Abstract
Context
Multiple studies suggest that adults who were normal weight at diabetes diagnosis are at higher risk for all-cause mortality than those who had overweight or obesity at diagnosis.
Objective
While obesity is a known risk factor for cardiometabolic disease, differences in body fat distribution in those without obesity are understudied, especially in African Americans.
Methods
In 1005 participants of the Jackson Heart Study, without cardiovascular disease at baseline, we used logistic regression to investigate the longitudinal association of body fat distribution by CT scan with metabolic syndrome (MetS) or type 2 diabetes (T2D). We used the harmonized International Diabetes Federation criteria to define MetS. We included only normal weight or overweight participants (BMI: 18.5 to < 30.0 kg/m2). We created separate models for MetS and T2D adjusted for a standard set of covariates. We excluded participants with prevalent MetS or T2D, respectively in sensitivity.
Results
Higher visceral fat, subcutaneous fat, BMI, and insulin resistance (HOMA-IR) were significantly associated with MetS and T2D after adjustment. Visceral fat was strongly associated with both outcomes (MetS OR = 2.07 [1.66-2.68]; T2D OR = 1.51 [1.21-1.88]), and the association for MetS persisted in the normal weight only group. Estimates were robust to sensitivity analysis and were only modestly mediated by insulin resistance. Physical activity was not associated with MetS or T2D.
Conclusion
Visceral fat is strongly associated with developing MetS, even in normal weight individuals, suggesting that excess visceral fat plays a role in cardiometabolic risk beyond that of overall adiposity and obesity in African Americans.
Keywords: type 2 diabetes, metabolic syndrome, visceral fat, normal weight, African American
Evidence suggests that those who were normal weight at diagnosis of type 2 diabetes (T2D) are at higher risk for all-cause mortality than those who had overweight or obesity at diagnosis, and this association was similar for white and non-white participants [1-3]. Some suggest that this finding is due to collider stratification bias from selecting only those with T2D into these studies [4]. In contrast, evidence suggests several mechanisms by which T2D could have an impact on morbidity and mortality even in those without obesity [5], and that these mechanisms may have the largest impact on racial/ethnic minority populations [6, 7]. Given the focus on obesity as a primary risk factor for T2D, prevention strategies for the normal weight group have been more limited. For instance, the U.S. Preventive Services Task Force continues to focus T2D screening recommendations on those with overweight or obesity [8]. Few studies have investigated risk factors for the development of T2D in the absence of obesity and the few studies that exist focus predominantly on genetic predisposition. Several conditions, such as polycystic ovary syndrome, are associated with high levels of visceral fat and metabolic dysregulation in individuals with normal weight; however, while insulin resistance has exhibited patterns within families in close connection to body fat distribution and metabolic syndrome, the drastic changes in the prevalence of obesity and T2D over the last several decades implicate systematic changes instead. Perhaps due to the overrepresentation of obesity and T2D in African Americans, the determinants of metabolic dysfunction in the absence of obesity have been poorly studied specifically in this group. This question is particularly pressing, as diabetes prevalence is higher for African Americans compared with white populations even in the normal weight and overweight categories [6]. Furthermore, there is some evidence to suggest that obesity-associated risk differs by race, at least partially due to differences in body fat composition [9-12]. This evidence combined with the concept of “normal weight obesity” offers a hypothesis to explain why some groups may be at higher cardiometabolic risk even in the lower body mass index (BMI) categories. Other work has focused on the role of visceral fat in transition to each risk factor separately, but it is well recognized that risk factor clustering has a synergistic effect on cardiovascular disease (CVD) outcomes [13]. Metabolic syndrome (MetS) provides a concise way of defining that risk factor clustering. Prior studies have shown that visceral fat is a stronger predictor of cardiometabolic outcomes (T2D and metabolic syndrome [MetS], for the purposes of this study) than BMI [14, 15], but few studies have investigated the role that body fat composition may play in risk stratification for individuals without obesity. This problem is exacerbated by the paucity of high-quality data on computed tomography (CT)-derived measurements of fat mass in cohorts with non-white participants [9-11, 16].
Further complicating this issue is the methodological concern about the current classification of body size phenotypes that focuses on metabolically healthy obesity and therefore includes BMI less than 30 kg/m2 in the “normal weight” or “non-obese” reference category [17, 18]. Combining overweight and normal weight groups may lead to biased estimates, as the literature suggests that the relationship between BMI and T2D or CVD is positive and linear [19]. For this reason, understanding the determinants of cardiometabolic risk, including body fat distribution, may require separate investigations by BMI category especially for those who fall into the true normal weight category.
