Abstract
Background
Awake surgery with intraoperative electrical mapping emerged as a gold-standard approach in newly diagnosed diffuse low-grade glioma (DLGG) to optimize the extent of resection (EOR) while sparing critical brain structures. However, no study has assessed to what extent cognitive recovery occurs following awake mapping-guided neurosurgery in a large, longitudinal, and homogeneous series of DLGG.
Methods
A longitudinal study on the cognitive status of 157 DLGG patients was performed. Neuropsychological assessments were done before and three months after awake mapping-based surgery. Z-scores and variations of Z-scores were computed to determine the number of patients with cognitive deficit(s) or decline. Clinical, surgical, and histopathological variables were studied to investigate factors contributing to neurocognitive outcomes.
Results
Eighty-seven patients (55.4%) had preoperative cognitive impairments. Statistical analysis between the preoperative (baseline) and postoperative assessments demonstrated a significant difference in three domains (Executive, Psychomotor Speed and Attention, Verbal Episodic Memory). Eighty-six percent of patients exhibited no postoperative cognitive decline, and among them, 10% exhibited cognitive improvement. The mean EOR was 92.3%±7.8%. The EOR, postoperative volume, and tumor lateralization had a significant association with cognitive decline. No patients demonstrated permanent postoperative neurologic deficits, but 5.8% did not resume their preoperative professional activities. The 5-year survival rate was 82.2%.
Conclusions
This is the largest series ever reported with systematic longitudinal neuropsychological assessment. 86% of patients demonstrated no cognitive decline despite large resections and only 5.8% did not return to work. This work supports the practice of awake surgery with cognitive mapping as safe and effective in DLGG patients.
Keywords: awake surgery, cognitive assessment, diffuse low-grade glioma, longitudinal assessment
Key Points.
Largest series of awake DLGG patients undergoing awake mapping surgery with serial, longitudinal cognitive evaluation.
Eighty-six percent (86%) of patients showed no cognitive decline despite mean EOR of 92.3% ± 7.8%.
Awake surgery with cognitive mapping is a safe and effective approach in DLGG.
Importance of the Study.
This study is the largest series to report on the systematic, longitudinal preoperative and postoperative (3 months) neuropsychological assessments performed of a cohort of 157 homogeneous, consecutive DLGG patients that underwent awake mapping resections. Patients in this study had excellent neurological results, with no permanent neurological deficits induced by surgery, and favorable neuropsychological outcomes, with 86% of patients showing no cognitive decline. Among these patients without cognitive decline,10 % of patients demonstrated significant cognitive improvement, meanwhile, only 5.8% of patients did not resume their professional activities. Favorable oncological outcomes were obtained with a mean EOR of 92.3 ± 7.8% and a 5-year survival rate of 82.2%. EOR was optimized while sparing critical neural structures by awake mapping resection of DLGG. Thus, this work effectively addresses the “onco-functional balance” challenge—that is, maximizing extent of tumor resection while preserving quality of life in DLGG patients.
Supratentorial diffuse low-grade gliomas (DLGG, ie WHO grade II gliomas), which account for about 15% of all gliomas, are a heterogeneous group of rare primitive brain neoplasms1 that arise from dysfunctional glial precursor cells.2 Their natural history demonstrates continuous infiltration of white matter pathways3 and a high propensity to transform into high-grade gliomas, thus conditioning patients’ neurological status and life expectancy A number of spontaneous and therapeutic prognostic factors impacting the natural history DLGG have been identified over the last two decades, including clinical,4 radiological5,6 and, histomolecular profiles.7 For example, pseudo-randomized studies demonstrated that an approach with early surgical resection compared to watchful waiting can significantly increase life expectancy.8 Furthermore, the volume of residual tumor following surgery has been shown to be as a major, independent predictor of poor prognosis.6,9 Furthermore, DLGGs are frequently located within “eloquent” areas, which provides an additional challenged to neuro-oncologist to find the optimal onco-functional balance, that is, maximizing extent of tumor resection while preserving cognitive abilities and quality of life (QOL).10
QOL is multifaceted and is made up of factors such as physical, emotional, and subjective wellbeing. Moreover, all these factors undoubtedly are related, and can have an impact on cognitive status.11 DLGG are most commonly found in young adults following onset of seizures but who otherwise enjoy an active, normal life. As such, preserving the QOL of an otherwise healthy and active patient is of utmost importance. Awake monitoring with intraoperative electrical mapping has emerged as the gold-standard approach in newly diagnosed DLGG as a means to maintain the QOL by maximizing the extent of resection (EOR) while sparing critical neural structures.12,13 In addition, multimodal techniques for cognitive mapping have been recently implemented. These techniques help map and preserve higher-order cognitive functions, in addition to their more conventional use in mapping motor and language functions.14 A number of studies performing awake-mapping surgeries have shown a drastic improvement in functional outcomes, with approximately 3% of surgery-associated permanent neurological deficits.15
Neurocognitive function indirectly reflects health-related QOL, social and professional abilities and has become an important outcome measure in neuro-oncology.16 Patients with DLGG may suffer from cognitive decline as a result of tumor infiltration per se (even in the earlier stages of the disease)17 as well as from implementation of oncologic therapies, including surgery,18 chemotherapy, and radiation therapy.19
However, no study has assessed the extent of cognitive recovery following awake mapping-guided neurosurgery in a large, longitudinal, and consecutive series of homogeneous DLGG patients. It is crucial to assess the ability of intraoperative cognitive monitoring to efficiently preserve the set of functions mapped. Moreover, as all functions cannot be intraoperatively mapped for clinical reasons (limited amount of time of the awake period, the patient’s tiredness which renders monitoring unreliable), it is necessary for the clinical practice to determine the degree of preservation of other unmapped functions. Here, we analyzed a cohort of patients that underwent an awake mapping-based resection and a comprehensive longitudinal neurocognitive assessment (ie. before and three months after awake surgical resection).
