Abstract
We adapted the RNA FISH Stellaris method to specifically detect the expression of Plasmodium genes by flow cytometry and ImageStream (Flow-FISH). This new method accurately quantified the erythrocytic forms of (1) Plasmodium falciparum and Plasmodium vivax and (2) the sexual stages of P vivax from patient isolates. ImageStream analysis of liver stage sporozoites using a combination of surface circumsporozoite protein (CSP), deoxyribonucleic acid, and 18S RNA labeling proved that the new Flow-FISH is suitable for gene expression studies of transmission stages. This powerful multiparametric single-cell method offers a platform of choice for both applied and fundamental research on the biology of malaria parasites.
Keywords: flow analyses, Plasmodium, RNA-FISH
Thanks to the combination of Plasmodium-specific RNA detection and surface and nucleus staining, the new Flow-FISH method allowed us to characterize and quantify asexual and sexual blood-stage parasite from P vivax clinical samples as well as liver-stage parasites.
Malaria remains a major health burden with more than 400 000 victims and 250 million clinical cases reported annually [1]. The disease is caused by 5 distinct parasite species of the Plasmodium genus. After transmission by mosquito bites, the parasite reaches the liver where it replicates, and then it infects red blood cells (RBCs). Blood stage infection is responsible for the symptoms of malaria, with the most virulent and best characterized parasite being Plasmodium falciparum. However, other human malaria species such as Plasmodium vivax, which is mostly spread outside Africa, cannot be cultivated, and their study depends mainly on clinical samples, making investigation of expressed genes laborious. Two developmental forms coexist in the blood circulation, the asexual and sexual stages, which are responsible for chronic infection and pathogenesis, and for transmission to mosquitoes, respectively. In contrast to erythrocytic stages, mosquito-derived salivary gland sporozoites are classically short-lived once injected into the human skin because they rapidly reach the liver to continue the life-cycle development within infected hepatocytes.
Flow cytometry-based approaches cumulate several advantages over current molecular or microscopic techniques, such as its single-cell analysis basis and the high number of cells analyzed (reviewed in [2]). Moreover, its coupling with imaging (ImageStream/AMNIS) allows us to add morphological analysis, which provides key information to study different processes such as parasite growth [3] or drug-induced leakage in its food vacuole [4]. However, parasite detection relies on either deoxyribonucleic acid (DNA) staining growth or transgenic parasites or on the availability of specific antibodies, which makes the study of nucleated infected cells difficult when transgenic parasites or antibodies are not available. The detection of parasite-specific ribonucleic acids (RNAs) by fluorescence, which may palliate the absence of the transgenic strains or specific antibodies, has been widely described for Plasmodium [5], but it is still laborious when a high number of analyzed cells are required. In this study, we report the establishment of a solid, single-cell, flow-based method for the detection, quantification, and gene expression studies of different human malaria species. We applied this method to quantify the presence of sexual stages of P vivax clinical samples using RNA-FISH combined with flow cytometry and flow imaging.
MATERIALS AND METHODS
Plasmodium Strains and Isolates
Plasmodium falciparum blood-stage 3D7 parasites were cultured as previously described [6]. Plasmodium falciparum NF54 (clone A11) salivary gland sporozoites were collected from adult-infected Anopheles stephensi females (Center for Production and Infection of Anopheles at Institut Pasteur) and isolated as described previously [7].
Clinical P vivax isolates were collected with written informed consent from Brazilian patients and cryopreserved in liquid nitrogen. Study protocols have been approved by the Institutional Review Board of the University Hospital of the University of São Paulo (1025/10) and by the National Human Research Ethics Committee of the Ministry of Health of Brazil (551/2010). They were thawed using a stepwise NaCl method as previously reported [8].
