and postpartum. Local inflammation is critical for embryonic implantation, suggesting that immune changes begin immediately upon fertilization [43, 44]. Another strength was the use of COMPASS. COMPASS analysis revealed 2 findings that were not observable using standard data analysis techniques. This sensitive analysis permitted us to identify diminished complexity of the M. tuberculosis-specific CD4+ T-cell response and increases in overall CD8+ activity. Taken together, no single cytokine response accounted for the changes to the M. tuberculosis-specific T-cell response, suggesting broad changes in the functional capacity of T cells during the third trimester.
Weaknesses of this study include the lack of tuberculosis disease outcomes data. This study used repository samples from a trial not originally designed to evaluate tuberculosis. However, we performed detailed immune characterization of the M. tuberculosis-specific immune response across pregnancy. Importantly, latent tuberculosis infection-positive women had increased T-cell activation postpartum, which is a validated biomarker of tuberculosis disease progression [22]. IGRA or TST was not performed in the parent study, which prevents correlation with clinically validated latent tuberculosis infection testing. We identified latent tuberculosis infection-positive participants using a well-accepted alternate approach that correlates with latent tuberculosis infection diagnosis [28, 29]. Generally, flow-based testing is more sensitive to detect prior exposure to M. tuberculosis antigens than IGRA, so this approach permits us to evaluate a broader range of individual immune responses. Errors introduced by including individuals without preexisting M. tuberculosis exposures may reduce the study sensitivity, but should not detract from the observations made here. M. tuberculosis-specific T-cell responses during pregnancy were generally independent of HIV status, although direct comparisons were limited due to small sample size. The Partners studies featured women with regular exposure to HIV [23]. This regular exposure may influence nonspecific T-cell activation, as HIV exposed, uninfected infants develop altered T-cell responses [45]. However, its role in systemic T-cell responses and its role in tuberculosis susceptibility in adults are unknown. We hypothesize that our findings reflect changes due to pregnancy, not HIV exposure, because they predominate in late pregnancy with return to pre- and early pregnancy levels postpartum.
Tuberculosis is the third leading cause of death among women of child-bearing age in high-burden areas and is associated with mortality in pregnant women and their children [1, 38]. Systemic changes in the T-cell response during the third trimester may contribute to increased risk for tuberculosis. T-cell cytokine responses are reduced and simplified, T-cell memory phenotypes skewed toward naive phenotypes, and nonspecific activation is enhanced, which all are independently associated with tuberculosis risk. Data suggest that isoniazid preventative therapy is associated with adverse pregnancy outcomes, with a signal that this may occur more often if initiated early in pregnancy [46]. Late pregnancy may be advantageous to target for shorter course regimens. Understanding host factors that place pregnant mothers at risk for tuberculosis may provide insight into optimal public health strategies for tuberculosis control in this high-risk population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the study participants and their families. We acknowledge the support of Harald Haugen on database management of clinical samples; and the clinical teams who obtained informed consent and collected samples for this biorepository. We also thank Chetan Seshadri for helpful technical conversations.
Author contributions. A. S. contributed to the design, data acquisition, analysis, and manuscript preparation. J. E. contributed data analysis, manuscript preparation, sample collection, and transport. T. L. contributed statistical analysis and software. B. R. contributed data analysis, statistical support, and manuscript editing. S. H. and H. H. contributed sample transport, sample storage, database management. N. M. and A. M. contributed sample collection/storage, participant enrollment. C. C. and J. B. contributed cohort enrollment, funding, sample storage, manuscript editing. J. L. contributed cohort enrollment, sample storage, funding, manuscript editing. G. J. S. contributed experimental design, data analysis, funding, manuscript editing. S. L. and J. A. S. contributed experimental design, data analysis, grant funding, manuscript preparation and editing.
Financial support. This work was supported by National Institute of Child Health and Human Development (grant number R21-HD098746); National Institute of Allergy and Infectious Diseases (grant numbers K23-AI120793 and R01-AI136921); and Bill and Melinda Gates Foundation (grant numbers 26469, 41185, and 47674).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 51st Union World Conference on Lung Health, virtual, 19 October 2020.