Materials and Methods
Participants
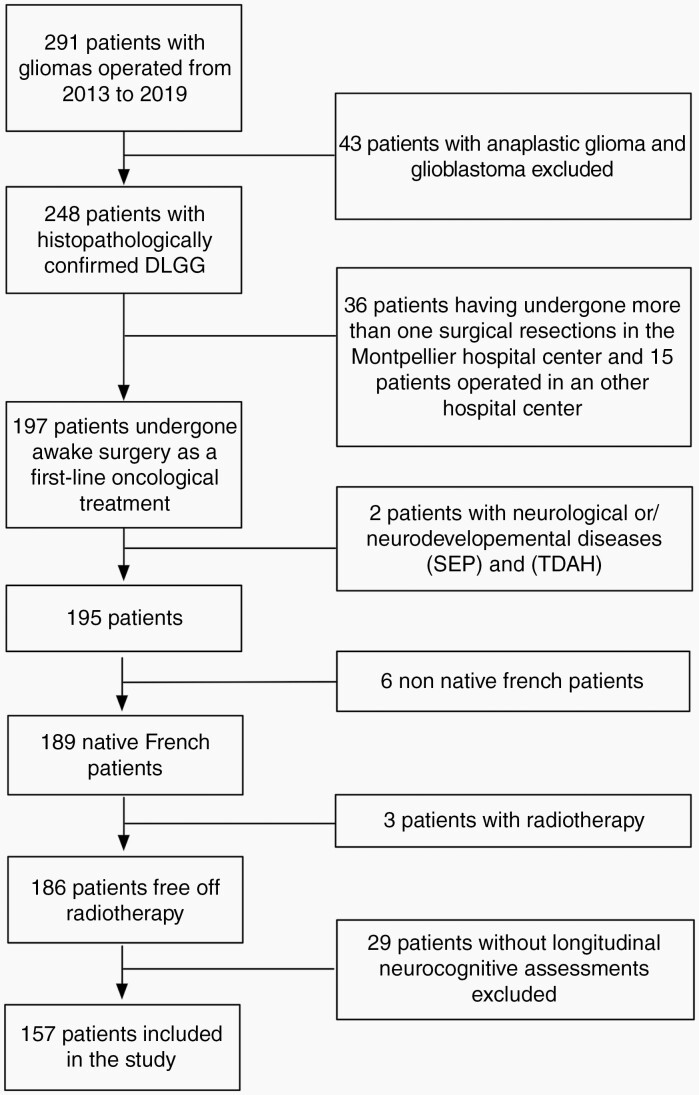
We analyzed data from 157 patients having undergone an awake mapping resection for DLGG at the Montpellier University Medical Center’s Department of Neurosurgery (Montpellier, France) over a period of seven years (2013–2019). Patients’ medical records were carefully screened by chart review (Figure 1). The following exclusion criteria was used: high-grade gliomas (based on histopathological reports), prior surgical resection, prior brain radiotherapy (which may influence cognitive performances), other neurological diseases (eg. stroke, traumatic brain injury, and multiple sclerosis), or neurodevelopmental diseases, non-native French patients, and patients for whom cognitive data was insufficiently documented. Patients provided informed consent for the surgical procedure. Approval for the study was granted by the Institutional Review Board of the ethical comity of research from the National French College of Neurosurgery (IRB N°00011687-2021/18).
Fig. 1.
Patient inclusion.
Measures
Neurocognitive assessment.
—Patients underwent a neuropsychological assessment at two times points: the day before surgery and three (3) months after surgery. The neurocognitive battery used consisted of a set of behavioral tasks that probe various aspects of cognition and language. The neuropsychological assessment was performed by a trained neuropsychologist (G.H. or A.-L.L.) and a dedicated speech therapist (S.M.G) for left-hemispheric tumors. The following cognitive domains were evaluated: (1) Executive Functions, assessed with the part B and the part B minus A of the Trail Making Test (TMT), the STROOP test (subtest Interference and Interference minus Naming subtest), forward and backward digit span from the Wechsler Adult Intelligent Scale (WAIS-IV) and phonological and semantic verbal fluencies; (2) Social Cognition evaluated with the Read the Mind in the Eyes test (RMET); (3) Psychomotor Speed and Attention assessed with the subtest “code” from the WAIS-IV, the subtests “color naming” and “reading” from the STROOP task, and the part A of the TMT; (4) Visuospatial Function evaluated with the copy of the Rey-Osterrieth complex figure (ROCF) or the Taylor complex figure (TCF), and the bell test; (5) language and semantic abilities, evaluated with the DO80 naming task, the PPTT task, reading of regular words, irregular words, pseudo-words and text; (6) verbal episodic memory, assessed with the “Rappel libre et rappel indicé à 16 items (RL RI-16)”, and (7) Nonverbal Memory, examined with the immediate and delayed recall of the Rey-Osterrieth complex figure or the Taylor complex figure. Note that, when available, alternate forms were used (version a and b of the RLRI-16, ROCF was alternated with the TCF). A detailed description of the neuropsychological tests is provided in Supplementary Table 1 and their rationales are provided in Supplementary references.
Raw scores for patients were Z-transformed (according to age, gender, and educational level) on the basis of published French normative data. Patients with a Z-score <1.65 were considered to have impaired performance, whereas those with Z-scores ranging from –1 to –1.65 were considered to have poor function. In addition, we calculated the difference between postsurgical Z-scores and presurgical Z-scores to derive a ΔZ-score. ΔZ-score provides a means to determine if a patient’s performance on a given neurocognitive test is stable (ΔZ-score = [–1; 1]), decreased (ΔZ-score <–1) or improved (ΔZ-score >1). A similar method was previously reported.17
Intraoperative monitoring.
—All patients were operated in an “awake” condition to enable intraoperative functional mapping using direct electrostimulation. Technical details of this approach have been extensively detailed in previous publications.12,20–22 Brain regions in which stimulation provoked three nonconsecutive identical functional perturbations were recorded as responsive23 and were labeled with sterile numbered tags on both the cortical surface and at the level of the white matter tracts.
During awake mapping surgery, we systematically assessed sensorimotor processes24–26 and language production (including speech articulation and lexical retrieval).27–29 Moreover, additional validated neurocognitive tasks were administrated according to the clinical and radiological characteristics (tumor localization, handedness) the patient’s presurgical language and neuropsychological assessment and patient’s expectations (eg. based on his/her job and hobbies and lifestyle). These additional tasks included: line bisection task to evaluate visuospatial cognition,30 nonverbal semantic association task,28 RME task to evaluate social cognition,22,31 visual tasks32 to evaluate visual fields (and avoid hemianopia) and reading aloud33 to evaluate reading abilities. A complete description of the surgical procedure is provided in the supplementary methods.
Clinical, radiological, and histomolecular measures.
—We calculated preoperative and 3-month postoperative tumor volumes (assessed by the same author (HD)) to investigate the association between the extent of cognitive recovery and the radiological data. Tumor volumes were calculated by measuring the volume of pre- and postoperative hyper FLAIR signal. Volumes were measured manually using a dedicated software (Myrian, Intrasense, Montpellier, France). EOR was characterized as “partial” (residual volume greater than ten (10) cm3), “subtotal” (residual volume less than ten (10) cm3) or “complete” (no residual tumor volume) based on the postoperative MRI.6 The histomolecular profile (based on the WHO 2016 classification2), IDH status, tumor location, history of previous oncological treatments (such as including type of chemotherapy, for example, Temozolomide or Procarbazine, Lomustine and Vincristine combination ie. PCV), use of preoperative anti-epileptic drugs (AED) (with or without), and occurrence of pre- and/or postoperative seizures were collected. All patients systematically received AEDs postoperatively up to three months after surgery. Standard clinical examinations were performed postoperatively at 3 months by the same surgeon (HD). Any of the following was considered a neurological deficit: aphasia, loss of muscle strength (grade 1 to 3 on the Medical Research Council Scale), or hemianopia. Clinical, radiological, and histomolecular data are provided in Table 1.
Table 1 .
