Abstract
Background
Spontaneous coronary artery dissection (SCAD) is still an underdiagnosed condition that requires a detailed assessment of angiographic signs. It also shares similar clinical presentations with Takotsubo syndrome (TTS). The concomitant presentation of SCAD with TTS is a possible occurrence, making it difficult for clinicians to treat and manage.
Case summary
This study included a 49-year-old woman with retrosternal chest pain who was admitted to the emergency department. Coronary angiography indicated Type 2A SCAD involving the middle part of the left anterior descending artery, while the left ventriculography indicated a typical left ventricular apical ballooning compatible with TTS. A conservative approach to the management of SCAD was observed. After a 3-month follow-up, the control coronary angiography showed a complete angiographic resolution. The results of the transthoracic echocardiogram (TTE) and cardiac magnetic resonance revealed a complete normalization of the pathological features. The patient remained asymptomatic and showed no recurrence of chest pain.
Discussion
Although TTS and SCAD are commonly observed in patients who share certain characteristics (women, without atheromatous terrain, stress-related factors), it is difficult to establish a pathophysiological link between them. This observation confirms the non-random association of two rare entities of myocardial infarction with no obstructive coronary arteries. Although TTS can be easily diagnosed via non-invasive imaging, the diagnosis of SCAD is more difficult. The findings of this study suggest a concomitant presentation between SCAD and TTS. Although the treatment approach to SCAD is usually conservative, severe forms of this disease require early diagnosis and appropriate treatment.
Keywords: Case report, Spontaneous coronary artery dissection, Takotsubo syndrome, Acute coronary syndrome, Left ventricular apical ballooning
Learning points.
The presence of Takotsubo syndrome may be concomitant with spontaneous coronary artery dissection (SCAD)
SCAD remains an underdiagnosed condition that requires a detailed assessment of angiographic signs.
Introduction
Spontaneous coronary artery dissection (SCAD) and Takotsubo syndrome (TTS) are two cardiovascular syndromes with similar clinical presentations that predominantly affect women, often occurring after a stressful emotional or physical event, and are transient with a reversion ad integrum.1 It is also likely that these conditions might co-occur in some patients. In contrast, SCAD is usually common in young subjects2 (mean age: 50 years), while TTS is common in women with a mean age of 67–70 years. Clinical features associated with SCAD tend to be extracoronary vascular abnormalities, such as fibromuscular dysplasia, pregnancy or postpartum, or connective tissue diseases, whereas TTS is associated with conditions of catecholamine excess3 (pheochromocytoma and central nervous system disorders) and endothelial dysfunction or abnormal vasomotor function, such as in postmenopausal patients or those with migraine or Raynaud’s phenomenon. The SCAD remains an underdiagnosed4,5 condition that requires a detailed assessment of angiographic signs. It can be concomitant with TTS, making it difficult for clinicians to treat and manage. The aim of this case report is to review the case of a 49-year-old woman with a history of stress disorder who presented with acute chest pain and was admitted to our medical centre. Paraclinical examinations have also demonstrated the potential co-existence of TTS and SCAD.
Timeline
| Time | Events |
| Day 1 Emergency department | Patient with history of smoking and very intense stress disorder admitted for retrosternal chest pain radiating to the left arm. |
| The electrocardiogram (ECG) indicated sinus tachycardia with no ST-T change (elevated hs-Tn level of 5883.4 ng/L; upper normal limit <15.6). | |
| Cardiac catheterization laboratory | The coronary angiography indicated Type 2A spontaneous coronary artery dissection (SCAD) involving the middle part of the left anterior descending artery, while the left ventriculography showed the typical left ventricular apical ballooning and a hypercontractile base compatible with a Takotsubo syndrome (TTS). |
| Day 2 Cardiovascular intensive care unit | The cardiac magnetic resonance (CMR) showed transmural myocardial oedema in T2-weighted sequence and the absence of myocardial necrosis in the late gadolinium enhancement images. |
| Management included medical therapy with the administration of bisoprolol and aspirin. | |
| Day 4 | Hospital discharge. |
| Three months after discharge | The patient remained asymptomatic and showed no reoccurrence of chest pain. |
| The coronary angiography showed normal coronaries. CMR and echocardiography revealed complete resolution. |
Case summary
A 49-year-old Caucasian woman with no history of cardiovascular disease was admitted to the emergency department presenting with retrosternal chest pain radiating to the left arm. A history of smoking is one of the risk factors for cardiovascular disease. No chronic diseases were recorded. Anamnestic questioning revealed a very intense stress disorder during the weeks preceding the acute event.