The determinants of MetS and T2D in the absence of obesity, and specifically the normal weight category, have been poorly studied in African Americans. This is especially the case for gold standard measurements of body fat distribution. We therefore hypothesized that body fat distribution, specifically higher visceral fat, along with lower physical activity, and higher insulin resistance are associated with MetS and T2D separately in Jackson Heart Study participants without obesity. Specifically, we hypothesized that the positive relationship between visceral fat and cardiometabolic outcomes is mediated by insulin resistance, but that C-reactive protein (CRP) will not be independently associated with the outcomes. We further hypothesized that the association for visceral fat would be present specifically in normal weight individuals and would not differ from that in overweight individuals.
Methods
Study Population
The Jackson Heart Study (JHS) began in 2000 by enrolling 5306 African American participants between the ages of 35 and 84 years from the Jackson, Mississippi metropolitan area [20]. Extensive data collection included demographics and socioeconomic status, lifestyle factors, traditional and suspected risk factors for T2D and CVD, laboratory assays conducted at a central laboratory, and imaging. Follow-up included 2 additional exams (Visit 2 in 2005-2008 and Visit 3 in 2009-2013), annual phone calls, and confirmation of vital statistics. Body fat composition, including visceral and subcutaneous fat, was measured from CT scans conducted at Visit 2. Therefore, we consider Visit 2 as baseline for this analysis. We excluded participants with obesity (n = 2822, BMI ≥ 30 kg/m2) or underweight (n = 40, BMI < 18.5 kg/m2). Then we excluded participants without data on visceral and subcutaneous fat (n = 1085), as well as those missing essential covariates like age, sex, socioeconomic status (income and education), and smoking status (n = 207). Since we were interested in MetS and T2D as separate outcomes, we created 2 separate analysis datasets. For the MetS dataset, we excluded participants with unknown MetS status due to missing data (n = 10), and participants with prevalent MetS at Visit 1 (n = 215). Similarly, for the T2D dataset, we excluded those missing data on T2D (n = 137), and cases of T2D that occurred before the measurement of body fat distribution at Visit 2 (n = 10). This left a final sample size of 927 for analysis of MetS and 1005 for T2D. Finally, to specifically investigate incident events, we excluded 132 participants with prevalent MetS and 61 with prevalent T2D at Visit 2 in sensitivity analysis. All participants provided written informed consent and all procedures were approved by the University of Mississippi Medical Center Institutional Review Board.
Measurement of Potential Determinants
BMI was calculated as weight in kg divided by height in meters squared, using a standardized measurement procedure. Absence of obesity was defined as a BMI between 18.5 and < 30 kg/m2 and all primary analyses focused on this group. Normal weight was defined as a BMI between 18.5 kg/m2 and < 25 kg/m2 and was used in secondary analysis. Visceral and subcutaneous fat were measured from CT scans using semi-automated volume analysis software (Advantage Windows; GE Healthcare, Waukesha, WI) at the core reading center. Fat was determined by a density of −190 to −30 Hounsfield units at the L4-L5 vertebral level [21].
Physical activity was self-reported. Physical activity was defined using the American Heart Association Life’s Simple 7 criteria with 3 categories: poor (0 minutes physical activity), intermediate (1-150 minutes of moderate activity or 1-75 minutes vigorous activity per week), and ideal (≥150 minutes of moderate activity or ≥ 75 minutes of vigorous activity per week), with ideal as the reference [22]. CRP and the homeostasis model assessment for insulin resistance (HOMA-IR) were measured from assays of fasting plasma. CRP was assayed by immunoturbidimetric CRP-Latex on a Hitachi 911 analyzer from Roche Diagnostics [23], and HOMA-IR was calculated as (insulin [μU/mL]* glucose [mmol/L])/22.5. Other covariates including age, sex, income, education, and smoking status were self-reported.
Measurement of Cardiometabolic Outcomes (Metabolic Syndrome and Type 2 Diabetes)
MetS was defined using the Harmonized International Criteria as 3 or more of the following: (1) fasting glucose ≥ 100 mg/dL or glucose-lowering treatment; (2) blood pressure ≥ 130 systolic and/or ≥ 85 diastolic or antihypertensive treatment; (3) HDL < 50 mg/dL for women or < 40 mg/dL for men or treatment for low HDL; (4) triglycerides ≥ 150 mg/dL or triglyceride-lowering treatment; and (5) waist circumference ≥ 88 cm for women or ≥ 102 cm for men [13]. MetS components were measured at every visit using standardized protocols. T2D was defined using the 2010-2021 American Diabetes Association criteria based on: (1) glucose-lowering medication use; (2) fasting glucose ≥ 126 mg/dL; and/or (3) glycated hemoglobin (HbA1c) ≥ 6.5%. The definition of T2D also included self-reported diagnosis. Oral glucose tolerance testing results are also part of the American Diabetes Association criteria but were not available in JHS. T2D was assessed at every visit using standardized protocols [24, 25].
