Abstract
Background
Takeda’s dengue vaccine is under evaluation in an ongoing phase 3 efficacy study; we present a 2-year update.
Methods
Children (20 099, 4–16 years old) were randomized to receive 2 doses of TAK-003 or placebo 3 months apart and are under surveillance to detect dengue by serotype-specific RT-PCR.
Results
Cumulative efficacy against dengue approximately 27 months since first dose was 72.7% (95% confidence interval [CI], 67.1%–77.3%), including 67.0% (95% CI, 53.6%–76.5%) in dengue-naive and 89.2% (95% CI, 82.4%–93.3%) against hospitalized dengue. In the second year, decline in efficacy was observed (56.2%; 95% CI, 42.3%–66.8%) with the largest decline in 4–5 year olds (24.5%; 95% CI, −34.2% to 57.5%); efficacy was 60.6% (95% CI, 43.8%–72.4%) in 6–11 year and 71.2% (95% CI, 41.0%–85.9%) in 12–16 year age groups. As TAK-003 efficacy varies by serotype, changes in serotype dominance partially contributed to efficacy differences in year-by-year analysis. No related serious adverse events occurred during the second year.
Conclusions
TAK-003 demonstrated continued benefit independent of baseline serostatus in reducing dengue with some decline in efficacy during the second year. Three-year data will be important to see if efficacy stabilizes or declines further.
Clinical Trials Registration. NCT02747927.
Takeda’s tetravalent dengue vaccine (TAK-003) continued to demonstrate benefit in reducing dengue independent of baseline serostatus up to 2 years after completing vaccination with some decline in efficacy during the second year in 4–16 year olds in dengue-endemic countries.
Keywords: dengue, vaccine, TAK-003, efficacy, immunogenicity, safety, persistence
The 4 serotypes of dengue viruses (DENV-1 to DENV-4) are primarily transmitted to humans by the Aedes aegypti mosquito and lead to approximately 390 million dengue infections every year, 96 million of which manifest clinically [1], with 500 000 cases requiring hospitalization [2]. Although tropical areas suffer the highest burden of disease, climate change is projected to expand the population at risk to new areas in Europe, high-altitude regions in the tropics, the United States, and Canada [3]. The only licensed dengue vaccine (CYD-TDV, Dengvaxia; Sanofi Pasteur) is recommended for use in individuals ≥ 9 years of age with evidence of previous dengue infection [4] because of an association with increased risk of severe dengue disease and hospitalization in seronegative individuals [5]. Hence, there is a substantial unmet need for a vaccine that can be administered more broadly.
Takeda’s tetravalent dengue vaccine candidate (TAK-003) is based on a live attenuated DENV-2 virus that provides the genetic backbone for all 4 of the vaccine viruses, which were originally designed and constructed by scientists at the US Division of Vector-Borne Diseases of the Centers for Disease Control and Prevention [6]. Phase 1 and 2 studies have demonstrated TAK-003 to be well tolerated, and to induce humoral responses against serotypes 1–4, long-term antibody persistence, and cross-reactive and multifunctional T-cell–mediated responses [7–16]. TAK-003 has a 0, 3-month dosing schedule and is currently under evaluation in a large phase 3 efficacy trial conducted in children 4–16 years of age. Previously we have reported the interim results through 18 months after the second dose [17]. In this timeframe, 2 doses of TAK-003 showed an overall efficacy of 73.3% (95% confidence interval [CI], 66.5%–78.8%) and an efficacy of 66.2% (95% CI, 49.1%–77.5%) in participants seronegative prior to immunization. Efficacy against hospitalized dengue was 90.4% (95 % CI, 82.6–94.7) and efficacy against dengue hemorrhagic fever (DHF) was 85.9% (95 % CI, 31.9–97.1). Efficacy varied against individual serotypes and the highest efficacy was seen against DENV-2.
Here we report results through 2 years after vaccination that includes the cumulative efficacy since first dose and the efficacy in each of the 2 years after completing vaccination. During a similar timeframe, analysis of another dengue vaccine (Dengvaxia) has identified a higher risk of hospitalization in younger children, which was subsequently attributed to their dengue-naive serostatus prior to immunization [5, 18]. Unfortunately, studies of Dengvaxia did not collect data on dengue cases that did not require hospitalization beyond 1 year after vaccination, precluding detailed comparisons between these vaccines. Subsequently, these data were collected from 5 years onwards in Dengvaxia trials in the surveillance expansion phase [19].
METHODS
Study Design and Participants
Healthy children aged 4–16 years inclusive were enrolled into a randomized, double-blind and placebo-controlled trial at 26 centers in 8 dengue-endemic countries. Informed assent or consent forms, and the study protocol and its amendments were reviewed and approved by applicable institutional review boards, independent ethics committees, and health authorities. Written informed assent or consent was obtained from all participants or their parents or legal guardians before enrolment. This trial is in accordance with the Declaration of Helsinki [20], and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use harmonized tripartite guidelines for good clinical practice [21]. Details of inclusion and exclusion criteria are provided in the Supplementary Material.
Trial Procedures
This study consists of 3 parts for each participant, with active surveillance during parts 1 and 2, and modified active surveillance during part 3 (see Supplementary Material for details of febrile illnesses assessment and the differences between active and modified active surveillance; Supplementary Figure 1). Each of these surveillance methods continues weekly contact with participants and detects both nonhospitalized and hospitalized dengue cases. Part 1 had 12 months of follow-up after the second dose to assess the primary endpoint, and part 2 had another 6 months to assess the secondary endpoints (previously reported) [17, 22]. Part 3 is an ongoing 3-year assessment of long-term efficacy and safety. At the time of this analysis, participants had completed 24 months since the second dose or approximately 27 months since the first dose.
One 0.5 mL dose of TAK-003 or placebo (saline solution) was administered subcutaneously into the upper arm at 0 and 3 months. Blood samples were collected from all participants at baseline and 1 month after the second dose to measure dengue neutralizing antibodies by microneutralization test (MNT). The results were expressed as the reciprocal of the highest dilution of test serum that shows a 50% reduction in plaque counts compared with that of virus controls. Additional MNT blood samples were taken from a randomly selected subset of participants in months 1, 3, 9, and 15, and then annually. For the entire trial duration, participants or their parents or guardians are contacted at least weekly reminding them to present for evaluation of febrile illness (defined as fever ≥ 38°C on any 2 of 3 consecutive days). Dengue cases are confirmed by serotype-specific quantitative reverse transcription polymerase chain reaction (RT-PCR) in acute samples (virologically confirmed dengue, VCD).