Flow FISH
The original manufacturer’s instructions (www.biosearchtech.com/stellarisprotocols) were modified as followed: all cells were fixed for 30 minutes at room temperature in 500 μL of a 4% formaldehyde (FA) methanol-free solution (Thermo Scientific) in phosphate-buffered saline (PBS) (Sigma). For RBCs, glutaraldehyde (0.0075% final) (Electron Microscopy Sciences) was added to 4% FA. Cells were washed 2 times with PBS 1X and hybridized according to Stellaris’ instructions with sets of anti-P falciparum and vivax 18S rRNA Cy5- or quasar-670-conjugated, and quasar-570-conjugated anti-P vivax Pvs25 RNA Stellaris FISH Probes (Biosearch Technologies). The RNA probes were designed using the Stellaris FISH Probe Designer available online at www.biosearchtech.com/stellarisdesigner. Hybridized cells were then processed for surface and nucleus staining. Cells were incubated for 15 minutes at room temperature in PBS 1% bovine serum albumin (BSA) (Sigma) and 2% goat serum (Biowest), followed by a 10-minute incubation on ice with anti-CD235a fluorescein isothiocyanate (FITC) (clone HIR2; Miltenyi Biotec), anti-CD71 AF700 (clone REA627; Miltenyi Biotec), anti-P falciparum circumsporozoite protein (PfCSP) FITC (MRA-183; BEI Resources) monoclonal antibodies (mAbs), and/or SYBR Green I Nucleic Acid Gel Stain (1/1000 of initial 10 000× in dimethyl sulfoxide; Thermo Fisher) in PBS .01% NaN3 (VWR) (fluorescence-activated cell sorting [FACS] buffer). After 2 washes in FACS buffer and before processing, 4’,6-diamidino-2-phenylindole (DAPI) (1/1000) (Sigma) was added to the cells. Cells were acquired on a BD LSRFortessa (BD Biosciences), and data were analyzed using FlowJo LLC software v10.6.1 (BD Biosciences). Alternatively, ≥4 × 105 hybridized cells in 50 μL FACS buffer were acquired on an Image stream ISX MkII flow cytometer (Amnis Corp., EMD Millipore). The channels used for reading the samples were channel 01 for BrightField, channel 02 for FITC-conjugated anti-CD235a mAbs and anti-PfCSP, and channel 07 for DAPI. Data were analyzed using IDEAS software (version 6.2).
Droplet Digital PCR
The DNA from P falciparum-infected RBCs was prepared using the QIAamp DNA blood kit (QIAGEN). Droplet generation was performed on a droplet generator according to manufacturer’s instructions (QX200 Droplet Generator; Bio-Rad). The PCRs were run using the following primers: CytBfwd (forward primer) TGGAGTGGATGGTGTTTTAGA, CytBrvs (reverse primer) TTGCACCCCAATAACTCATTT; and r18Sfwd (forward primer) CAATTGGAGGGCAAGTCTGG, r18Srvs (reverse primer) ATGCGCACAAAGTCGATACG. The DNA copy numbers per microliter were analyzed using QuantaSoft (v.1.7.4). Triplicates were performed. Means were used for drawing correlation curves.
Statistical Analyses
Spearman correlation coefficients rs and respective P values were calculated using R; significance was set at the 5% level.
RESULTS
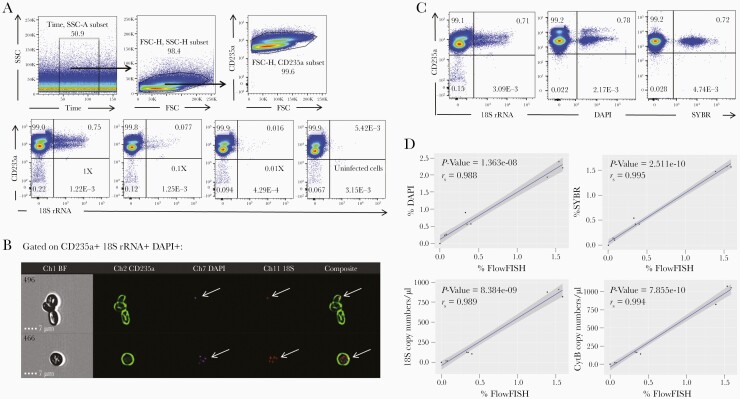
We have adapted a commercial RNA-FISH assay (Stellaris) for the flow cytometric and ImageStream detection and quantification of human Plasmodium species. First, we assessed the specificity, reproducibility, and sensibility of the new Flow-FISH technique by quantifying parasites after serial dilutions of asynchronous cultures of P falciparum (3D7)-infected RBCs. For this, we have targeted the small-subunit 18S rRNA, ubiquitously expressed by all parasite stages, using a set of specific Cy5-conjugated probes (Figure 1A). The probes were combined with DAPI and anti-CD235a mAbs labeling for the respective detection of parasite’s DNA and the RBC surface protein glycophorin A. The strategy of cell analysis is shown on upper dot plots. Parasite labeling with the r18S-probe was specific, because no signal was detected in CD235a+ uninfected cells. In contrast, Cy5+CD235a+ cells were detected in all parasite cultures, with a percentage of positive cells proportional to the culture dilutions tested. Infected cell staining with DAPI and 18S rRNA shows that the label overlaps (Figure B, dot plot), consistent with the ImageStream analysis of gated CD235a+ 18S rRNA+ cells (Figure B). It is interesting to note that the latter also enables us to distinguish between different stages of P falciparum because we can identify both rings (upper cells) and schizonts (lower cells). We found that the percentage of positive cells obtained by Flow-FISH, DAPI, and SYBR Green staining was similar for all culture dilutions with a strong correlation between the Flow-FISH method and the other 2 flow cytometric methods used for Plasmodium quantification (Figure 1C and D, upper curves). Finally, we also found a strong correlation between the percentage of parasitized RBCs measured by Flow-FISH and parasite 18S and CytB DNA copy numbers obtained after Droplet Digital PCR (ddPCR) amplification of 18S and CytB genes (Figure C, lower curves).