Statistical Analysis
To investigate determinants of cardiometabolic dysfunction in those without obesity, we assessed associations of body fat distribution, insulin resistance, physical activity, and CRP with MetS and T2D. For all models, MetS and T2D were modeled separately as distinct outcomes. Continuous variables were standardized to investigate outcomes per 1 standard deviation difference in exposures. We described the Visit 2 characteristics of the non-obese Jackson Heart Study participants without obesity by MetS and T2D status at follow-up. We then used logistic regression to model the longitudinal association between potential determinants and cardiometabolic dysfunction, with body fat distribution (visceral and subcutaneous fat) as the primary determinants of interest. For all models we adjusted for confounding by including age, sex, education, income, and smoking status followed by further mutual adjustment for other body fat composition variables. Final models included full adjustment, except that models included either BMI or subcutaneous fat but not both. Based on a priori understanding of the causal pathway, we used the Valeri and VanderWeele mediation approach and PROC CAUSALMED with only main effects to assess how much of the association between visceral fat and cardiometabolic outcomes (MetS and T2D, respectively) was mediated by insulin resistance (HOMA-IR) [26, 27].
We investigated these associations in the normal weight and overweight groups separately in order to determine whether discrepancies in prior associations could be explained by considering the inclusion of the overweight category (BMI between 25 and 30 kg/m2) into the non-obese group as misclassification of normal weight. We formally investigated heterogeneity in the estimates for visceral fat and HOMA-IR by BMI category by including an interaction term. We also formally tested for interaction with visceral fat by age and sex by including an interaction term in the fully adjusted model. Finally, we used sensitivity analysis to test the robustness of our results by: (1) excluding participants known to have taken hormone replacement therapy (HRT) or with unknown HRT status, as HRT is associated with body fat composition and with cardiometabolic disease; (2) using an abbreviated definition of MetS with waist circumference criterion removed and requiring 2 or more of the 4 remaining criteria to eliminate concern about the overlap between BMI and waist circumference; and (3) excluded participants with prevalent MetS or T2D, respectively, to investigate incident outcomes specifically.
Results
All baseline characteristics (Visit 2 for this analysis) except smoking and physical activity differed significantly by visceral fat quartile (Table 1). Trends were generally in the expected direction, with participants in higher visceral fat quartiles characterized on average by older age, higher percentage male, higher insulin resistance, and higher prevalence of MetS criteria. Average weight was similar between Visit 2 and 3 (mean = 78.2 kg for both visits), but some increases were noted in the groups in the third and fourth quartile of visceral fat. Similarly, prevalence of MetS components was also higher at Visit 3 (Table 1). Those with higher visceral fat quartile at baseline (Visit 2) also generally had higher incidence of MetS and T2D at follow-up (Visit 3). There were 212 MetS cases and 116 T2D cases across a median of 5 years of follow-up. Of those with MetS, 44 (21%) also had T2D.
Table 1.
Baseline characteristics (mean [SD] or percentage) in 1005 Jackson Heart participants without obesity and type 2 diabetes at baseline, by visceral fat quartile
| Characteristic at baseline (visit 2) | Visceral Fat Quartile* | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value for trend | |
| N** | 251 | 251 | 252 | 251 | |
| Visceral fat, cm3 | 329.7 | 541.4 | 731.6 | 1089.2 | |