Outcomes
The exploratory analysis presented in this report was conducted to assess cumulative efficacy since the first dose and efficacy in the first 12-month interval after completing part 1.
Additional details of study materials, methods, statistical methodology, trial procedures, and safety data reporting are available in the Supplementary Material and in previously published reports [17, 22].
RESULTS
Participants
The trial was initiated in September 2016, and part 3 is scheduled for completion in January 2022. A total of 20 099 participants were randomly assigned in a ratio of 2:1 to receive either TAK-003 (n = 13 401) or placebo (n = 6698; Supplementary Figure 2). Of the total participants, 20 071 (99.9%) received at least 1 dose and were included in the safety set, of which 19 741 (98.4%) received 2 doses, and 19 330 (96.3%) completed 2 years of follow-up after the second dose (safety set data). In the per protocol set, 19 021 participants (94.6%) were included, which had 27.7% (5259/19 014) of the participants seronegative at baseline to all 4 serotypes (MNT titer < 10). Further information on the baseline characteristics of the study population is available in previous reports [17, 22].
Febrile Illnesses and VCD
In the approximately 27 months after initiating vaccination, 17 207 febrile illnesses have been reported and acute samples were taken in 98.5% of these cases for RT-PCR testing (94.2% within 5 days of fever onset). A total of 487 VCD cases were detected, of which 111 cases required hospitalization (Supplementary Table 2). In the per protocol set, 414 VCD cases (91 hospitalized) occurred in the 23-month period starting 1 month after second dose and were included in the year-by-year analysis. Year 1 refers to the first 11 months with 210 cases and year 2 refers to the next 12 months with 204 cases (Table 1 and Table 2). An increase in VCD cases was observed in Latin America from year 1 (n = 29) to year 2 (n = 77), while cases decreased in Asia from year 1 (n = 181) to year 2 (n = 127; Table 1).
Table 1.
Serotype Distribution Among Cases of VCD and Hospitalized VCD Cases Occurring Between 30 Days After Second Dose and End of Part 1 (Year 1, Month 4–15), and Over 12 Months After End of Part 1 (Year 2, Month 16–27) Per Protocol Set Data
| Recipients | Row Total of VCD Cases | DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| All participants | 210 (58) | 204 (33) | 46 (5) | 90 (19) | 67 (43) | 42 (7) | 90 (10) | 63 (6) | 7 (0) | 9 (1) |
| Age 4–5 y | 36 (4) | 49 (9) | 5 (0) | 19 (4) | 4 (3) | 5 (2) | 25 (1) | 21 (3) | 2 (0) | 4 (0) |
| Age 6–11 y | 119 (35) | 123 (20) | 32 (4) | 55 (11) | 41 (25) | 32 (5) | 42 (6) | 32 (3) | 4 (0) | 4 (1) |
| Age 12–16 y | 55 (19) | 32 (4) | 9 (1) | 16 (4) | 22 (15) | 5 (0) | 23 (3) | 10 (0) | 1 (0) | 1 (0) |
| Philippines | 109 (10) | 87 (8) | 13 (1) | 8 (1) | 2 (0) | 9 (1) | 87 (9) | 63 (6) | 7 (0) | 7 (0) |
| Sri Lanka | 60 (43) | 11 (3) | 5 (3) | 6 (1) | 54 (40) | 5 (2) | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| Thailand | 12 (4) | 29 (13) | 3 (1) | 19 (10) | 7 (2) | 8 (2) | 2 (1) | 0 (0) | 0 (0) | 2 (1) |
| Brazil | 3 (0) | 13 (2) | 1 (0) | 10 (2) | 2 (0) | 3 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Colombia | 5 (1) | 34 (5) | 3 (0) | 34 (5) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dominican Republic | 0 | 6 (0) | 0 | 6 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Nicaragua | 0 | 17 (2) | 0 | 0 (0) | 0 | 17 (2) | 0 | 0 (0) | 0 | 0 (0) |
| Panama | 21 (0) | 7 (0) | 21 (0) | 7 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Data are number of cases of each serotype and hospitalized VCD cases in parentheses. Data are pooled for TAK-003 and placebo recipients.
Abbreviations: DENV, dengue virus; VCD, virologically confirmed dengue.
Table 2.
Vaccine Efficacy (95% CI) of TAK-003 in Prevention of VCD Occurring Between 30 Days After Second Dose and End of Part 1 (Year 1, Month 4–15), and over 12 Months After End of Part 1 (Year 2, Month 16–27) Per Protocol Set Data
| TAK-003 VCD Casesa | Placebo VCD Casesa | Vaccine Efficacy, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| Overall | 61/12 700 (0.5) | 97/12 577 (0.8) | 149/6316 (2.6) | 106/6268 (1.8) | 80.2 (73.3–85.3) | 56.2 (42.3–66.8) |
| SP | 41/9165 (0.5) | 64/9077 (0.7) | 110/4587 (2.7) | 77/4552 (1.8) | 82.2 (74.5–87.6) | 60.3 (44.7–71.5) |