Upon admission, the patient was apyrexial and tachycardic with a heart rate of 110 b.p.m. and a blood pressure of 130/87 mmHg. The results of the physical examination were insignificant. Cardiovascular examination revealed a regular rate and rhythm with clear S1 and S2 and no murmurs or rubs. The lungs were clear to auscultation and the lower extremities were warm without oedema bilaterally. A 12-lead electrocardiogram (ECG) revealed sinus tachycardia, normal axis, no ST-T change, and a normal QTc interval (Figure 1). The laboratory results showed elevated cardiac enzyme values, including a hs-Tn I level of 5883.4 ng/L (upper normal limit <15.6). The NT-proBNP (45 pg/mL; upper normal limit <450) and D-dimer (138 ng/mL; upper normal limit <500) levels were within normal limits. The InterTAK Diagnostic Score6 was 61 out of 100 with a TTS probability of 58.6%. The items checked were ‘female sex,’ ‘emotional stress,’ and ‘no ST-segment depression.’
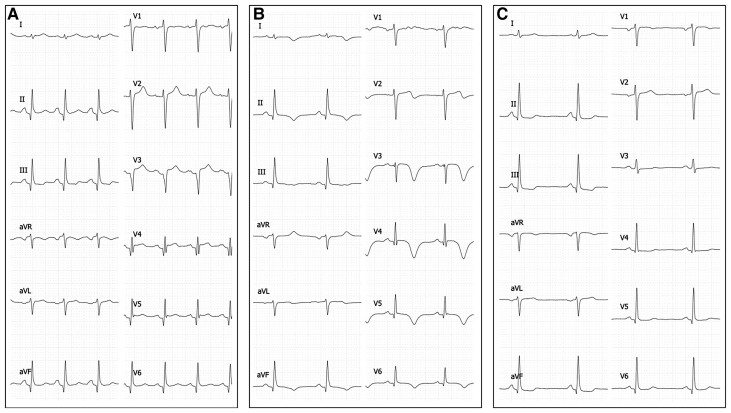
Figure 1.
Twelve-lead electrocardiogram performed upon admission (A) indicated sinus tachycardia, normal axis, and no ST-T change. (B) 24 h post-admission: T-wave inversions in lead V2–V6 with QTc prolongation. (C) At 3 months.
The patient was transferred to the cardiac catheterization laboratory due to refractory chest pain despite medical treatment with nitroglycerin and P2Y12 inhibition. Coronary angiography indicated Type 2A SCAD involving the middle part of the left anterior descending (LAD) artery with a diffuse smooth stenosis of 26 mm. The thrombolysis in myocardial infarction (TIMI) flow was Grade 3 (Figure 2 and Supplementary material online, Figure S1). Left ventriculography demonstrated a typical left ventricular apical ballooning and a hypercontractile base compatible with a TTS (Supplementary material online, Video 1). Given the typical appearance of SCAD, we did not use intracoronary imaging and opted for a conservative approach in accordance with the current guidelines.7
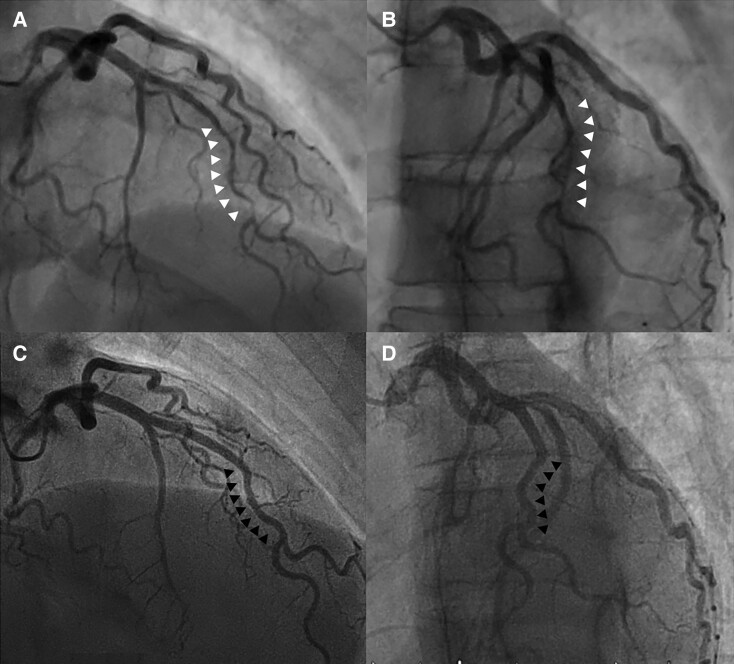
Figure 2.
(A and B) Coronary angiography in the right anterior oblique cranial view indicated Type 2A spontaneous coronary artery dissection based on the classification system published by Saw et al.9 involving the middle portion of the left anterior descending artery with a ‘stick insect’ appearance bordered by normal artery segment (white arrows) (C and D). Control angiography at 3M showed complete resolution of the previous pathological findings (black arrows). (A and C) Face cranial view. (B and D) Left anterior oblique cranial view.