| Age, years | 51.6 (11.4) | 54.8 (11.2) | 54.8 (10.8) | 59.1 (10.3) | <0.01 |
| Sex, % female | 67.7% | 58.6% | 55.6% | 37.5% | <0.01 |
| Education, % high school graduate | 91.6% | 89.2% | 88.5% | 80.1% | <0.01 |
| Income, % >lower-middle | 86.5% | 94.0% | 91.7% | 88.8% | 0.03 |
| Smoker, % current) | 16.3% | 8.4% | 13.9% | 13.5% | 0.06 |
| Weight, kg | 70.0 (10.5) | 76.0 (10.7) | 77.9 (10.1) | 82.7 (10.2) | <0.01 |
| Height, cm | 167.8 (9.0) | 170.0 (9.4) | 170.3 (9.3) | 173.9 (9.4) | <0.01 |
| Body mass index, kg/m2 | <0.01 | ||||
| Normal weight | 52.0% | 20.2% | 13.6% | 8.4% | |
| Overweight | 48.0% | 79.8% | 86.4% | 91.6% | |
| Subcutaneous fat, cm3 | 1374 (651.6) | 1682 (565.8) | 1729 (581.8) | 1732 (525.7) | <0.01 |
| HOMA-IR | 2.2 (1.0) | 2.9 (3.4) | 3.0 (1.4) | 3.2 (1.6) | <0.01 |
| CRP, mg/L | 0.3 (0.4) | 0.3 (0.5) | 0.4 (0.8) | 0.4 (0.5) | <0.01 |
| Physical activity | 0.53 | ||||
| Poor | 41.0% | 35.5% | 38.1% | 43.4% | |
| Intermediate | 33.5% | 39.8% | 35.3% | 31.9% | |
| Ideal | 25.5% | 24.7% | 26.6% | 24.7% | |
| MetS criteria at baseline (visit 2) | |||||
| Fasting glucose, % ≥100 mg/dL or glucose-lowering treatment) | 13.6% | 21.4% | 37.3% | 44.4% | <0.01 |
| Blood pressure, % ≥130 mmHg systolic and/or ≥85 mmHg diastolic or antihypertensive treatment | 62.9% | 64.5% | 69.8% | 80.5% | <0.01 |
| HDL, % <50 mg/dL in women or <40 mg/dL in men, or treatment | 9.0% | 14.6% | 29.9% | 26.9% | <0.01 |
| Triglycerides, % ≥150 mg/dL or triglyceride-lowering treatment | 2.8% | 9.9% | 15.5% | 22.2% | <0.01 |
| Waist circumference (% ≥88 cm in women or ≥102 cm in men) |
17.9% | 23.1% | 27.4% | 40.6% | <0.01 |
| Weight and MetS criteria at follow-up (visit 3) | |||||
| Weight, kg | 70.2 (11.8) | 77.3 (11.5) | 79.9 (11.7) | 78.2 (12.8) | <0.01 |
| Fasting glucose, % ≥100 mg/dL or glucose-lowering treatment | 18.0% | 21.9% | 42.1% | 44.9% | <0.01 |
| Blood pressure, % ≥130 mmHg systolic and/or ≥ 85 mmHg diastolic or antihypertensive treatment | 69.3% | 73.2% | 77.7% | 83.8% | <0.01 |
| HDL, % <50 mg/dL in women or <40 mg/dL in men, or treatment | 4.9% | 10.0% | 18.3% | 23.3% | <0.01 |
| Triglycerides, % ≥150 mg/dL or triglyceride-lowering treatment | 5.8% | 9.0% | 10.5% | 20.7% | <0.01 |
| Waist circumference, % ≥88 cm in women or ≥102 cm in men | 22.5% | 38.7% | 47.6% | 56.2% | <0.01 |
| Metabolic syndrome, % | 4.8% | 9.2% | 21.6% | 23.8% | < 0.01 |
| Type 2 diabetes, % | 4.8% | 6.4% | 12.7% | 13.9% | < 0.01 |
*Visceral fat quartiles: Quartile 1 < 461.034; 461.034 ≤ Quartile 2 ≤ 627.875; 627.875 ≤ Quartile 3 ≤ 849.101; 849.101 ≤ Quartile 4.
**Sample size is for those participants without type 2 diabetes at baseline, and therefore includes 78 participants with prevalent metabolic syndrome. Those participants were further excluded for the analysis of metabolic syndrome.
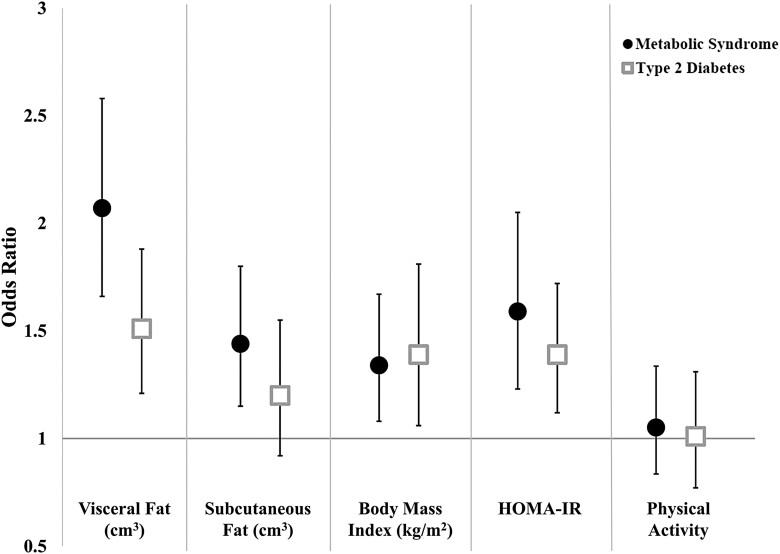
MetS and T2D were modeled separately, but results were generally similar between the 2 outcomes. Higher visceral fat was significantly associated with both MetS and T2D (Fig. 1 and Table 2). MetS and T2D estimates for subcutaneous fat were partially attenuated by adjustment for visceral fat (Table 2). HOMA-IR estimates were mildly attenuated by the inclusion of visceral fat in the model and visceral fat estimates were mildly attenuated by the inclusion of HOMA-IR (Table 2), suggesting weak mediation of the relationship of visceral fat with MetS and T2D by insulin resistance. In formal mediation analysis, HOMA-IR mediated 15% (6%-24%) of the association between visceral fat and MetS and 19% (5%-34%) of the association between visceral fat and T2D. In contrast, physical activity and CRP were not significantly associated with either MetS or T2D in any model (For poor physical activity compared to ideal: Fig. 1 and OR = 0.97 [0.69-1.38] for MetS and OR = 1.05 [0.68-1.63] for T2D; for CRP: OR = 1.13 [0.98-1.30] for MetS and OR = 1.01 [0.90-1.14] for T2D adjusted for demographics and smoking).