Clinical, Radiological, and Histopathological Characteristics of the Patients’ Sample
| Total patients | |
|---|---|
| Mean (SD) or % | |
| Demographics | |
| Age | 39.08 (10.49) |
| Mean education in years | 14.45 (3.08) |
| Gender H/F, n (%) | 85 (54.14%)/72 (45.86.0%) |
| Right-handed, n (%) | 130 (83.0%) |
| Left-handed, n (%) | 16 (10.0%) |
| Ambidextrous, n (%) | 11 (7.0%) |
| Tumor location | |
| Left, n (%) | 78 (49.68%) |
| Right, n (%) | 79 (50.32%) |
| Frontal, n (%) | 94 (59.99%) |
| FTI, n (%) | 34 (21.66%) |
| F, n (%) | 25 (15.92%) |
| SMA, n (%) | 9 (5.73%) |
| Premotor, n (%) | 13 (8.28%) |
| Fronto-insular (Predom. F). n (%) | 13 (8.28%) |
| Temporal | 36 (22.92%) |
| Temporal, n (%) | 17 (10.83%) |
| Temporo-insular (Predom, T), n (%) | 12 (7.64%) |
| Temporal (posterior), n (%) | 4 (2.58%) |
| Temporo-basal, n (%) | 1 (0.64%) |
| JTO, n (%) | 2 (1.27%) |
| Insular | 10 (6.43%) |
| Insular, n (%) | 9 (5.73%) |
| Insular-frontal (Predom. Ins), n (%) | 1 (0.64%) |
| Parietal | 17 (10.8%) |
| Parietal, n (%) | 14 (8.92%) |
| Parieto-insular (Predom,P), n (%) | 2 (1.27%) |
| Parieto-temporal (Predom,P, n (%) | 1 (0.64) |
| Tumor radiological characteristics | |
| Presurgical volume, cm3 | 53.18 (46.73) |
| Postsurgical volume, cm3 | 5.54 (7.88) |
| Extent of Resection (%) | 92.28% |
| Total resection, n (%) | 44 (28.02%) |
| Subtotal resection, n (%) | 80 (50.96%) |
| Partial resection, n (%) | 33 (21.02%) |
| Tumor Histopathological characteristics | |
| Astrocytoma, IDH mutated, n (%) | 85 (54%) |
| Astrocytoma, IDH wild type, n (%) | 25 (16%) |
| Oligodendroglioma IDH mutated 1p19qcodeleted, n (%) | 47 (30%) |
| Oncological treatment | |
| Previous chemotherapy, n (%) | 11 (7%) |
Statistical Analyses
We used either a parametric t-test or a nonparametric Wilcoxon signed-rank test to compare paired preoperative vs. postoperative Z-scores for each cognitive test. The normality of the distribution was determined with the Kolmogorov-Smirnov test.
To probe the relationship between clinical and histopathological variables and neurocognitive recovery (quantified by use of the ΔZ-score), we computed nonparametric correlations (Spearman’s Rho) by means of a subgroup analysis using Statistica Software (version 6.0) (http://www.statsoft.com/). Since language assessment was only performed on left-sided tumors, statistical analyses using language subtests were only performed on these patients. To decrease the risk of type-I error, we performed a Bonferroni correction.34 This correction led to a critical alpha level of 0.00156 (ie. the α-level divided by the number of predictors, referring here to the number of neurocognitive tests: α/32) and only significant results after the Bonferroni correction are discussed.
We computed the number of patients that displayed normal performance (Z-score > –1), impaired performance (Z-scores < 1.65), or poor function [–1.65; –1] to identify the most frequent neuropsychological deficits. Likewise, to identify patients with the most impaired cognitive performance, we computed the frequency of patients with a stable, decreased, or improved ΔZ-score. Frequency calculations were performed individually for each cognitive task.
It is important to note that multivariate analyses were not performed in this study since the key assumptions underlying parametric statistical models were not fulfilled.35
Results
Participants
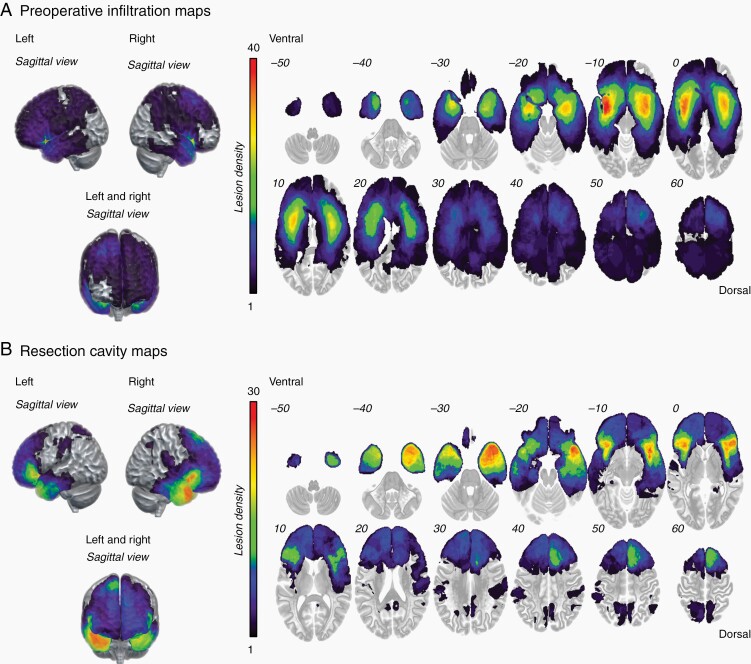
The patient population consisted of 72 females and 85 males (with a mean ± standard deviation age of 39.1 ± 10.5 years; range: 18–67). Seventy-eight (78) patients had left-sided and 79 patients had a right-sided DLGG. The most infiltrated region was the fronto-temporal-insular region (21.7% of the patients) (Figure 2A). The maximum overlap of the resection cavity maps occurred in the right and the left fronto-temporal-insular region (n = 30) (Figure 2B). Note that eleven patients (7%) were administered chemotherapy (Temozolomide in 10 patients, and PCV in 1 patient) before surgical resection.
Fig. 2.
Glioma spatial distribution. (A) Preoperative tumor infiltration maps, and (B) resection cavity maps.
One-hundred seventeen (117) patients (74.5%) had seizures preoperatively and 22 (14.1%) postoperatively. Further, of the 22 patients with postoperative seizures, 20 already had preoperative seizures and only 2 patients developed seizures for the first time postoperatively. Additional information regarding AED usage is provided in the Supplementary Table 2. No patients had a postoperative neurological deficit (eg. motor deficit, aphasia, hemianopia) based on a standard clinical examination at three months.
In terms of return to professional activity, 139 patients were employed at the moment of the surgery, the others were unemployed, retired or stay at home. 115 patients (82.7%) resumed their preoperative professional activities. The reasons why the other patients did not resume their professional life were mainly: legal issues related to seizures or to the administration of AED (n = 9, 6.5%) or introduction of chemotherapy (n = 7; 5.0%). Only 8 patients (5.8%) did not return to their professional life at one year after the surgery.
Surgical Results
The average presurgical tumor volume was 53.2 ± 46.7 cm3 (range 1.3–230.0 cm3) and the mean postoperative residual tumor volume was 5.5 ± 7.9 cm3 (range 0.0–50.0 cm3). The mean EOR was 92.3 ± 7.8% (range 55.0–100%) with 44 (28%) total resections, 80 (51%) subtotal resections and 33 (21%) partial resections. Radiological characteristics are provided in Table 1 and additional information is provided in the Supplementary Materials.
Clinical and Oncological Follow-up
Five patients (3.2%) received oncological treatment immediately after the surgery (two patients underwent chemotherapy with temozolomide and three with PCV). The three-year and five-year survival rates were 94.8% and 82.2%, respectively, with 144 patients still alive at the end of follow-up. Kaplan-Meier survival analyses are provided in Supplementary Figure 2.
