ABSTRACT
Background
The associations between specific types of fat and head and neck squamous cell carcinoma (HNSCC) recurrence and mortality rates have not yet been examined.
Objectives
The purpose of this study was to determine how intakes of various fat subtypes before cancer treatment are associated with recurrence and mortality in adults diagnosed with HNSCC.
Methods
This was a secondary analysis longitudinal cohort study of data collected from 476 newly diagnosed patients with HNSCC. Patients completed baseline FFQs and epidemiologic health surveys. Recurrence and mortality events were collected annually. Fat intakes examined included long-chain fatty acids (LCFAs), unsaturated fatty acids (FAs), PUFAs, ω-3 (n–3) PUFAs, ω-6 (n–6) PUFAs, MUFAs, animal fats, vegetable fats, saturated FAs, and trans fats. Associations between fat intake (categorized into tertiles) and time to event were tested using multivariable Cox proportional hazards models, adjusting for age, sex, smoking status, human papillomavirus status, tumor site, cancer stage, and total caloric intake. Intake of fats was compared with the lowest tertile.
Results
During the study period, there were 115 recurrent and 211 death events. High LCFA intake was associated with a reduced all-cause mortality risk (HR: 0.55; 95% CI: 0.34, 0.91; P-trend = 0.02). High unsaturated FA intake was associated with a reduced all-cause mortality risk (HR: 0.62; 95% CI: 0.40, 0.97; P-trend = 0.04) and HNSCC-specific mortality risk (HR: 0.51; 95% CI: 0.29, 0.90; P-trend = 0.02). High intakes of ω-3 PUFAs (HR: 0.56; 95% CI: 0.35, 0.91; P-trend = 0.02) and ω-6 PUFAs (HR: 0.57; 95% CI: 0.34, 0.94; P-trend = 0.02) were significantly associated with a reduced all-cause mortality risk. There were no significant associations between other fat types and recurrence or mortality risk.
Conclusions
In this prospective survival cohort of 476 newly diagnosed patients with HNSCC, our data suggest that HNSCC prognosis may vary depending on the fat types consumed before cancer treatment. Clinical intervention trials should test these associations.
Keywords: head and neck cancer, nutrition, fats, survival, recurrence
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a diverse disease that develops in the oral cavity, oropharynx, nasopharynx, hypopharynx, or larynx areas (1). HNSCC is ranked the seventh most common type of cancer worldwide, with more than 650,000 newly diagnosed cases and 330,000 deaths each year (2). Despite the advancements of therapeutic modalities, the HNSCC 5-y survival rate remains unacceptably low at 65% due to the delays in detecting the disease and the high rates of its recurrence (3). HNSCC develops mainly due to smoking, alcohol consumption (4–6), and human papillomavirus (HPV) (7). Smoking cessation and reduced alcohol intake are 2 modifiable lifestyle factors that can decrease the risk of recurrence and death after diagnosis. Still, identification of other modifiable factors to improve outcomes after diagnosis is needed. Dietary interventions hold promise for improving response to treatment and prognosis in patients with cancer; however, the role of diet remains understudied in this population.
Patients with HNSCC frequently experience malnutrition before and during the acute phase of treatment due to side effects of the disease itself and oncologic treatment that negatively affect their ability and desire to eat (8). This leaves patients highly susceptible to infections and complications during and after treatment or postoperatively (9). Therefore, better nutrition early in the disease trajectory may improve cancer prognosis. Our research team recently conducted a secondary analysis of a longitudinal cohort study examining how self-reported carbohydrate intake was associated with mortality and recurrence after HNSCC diagnosis (10). Findings from that study showed that participants who consumed a baseline diet high in carbohydrates, total sugar, and simple carbohydrates had a higher risk for disease mortality after HNSCC diagnosis. In contrast, participants who reported moderate total fat intake posttreatment had a reduced risk of recurrence. In addition, patients who reported consuming high intakes of fiber before initiation of treatment had a significantly lower risk of all-cause mortality compared with low intake (11).
Usually, a diet high in fats is known to have an adverse effect on various pathologies, including cancer (12). However, research on human subjects has not yet proved that an approach of lower intake of fats is effective in fighting cancer (13), and there may be differing impacts of various fat subtypes on cancer prognosis than when examining total fat in isocaloric diets (13). Preclinical reports examining cancer suggest that saturated fats and trans fats have harmful effects. At the same time, both MUFAs and PUFAs, especially from plant sources, may be protective (13). Human studies of diet and cancer have yielded inconsistent results. As such, the current study aims to determine how baseline intake (intakes after diagnosis and before the start of treatment) of various fat subtypes (as totals and as subsets) is associated with all-cause mortality, HNSCC-specific mortality, and recurrence in adults diagnosed with HNSCC. To our knowledge, this is the only study to date that has examined associations between dietary fat intake and HNSCC prognosis. The study hypothesis was that mortality and recurrence rates differ by the type of fats consumed before treatment. For example, that unsaturated fats and omega-3 (ω-3) PUFAs would be associated with improved outcomes, whereas omega-6 (ω-6) PUFAs, saturated fatty acids (SFAs), and trans unsaturated fats would be associated with poorer outcomes.