Figure 1.
Determinants of metabolic syndrome and type 2 diabetes in Jackson Heart Study participants without obesity: odds ratio and 95% CI per 1 SD difference*. All models are mutually adjusted, except for BMI, which is adjusted for visceral but not subcutaneous fat. Models are also adjusted for age, sex, education, income, and smoking. *Per 1 SD difference for visceral fat, subcutaneous fat, BMI, and HOMA-IR. Per 1 category difference for physical activity (Ideal, Intermediate, or Poor).
Table 2.
Association of baseline characteristics with metabolic syndrome and type 2 diabetes in participants without obesity in the Jackson Heart Study: odds ratio and 95% CI*
| Normal weight and overweight combined | Metabolic syndrome | Type 2 diabetes | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Adjusted for age, sex, education, income, and smoking | ||||
| Visceral fat, cm^3 | 2.49 | 2.05-3.02 | 1.68 | 1.38-2.05 |
| Subcutaneous fat, cm^3 | 1.82 | 1.51-2.19 | 1.44 | 1.15-1.81 |
| Body mass index, kg/m2 | 1.34 | 1.08-1.67 | 1.39 | 1.06-1.81 |
| HOMA-IR | 1.84 | 1.50-2.26 | 1.57 | 1.34-1.85 |
| Adjusted for age, sex, education, income, smoking, subcutaneous fat, and physical activity | ||||
| Visceral fat, cm^3 | 2.27 | 1.86-2.78 | 1.59 | 1.29-1.97 |
| HOMA-IR | 1.88 | 1.46-2.42 | 1.52 | 1.23-1.87 |
| Adjusted for age, sex, education, income, smoking, visceral fat, subcutaneous fat, HOMA-IR, and physical activity ** | ||||
| Visceral fat, cm^3 | 2.07 | 1.66-2.58 | 1.51 | 1.21-1.88 |
| Subcutaneous fat, cm^3 | 1.44 | 1.15-1.80 | 1.20 | 0.92-1.55 |
| Body mass index, kg/m2 | 1.34 | 1.08-1.67 | 1.39 | 1.06-1.81 |
| HOMA-IR | 1.59 | 1.24-2.05 | 1.39 | 1.12-1.72 |
| Restricted to Normal Weight | ||||
| Adjusted for age, sex, education, income, smoking, visceral fat, subcutaneous fat, HOMA-IR, and physical activity ** | ||||
| Visceral fat, cm^3* | 2.08 | 1.25-3.45 | 1.06 | 0.57-1.98 |
| Subcutaneous fat, cm^3* | 1.90 | 1.02-3.54 | 1.11 | 0.50-2.47 |
| Body mass index, kg/m2 | 2.11 | 1.13-3.95 | 0.96 | 0.50-1.85 |
| HOMA-IR* | 1.22 | 0.77-1.93 | 1.52 | 0.92-2.49 |
| Restricted to Overweight | ||||
| Adjusted for age, sex, education, income, smoking, visceral fat, subcutaneous fat, HOMA-IR, and physical activity ** | ||||
| Visceral fat, cm^3* | 1.97 | 1.55-2.51 | 1.57 | 1.24-2.00 |
| Subcutaneous fat, cm^3* | 1.24 | 0.96-1.60 | 1.18 | 0.88-1.58 |
| Body mass index, kg/m2 | 1.00 | 0.82-1.22 | 1.29 | 1.02-1.64 |
| HOMA-IR* | 1.61 | 1.18-2.21 | 1.35 | 1.05-1.73 |
Bold indicates significant at the P < 0.05 level
*Per 1 SD for all variables except physical activity.
**Except for body mass index (BMI) model which does not include adjustment for subcutaneous fat.
***Number of incident events/n: Normal weight participants (35/252 for MetS & 15/272 for T2D); Overweight participants (177/675 for MetS & 101/733 for T2D); Combined numbers are added.