| SN | 20/3531 (0.6) | 33/3498 (1.0) | 39/1726 (2.5) | 29/1715 (1.8) | 74.9 (57.0–85.4) | 45.3 (9.9–66.8) |
| DENV-1 | 16/12 700 (0.1) | 41/12 577 (0.3) | 30/6316 (0.5) | 49/6268 (0.8) | 73.7 (51.7–85.7) | 59.4 (38.5–73.2) |
| DENV-2 | 3/12 700 (< 0.1) | 14/12 577 (0.1) | 64/6316 (1.1) | 27/6268 (0.4) | 97.7 (92.7–99.3) | 75.0 (52.3–86.9) |
| DENV-3 | 39/12 700 (0.3) | 37/12 577 (0.3) | 51/6316 (0.9) | 26/6268 (0.4) | 62.6 (43.3–75.4) | 32.8 (−10.9 to 59.3) |
| DENV-4 | 3/12 700 (< 0.1) | 5/12 577 (< 0.1) | 4/6316 (< 0.1) | 4/6268 (< 0.1) | 63.2 (−64.4 to 91.8) | 41.2 (−119.0 to 84.2) |
| SP | ||||||
| DENV-1 | 7/9165 (< 0.1) | 26/9077 (0.3) | 17/4587 (0.4) | 31/4552 (0.7) | 79.8 (51.3–91.6) | 59.1 (31.1–75.7) |
| DENV-2 | 3/9165 (< 0.1) | 11/9077 (0.1) | 42/4587 (1.0) | 22/4552 (0.5) | 96.5 (88.8–98.9) | 75.5 (49.5–88.1) |
| DENV-3 | 28/9165 (0.3) | 25/9077 (0.3) | 47/4587 (1.1) | 21/4552 (0.5) | 71.4 (54.3–82.1) | 44.9 (1.5–69.1) |
| DENV-4 | 3/9165 (< 0.1) | 2/9077 (< 0.1) | 4/4587 (< 0.1) | 3/4552 (< 0.1) | 63.8 (−61.8 to 91.9) | 69.0 (−85.8 to 94.8) |
| SN | ||||||
| DENV-1 | 9/3531 (0.3) | 15/3498 (0.4) | 13/1726 (0.8) | 18/1715 (1.1) | 67.2 (23.2–86.0) | 60.7 (22.1–80.2) |
| DENV-2 | 0/3531 (0) | 3/3498 (< 0.1) | 22/1726 (1.4) | 5/1715 (0.3) | 100 (–) | 70.5 (−23.4 to 93.0) |
| DENV-3 | 11/3531 (0.3) | 12/3498 (0.3) | 4/1726 (0.3) | 5/1715 (0.3) | −38.7 (−335.7 to 55.8) | −18.5 (−236.2 to 58.3) |
| DENV-4 | 0/3531 (0) | 3/3498 (< 0.1) | 0/1726 (0) | 1/1715 (< 0.1) | … | −47.6 (−1319.1 to 84.6) |
| Age 4–5 y | 13/1619 (0.9) | 30/1603 (1.9) | 23/801 (3.2) | 19/795 (2.5) | 72.8 (46.4–86.2) | 24.5 (−34.2 to 57.5) |
| Age 6–11 y | 34/7009 (0.5) | 55/6961 (0.8) | 85/3491 (2.7) | 67/3476 (2.0) | 80.7 (71.3–87.0) | 60.6 (43.8–72.4) |
| Age 12–16 y | 14/4072 (0.4) | 12/4013 (0.3) | 41/2024 (2.2) | 20/1997 (1.0) | 83.3 (69.4–90.9) | 71.2 (41.0–85.9) |
| SP | ||||||
| Age 4–5 y | 7/957 (0.8) | 15/948 (1.6) | 18/464 (4.3) | 13/461 (3.0) | 81.9 (56.6–92.4) | 46.4 (−12.7 to 74.5) |
| Age 6–11 y | 22/4806 (0.5) | 39/4775 (0.8) | 58/2423 (2.7) | 48/2415 (2.1) | 82.0 (70.7–89.0) | 60.8 (40.2–74.3) |
| Age 12–16 y | 12/3402 (0.4) | 10/3354 (0.3) | 34/1700 (2.2) | 16/1676 (1.0) | 82.7 (66.5–91.0) | 69.9 (33.7–86.3) |
| SN | ||||||
| Age 4–5 y | 6/662 (1.0) | 15/655 (2.4) | 5/337 (1.6) | 6/334 (1.9) | 39.1 (−99.8 to 81.4) | −23.7 (−219.1 to 52.0) |
| Age 6–11 y | 12/2200 (0.6) | 16/2184 (0.7) | 27/1065 (2.8) | 19/1060 (1.9) | 78.4 (57.3–89.1) | 59.7 (21.6–79.3) |
| Age 12–16 y | 2/669 (0.3) | 2/659 (0.3) | 7/324 (2.4) | 4/321 (1.3) | 86.2 (33.8–97.1) | 75.9 (−31.6 to 95.6) |
| Hospitalized | ||||||
| Overall | 5/12 700 (< 0.1) | 11/12 577 (< 0.1) | 53/6316 (0.9) | 22/6268 (0.4) | 95.4 (88.4–98.2) | 76.1 (50.8–88.4) |
| SP | 4/9165 (< 0.1) | 5/9077 (< 0.1) | 35/4587 (0.8) | 16/4552 (0.4) | 94.4 (84.4–98.0) | 85.2 (59.6–94.6) |
| SN | 1/3531 (< 0.1) | 6/3498 (0.2) | 18/1726 (1.2) | 6/1715 (0.4) | 97.2 (79.1–99.6) | 51.4 (−50.7 to 84.3) |
| Age 4–5 y | 1/1619 (< 0.1) | 5/1603 (0.3) | 3/801 (0.4) | 4/795 (0.5) | 83.7 (−56.5 to 98.3) | 40.1 (−123.0 to 83.9) |
| Age 6–11 y | 2/7009 (< 0.1) | 5/6961 (< 0.1) | 33/3491 (1.0) | 15/3476 (0.4) | 97.1 (87.8–99.3) | 84.1 (56.1–94.2) |
| Age 12–16 y | 2/4072 (< 0.1) | 1/4013 (< 0.1) | 17/2024 (0.9) | 3/1997 (0.2) | 94.2 (74.9–98.7) | 84.2 (−52.1 to 98.4) |
| SP age 4–5 y | 1/957 (0.1) | 2/948 (0.2) | 1/464 (0.2) | 3/461 (0.7) | 52.3 (−662.3 to 97.0) | 68.7 (−87.1 to 94.8) |
| SP age 6–11 y | 1/4806 (< 0.1) | 2/4775 (< 0.1) | 19/2423 (0.9) | 11/2415 (0.5) | 97.5 (81.5–99.7) | 91.4 (61.0–98.1) |
| SP age 12–16 y | 2/3402 (< 0.1) | 1/3354 (< 0.1) | 15/1700 (1.0) | 2/1676 (0.1) | 93.4 (71.0–98.5) | 75.9 (−166.4 to 97.8) |
| SN age 4–5 y | 0/662 (0) | 3/655 (0.5) | 2/337 (0.7) | 1/334 (0.3) | 100 (…) | −54.6 (−1387.6 to 83.9) |
| SN age 6–11 y | 1/2200 (< 0.1) | 3/2184 (0.1) | 14/1065 (1.5) | 4/1060 (0.4) | 96.4 (72.5–99.5) | 64.4 (−59.0 to 92.0) |
| SN age 12–16 y | 0/669 (0) | 0/659 (0) | 2/324 (0.7) | 1/321 (0.3) | 100 (…) | 100 (…) |
For serotype-specific vaccine efficacy calculations, only the first instance of VCD due to the individual serotype in question was included, regardless of previous instances of VCD due to other serotypes; some data may differ from that previously published [22] due to the inclusion of updated datasets and correction of an error involving censoring of 37 participants in the earlier analysis.