Preoperative Individual Level of Cognitive Functioning
Prior to surgery, 87 patients (55.4 %) demonstrated cognitive impairments (ie. at least one Z-score inferior to –1.65 in at least one cognitive test) and 40 patients (25.5%) demonstrated weak performance (ie. Z-scores ranging from –1.65 to –1). Cognitive performance was within the normal range in all tests in 30 patients (19.1%). Table 2 shows the deficit (Z < –1.65), poor function (Z < –1), and normal function rates on a task-by-task basis. The highest deficit rate occurred in (1) Language (PPTT); (2) Verbal Episodic Memory (FR 1,2,3 and DFR); (3) Executive Functions (phonological and categorical fluency); (4) Psychomotor Speed and Attention, specifically the Naming and Reading part of the STROOP test (range: 10% to 22%).
Table 2 .
Frequency of Preoperative and Postoperative Deficits, poor Performances and Normal Performances for Each Neurocognitive Task
| TESTS | Frequency (preoperative) | Frequency (postoperative) | ||||
|---|---|---|---|---|---|---|
| Deficit | Poor functioning | Normal range | Deficit | Poor functioning | Normal range | |
| Z < –1.65 | Z [–1.65; –1] | –1 < Z | Z < –1.65 | Z [–1.65; –1] | –1 < Z | |
| Executives functions (Average of the domain) | 5.63 | 10.13 | 84.24 | 9.74 | 11.43 | 78.84 |
| TMTB | 4.52 | 3.87 | 91.61 | 4.46 | 9.55 | 85.99 |
| TMT B-A | 4.52 | 10.32 | 85.16 | 9.55 | 7.64 | 82.80 |
| STROOP interference | 5.84 | 7.14 | 87.01 | 11.18 | 9.87 | 78.95 |
| STROOP I-N | 5.84 | 7.79 | 86.36 | 11.84 | 7.89 | 80.26 |
| Forward span | 4.29 | 11.43 | 84.29 | 5.56 | 9.72 | 84.72 |
| Backward span | 0.71 | 6.43 | 92.86 | 3.47 | 9.72 | 86.81 |
| Phonological fluency | 10.32 | 15.48 | 74.19 | 15.58 | 18.18 | 66.23 |
| Categorical fluency | 8.97 | 18.59 | 72.44 | 16.23 | 18.83 | 64.94 |
| Social cognition | ||||||
| RMET | 8.79 | 10.99 | 80.22 | 12.09 | 7.69 | 73.63 |
| Psychomotor speed and attention (Average of the domain) | 6.07 | 8.05 | 85.89 | 10.25 | 8.18 | 81.57 |
| Codes | 4.96 | 7.09 | 87.94 | 5.84 | 8.03 | 86.13 |
| TMT A | 0.65 | 1.29 | 98.06 | 1.91 | 3.18 | 94.90 |
| STROOP naming | 8.33 | 10.90 | 80.77 | 13.64 | 9.74 | 76.62 |
| STROOP reading | 10.32 | 12.90 | 76.77 | 19.61 | 11.76 | 68.63 |
| Visuospatial functioning (Average of the domain) | 2.14 | 0.36 | 97.50 | 1.03 | 2.07 | 96.90 |
| R&T: Copy | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 | 100.00 |
| Bell test: omissions | 4.29 | 0.71 | 95.00 | 2.07 | 4.14 | 93.79 |
| Language (Average of the domain) | 6.60 | 7.81 | 85.59 | 10.80 | 7.85 | 81.35 |
| DO80 | 1.30 | 3.90 | 94.81 | 10.14 | 7.25 | 82.61 |
| PPTT | 22.08 | 6.49 | 71.43 | 15.94 | 4.35 | 79.71 |
| Reading text | 4.05 | 16.22 | 79.73 | 14.52 | 17.74 | 67.74 |
| Regular words | 1.35 | 12.16 | 86.49 | 3.23 | 6.45 | 90.32 |
| Irregular words | 1.35 | 1.35 | 97.30 | 3.23 | 1.61 | 95.16 |
| Pseudo-words | 9.46 | 6.76 | 83.78 | 17.74 | 9.68 | 72.58 |
| Verbal Episodic Memory (Average of the domain) | 9.18 | 12.56 | 78.25 | 13.15 | 9.90 | 76.96 |
| Encoding | 2.68 | 6.25 | 91.07 | 5.36 | 2.68 | 91.96 |
| Immediate free recall 1 (FR1) | 14.29 | 23.21 | 62.50 | 16.96 | 16.07 | 66.96 |
| Immediate free recall 2 (FR2) | 10.71 | 18.75 | 70.54 | 16.51 | 11.01 | 72.48 |
| Immediate free recall 3 (FR3) | 11.71 | 17.12 | 71.17 | 15.38 | 13.46 | 71.15 |
| Total recall 1 (TR1) | 7.21 | 9.91 | 82.88 | 16.36 | 1.82 | 81.82 |
| Total recall 2 (TR2) | 6.31 | 9.01 | 84.68 | 8.33 | 5.56 | 86.11 |
| Total recall 3 (TR3) | 9.01 | 9.01 | 81.98 | 7.69 | 6.73 | 85.58 |
| Delayed free recall (DFR) | 12.61 | 10.81 | 76.58 | 21.15 | 18.27 | 60.58 |
| Delayed total recall (DTR) | 8.11 | 9.01 | 82.88 | 10.58 | 13.46 | 75.96 |
| Nonverbal memory (Average of the domain) | 3.93 | 9.17 | 86.90 | 7.86 | 10.05 | 78.61 |
| R&T: immediate recall | 3.51 | 8.77 | 87.72 | 7.89 | 11.40 | 79.82 |
| R&T: delayed recall | 4.35 | 9.57 | 86.09 | 7.83 | 8.70 | 77.39 |
| Overall, all domain | 6.05 | 8.44 | 85.51 | 9.27 | 8.17 | 81.12 |
DO80: Dénomination orale d’images, PPTT: Pyramid and palm tree test, TMT: Trail making test, R&T Rey or Complex Figure, WAIS-IV: Wechsler adult intelligence scale IV, RMET: Read the mind in the eyes task, FR: Free Recall, TR: Total recall, DFR: Delayed Free Recall; DTR: Delayed Total Recall
The majority of patients (minimal of 75% of the patients) displayed a normal Z-score in other subtests including Executive Functions, Psychomotor Speed and Attention, Language, Verbal Episodic Memory (Encoding, TR 1,2,3 and DTR), Visuospatial Function, Nonverbal Memory and, Social Cognition.
Postoperative Level of Cognitive Function per Individual
The cognitive domains most affected when evaluated at the postsurgical neuropsychological assessment (Table 2), (range: 13.6% to 21.2%) were: (1) Verbal Episodic Memory (FR1,2,3, TR1 and DFR), (2) Psychomotor Speed and Attention with the Reading subpart of the STROOP, (3) Language (Text Reading and the subtest Nonwords reading), (4) Executive Functions (fluency tasks).