Table 2 shows that estimates for the normal weight (18.5 kg/m2 ≤ BMI < 25 kg/m2) and overweight groups (25 kg/m2 ≤ BMI < 30 kg/m2) analyzed separately produced similar results with higher visceral fat significantly associated with MetS. The estimate for HOMA-IR was partially attenuated and not significant in the normal weight group, and the T2D estimates for visceral fat and HOMA-IR were almost completely attenuated when restricted to the normal weight group (Table 2); however, there were only 35 MetS and 15 T2D events in the normal weight group. There was no statistically significant difference between normal weight and overweight estimates (interaction between BMI category and visceral fat for MetS P = 0.52, and for T2D P = 0.50; interaction between BMI category and HOMA-IR for MetS P = 1.00, and for T2D P = 0.27). Other sensitivity analyses also produced similar results for visceral fat when restricting to participants known not to have taken HRT (n = 923), using a definition of MetS that does not include waist circumference, and using only incident cases (Table 3). There was no statistically significant heterogeneity by age (P = 0.28 for MetS and P = 0.32 for T2D), but there was some evidence of heterogeneity by sex, with a stronger association for women than for men for both MetS and T2D; however, the estimates for interaction were not significant (Table 3).
Table 3.
Sensitivity analyses (odds ratios and 95% CI) for association of 1 SD difference in visceral fat with metabolic syndrome and type 2 diabetes in participants without obesity in the Jackson Heart Study
| Model | Metabolic syndrome | Type 2 diabetes | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Primary analysis * | 2.07 | 1.66-2.58 | 1.51 | 1.21-1.88 |
| Incident cases only | 2.00 | 1.49-2.69 | 1.70 | 1.29-2.25 |
| Excluding waist circumference from MetS definition ** | 1.74 | 1.41-2.15 | 1.51 | 1.21-1.88 |
| No hormone replacement therapy usage in women | 1.88 | 1.47-2.40 | 1.57 | 1.23-2.01 |
| By sex | ||||
| Women | 2.60 | 1.83-3.70 | 1.80 | 1.28-2.54 |
| Men | 1.74 | 1.30-2.33 | 1.29 | 0.96-1.73 |
| P value for difference by sex | 0.06 | 0.13 |
BOLD indicates significant at the P < 0.05 level.
*Primary analysis includes adjustment for age, sex, education, income, smoking, visceral fat, subcutaneous fat, HOMA-IR, and physical activity.
**Sample size for incident analysis is 795 for MetS and 944 for T2D, and analysis excluding women with known hormone replacement therapy is 923.
Discussion
Higher visceral fat was strongly and significantly associated with higher odds of incident MetS and T2D in normal weight and overweight participants from the Jackson Heart Study. The MetS association was consistent when restricted to normal weight participants and there was no significant heterogeneity between the normal weight and overweight groups with regards to either visceral fat or HOMA-IR. This association was also consistent after adjustment for BMI or subcutaneous fat as markers of overall adiposity. Visceral fat and HOMA-IR were more strongly associated with MetS and T2D than other indices of obesity (BMI or subcutaneous fat) and other determinants such as physical activity. Further, visceral fat and HOMA-IR were both strongly and significantly associated with cardiometabolic disease, with only moderate evidence that HOMA-IR mediated the association of visceral fat with MetS or T2D.
Liu et al have previously shown a cross-sectional association of higher visceral and subcutaneous fat with MetS and T2D in the Jackson Heart Study [28]. This analysis builds on that work by adding the longitudinal associations and addressing the specific question of the role that adiposity plays in individuals without obesity. This fills a meaningful gap in the literature, as several studies have shown that higher visceral fat is associated with MetS, but do not offer evidence of this association specifically in the context of overweight or normal weight. This disambiguation between general obesity measured using BMI and visceral fat level is particularly important for understanding the development of metabolic risk in individuals without obesity. Also noteworthy is that while this study is consistent with the bulk of the literature, most of the evidence in this area is cross-sectional [29-36] or provides evidence only on obesity or aggregated across BMI categories [15, 37].
This work is also consistent with prior longitudinal analyses showing that higher visceral fat is significantly associated with incident MetS and T2D in 2 prior studies. Shah et al report that visceral fat is associated with incident MetS in the Multi-Ethnic Study of Atherosclerosis, but do not provide specific evidence of this longitudinal association persisting in non-obese or normal weight participants [14]. Kuwahara et al similarly link higher visceral fat with incident T2D in the Japan Epidemiology Collaboration on Occupational Health Study and suggest that this association is independent of BMI, but also do not provide specific evidence about the normal weight group [15]. Consistent with our finding of moderate mediation in the relationship between visceral fat and incident cardiometabolic disease by insulin resistance, Kuwahara et al also report that higher HOMA-IR and increasing HOMA-IR are associated with incident T2D, suggesting that visceral fat increases insulin resistance as a mediator leading to T2D. Both studies also found that change in visceral fat over time may confer additional risk. Other hypothesized mechanisms include CRP as an intermediary in the development of MetS and CVD risk, and CRP is even included in CVD risk assessments like the Reynolds Risk Score. In contrast, our results are consistent with those suggesting that while CRP is a strong marker of inflammation, it is likely not on the causal pathway between body fat distribution and metabolic dysfunction. Our findings further support the idea that insulin resistance plays a role in the causal pathway between visceral adiposity and cardiometabolic disease, but the modest amount of mediation (15% and 19%, respectively) suggests that investigation of other pathways between excess visceral fat and cardiometabolic dysfunction in individuals without obesity is warranted.