Abbreviations: CI, confidence interval; DENV, dengue viruses; SN, seronegative at baseline; SP, seropositive at baseline; VCD, virologically confirmed dengue.
aData under the TAK-003 and placebo groups are number of VCD/ number of evaluated participants (number of VCD per 100 person-years at risk).
Distribution of Dengue Serotypes in Year-by-Year Analysis (Per Protocol Set)
In the placebo group, the number of cases per 100 person-years decreased from 2.6 in year 1 to 1.8 in year 2 (Table 2). In this group, DENV-2 was reported in the highest proportions during year 1 with 1.1 cases per 100 person-years, while in year 2 DENV-1 (0.8 cases per 100 person years) was most frequently reported. In Latin America, no DENV-3 or DENV-4 was identified in either year. The vast majority of DENV-3 cases (87/90 in year 1 and 63/63 in year 2) were identified in the Philippines. DENV-4 was the least reported serotype in both years. Across both the placebo and vaccine groups, the dominant serotype resulting in hospitalization was DENV-2 (43 of 58 VCD, 74%) during year 1 and DENV-1 (19 of 33 VCD, 58%) in year 2 (Table 1). Serotype distribution data between first dose and month 27 in the safety set is presented in Supplementary Table 2.
Vaccine Efficacy
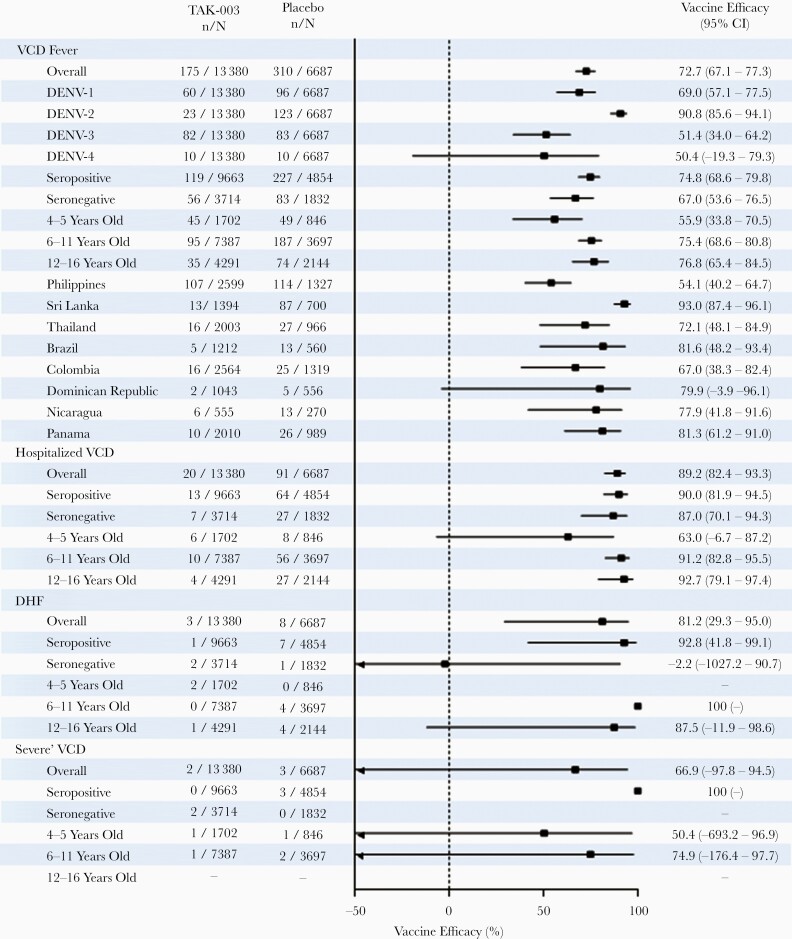
Cumulative overall efficacy of TAK-003 over the 27 months after initiating vaccination and the subgroup analysis is presented in Figures 1, 2, and 3. Overall efficacy was 72.7% (95% CI, 67.1%–77.3%), 74.8% (95% CI, 68.6%–79.8%) in baseline seropositives, and 67% (95% CI, 53.6%–76.5%) in baseline seronegatives; efficacy against hospitalized dengue was 90% (95% CI, 81.9%–94.5%) and 87% (95% CI, 70.1%–94.3%), respectively. Cumulative serotype-specific efficacy remained variable against DENV-1 (69.0%), DENV-2 (90.8%), and DENV-3 (51.4%), and was inconclusive against DENV-4 (50.4%; 95% CI, −19.3% to 79.3%). Analysis in the 3 predefined age groups showed similarly high efficacy in the 2 upper age groups (75.4% in 6–11 years and 76.8% in 12–16 years groups), while modest efficacy (55.9%) was seen in the 4–5 years group.
Figure 1.
Vaccine efficacy (95% CI) in prevention of VCD fever, hospitalization due to VCD, severe VCD, and DHF between first dose administration and 12 months after end of part 1 (month 27, year 2 after second dose). Forest plot shows efficacy according to serotype, baseline serostatus, age, and region (safety set data; lower bound 95% CI values < minus 50 not shown on x-axis). For serotype-specific efficacy calculations, only the first instance of VCD due to the individual serotype in question was included, regardless of previous instances of VCD due to other serotypes. Participants were classified as seronegative when testing seronegative for all dengue serotypes at baseline. Participants were classified as seropositive when demonstrating a reciprocal neutralizing antibody titer ≥ 10 against at least 1 dengue serotype at baseline. Cases of severe VCD were determined according to Dengue Case Adjudication Committee criteria. Cases of DHF were determined according to World Health Organization 1997 criteria [23]. Abbreviations: CI, confidence interval; DENV, dengue virus; DHF, dengue hemorrhagic fever; TAK-003, Takeda dengue vaccine candidate; VCD, virologically confirmed dengue.