Seventy-five percent (75%) of patients did not show disorders in neurocognitive subtasks embedded in the Executive Functions, Psychomotor Speed and Attention (Codes, part A of the TMT), Language (DO80, Text reading, and regular irregular reading), Verbal Episodic Memory (Encoding and DTR), Visuospatial function, Nonverbal Memory and Social Cognition.
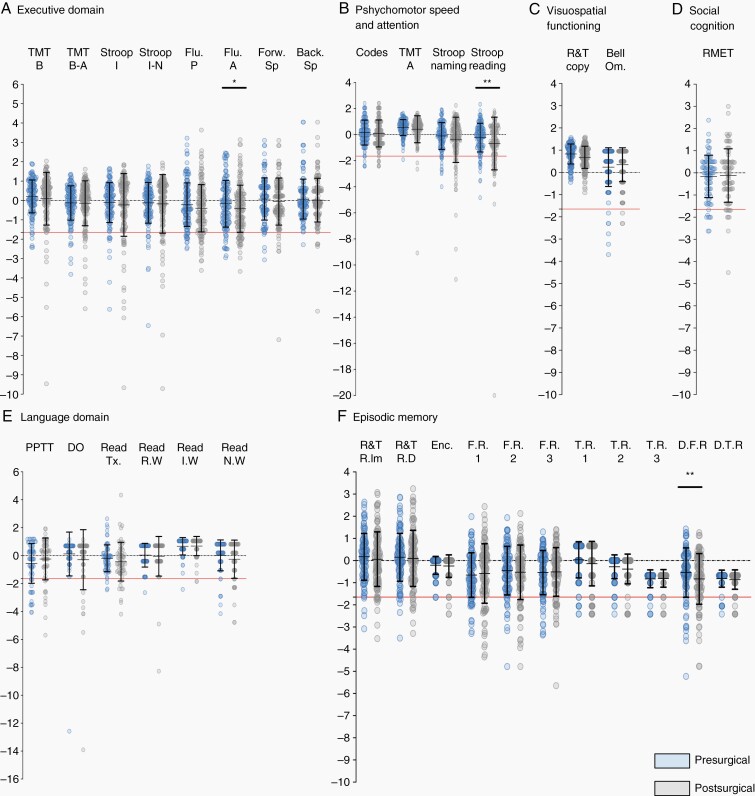
Comparison Between Preoperative (Baseline) vs. Postoperative Performance
We found a significant difference for the following tasks: categorical fluency (pre: –0.16 ±1.21 vs. post: –0.43 ±1.22; t(154) = 3.566, pcorrected = 0.016, mean difference = 0.266, 95% CI [–0.41; –0.119]), the reading part of the STROOP task (pre: –0.23 ±1.10 vs. post: –0.68 ± 2.02; Z(151) = 4.28, pcorrected = 0.0003), and the DFR of the episodic memory test (pre: –0.54 ± 1.12 vs. post: –0.82 ± 1.15; Z(81) = 4.062, pcorrected = 0.0016).
We found no significant differences with the other cognitive tasks. Comparisons are summarized in Figure 3 and in Supplementary Table 3.
Fig. 3.
Statistical comparison between presurgical versus postsurgical Z-score for each neurocognitive domain. (A) Executive Functions, (B) Psychomotor Speed and Attention, (C)Visuospatial Functioning, (D) Social Cognition (E) Language domain (F) Verbal Episodic Memory and Nonverbal Memory. All neuropsychological Z-scores are shown. The black lines denotes to one standard deviation and the red line to the pathological thresholds (Z-score < –1.65). *: P < .05; **: P < .01.
Hemispheric Dominance Analyses on Pre- and Postoperative Assessment
Subgroup comparisons showed a significant difference between tumor lateralization and postoperative Z-scores in Executive Functions and Verbal Episodic Memory. For the Executive domain, there was a significant difference in the TMT B (Left: –0.36 ± 1.6 vs. Right: 0.54 ±0.86; Z(155) = –4.80, pcorrected = 0.000064) and the TMTB-A (Left: –0.56 ± 1.28 vs. Right: 0.27 ±0.85; Z(155) = –5, pcorrected = 0.000032). In the Verbal Episodic Memory, there was a significant difference for postoperative FR1 (Left: –0.98 ±1.54 vs. Right: –0.12 ±0.87; Z(110) = –3.39, pcorrected = 0.02).
Correlations analyses were performed separately based on tumor lateralization and results are summarized in the Supplementary Figure 1. First, in patients with a left-sided-lesion, those with a high socio-educational level had significantly higher Z-scores in Executive Functions (TMT B, in the pre- and postsurgical phase), Language (PPTT preoperatively, Reading text pre- and postoperatively), and in the Psychomotor Speed and Attention (Code preoperatively). In contrast, a high socio-educational level was negatively correlated with Z-scores in the Visuospatial function (R&T Copy in the presurgical step). Our results showed that the preoperative volume was significantly correlated with a lower Z-score in the Verbal Episodic Memory (preoperative Z-scores in Encoding). However, for the Executive Functions (phonological fluency) and Verbal Episodic Memory (encoding, FR1, FR2, and TR1), the postoperative Z-scores were negatively correlated with postoperative volume, while the extent of resection were positively correlated.
In patients with a right-sided tumor, a high socio-educational level was positively correlated with a higher score in Executive Functions (categorical fluency in pre- and postsurgical) and Psychomotor Speed and Attention (Codes preoperatively) but negatively correlated with Visuospatial function (R&T Copy preoperatively). In addition, greater Age was positively correlated with Visuospatial function (R&T Copy both for pre- and postsurgical assessment). Correlation analyses are summarized in the Supplementary Figure 1.
Task-by-Task Evaluation of Cognitive Performance
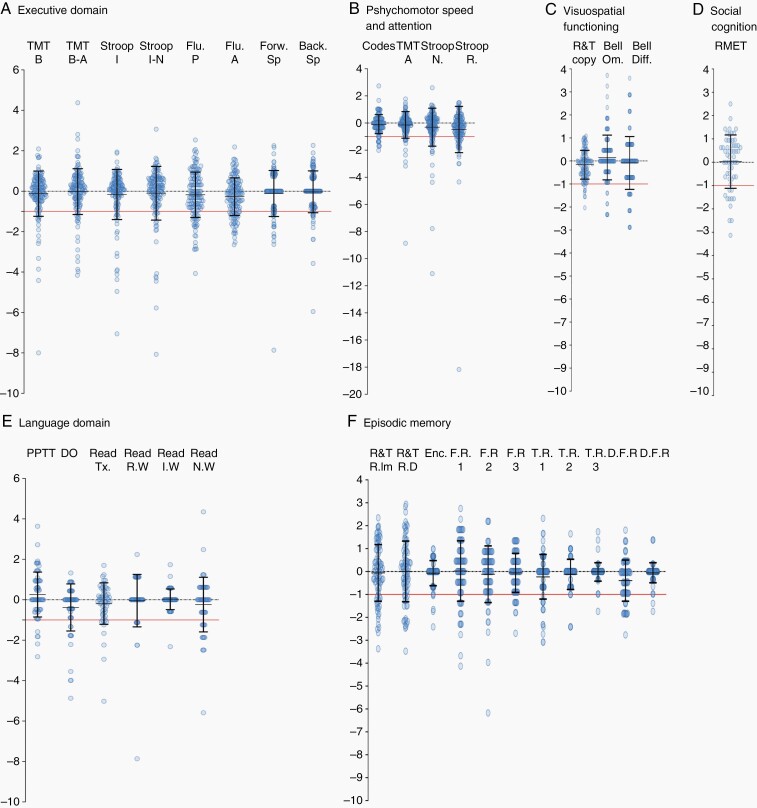
The task-by-task average of all patients with preserved cognitive performance was estimated to 86.2% (range: 75.8% to 98.8%). Thus, patients did not have a decreased performance in most of cognitive tasks at the three months postoperative assessment. Among them, the average of all patients with improved performance was 10.8% (range: 2.4% to 24.2%). The main domains in which patients demonstrated the most improvement was Nonverbal Memory (Immediate and Delayed Recall of the R&T figure), Verbal Episodic Memory (the first Free Recall), Language (PPTT), Executive Functions (phonological fluency), and Social Cognition. Detailed ΔZ-scores are shown in Table 3 and in Figure 4.