Given the known dimorphisms by sex in body fat distribution and function, investigating heterogeneity by sex in associations with visceral and subcutaneous fat is warranted [38]. Shah et al did not find evidence of heterogeneity by sex or race/ethnicity [14], and Kuwahara et al utilized a cohort that was predominantly male, and the authors specify that generalizing to women many be inappropriate [15]. In studies that investigated the role of body fat composition on cardiometabolic risk across the full BMI range or only in those with obesity, there has been inconsistency with some finding heterogeneity by sex [35], and others not [37]. Our results are consistent with those showing a qualitatively stronger association for women than for men for the association of visceral fat with incident MetS [35], but the lack of a statistically significant finding may be the result of being underpowered to detect heterogeneity. In addition to differences by sex, differences by race/ethnicity should also be considered. While distributions of visceral fat have been shown to be lower for African American individuals at the same BMI compared to white cohorts [11], this does not necessarily mean that the underlying relationship between body fat distribution and cardiometabolic disease differs by race/ethnicity. While the strong focus of research in African American cohorts has been on the high prevalence and impact of obesity in the community, evidence for the importance of cardiometabolic risk in normal weight is increasing for African Americans as well as other underserved racial/ethnic populations [2, 6, 7]. Our results contribute to understanding the source of these inequities by showing that even in the absence of obesity, higher levels of visceral fat may contribute to MetS and T2D risk in the African American participants of the Jackson Heart Study. While it has long been argued that BMI categories alone are insufficient for risk stratification, our findings further suggest that interventions to prevent cardiometabolic disease are needed across the full range of BMI.
The limitations of this study include relatively short follow-up for events and self-reported data for physical activity. The small number of cases, especially in the normal weight group and for the incident analysis, creates additional uncertainty around those estimates. Furthermore, JHS does not have repeated measurements of visceral and subcutaneous fat and we cannot assess the added risk of change over time. Strengths of this study include the novel approach to understanding MetS and T2D in participants without obesity and the direct measurement of visceral and subcutaneous fat separately. Also, this study uses gold standard measurements of body fat composition in an African American cohort, a population that is understudied. Finally, this study adds additional prospective evidence of the association between adiposity and insulin resistance with the development of cardiometabolic disease.
Given compelling evidence suggesting that those who are normal weight when diagnosed with T2D have the highest risk of mortality [1-3], the need to understand what causes cardiometabolic disease in normal weight and overweight individuals is clear. Our findings show that adiposity, particularly visceral fat, plays a role in development of cardiometabolic dysfunction even in the absence of obesity. This suggests that there is a missed prevention opportunity if we only focus on individuals with generalized obesity. Thus, we need to determine how we can identify these high-risk individuals who would not be detected by traditional measures. Advances in dual-energy X-ray absorptiometry (DEXA) technology for measuring body composition may offer a more accessible and lower radiation option for risk stratification.
Acknowledgments
The authors wish to thank the staffs and participants of the JHS.
Glossary
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- CT
computed tomography
- CVD
cardiovascular disease
- HOMA-IR
homeostasis model assessment of insulin resistance
- JHS
Jackson Heart Study
- MetS
metabolic syndrome
- T2D
type 2 diabetes
Funding
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD).
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Conflict of Interest
The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of all data from the Jackson Heart Study to preserve patient confidentiality. Data access procedures are detailed on the Jackson Heart Study website https://www.jacksonheartstudy.org/Research/Study-Data/Data-Access.