Figure 1.
Flow-FISH allows us to accurately detect and quantify blood-stage Plasmodium falciparum in vitro. Serial 1:10 dilutions were performed from an original 3D7 P falciparum-infected red blood cells (RBCs) culture at 0.75%. Each culture was then processed by fluorescence-activated cell sorting (FACS) or AMNIS after staining with anti-CD235a monoclonal antibodies, 4’,6-diamidino-2-phenylindole (DAPI), and 18S rRNA-specific probes. (A) Upper dot plots show the gating strategy for the Flow-FISH expression analysis of Plasmodium-specific-18S rRNA probes by CD235a+ cells in the different cultures and the noninfected control (right panel). (B) ImageStream analyses of gated 18S+DAPI+CD235a+ cells (CD235a in green, DAPI in violet, and 18S rRNA probes in red, all indicated with arrows). (C) The dot plots show the coexpression by parasitized cells of CD235a and 18S rRNA probes (left), DAPI (middle), or SYBR Green (right). (D) Correlation between 18S rRNA+ percentages and both DAPI or SYBR percentages (upper panels), and 18S or CytB DNA copy numbers quantified by Droplet Digital PCR (ddPCR) (lower panels). For all correlation curves, the P values and Pearson’s correlation coefficient rs are indicated. BF, bright field; FSC, forward scatter; SSC, side scatter.
We then applied this new method to tackle biological questions highly relevant for epidemiological studies. First, we studied intraerythrocytic parasites from P vivax stages from clinical isolates using 2 different assays. For this, P vivax-infected RBCs isolates were labeled with DAPI, anti-CD235a, and anti-CD71 mAbs together with 18S- and Pvs25-specific probes, the latter specifically recognizing P vivax sexual parasites (Figure 2A–C). The percentages of P vivax-infected 18S rRNA+ among total and immature CD71+ RBCs were first determined by flow cytometry (Figure 2A, upper and lower dot plots). For 5 different P vivax isolates (right graph), parasitemia (18S+CD235a+ RBCs) ranged from 0.045% to 0.13%, with a high proportion—from 19% to 38%—of immature CD71+-infected cells. Parasitemia determined by microscopy using GIEMSA-stained thin smears were much lower, ranging from 0.03% to 0.045% (data not shown). Second, we analyzed the proportion of sexual Pvs25+ among the total 18S+ parasites (Figure 2B and C). As shown on ImageStream analyses, sexual parasitized cells are costained by 18S rRNA probes, Pvs25 probes, and DAPI (Figure 2B), confirming the reliability of both 18S- and Pvs25-specific probes to detect ex vivo P vivax sexual and asexual parasites. The flow cytometric analyses of the 5 isolates showed proportions of sexual stages, ranging from 11.5% to 40.5%; these were not associated with parasitemia levels (Figure 2C).
Figure 2.
Applications of the Flow-FISH technique. (A and B) red blood cells (RBCs) from Plasmodium vivax isolates from 5 patients were analyzed for CD235a, CD71, Plasmodium-specific 18S rRNA, Pvs25 RNA, and 4’,6-diamidino-2-phenylindole (DAPI). (A) The dot plots show the RBCs gating strategy for 18S rRNA expression by the total CD235a+ cells (upper panels) and the immature CD235a+CD71+ cells (lower panels). The percentage of CD71+-infected RBCs (histogram bars) together with the percentage of infected 18S+ RBCs (•) for all individual isolates are plotted on right panel with histograms corresponding to the left y-axis and dot marks corresponding to right y-axis (right graph). (B) Two representative ImageStream analysis of parasitized RBCs from the same clinical isolates are shown (bright field image on the left, Pvs25 in yellow, DAPI in violet, 18S rRNA in red) (left panels). (C) The isolates were analyzed for the proportion of sexual Pvs25+ stages among all 18S+ parasites by flow cytometry (middle dot plots). The percentage of Pvs25+-infected RBCs (histogram bars, left y-axis) together with the percentage of infected 18S+ RBCs (dot marks [•], right y-axis) for all individual isolates are plotted on the right graph. (D) Representative ImageStream analyses of sporozoites isolated from mosquitoes infected by Plasmodium falciparum NF54. Green for P falciparum circumsporozoite protein (PfCSP), red for 18S rRNA, and violet for nucleus. SSC, side scatter.