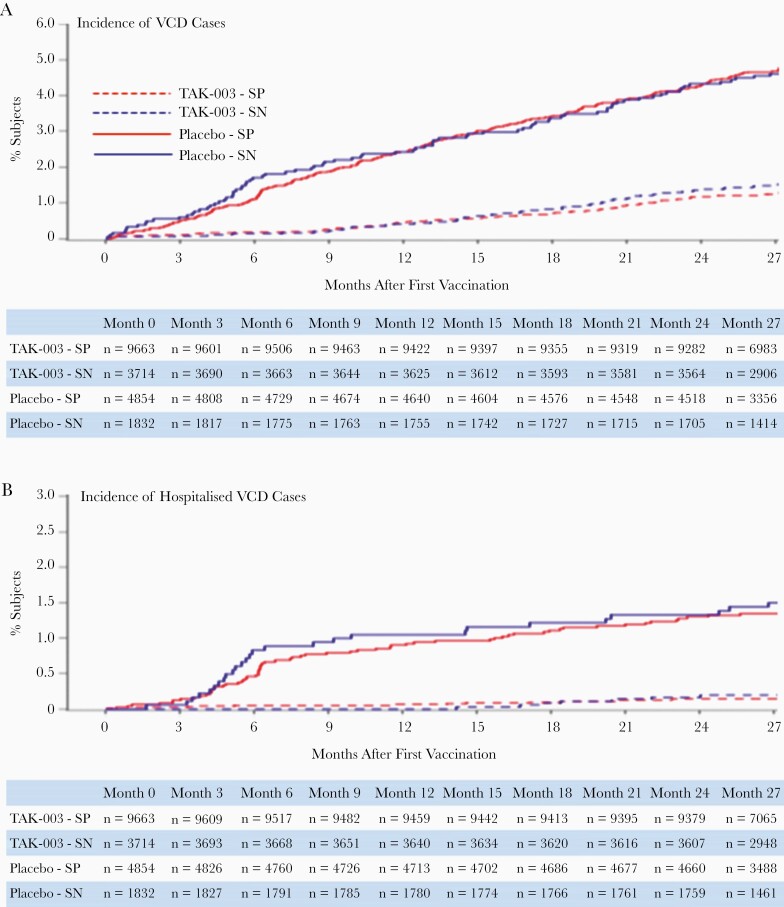
Figure 2.
Cumulative incidence of (A) VCD cases and (B) hospitalized VCD cases (safety set data), occurring between first vaccination and 12 months after the end of part 1 (month 27, year 2 after second dose). Abbreviations: SP, seropositive; SN, seronegative; TAK-003, Takeda dengue vaccine candidate; VCD, virologically confirmed dengue.
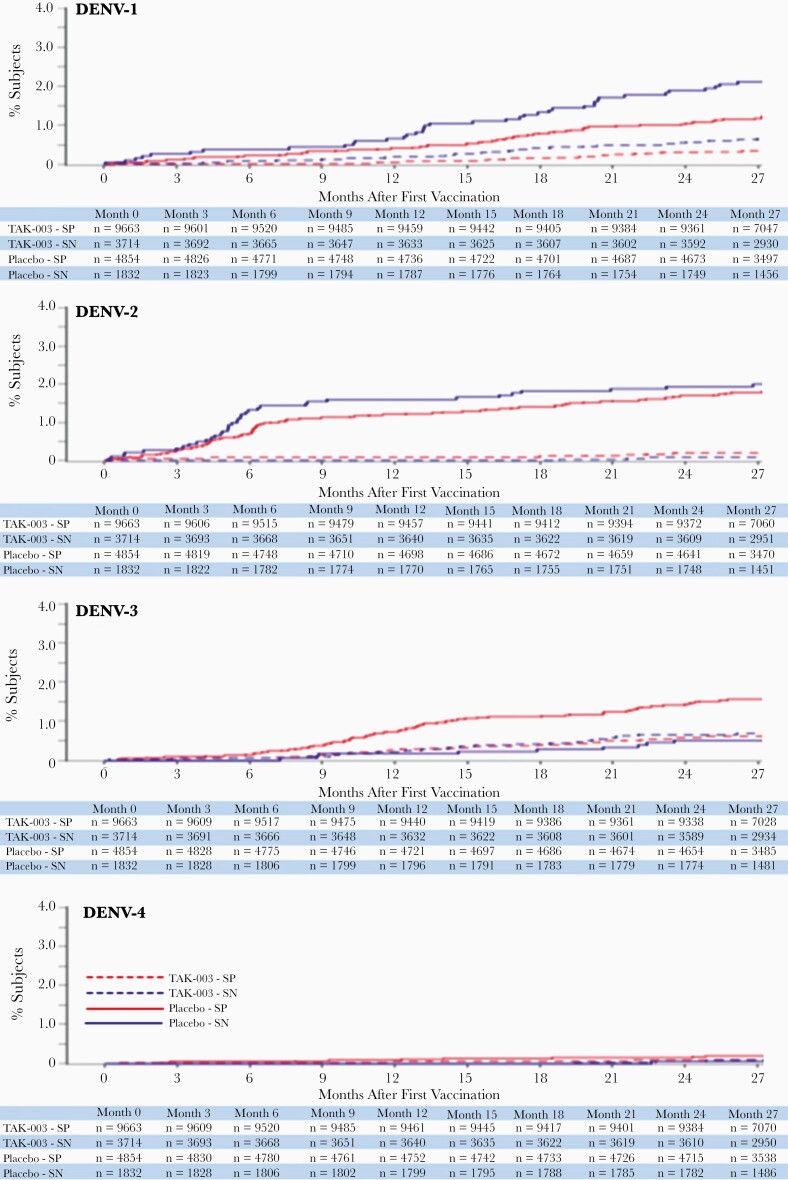
Figure 3.
Cumulative incidence of VCD cases for each serotype (safety set data), occurring between first vaccination and 12 months after the end of part 1 (month 27, year 2 after second dose). Abbreviations: DENV, dengue virus; SP, seropositive; SN, seronegative; TAK-003, Takeda dengue vaccine candidate; VCD, virologically confirmed dengue.