References
- 1. Carnethon MR, Rasmussen-Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep. 2014;16(2):446. doi: 10.1007/s11886-013-0446-3 [DOI] [PubMed] [Google Scholar]
- 2. Gujral UP, Weber MB, Staimez LR, Narayan KMV. Diabetes among non-overweight individuals: an emerging public health challenge. Curr Diab Rep. 2018;18(8):60. doi: 10.1007/s11892-018-1017-1 [DOI] [PubMed] [Google Scholar]
- 3. Kwon Y, Kim HJ, Park S, Park YG, Cho KH. Body mass index-related mortality in patients with type 2 diabetes and heterogeneity in obesity paradox studies: a dose-response meta-analysis. PLoS One. 2017;12(1):e0168247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lajous M, Bijon A, Fagherazzi G, et al. Body mass index, diabetes, and mortality in French women: explaining away a “paradox”. Epidemiology. 2014;25(1):10-14. doi: 10.1097/EDE.0000000000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: An emerging entity in the era of obesity. World J Diabetes. 2015;6(4):613-620. doi: 10.4239/wjd.v6.i4.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Y, Sidell MA, Arterburn D, et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care. 2019;42(12):2211-2219. doi: 10.2337/dc19-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gujral UP, Narayan KMV. Diabetes in normal-weight individuals: high susceptibility in nonwhite populations. Diabetes Care. 2019;42(12):2164-2166. doi: 10.2337/dci19-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(8):736-743. [DOI] [PubMed] [Google Scholar]
- 9. Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16(3):600-607. doi: 10.1038/oby.2007.92 [DOI] [PubMed] [Google Scholar]
- 10. Mongraw-Chaffin ML, Golden SH, Allison MA, et al. The sex and race specific relationship between anthropometry and body fat composition determined from computed tomography: Evidence from the Multi-Ethnic Study of Atherosclerosis. PLoS One. 2015;10(10):e0139559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69(3):381-387. [DOI] [PubMed] [Google Scholar]
- 12. Lutsey PL, Pereira MA, Bertoni AG, Kandula NR, Jacobs DR Jr. Interactions between race/ethnicity and anthropometry in risk of incident diabetes: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010;172(2):197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645. [DOI] [PubMed] [Google Scholar]
- 14. Shah RV, Murthy VL, Abbasi SA, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7(12):1221-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuwahara K, Honda T, Nakagawa T, Yamamoto S, Hayashi T, Mizoue T. Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci Rep. 2017;7:43521-43521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Araneta MRG, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: filipino, African-American, and white women. Obesity Res. 2005;13(8):1458-1465. doi: 10.1038/oby.2005.176 [DOI] [PubMed] [Google Scholar]
- 17. Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97(7):2482-2488. doi: 10.1210/jc.2011-3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(9):956-966. doi: 10.1177/2047487315623884 [DOI] [PubMed] [Google Scholar]
- 19. Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085-1095. doi: 10.1016/S0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor HA Jr., Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4Suppl 6):S6-4-S6-17. [PubMed] [Google Scholar]
- 21. Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31(11):2715-2722. doi: 10.1161/ATVBAHA.111.234062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45(5):901-907. doi: 10.1249/MSS.0b013e31827d87ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fox ER, Benjamin EJ, Sarpong DF, et al. Epidemiology, heritability, and genetic linkage of C-reactive protein in African Americans (from the Jackson Heart Study). Am J Cardiol. 2008;102(7):835-841. doi: 10.1016/j.amjcard.2008.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021 [published correction appears in Diabetes Care. 2021 Sep;44(9):2182]. Diabetes Care. 2021;44(Suppl 1):S15-S33. [DOI] [PubMed] [Google Scholar]
- 26. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137-150. doi: 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. 2014;25(5):749-761. doi: 10.1097/EDE.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding C, Chan Z, Magkos F. Lean, but not healthy: the “metabolically obese, normal-weight” phenotype. Curr Opin Clin Nutr Metab Care. 2016;19(6):408-417. [DOI] [PubMed] [Google Scholar]
- 30. Stefan N, Schick F, Häring H-U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017;26(2):292-300. doi: 10.1016/j.cmet.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 31. Nomura K, Eto M, Kojima T, et al. Visceral Fat Accumulation and Metabolic Risk Factor Clustering in Older Adults. J Am Geriatr Soc. 2010;58(9):1658-1663. doi: 10.1111/j.1532-5415.2010.03018.x [DOI] [PubMed] [Google Scholar]
- 32. Hermans MP, Amoussou-Guenou KD, Bouenizabila E, Sadikot SS, Ahn SA, Rousseau MF. The normal-weight type 2 diabetes phenotype revisited. Diabetes Metab Syndr. 2016;10(2Suppl 1):S82-S88. [DOI] [PubMed] [Google Scholar]
- 33. Tatsumi Y, Nakao YM, Masuda I, et al. Risk for metabolic diseases in normal weight individuals with visceral fat accumulation: a cross-sectional study in Japan. BMJ Open. 2017;7(1):e013831. doi: 10.1136/bmjopen-2016-013831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lv X, Zhou W, Sun J, et al. Visceral adiposity is significantly associated with type 2 diabetes in middle-aged and elderly Chinese women: a cross-sectional study. J Diabetes. 2017;9(10):920-928. doi: 10.1111/1753-0407.12499 [DOI] [PubMed] [Google Scholar]
- 35. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39-48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 36. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616-627. doi: 10.1016/S2213-8587(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 37. Hwang Y-C, Hayashi T, Fujimoto WY, et al. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes. 2015;39:1365-1370. doi: 10.1038/ijo.2015.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2(3):321-327. doi: 10.1007/s12265-009-9101-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of all data from the Jackson Heart Study to preserve patient confidentiality. Data access procedures are detailed on the Jackson Heart Study website https://www.jacksonheartstudy.org/Research/Study-Data/Data-Access.