We also assessed 18S rRNA detection by salivary gland P falciparum sporozoites (Figure 2D) by labeling P falciparum sporozoites with anti-PfCSP (ubiquitously expressed at the surface of sporozoites) mAbs, DAPI, and 18S-specific rRNA probes, and we analyzed cells by ImageStream. As shown on Figure 2D in bright field (BF), the Flow-FISH method allows the maintenance of the morphological integrity of sporozoites for antibody staining and RNA FISH analysis. This analysis was also successfully performed on Plasmodium berghei salivary gland sporozoites (data not shown). Therefore, the Flow-FISH method applied to malaria parasites offers a reliable tool for the quantification of this pathogen and the study of gene expression in different life-cycle stages.
DISCUSSION
We have developed a new Flow-FISH method that, through the labeling of Plasmodium-specific transcripts, allows the accurate single-cell detection, quantification, and characterization of parasitized cells by flow cytometry and flow imaging. For this, the initial fixation protocol was mainly modified in the case of RBC processing by adding glutaraldehyde to maintain cell integrity. Second, surface and nucleus staining were performed after saturation of hybridized cells in a mix of goat serum and BSA, a required step to avoid nonspecific binding by mAbs. In addition to its ability to process thousands of cells shared by other flow cytometric methods of Plasmodium detection, Flow-FISH has proven to be highly specific, while bypassing the requirement of parasite-specific antibodies or transgenic parasites expressing fluorescent reporter. This feature is particularly attractive for malaria parasites that cannot be cultured continuously, such as P vivax, Plasmodium malariae, or Plasmodium ovale, and for which many tools are still lacking. When applied to P vivax-infected patient isolates, Flow-FISH significantly improved the quantification of parasitized cells, when compared with microscopy-based counting. Furthermore, we observed P vivax infection of CD71+ immature RBCs consistent with the selective tropism of P vivax [9]. More importantly, the Flow-FISH technique allowed P vivax sexual staging. Our results confirm the high representation of transmissible parasites found by single-cell RNA sequencing of P vivax parasite strains from infected monkeys [10]. The variability among isolates, with some of them reaching more than 40%, may represent a snapshot of a highly dynamic process of sexual differentiation involving different locations, including the spleen and bone marrow [11]. Therefore, Flow-FISH is a highly valuable tool for epidemiological transmission studies, especially when sexual versus asexual morphological determination is challenging. This method works with small sample sizes from patients and allows the detection of a subset of pathogens expressing distinct genes.
More importantly, the Flow-FISH method, through its ability to compile multiple parameters (RNA, surface and intracellular protein expression, nuclear staining) for both parasites and target cells, should open new avenues to address challenging questions. Recent reports have used this assay to characterize Epstein-Barr virus-infected cells [12] or unveil rare subsets of human immunodeficiency virus-infected cells [13] from patients’ blood. Similarly, one important issue of Plasmodium biology is the infection of bone marrow (BM) developmental erythroid cells, which may explain in part the malaria-induced anemia. Although infection of immature erythroid cells by human Plasmodium has been reported for in vitro-developing RBCs [14, 15], the demonstration in humans of ex vivo infection of nucleated erythrocytes from patients’ BM biopsies is still challenging. The new Flow-FISH technique should (1) allow for direct simultaneous ex vivo characterization of parasitic stages and host cells and (2) extend this analysis to any Plasmodium spp. This is especially valuable for complex cellular environments of interest during malaria, such as the BM, spleen, skin, or liver.
CONCLUSIONS
Finally, the use of ImageStream to process Flow-FISH stained cells is the method of choice to combine morphological studies with RNA expression. For example, the distinction between rings from schizonts reported here may be useful to study key transitional blood stage transcripts. In addition, exoerythrocytic stage (including vector stages) should also be easily identified and studied.