The patient was admitted to the cardiovascular intensive care unit. Management included medical therapy with the administration of beta-blockers (bisoprolol 2.5 mg daily) and antiplatelet agents (aspirin 75 mg daily).
The transthoracic echocardiogram (TTE) revealed broad, non-systematized hypokinesia of the left ventricular apex with typical apical balloonization with hypercontractile basal segments and no dynamic intraventricular obstruction. Left ventricular systolic function was impaired, with an ejection fraction of 45%. No valvular abnormalities or pericardial effusion was observed (Supplementary material online, Video 2).
Cardiac magnetic resonance (CMR) imaging on Day 1 showed typical apical ballooning with transmural myocardial oedema in T2-weighted sequence and the absence of myocardial necrosis based on the late gadolinium enhancement images (Figure 3A–D and Supplementary material online, Figure S2). Additional ECG on Day 1 showed T-wave inversions in lead V2–V6 with QTc prolongation to 464 ms. The patient did not experience a recurrence of chest pain during hospitalization and was discharged on the fourth day after the procedure.
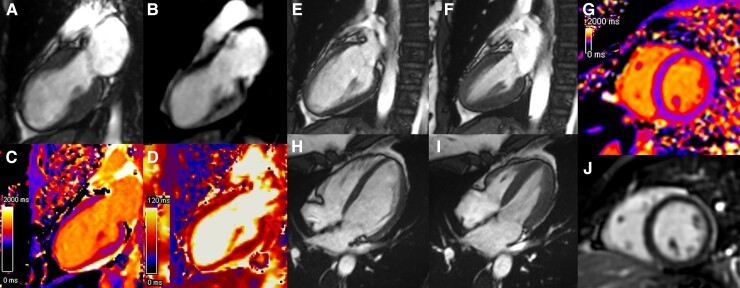
Figure 3.
(A) Cine cardiac magnetic resonance two-chamber showed typical apical ballooning in end-systole. (B) Late gadolinium enhancement excluded myocardial necrosis in the dyskinetic anterior wall. (C) T1 mapping revealed a focal elevation in the T1 values of the distal anterior, apical, and distal inferior walls (T1 1300 ms vs. 1000 ms in other myocardial regions). (D) T2 mapping showed an elevation in the T2 values of the apical wall (60 ms vs. 50 ms in the other territories) indicating myocardial oedema. At follow-up, Cine cardiac magnetic resonance imaging in the vertical long axis (E and F) and four-chamber view (H and I) showed a normalization of global left ventricular systolic function. (G) T1 cartography in the short axis showed a homogeneous aspect with normal T1 at 1030 ms. (J) Late gadolinium enhancement-cardiac magnetic resonance imaging could not detect significant late gadolinium enhancement.
The control coronary angiography performed 3 months after the acute event showed a complete angiographic resolution (Figure 2C and D and Supplementary material online, Figure S3). The TTE revealed complete normalization of the left ventricular function and a global longitudinal strain score (−22.4%). The CMR revealed a complete resolution of the pathological features described above (Figure 3E–J and Supplementary material online, Figure S4). The patient remained asymptomatic and had no recurrence of chest pain.
Discussion
As described in the revised Mayo Clinic Diagnostic Criteria (2008), the presence of coronary artery disease may be concomitant with TTS.3 Chou et al.8 found that in up to 8% of patients with diagnosed or suspected TTS, SCAD is misdiagnosed. SCAD remains an underdiagnosed condition that requires a detailed assessment of angiographic signs. The treatment and management of SCAD remains a challenge for clinicians as the therapeutic approach to this disease varies greatly depending on the diagnosis. Since the classification of Saw et al.9 published in 2017, new angiographic signs have been described5 as for the onset and/or termination of the angiographic ambiguity on a side branch, narrowing of the lumen diameter mimicking a ‘radish’ aspect, or a ‘stick insect’ as in this case.
Although TTS and SCAD are commonly observed in patients who share certain characteristics (women, without atheromatous terrain, stress-related factors), it is difficult to establish a pathophysiological link. TTS is caused by myocardial sideration after catecholamine release, which explains cases complicating endocrinopathies, such as pheochromocytoma. SCAD is known to be caused by mechanical stress on constitutional arterial fragility, hence the association with fibromuscular dysplasia found in almost 50% of cases.2 This mechanical stress can be promoted not only by exertion but also by emotion, leading to the release of catecholamines, affecting the inotropism and blood pressure levels, as encountered in TTS. Hyperkinesis in some parts of the myocardium and apical ballooning could also contribute to the mechanical stress of epicardial coronaries as hypothesized by Madias et al.10 Conversely, during SCAD, the sudden onset of pain in a young patient with no risk factors may be complicated by emotional shock, a panic attack similar to that described as a risk factor for TTS. Finally, a third hypothesis cannot be ruled out, which would assume that a catecholaminergic discharge could simultaneously or almost simultaneously lead to both stress cardiomyopathy and SCAD.