References
- 1. Mathad JS, Gupta A.. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathad JS, LaCourse SM, Gupta A.. TB prevention strategies and unanswered questions for pregnant and postpartum women living with HIV: the need for improved evidence. J Int AIDS Soc 2020; 23:e25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonsson J, Kuhlmann-Berenzon S, Berggren I, Bruchfeld J.. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. Eur Respir J 2020; 55:1901886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zenner D, Kruijshaar ME, Andrews N, Abubakar I.. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. [DOI] [PubMed] [Google Scholar]
- 5. Mor G, Aldo P, Alvero AB.. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017; 17:469–82. [DOI] [PubMed] [Google Scholar]
- 6. Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol 2013; 31:387–411. [DOI] [PubMed] [Google Scholar]
- 7. Shah NM, Herasimtschuk AA, Boasso A, et al. Changes in T cell and dendritic cell phenotype from mid to late pregnancy are indicative of a shift from immune tolerance to immune activation. Front Immunol 2017; 8:1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aghaeepour N, Ganio EA, McIlwain D, et al. An immune clock of human pregnancy. Sci Immunol 2017; 2:eaan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kourtis AP, Read JS, Jamieson DJ.. Pregnancy and infection. N Engl J Med 2014; 370:2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Racicot K, Mor G.. Risks associated with viral infections during pregnancy. J Clin Invest 2017; 127:1591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kay AW, Fukuyama J, Aziz N, et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci USA 2014; 111:14506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leveton C, Barnass S, Champion B, et al. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun 1989; 57:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakai S, Mayer-Barber KD, Barber DL.. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr Opin Immunol 2014; 29:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin PL, Rutledge T, Green AM, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses 2012; 28:1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Badri M, Wilson D, Wood R.. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002; 359:2059–64. [DOI] [PubMed] [Google Scholar]
- 16. Sakai S, Kauffman KD, Sallin MA, et al. CD4 T cell-derived IFN-gamma plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog 2016; 12:e1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008; 181:4955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindenstrom T, Knudsen NP, Agger EM, Andersen P.. Control of chronic Mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol 2013; 190:6311–9. [DOI] [PubMed] [Google Scholar]
- 19. Andersen P, Urdahl KB.. TB vaccines; promoting rapid and durable protection in the lung. Curr Opin Immunol 2015; 35:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grotzke JE, Lewinsohn DM.. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect 2005; 7:776–88. [DOI] [PubMed] [Google Scholar]
- 21. Lancioni C, Nyendak M, Kiguli S, et al. CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Resp Crit Care 2012; 185:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 2016; 7:11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tesfa L, Koch FW, Pankow W, Volk HD, Kern F.. Confirmation of Mycobacterium tuberculosis infection by flow cytometry after ex vivo incubation of peripheral blood T cells with an ESAT-6-derived peptide pool. Cytometry B Clin Cytom 2004; 60:47–53. [DOI] [PubMed] [Google Scholar]
- 25. Papageorgiou CV, Anastasopoulos A, Ploussi M, et al. Flow cytometry analysis of CD4+IFN-gamma+ T-cells for the diagnosis of Mycobacterium tuberculosis infection. Cytometry B Clin Cytom 2016; 90:303–11. [DOI] [PubMed] [Google Scholar]
- 26. Lin L, Finak G, Ushey K, et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 2015; 33:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah JA, Musvosvi M, Shey M, et al. A functional TOLLIP variant is associated with BCG-specific immune responses and tuberculosis. Am J Respir Crit Care Med 2017; 196:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Lee SY, Won DI, Cha SI, Park JY, Kim CH.. Comparison of whole-blood interferon-gamma assay and flow cytometry for the detection of tuberculosis infection. J Infect 2013; 66:338–45. [DOI] [PubMed] [Google Scholar]
- 29. Sauzullo I, Scrivo R, Mengoni F, et al. Multi-functional flow cytometry analysis of CD4+ T cells as an immune biomarker for latent tuberculosis status in patients treated with tumour necrosis factor (TNF) antagonists. Clin Exp Immunol 2014; 176:410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu LL, Smith MT, Yu KKQ, et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med 2019; 25:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin PL, Flynn JL.. CD8 T cells and Mycobacterium tuberculosis infection. Semin Immunopathol 2015; 37:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuyuzaki M, Igari H, Okada N, Suzuki K.. Role of CD8 T-cell in immune response to tuberculosis-specific antigen in QuantiFERON-TB gold plus. J Infect Chemother 2020; 26:570–4. [DOI] [PubMed] [Google Scholar]
- 33. LaCourse SM, Cranmer LM, Matemo D, et al. Effect of pregnancy on interferon gamma release assay and tuberculin skin test detection of latent TB infection among HIV-infected women in a high burden setting. J Acquir Immune Defic Syndr 2017; 75:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seder RA, Darrah PA, Roederer M.. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8:247–58. [DOI] [PubMed] [Google Scholar]
- 35. Jameson SC, Masopust D.. Understanding subset diversity in T cell memory. Immunity 2018; 48:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vogelzang A, Perdomo C, Zedler U, et al. Central memory CD4+ T cells are responsible for the recombinant bacillus Calmette-Guerin Δ ureC:: hly vaccine’s superior protection against tuberculosis. J Inf Dis 2014; 210:1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arenas-Hernandez M, Romero R, Xu Y, et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J Immunol 2019; 202:2585–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mathad JS, Bhosale R, Sangar V, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PLoS One 2014; 9:e92308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathad JS, Bhosale R, Balasubramanian U, et al. Quantitative IFN-gamma and IL-2 response associated with latent tuberculosis test discordance in HIV-infected pregnant women. Am J Respir Crit Care Med 2016; 193:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lewinsohn DM, Tydeman IS, Frieder M, et al. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect 2006; 8:2587–98. [DOI] [PubMed] [Google Scholar]
- 41. Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J.. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol 2007; 179:3973–81. [DOI] [PubMed] [Google Scholar]
- 42. Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM.. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Resp Crit Care 2003; 168:1346–52. [DOI] [PubMed] [Google Scholar]
- 43. Zenclussen AC, Hämmerling GJ.. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front Immunol 2015; 6:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gnainsky Y, Granot I, Aldo P, et al. Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction 2015; 149:75–85. [DOI] [PubMed] [Google Scholar]
- 45. Jalbert E, Williamson KM, Kroehl ME, et al. HIV-exposed uninfected infants have increased regulatory T cells that correlate with decreased T cell function. Front Immunol 2019; 10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta A, Montepiedra G, Aaron L, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med 2019; 381:1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.