Notes
Acknowledgments. We thank the following: Julien Cherfils Vicini (Faculté de Médecine de Nice); the Unité de Technologie et Service Cytometrie et Biomarqueurs (UTechS CB) of the Institut Pasteur Paris for their assistance with ImageStream; and Patty Chen (Biology of Host-Parasite Interactions Unit, Institut Pasteur Paris) and Didier Ménard (Malaria Genetics and Resistance Unit, Institut Pasteur, Paris) for providing help with the Stellaris probes. The anti-Plasmodium falciparum circumsporozoite protein (PfCSP) was obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health: Hybridoma 2A10 anti-Plasmodium falciparum Circumsporozoite Protein (CSP), MRA-183, contributed by Elizabeth Nardin. We are grateful to Vanessa C. Nicolete and Maria José Menezes for expert help with parasite sample collection and processing.
Author contributions. C. L.-B. and S. G. conceived and designed experiments. C. L.-B., F. N., S. T., and M. S.-H. performed experiments. C. L.-B. an S. G. analyzed data. M. U. F. collected and provided clinical samples. S. G. and A. S. wrote the manuscript. All authors reviewed the manuscript for intellectual content and approved the final version to be submitted for publication.
Financial support. C. L.-B. is part of the Pasteur - Paris University (PPU) International PhD Program. This project has received funding from the French Parasitology Consortium ParaFrap (Grant ANR-11-LABX0024), Fondation Pasteur Suisse and a Pasteur Cantarini-Roux postdoc fellowship (to F. N.). Field work has been supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (2016/18740-9; to M. U. F.).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Malaria Report, WHO, 2020. https://www.who.int/malaria [Google Scholar]
- 2. Grimberg BT. Methodology and application of flow cytometry for investigation of human malaria parasites. J Immunol Methods 2011; 367:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chua ACY, Ong JJY, Malleret B, et al. Robust continuous in vitro culture of the Plasmodium cynomolgi erythrocytic stages. Nat Commun 2019; 10:3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tong JX, Chandramohanadas R, Tan KS. High -content screening of the medicines for malaria venture pathogen box for Plasmodium falciparum digestive vacuole-disrupting molecules reveals valuable starting points for drug discovery. Antimicrob Agents Chemother 2018; 62:e02031-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mancio-Silva L, Scherf A. In situ fluorescence visualization of transcription sites and genomic loci in blood stages of Plasmodium falciparum. Methods Mol Biol 2013; 923:335–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trager W, Jensen JB. Human malaria parasites in continuous culture. 1976. J Parasitol 2005; 91:484–6. [DOI] [PubMed] [Google Scholar]
- 7. Ponnudurai T, Lensen AH, Meuwissen JH. An automated large-scale culture system of Plasmodium falciparum using tangential flow filtration for medium change. Parasitology 1983; 87 (Pt 3):439–45. [DOI] [PubMed] [Google Scholar]
- 8. Noulin F, Borlon C, van den Eede P, et al. Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS One 2012; 7:e40798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gruszczyk J, Kanjee U, Chan LJ, et al. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science 2018; 359:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sà JM, Cannon MV, Caleon RL, Wellems TE, Serre D. Single-cell transcription analysis of Plasmodium vivax blood-stage parasites identifies stage- and species-specific profiles of expression. PLoS Biol 2020; 18:e3000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obaldia N 3rd, Meibalan E, Sa JM, et al. Bone marrow is a major parasite reservoir in Plasmodium vivax infection. mBio 2018; 9:e00625-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fournier B, Tusseau M, Villard M, et al. DEF6 deficiency, a mendelian susceptibility to EBV infection, lymphoma, and autoimmunity. J Allergy Clin Immunol 2021; 147:740–3.e9. [DOI] [PubMed] [Google Scholar]
- 13. Baxter AE, Niessl J, Fromentin R, et al. Multiparametric characterization of rare HIV-infected cells using an RNA-flow FISH technique. Nat Protoc 2017; 12:2029–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neveu G, Richard C, Dupuy F, et al. Plasmodium falciparum sexual parasites develop in human erythroblasts and affect erythropoiesis. Blood 2020; 136:1381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamez PA, Liu H, Fernandez-Pol S, Haldar K, Wickrema A. Stage-specific susceptibility of human erythroblasts to Plasmodium falciparum malaria infection. Blood 2009; 114:3652–5. [DOI] [PMC free article] [PubMed] [Google Scholar]