In this case, it was difficult to determine the risk factors of the patient’s condition. In this study, the evidence gathered is insufficient. The kinetics of biology showing elevated and decreased levels of troponin upon admission, while NT-proBNP increased only secondarily, might suggest that SCAD was the initial component complicated by TTS.
Conclusions
This observation confirms the non-random association of two rare entities of myocardial infarction with no obstructive coronary arteries. Once the acute phase is over, the evolution of both is usually spontaneously favourable. While TTS can be easily diagnosed via non-invasive imaging (CMR, TTE), the diagnosis of SCAD is more difficult. The findings of this study suggest a concomitant presentation between SCAD and TTS and the important role of diagnostic angiography in diagnosing these conditions. If needed, endocoronary imaging can also be used. Although the treatment approach to SCAD is usually conservative,2,11 severe forms of this disease require early diagnosis and appropriate treatment.
Lead author biography
Simon Fitouchi is a cardiologist at the University Hospital of Strasbourg (France). He graduated from the University of Strasbourg in Cardiovascular Medicine in 2021 and is currently training in interventional rhythmology.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case report including images and associated text has been obtained from the patient in accordance with the COPE guidelines.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
Contributor Information
Simon Fitouchi, Division of Cardiovascular Medicine, Cardiovascular Institute, Strasbourg, France; Division of Cardiovascular Medicine, University Hospital of Strasbourg, Strasbourg, France.
Paola Di Marco, Division of Cardiovascular Medicine, Cardiovascular Institute, Strasbourg, France.
Pascal Motreff, Department of Cardiology, Gabriel Montpied Hospital, Clermont-Ferrand University Hospital, Clermont-Ferrand, France; Clermont Université, Université d’Auvergne, Cardio Vascular Interventional Therapy and Imaging (CaVITI), Image Science for Interventional Techniques (ISIT), Clermont-Ferrand, France.
Nicolas Lhoest, Division of Cardiovascular Medicine, Cardiovascular Institute, Strasbourg, France.
References
- 1. Gori T, Anadol R, Jung F. Tako-Tsubo syndrome, spontaneous coronary dissection and microvascular disease: sex-differences. Clin Hemorheol Microcirc 2019;70:375–379. [DOI] [PubMed] [Google Scholar]
- 2. Combaret N, Gerbaud E, Dérimay F, Souteyrand G, Cassagnes L, Bouajila S, Berrandou T, Rangé G, Meneveau N, Harbaoui B, Lattuca B, Bouatia-Naji N, Motreff P. National French registry of spontaneous coronary artery dissections: prevalence of fibromuscular dysplasia and genetic analyses. EuroIntervention 2021;17:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghadri J-R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y.-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International expert consensus document on Takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tweet MS, Gulati R, Hayes SN. Spontaneous coronary artery dissection. Curr Cardiol Rep 2016;18:60. [DOI] [PubMed] [Google Scholar]
- 5. Motreff P, Malcles G, Combaret N, Barber-Chamoux N, Bouajila S, Pereira B, Amonchot A, Citron B, Lusson J-R, Eschalier R, Souteyrand G. How and when to suspect spontaneous coronary artery dissection: novel insights from a single-centre series on prevalence and angiographic appearance. EuroIntervention 2017;12:e2236–e2243. [DOI] [PubMed] [Google Scholar]
- 6. Ghadri JR, Cammann VL, Jurisic S, Seifert B, Napp LC, Diekmann J, Bataiosu DR, D’Ascenzo F, Ding KJ, Sarcon A, Kazemian E, Birri T, Ruschitzka F, Lüscher TF, Templin C. A novel clinical score (InterTAK Diagnostic Score) to differentiate Takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail 2017;19:1036–1042. [DOI] [PubMed] [Google Scholar]
- 7. Tweet MS, Eleid MF, Best PJM, Lennon RJ, Lerman A, Rihal CS, Holmes DR, Hayes SN, Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–786. [DOI] [PubMed] [Google Scholar]
- 8. Chou AY, Sedlak T, Aymong E, Sheth T, Starovoytov A, Humphries KH, Mancini GBJ, Saw J. Spontaneous coronary artery dissection misdiagnosed as Takotsubo cardiomyopathy: a case series. Canadian J Cardiol 2015;31:1073.e5–1073.e8. [DOI] [PubMed] [Google Scholar]
- 9. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection. J Am Coll Cardiol 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 10. Madias JE. A possible amphidromic relation between spontaneous coronary artery dissection and Takotsubo syndrome. Am J Cardiol 2017;120:e69. [DOI] [PubMed] [Google Scholar]
- 11. Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, Joerg L, Rickli H. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv 2017;89:59–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.