In year 1, TAK-003 had demonstrated an overall efficacy of 80.2% (95% CI, 73.3%–85.3%) against VCD (per protocol set data); in year 2, overall efficacy against VCD was 56.2% (95% CI, 42.3%–66.8%). It should be noted that some of the differences can be attributed to changes in background serotype dominance in the environment (see “Discussion” section). A descriptive account of year-by-year case distribution and efficacy in the various subgroups are presented in Table 2. Efficacy against VCD was lower in year 2 than year 1 in all 3 age groups, although the largest decrease was observed in 4–5 year olds, from 72.8% (95% CI, 46.4%–86.2%) in year 1 to 24.5% (95% CI, −34.2% to 57.5%) in year 2. Efficacy against VCD by individual age is presented in Supplementary Figure 3. In year 1 and year 2, efficacy was similar regardless of serostatus against either DENV-1 or DENV-2, and no efficacy was demonstrated against DENV-3 in baseline seronegatives, while it was shown in baseline seropositives. The efficacy against DENV-4 remained inconclusive due to the few cases identified.
A total of 15 cases of Dengue Case Severity Adjudication Committee (DCAC)-defined severe dengue (n = 5) or DHF by World Health Organization 1997 criteria (n = 11) have been reported up to 2 years after the second dose, including 1 case which met both criteria (11 in placebo versus 4 in TAK-003 groups). Overall, cumulative efficacy against DHF was 81.2% (95% CI, 29.3%–95.0%) and remained inconclusive against DCAC-defined severe dengue at 66.9% (95% CI, −97.8% to 94.5%). Additional data in subgroup analysis and details of individual cases are provided in Figure 1 and Supplementary Table 3.
Clinical Characteristics of Dengue Cases
Details on the clinical presentation of VCD cases are provided in Supplementary Table 4. Plasma leakage was observed in 1 (0.8%) of 119 in TAK-003 versus 14 (6.1%) of 228 in placebo seropositive groups; and 3 (5.4%) of 56 in TAK-003 versus 3 (3.6%) of 84 in placebo seronegative groups. Bleeding was observed in 5 (4.2%) of 119 participants in the TAK-003 seropositive group versus 19 (8.3%) of 228 in the placebo seropositive group; and 3 (5.4%) of 56 in TAK-003 versus 6 (7.1%) of 84 in placebo seronegative groups. Platelet counts of ≤ 100 × 109/L were observed in 5 (4.7%) of 107 in TAK-003 versus 54 (24.8%) of 218 in placebo seropositive groups; and 4 (8.2%) of 49 in TAK-003 versus 11 (14.3%) of 77 in the placebo seronegative groups. Further details on DENV-3 VCD cases are provided in Supplementary Table 5.
Safety
Three deaths occurred during this 12-month period between month 16 and month 27. Causes were 2 road traffic accidents and 1 case of adenocarcinoma of the colon and none were considered to be related to any study procedures or the investigational product by the investigators or the sponsor. In this time period, serious adverse events (SAEs) were experienced by 422 (271 [2.0%] and 151 [2.3%] participants in the TAK-003 and placebo groups, respectively; Table 3). None of the SAEs reported were considered to be related to any study procedures or the investigational product. Additional SAE data are provided in Supplementary Table 1.
Table 3.
Numbers of Participants Experiencing Serious Adverse Events After Any Vaccination During 12 Months of End of Part 1 (Month 16 to Month 27; Safety Set Data)
| Adverse Events | TAK-003, No. (%) (n = 13 380) | Placebo, No. (%) (n = 6687) |
|---|---|---|
| Any | 271 (2.0) | 151 (2.3) |
| Mild | 25 (0.2) | 12 (0.2) |
| Moderate | 199 (1.5) | 108 (1.6) |
| Severe | 47 (0.4) | 31 (0.5) |
| Related to investigational producta | 0 | 0 |
| Related to trial procedures | 0 | 0 |
| Leading to withdrawal of investigational product or trial discontinuation | 2 (< 0.1) | 2 (< 0.1) |
| Deaths | 2 (< 0.1) | 1 (< 0.1) |
aAs assessed by investigator and sponsor.
Immunogenicity
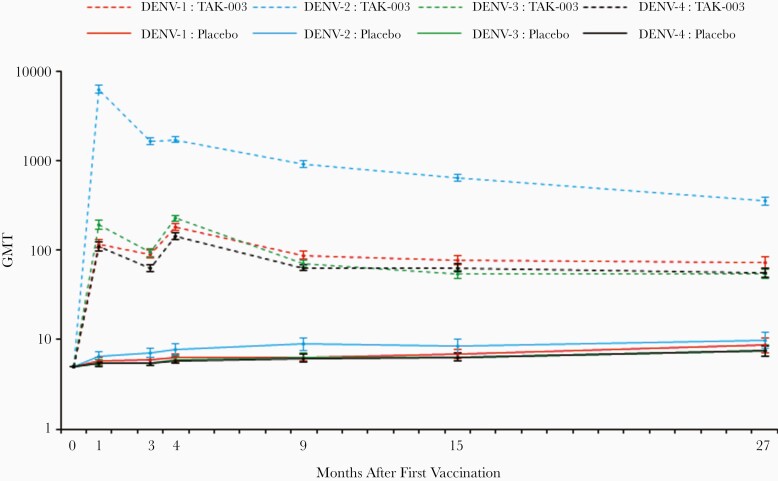
In the baseline seronegative group, analysis of geometric mean antibody titers (GMTs) from the last 3 timepoints (months 9, 15, and 27) found a general trend of decreasing DENV-2 GMTs over time, whereas GMTs for DENV-1, -3, and -4 remained relatively stable in TAK-003 recipients (Figure 4 and Supplementary Table 6). There was minimal change in the seropositivity rates (Supplementary Table 7) with tetravalent seropositivity rates of 91.3% at month 9 and 85.9% at month 27. Further exploration did not suggest any notable difference in GMTs or seropositivity rates between the 3 age groups seronegative at baseline (Supplementary Tables 8 and 9). Additional data on the baseline seropositive group are provided in Supplementary Tables 6 and 7.
Figure 4.
Serotype-specific GMTs (95% CI) in participants seronegative at baseline (per protocol set for immunogenicity data). Abbreviations: CI, confidence interval; DENV, dengue virus; GMT, geometric mean titer; TAK-003, Takeda dengue vaccine candidate.