Table 3 .
Frequency of Patients Showing a Decrease, Stability or Increase of Their Cognitive Performances in Each Neurocognitive Task
| TESTS | N | Frequency | |||
|---|---|---|---|---|---|
| Decrease and no decrease | Stability and improvement | ||||
| ΔΖ<−1 | ΔΖ>−1 | ΔΖ[−1;1] | ΔΖ>1 | ||
| Executives functions (Average of the domain) | 14.71 | 85.30 | 76.15 | 9.14 | |
| TMTB | 155 | 10.97 | 89.03 | 84.52 | 4.52 |
| TMT B-A | 155 | 12.26 | 87.74 | 78.06 | 9.68 |
| STROOP interference | 149 | 12.75 | 87.25 | 82.55 | 4.70 |
| STROOP I-N | 149 | 12.75 | 87.25 | 77.85 | 9.40 |
| Forward span | 131 | 15.27 | 84.73 | 76.34 | 8.40 |
| Backward span | 131 | 10.69 | 89.31 | 76.34 | 12.98 |
| Phonological fluency | 153 | 19.61 | 80.39 | 65.36 | 15.03 |
| Categorical fluency | 154 | 23.38 | 76.62 | 68.18 | 8.44 |
| Social cognition | |||||
| RMET | 59 | 18.64 | 81.36 | 66.10 | 15.25 |
| Psychomotor speed and attention (Average of the domain) | 11.21 | 88.79 | 84.34 | 4.45 | |
| Codes | 123 | 8.13 | 91.87 | 87.80 | 4.07 |
| TMT A | 155 | 7.74 | 92.26 | 88.39 | 3.87 |
| STROOP naming | 153 | 13.07 | 86.93 | 82.35 | 4.58 |
| STROOP reading | 151 | 15.89 | 84.11 | 78.81 | 5.30 |
| Visuospatial functioning (Average of the domain) | 6.28 | 93.72 | 86.37 | 7.35 | |
| R&T: Copy | 125 | 6.40 | 93.60 | 91.20 | 2.40 |
| Bell test: omissions | 130 | 6.15 | 93.85 | 81.54 | 12.31 |
| Language (Average of the domain) | 12.91 | 87.09 | 77.15 | 9.94 | |
| DO80 | 69 | 15.94 | 84.06 | 82.61 | 1.45 |
| PPTT | 69 | 5.80 | 94.20 | 75.36 | 18.84 |
| Reading text | 61 | 16.39 | 83.61 | 78.69 | 4.92 |
| Regular words | 61 | 14.75 | 85.25 | 62.30 | 22.95 |
| Irregular words | 61 | 1.64 | 98.36 | 95.08 | 3.28 |
| Pseudo-words | 61 | 22.95 | 77.05 | 68.85 | 8.20 |
| Verbal episodic memory (Average of the domain) | 9.61 | 90.39 | 84.23 | 6.16 | |
| Encoding | 88 | 4.55 | 95.45 | 94.32 | 1.14 |
| Immediate free recall 1 (FR1) | 88 | 17.05 | 82.95 | 62.50 | 20.45 |
| Immediate free recall 2 (FR2) | 85 | 12.94 | 87.06 | 80.00 | 7.06 |
| Immediate free recall 3 (FR3) | 81 | 11.11 | 88.89 | 79.01 | 9.88 |
| Total recall 1 (TR1) | 86 | 16.28 | 83.72 | 79.07 | 4.65 |
| Total recall 2 (TR2) | 84 | 3.57 | 96.43 | 95.24 | 1.19 |
| Total recall 3 (TR3) | 81 | 1.23 | 98.77 | 96.30 | 2.47 |
| Delayed free recall (DFR) | 81 | 17.28 | 82.72 | 77.78 | 4.94 |
| Delayed total recall (DTR) | 81 | 2.47 | 97.53 | 93.83 | 3.70 |
| Nonverbal memory (Average of the domain) | 23.20 | 76.80 | 53.60 | 23.20 | |
| R&T: immediate recall | 91 | 24.18 | 75.82 | 51.65 | 24.18 |
| R&T: delayed recall | 90 | 22.22 | 77.78 | 55.56 | 22.22 |
| Overall, all domain | 13.80 | 86.21 | 75.42 | 10.80 |
DO80: Dénomination orale d’images, PPTT: Pyramid and palm tree test, TMT: Trail making test, R&T Rey or Complex Figure, WAIS-IV: Wechsler adult intelligence scale IV, RMET: Read the mind in the eyes task, FR: Free Recall, TR: Total recall, DFR: Delayed Free Recall; DTR: Delayed Total Recall.
Fig. 4.
ΔZ-scores in each neurocognitive domain. (A) Executive Functions, (B) Psychomotor Speed and Attention, (C)Visuospatial Functioning, (D) Social Cognition, (E) Language domain, (F) Verbal Episodic Memory and Nonverbal Memory. All ΔZ -scores are shown. The black denotes refer to one standard deviation and the red line to the pathological thresholds (ΔZ-scores < –1).
The average of all patients with decreased cognitive performances was 13.8% (range: 1.2% to 24.2%). Impaired performance was mostly noted in Nonverbal Memory (Immediate and delayed Recall of the R&T figure), Language (text and nonwords reading, naming task), Verbal Episodic Memory (FR1, TR1, and DFR), and Executive Functions (fluency tasks).
Influence of Sociodemographic, Clinical, Radiological and Histomolecular Factors on ΔZ-scores
We performed subgroup analysis to ascertain the extent to which clinical/histopathological variables had a significant impact on cognitive recovery (ie. ΔZ-scores). We found a significant difference only for forward digit span when considering the quality of resection (H(2,131) = 12.77, pcorrected = 0.03). We performed posthoc analyses with a Dunn’s test for multiple comparisons. Patients with a partial resection had a greater decrease in ΔZ-score compared to patients that underwent either a subtotal (Partial ΔZ: –0.855 ± 1.66 vs. SBT ΔZ: 0.064 ± 0.73; P < .01) or complete resection (Partial ΔZ: -0.855 ±1.66 vs. Complete ΔZ: 0.12 ±1.085; P < .01). Meanwhile, there was no significant difference in ΔZ-score between subtotal and complete resection (SBT ΔZ: 0.064 ± 0.73; vs. Complete ΔZ: 0.12 ± 1.085; P > .05). There were no significant differences when we used tumor location, seizure (use of preoperative AED, pre- and postoperative seizures), histomolecular parameters, or IDH status as predictors (Supplementary Tables 4-7). We found a significant difference for the Naming subtest of the STROOP task with respect to tumor lateralization, (LeftΔZ = –0.679 ± 1.84, RightΔZ = 0.054 ± 0.617; Z(153) = –3.35, pcorrected = 0.026).