DISCUSSION
This planned exploratory analysis enabled us to assess cumulative vaccine efficacy over 2 years and to compare efficacy in years 1 and 2. Overall, approximately 27 months after initiating vaccination, TAK-003 has prevented 72.7% of cases of symptomatic dengue, 89.2% of hospitalized dengue, and 81.2% of DHF in a population aged 4–16 years. Importantly, these benefits accrued regardless of baseline serostatus. The cumulative efficacy against symptomatic dengue was 74.8% in baseline seropositives and 67% in baseline seronegatives, with corresponding reductions in hospitalization rates of 90% and 87%. These estimates in the baseline seronegative population translate to the prevention of 3023 cases of symptomatic dengue and 1285 cases of hospitalized dengue per 100 000 vaccinees. Although the detailed subgroup analyses would suggest some potential variation of impact in different epidemiological conditions, these data over 2 years in diverse settings underscore the likely overall benefit within a community. The data at this point suggests that prevaccination screening would not be necessary.
There are several challenges in developing a dengue vaccine, necessitating not only the complexities of formulating a tetravalent vaccine, but conducting and analyzing a pivotal efficacy trial against all 4 serotypes. Multiple endpoints as well as several potential effect modifiers lead to a vast number of subgroup analyses. Our trial design and meticulous collection of data enables granular analyses to explore important subgroups previously identified during dengue vaccine development. However, the known limitations of extensive subgroup analyses may result in misleading random findings and invites overinterpretation. It is likely that at a very granular level, the trial population is no longer randomly distributed between the TAK-003 and placebo groups, and the risk of symptomatic infection and its manifestation are more dependent on each participant’s individual circumstances. Therefore, each subgroup or timeframe may not display similar efficacy, which warrants careful interpretation of the data.
The key finding of the year-by-year analysis was the indication of waning efficacy in the second year. This was seen across multiple serotypes, in both seronegatives and seropositives, and across all 3 age groups. The waning of efficacy against DENV-2 is noteworthy. While some decline in efficacy against the non–DENV-2 serotypes may be intuitive, waning of efficacy against DENV-2, which forms the backbone of TAK-003, was unexpected. This finding has implications for the field of dengue vaccine development as it may suggest that a nonchimeric attenuated construct may not fully replicate the decades of protection provided by natural dengue infection. It will be important to see if vaccine performance stabilizes in the third year. In addition, evaluation of a booster dose is under consideration. Interestingly, little or no decline in efficacy was noted in year 2 against DENV-1 in baseline seronegatives (67.2% vs 60.7%) or against DENV-4 in baseline seropositives (63.8% vs 69%). These data support the role of non–DENV-2 components (ie, the serotype-specific premembrane and envelope gene proteins) in the tetravalent TAK-003 formulation.
With TAK-003 efficacy varying by serotype, dengue serotype distribution plays a role in determining the overall efficacy against all serotypes in a given year. This was particularly evident in the context of hospitalized dengue, for which the lower efficacy in the second year was largely driven by a lower proportion of DENV-2 (43/58 in year 1 versus 7/33 in year 2). The lack of efficacy against DENV-3 in baseline seronegatives continued in the second year and was reflected in the overall lower efficacy against both VCD and hospitalized VCD compared with baseline seropositives. Importantly, the relative risk against DENV-3 in this population was similar in both years (1.18 in year 2 versus 1.34 in the first year). During the first year, hospitalization was necessary for 1 of the 4 DENV-3 VCD in the placebo group versus 1 of 11 VCD in the TAK-003 group; 3 of the 12 VCD in the second year required hospitalization versus none of the 5 VCD in the placebo group. In this context, the unbalanced randomization ratio of 2:1 (TAK-003 to placebo) complicates data interpretation, and the small number of cases limits any robust conclusion, while the noted proportions of hospitalization may not be unusual for dengue. Therefore, continued monitoring is essential, and background rate of hospitalization in the participating countries will be an important consideration.
The year-by-year analysis provides an update on the performance of TAK-003 in different age groups. In year 1, efficacy in the 3 age groups by serostatus was generally similar (78.4%–86.2%) except for the seronegative 4–5-year-old population (39.1%). This difference could be largely explained by serotype distribution (ie, a higher proportion of DENV-3 and a lower proportion of DENV-2) and thus no clear indication of an age effect could be inferred. However, year 2 data showed a higher persistence of efficacy in the 2 upper age groups (60.6% in 6–11 years and 71.2% in 12–16 years), as well as similar efficacy in baseline seropositives and baseline seronegatives. In comparison, efficacy was only 24.5% in 4–5 year olds and varied by baseline serostatus; 46.4% in baseline seropositives and −23.7% in baseline seronegatives. It is important to note that the 4–5 years age group was the smallest of the 3 age groups (12.7% of the study population) and had fewer cases, which precludes clear interpretation in a way that distinguishes the serotype-linked variation as described in this report from a true age effect. In further detailed analysis (not shown), we could not establish either a definitive age effect or an age threshold separating benefit from no benefit.
In line with our earlier reports [17, 22], the encouraging trend of a favorable disease-modifying effect on dengue clinical presentation continued (eg, bleeding, signs of plasma leakage, platelet count), suggesting some impact of vaccination on dengue pathophysiology. Unsurprisingly, this trend seemed to be mostly driven by the baseline seropositive group, but it is reassuring that the data in baseline seronegatives did not indicate any increased severity in clinical presentation. In the baseline seronegative group, while the small number of participants with dengue-relevant clinical signs prevents a meaningful direct comparison with the placebo group, the low proportion of vaccine recipients with these clinical presentations overall is encouraging.
Two years after vaccination, the trial has answered several key questions, but some areas require continued monitoring, specifically the performance of the vaccine against DENV-3 and DENV-4 in initially seronegative participants. This requires additional cases of DENV-4 and severe dengue, as well as DENV-3 outside the Philippines, to allow for evaluation. Data from year 3 of monitoring will allow further exploration of the persistence of efficacy, effect of age, and long-term safety. As we previously noted, the complexities of dengue vaccines mean that this reasonably large trial involving over 20 000 participants in 8 countries over 2 continents has not been able to guarantee a robust evaluation of certain endpoints in a reasonable timeframe of 2 years. Hence, while we remain optimistic that the remaining 2.5 years of follow-up will answer these questions, some may require further specific evaluation in the postlicensure period, assuming TAK-003 secures marketing authorization and is used in a wider population. Regulatory and public health authorities will have to carefully balance these uncertainties against the broader profile of TAK-003 and the available options for dengue prevention.