Further, we performed correlation analyses using the Spearman’s Rho. In patients with a left-sided tumor, we found a significant negative correlation between ΔZ-scores and postoperative volume with the encoding part of Verbal Episodic Memory (ΔZ Encoding r50 = –0.47, pcorrected = 0.016). The EOR had a positive correlation with the same memory measure (ΔZ Encoding r50 = 0.474, pcorrected = 0.017). Correlation analyses are summarized in Supplementary Table 5 (right-sided tumors) and Supplementary Table 6 (left-sided tumors). No were no other significant correlations found in this study.
Discussion
The purpose of this work was to study the effects on neurocognition of awake mapping resection in a large cohort of DLGG patients that underwent comprehensive longitudinal neurocognitive assessment. Here we demonstrate that the use of awake cognitive monitoring for the resection of DLGG provides a high level of safety. First, no patients had a permanent neurological deficit. Second, from a neurocognitive standpoint, 86% of patients demonstrated stable cognitive performance, 10 % had improvement in their cognitive status, and 13% had a cognitive decline at the 3-month postoperative follow-up. Finally, 5.8% of patients did not return to their previous professional activities within the first year of surgery. The strengths of this study are the following: 1) this is the largest, homogeneous, and consecutive series of DLGG with systematic neuropsychological assessment to date; 2) only few patients received confounding adjuvant therapies (eg. chemotherapy); 3) and all patients were operated on with the same surgical technique.
Survival of patients with DLGG has drastically improved over the last two decades, as a result of improved surgical management and oncologic therapies. Recent surgical studies have established a survival benefit in patients with residual tumor volume (ie. residual FLAIR infiltration) <10–15 cm3. Therefore, maximal surgical resection is the gold-standard therapy in newly diagnosed DLGG patients.36 Furthermore, the balance between cytoreduction and neurological morbidity (previously reported to account for 3.4% of DLGG surgeries)15 has been considerably refined with the advent of functional-based surgery performed under awake conditions with direct electrostimulation.37Online cognitive monitoring allows the oncological surgeon to determine each individual patient’s functional boundaries both at the cortical and subcortical levels thus sparing critical neural circuits.12,14
Recent meta-analyses demonstrate cognitive changes in executive, attentional, verbal memory and, language domains following glioma surgery. These changes can occur as early as the immediate postoperative period with sustained long term decline.38,39 Nevertheless, current literature does not provide a comprehensive overview of the effects of surgery on neurocognition in DLGG patients. The 3 main reasons are: (i) available studies frequently include a mixture of heterogeneous glioma histologies (including significant subsets of high-grade gliomas, metastatic lesions, and meningiomas); (ii) neurocognitive outcomes are frequently measured at different time endpoints following surgery; and (iii) intraoperative awake mapping is used inconsistently in tumors invading “noneloquent areas”. Cognitive sequelae result from tumor progression and oncological treatment can have a significant impact on health-related QOL. Nevertheless, no study has investigated the effects of awake surgery on cognition in a large cohort of DLGG patients.
The prevalence of cognitive impairment prior to treatment varies widely (31 to 75%) in patients with a DLGG.38,39 However, baseline cognitive impairments can have a significant impact in selected populations of asymptomatic patients.17 Our findings suggest that 55.4 % of patients may suffer from different forms of cognitive decline prior to surgery. Executive, language attention, and verbal episodic memory were the most impaired cognitive domains in the preoperative setting, with 10% to 22% of patients showing impairment in neuropsychological tasks. These results support the view that extensive neuropsychological assessment should be introduced in routine neurosurgical practice from the moment of diagnosis. Therefore, our data highlight that cognitive decline induced by surgery (eg. forward digit span, subtest Naming from the STROOP and encoding), may be associated with a specific surgical condition and tumor characteristic (eg. EOR, tumor lateralization, and postoperative volume). Surprisingly, a greater EOR did not correlate with cognitive deficits but instead with improvement in certain tasks (eg. memory encoding). These results may be due to better control of mass effect and/or seizure control in the early postoperative setting. The challenge in addressing the “onco-functional balance” may be accomplished with an awake mapping surgery, which will optimize extent of surgical resection while sparing critical neural structures.
These results suggest that cognitive domains may be affected by surgery (eg. forward digit span, subtest Naming from the STROOP, and encoding). Dedicated rehabilitation programs in the early postoperative course may help minimize any potential negative impact surgery might have on these cognitive domains and optimize a patients ability to resume an active life.14 For example, the benefit of rehabilitation has been reported for attentional, verbal memory, and mental fatigue domains.40–42 Multiple rehabilitation strategies, based on longitudinal neuropsychological assessments, subjective complaints, patient characteristics (eg. socio-professional life, bilingualism) may enable development of compensatory strategies that could be implemented in everyday life and ultimately, in preserving QOL.
This study has limitations that should be mentioned. First, cognitive changes were assessed only 3 months after surgery, which is a short postoperative endpoint. Indeed, neurocognitive recovery may take up to one year after surgical resection.43 Therefore, the cognitive recovery observed here might have probably been even better if an extensive neurocognitive assessment had been performed one year after surgery. Second, neuropsychological subtests were selected according to tumor location and thus, specific cognitive tasks were not displayed for each patient (eg. lexical retrieval tasks in patients with a right DLGG). Third, most of our patients received AEDs before (n = 115, 73.2%) and, systematically after surgery, which may have substantially modified mood and cognitive performance.
Conclusion
To our knowledge, this is the largest series ever reported describing a homogeneous and consecutive cohort of DLGG patients that underwent awake mapping surgery as well as systematic longitudinal (pre- and postoperative) neuropsychological assessments. The present study supports the idea that awake surgery with cognitive mapping is a safe and effective therapeutic approach in newly diagnosed DLGG patients This claim is supported by the favorable oncological results (mean EOR, 92.3 ± 7.8%, 5-year survival rate 82.2%), excellent neurological outcomes (no permanent neurological deficits) and low negative cognitive impact at 3 months (range of 75.0% to 96.30% of the patients with stabile performance in 75% of cognitive tasks). Only 5.8% of patients did not resume their professional activities. However, 82.7% of patients returned to work at one year after awake surgery, which is greater than 52% return to work reported in previous research.44
Supplementary Material
Acknowledgments
The authors would like to acknowledge the patients for their participation in the present study. The authors are grateful to Dr Pablo Valdes for the English correction.
Contributor Information
Anne-Laure Lemaitre, Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Center, Montpellier, France; Institute of Functional Genomics, University of Montpellier, CNRS, INSERM, Montpellier, France.
Guillaume Herbet, Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Center, Montpellier, France; Institute of Functional Genomics, University of Montpellier, CNRS, INSERM, Montpellier, France.
Sam Ng, Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Center, Montpellier, France; Institute of Functional Genomics, University of Montpellier, CNRS, INSERM, Montpellier, France.
Sylvie Moritz-Gasser, Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Center, Montpellier, France; Institute of Functional Genomics, University of Montpellier, CNRS, INSERM, Montpellier, France.
Hugues Duffau, Department of Neurosurgery, Gui de Chauliac Hospital, Montpellier University Medical Center, Montpellier, France; Institute of Functional Genomics, University of Montpellier, CNRS, INSERM, Montpellier, France.
Funding
The authors report no funding.
Conflict of interest statement. The authors report no conflict of interest.
Authorship statement. H.D. was the chief investigator and had supervised and conceived the study concept and design. A.L.L., G.H., S.M.G., and H.D. collected data. ALL did the literature review and wrote the original draft of the manuscript. A.L.L., G.H., and S.N. had access to the study data and contributed to the analysis and interpretation of the data. A.L.L. made the figures and tables. All authors reviewed and approved the final manuscript.
Previous presentation. None.
References
- 1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncol. 2020;22(Supplement_1):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Pallud J, Fontaine D, Duffau H, et al. Natural history of incidental World Health Organization grade II gliomas. Ann Neurol. 2010;68(5):727–733. [DOI] [PubMed] [Google Scholar]
- 4. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group . Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 5. Pallud J, Blonski M, Mandonnet E, et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2013;15(5):595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capelle L, Fontaine D, Mandonnet E, et al. ; French Réseau d’Étude des Gliomes . Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 7. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 9. Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. [DOI] [PubMed] [Google Scholar]
- 10. Duffau H, Mandonnet E. The “onco-functional balance” in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien). 2013;155(6):951–957. [DOI] [PubMed] [Google Scholar]
- 11. Taphoorn MJ, Heimans JJ, Snoek FJ, et al. Assessment of quality of life in patients treated for low-grade glioma: a preliminary report. J Neurol Neurosurg Psychiatry. 1992;55(5):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4(8):476–486. [DOI] [PubMed] [Google Scholar]
- 13. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 14. Duffau H. New philosophy, clinical pearls, and methods for intraoperative cognition mapping and monitoring “à la carte” in brain tumor patients. Neurosurgery. 2021;88(5):919–930. [DOI] [PubMed] [Google Scholar]
- 15. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 16. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 17. Ng S, Herbet G, Lemaitre A-L, Cochereau J, Moritz-Gasser S, Duffau H. Neuropsychological assessments before and after awake surgery for incidental low-grade gliomas. J Neurosurg. 2020;1(aop):1–10. [DOI] [PubMed] [Google Scholar]
- 18. Klein M, Duffau H, De Witt Hamer PC. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108(2):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 20. Tate MC, Herbet G, Moritz-Gasser S, Tate JE, Duffau H. Probabilistic map of critical functional regions of the human cerebral cortex: Broca’s area revisited. Brain. 2014;137(Pt 10):2773–2782. [DOI] [PubMed] [Google Scholar]
- 21. Herbet G, Maheu M, Costi E, Lafargue G, Duffau H. Mapping neuroplastic potential in brain-damaged patients. Brain. 2016;139(Pt 3):829–844. [DOI] [PubMed] [Google Scholar]
- 22. Yordanova YN, Cochereau J, Duffau H, Herbet G. Combining resting state functional MRI with intraoperative cortical stimulation to map the mentalizing network. Neuroimage. 2019;186:628–636. [DOI] [PubMed] [Google Scholar]
- 23. Ojemann GA. Individual variability in cortical localization of language. J Neurosurg. 1979;50(2):164–169. [DOI] [PubMed] [Google Scholar]
- 24. Schucht P, Moritz-Gasser S, Herbet G, Raabe A, Duffau H. Subcortical electrostimulation to identify network subserving motor control. Hum Brain Mapp. 2013;34(11):3023–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rech F, Herbet G, Moritz-Gasser S, Duffau H. Disruption of bimanual movement by unilateral subcortical electrostimulation. Hum Brain Mapp. 2014;35(7):3439–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almairac F, Herbet G, Moritz-Gasser S, Duffau H. Parietal network underlying movement control: disturbances during subcortical electrostimulation. Neurosurg Rev. 2014;37(3):513–6; discussion 516. [DOI] [PubMed] [Google Scholar]
- 27. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4(8):476–486. [DOI] [PubMed] [Google Scholar]
- 28. Moritz-Gasser S, Herbet G, Duffau H. Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: a brain electrostimulation study. Neuropsychologia. 2013;51(10):1814–1822. [DOI] [PubMed] [Google Scholar]
- 29. Herbet G, Moritz-Gasser S, Duffau H. Electrical stimulation of the dorsolateral prefrontal cortex impairs semantic cognition. Neurology. 2018;90(12):e1077–e1084. [DOI] [PubMed] [Google Scholar]
- 30. Thiebaut de Schotten M, Urbanski M, Duffau H, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309(5744):2226–2228. [DOI] [PubMed] [Google Scholar]
- 31. Herbet G, Lafargue G, Moritz-Gasser S, Bonnetblanc F, Duffau H. Interfering with the neural activity of mirror-related frontal areas impairs mentalistic inferences. Brain Struct Funct. 2015;220(4):2159–2169. [DOI] [PubMed] [Google Scholar]
- 32. Gras-Combe G, Moritz-Gasser S, Herbet G, Duffau H. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012;117(3):466–473. [DOI] [PubMed] [Google Scholar]
- 33. Zemmoura I, Herbet G, Moritz-Gasser S, Duffau H. New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Hum Brain Mapp. 2015;36(6):2215–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Curtin F, Schulz P. Multiple correlations and Bonferroni’s correction. Biol Psychiatry. 1998;44(8):775–777. [DOI] [PubMed] [Google Scholar]
- 35. Hardin JW, Hardin JW, Hilbe JM, Hilbe J. Generalized Linear Models and Extensions. Texas: Stata press; 2007. [Google Scholar]
- 36. Sanai N, Berger MS. Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol. 2018;15(2):112–125. [DOI] [PubMed] [Google Scholar]
- 37. Duffau H, Lopes M, Arthuis F, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76(6):845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ng JCH, See AAQ, Ang TY, Tan LYR, Ang BT, King NKK. Effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post-operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2019;141(1):167–182. [DOI] [PubMed] [Google Scholar]
- 40. Zucchella C, Capone A, Codella V, et al. Cognitive rehabilitation for early post-surgery inpatients affected by primary brain tumor: a randomized, controlled trial. J Neurooncol. 2013;114(1):93–100. [DOI] [PubMed] [Google Scholar]
- 41. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 42. Weyer-Jamora C, Brie MS, Luks TL, Smith EM, Hervey-Jumper SL, Taylor JW. Postacute cognitive rehabilitation for adult brain tumor patients. Neurosurgery. 2021;89(6):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Satoer D, Visch-Brink E, Smits M, et al. Long-term evaluation of cognition after glioma surgery in eloquent areas. J Neurooncol. 2014;116(1):153–160. [DOI] [PubMed] [Google Scholar]
- 44. Rydén I, Carstam L, Gulati S, et al. Return to work following diagnosis of low-grade glioma: a nationwide matched cohort study. Neurology. 2020;95(7):e856–e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.