In the context of a dengue vaccine efficacy trial, long-term data is essential, and is critically dependent on compliance with trial procedures. When designing the trial, we carefully planned to optimize the surveillance methodology once primary and secondary efficacy endpoints had been evaluated (ie, after part 2) with the intention to reduce the burden on participants and thereby facilitate higher compliance. Modified active surveillance in part 3 will still detect all cases of dengue while limiting noncritical visits and blood collections. For this reason, year 2 uses both surveillance methods, that is, active febrile surveillance in the first 6 months and modified active surveillance in the last 6 months. We believe this would not have introduced a bias because of the placebo control. SAE reporting in the last 6 months (ie, after part 2 of the trial) was limited to those assessed as related or relevant in the context of a dengue vaccine trial and the safety analysis did not suggest any new important findings.
In summary, TAK-003 continues to demonstrate benefit up to 2 years after completing vaccination in a trial population of 4–16 year olds in 8 dengue-endemic countries. The evolving profile of TAK-003 continues to support this vaccine as a component of a multimodal approach to dengue control. The remaining years of follow-up and the booster data will be valuable to further our understanding of this promising vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Investigators were E. L.-M., X. S.-L., C. B.-T., L. B., C. S., L. M. V., M. T. A., H. V., H. R., L. R., V. W., E. R.-A., D. Y., F. E., R. D., L. F., P. W., E. D. M., A. F., D. G., K. L., R. V. da C., and P. K. Study design was carried out by S. B., A. B., M. R., and D. W. Data analysis and interpretation was carried out by S. B., A. B., M. R., V. T., M. L., I. L., and D. W. Publication management was by S. B. All authors had full access to the presented data, provided critical input during manuscript preparation, and approved the manuscript for submission.
Acknowledgments. The authors sincerely thank the trial participants and their parents/guardians who agreed to take part in the trial; the data monitoring committee; the Dengue Case Adjudication Committee members; the Takeda expanded study team; the study team at PPD, Wilmington, NC, USA; and all the trial staff in each of the countries. The authors also acknowledge the contribution of the late Dan Stinchcomb PhD and Inviragen Inc., Fort Collins, CO in the initial developmental work of TAK-003 (Inviragen Inc. was subsequently acquired by Takeda). The authors are grateful to Dr Jamie Stirling (OLC Bioscience Ltd, London, UK) and Dr Keith Veitch (keithveitch communications, Amsterdam) for editorial assistance in the preparation of this manuscript.
Financial support. This work was supported by Takeda Vaccines Inc.
Potential conflicts of interest. S. B., V. T., M. R., M. L., I. L., D. W., and A. B. are permanent employees of the Takeda group of companies. D. W. has patents WO2017/179017 and WO2020/051334 pending. X. S.-L., P. K., and L. B. have served as advisory board members for Takeda. All other authors have no potential conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data sharing. Access to the study protocol, statistical analysis plan, and deidentified patient data will be provided to suitably qualified researchers at the discretion of an independent committee using a data sharing platform on written request when appropriate marketing authorization has been received.
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol 2010; 8:S7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis 2019; 13:e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Dengue vaccine: WHO position paper—September 2018. Wkly Epidemiol Rec 2018; 93:457–76. [Google Scholar]
- 5. Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379:327–40. [DOI] [PubMed] [Google Scholar]
- 6. Huang CY, Kinney RM, Livengood JA, et al. Genetic and phenotypic characterization of manufacturing seeds for a tetravalent dengue vaccine (DENVax). PLoS Negl Trop Dis 2013; 7:e2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu H, George SL, Stinchcomb DT, Osorio JE, Partidos CD. CD8+ T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 2015; 212:1618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. George SL, Wong MA, Dube TJ, et al. Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis 2015; 212:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson LA, Rupp R, Papadimitriou A, Wallace D, Raanan M, Moss KJ. A phase 1 study of safety and immunogenicity following intradermal administration of a tetravalent dengue vaccine candidate. Vaccine 2018; 36:3976–83. [DOI] [PubMed] [Google Scholar]
- 10. Osorio JE, Velez ID, Thomson C, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rupp R, Luckasen GJ, Kirstein JL, et al. Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a phase 1b randomized study. Vaccine 2015; 33:6351–9. [DOI] [PubMed] [Google Scholar]
- 12. Sáez-Llorens X, Tricou V, Yu D, et al. Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2–17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2018; 18:162–70. [DOI] [PubMed] [Google Scholar]
- 13. Sáez-Llorens X, Tricou V, Yu D, et al. Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2017; 17:615–25. [DOI] [PubMed] [Google Scholar]
- 14. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis 2016; 213:1562–72. [DOI] [PubMed] [Google Scholar]
- 15. Tricou V, Sáez-Llorens X, Yu D, et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2–17 years: a randomised, placebo-controlled, phase 2 trial. Lancet 2020; 395:1434–43. [DOI] [PubMed] [Google Scholar]
- 16. Turner M, Papadimitriou A, Winkle P, et al. Immunogenicity and safety of lyophilized and liquid dengue tetravalent vaccine candidate formulations in healthy adults: a randomized, phase 2 clinical trial. Hum Vaccin Immunother 2020; 16:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biswal S, Borja-Tabora C, Martinez Vargas L, et al. ; TIDES Study Group . Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: a randomised, placebo-controlled, phase 3 trial. Lancet 2020; 395:1423–33. [DOI] [PubMed] [Google Scholar]
- 18. Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. ; CYD-TDV Dengue Vaccine Working Group . Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. [DOI] [PubMed] [Google Scholar]
- 19. Dayan GH, Langevin E, Gilbert PB, et al. Assessment of the long-term efficacy of a dengue vaccine against symptomatic, virologically-confirmed dengue disease by baseline dengue serostatus. Vaccine 2020; 38:3531–6. [DOI] [PubMed] [Google Scholar]
- 20. World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 10 September 2020.
- 21. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guideline for Good Clinical Practice E6(R2). https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice. Accessed 10 September 2020.
- 22. Biswal S, Reynales H, Saez-Llorens X, et al. ; TIDES Study Group . Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med 2019; 381:2009–19. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization (WHO). Dengue haemorrhagic fever: diagnosis, treatment, prevention, and control. 2nd ed. Geneva: WHO, 1997. https://www.who.int/csr/resources/publications/dengue/Denguepublication/en/. Accessed 10 September 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.