Methods
Study design and recruitment
This study included a secondary analysis of data collected from newly diagnosed patients with HNSCC enrolled in the prospective University of Michigan Head and Neck Specialized Program of Research Excellence survival cohort. Participants diagnosed with laryngeal, oropharyngeal, oral cavity, or hypopharyngeal cancer were recruited from University of Michigan clinics prior to treatment between November 2008 and October 2014. Exclusion criteria included 1) <18 y of age, 2) pregnant, 3) non-English speaking, 4) diagnosed as mentally unstable, 5) diagnosed with another non–upper aerodigestive tract cancer, or 6) diagnosed with any other primary HNSCC within the past 5 y. The institutional review board of the University of Michigan Medical School approved all study activities and met the terms of the 1975 Declaration of Helsinki as revised in 1983.
Baseline and annual medical record review
Upon enrollment into the study, consented participants completed detailed surveys on health behaviors and dietary intake using the self-administered and validated 2007 Harvard FFQ (14). Medical record reviews were conducted at baseline and annually to collect data on clinical variables. Data on health behaviors included demographics (age, sex, and race) and epidemiologic characteristics (baseline BMI, smoking status, alcohol use, and education). Clinical variables included tumor site and stage, comorbidities, HPV status, treatment modalities, cancer recurrence, and survival status. Of the original 1137 eligible participants, 582 (51.2%) filled out baseline FFQs. Participants were excluded from the analysis if they 1) did not meet eligibility criteria (n = 32); 2) were diagnosed with a tumor at the skull bones, unknown primary, nasopharynx, salivary gland, or other sites (n = 44); 3) had left whole missing pages of the FFQ or had >70 missing items on the FFQ (n = 20); or 4) reported a total energy intake >5000 kcal/d or < 200 kcal/d (n = 6). Participants who were missing a covariate, including age, sex, smoking status, tumor site, and cancer stage, were excluded (n = 4). The final sample size was N = 476, which was used in the current study.
Predictors: fat intake
Newly diagnosed HNSCC participants’ usual dietary intake over the past year (referred to as “baseline intake”) was estimated using the semiquantitative 2007 Harvard FFQ (14). The FFQ, collected after diagnosis and before the start of treatment, was intended to reflect the year prior to the patient's diagnosis. The reproducibility and validity of this FFQ have been previously reported (15, 16). Types of fat intake examined were both as totals and subsets and included long-chain fatty acids (LCFAs), unsaturated fatty acids (FAs), PUFAs, ω-3 PUFAs, ω-6 PUFAs, MUFAs, animal fats, vegetable fats, SFAs, trans unsaturated fats, the ratio between ω-6 and ω-3 (ω-6/ω-3) PUFAs, and total fats. The Harvard nutrient database was used to calculate fat intakes (17). Total fats were defined as the sum of LCFAs, unsaturated FAs, PUFAs, ω-3 PUFAs, ω-6 PUFAs, MUFAs, animal fats, vegetable fats, SFAs, and trans unsaturated fats. All fat variables were energy adjusted using the residual method described by Willet et al. (18). Fat intakes were categorized into tertiles (high, medium, low) to maintain statistical power and for ease of interpretability.
Covariates
Covariates included in statistical models were age, sex, race, baseline BMI, tumor site (involving larynx, oral cavity, hypopharynx, or oropharynx), cancer stage (ranging from I–IV), treatment modality [categorized as surgery alone, radiation only, chemotherapy only, chemoradiation (CRT), surgery + adjuvant radiation or CRT, and unknown or palliative], HPV status for all cases (categorized as positive, negative, or unknown), Adult Comorbidity Evaluation (ACE) score (categorized as none or missing, mild, moderate, and severe), smoking status (categorized as current in the past 12 mo, former for >12 mo ago, and never), drinking status (categorized as current, former for >12 mo ago, and never), daily fruit and vegetable (FV) intake [categorized into tertiles and computed for each participant using an average of the following nutrient variables (servings/d): fruit without juice, total vegetables, and total legumes], and education level (less than college and some college or higher). Covariates included were selected according to previous knowledge of the factors that affect the outcomes of interest (mortality and recurrence). Treatment modality was excluded from the final model due to a significant correlation with cancer stage, site, HPV status, and age. BMI was not included due to a significant correlation with smoking status and for not being a common risk factor for HNSCC. ACE was not included due to its significant correlation with HPV status, tumor site and stage, and age. Drinking status was excluded due to its significant correlation with smoking status, tumor site, and age. FV intake was not included in the final model due to the variable's significant correlation with tumor site and smoking status. Education level was excluded due to its significant correlation with tumor site, HPV status, and smoking status. Race was not included because our study population is 95% non-Hispanic white. Final models included adjustment for age, sex, smoking status, HPV status, tumor site, cancer stage, and total caloric intake.
Outcomes: recurrence and mortality
Outcomes of interest were 1) all-cause mortality, 2) HNSCC-specific mortality, and 3) recurrence. The outcomes were measured as time to event and were obtained from annual medical reviews. They were assessed using available data on time to recurrence, survival time, recurrence status, and death status. Death status was detected through the Social Security Death Index and LexisNexis, updates to medical and survey data during follow-ups, and/or through reporting by family, physicians, or medical record reviews. The date of diagnosis was the start of the follow-up period, which was used to measure time to recurrence and survival times. The cutoff date used to determine survival was February 1, 2014, and the time to determine “no recurrence” was based on the date of the last known medical record review for a given participant. The loss to follow-up was adjusted by using the last date for lost participants’ reported status. The median patient follow-up was 36.22 mo.
Statistical analysis
Demographics and clinical and behavioral characteristics were examined using descriptive statistics. Kruskal–Wallis test was used to determine significant differences between fat subtype tertiles when specific characteristics were selected. Pearson correlation coefficients, Spearman correlation coefficients, χ2 test, and ANOVA test were used to assess collinearity among the chosen continuous, ordinal, and categorical covariates. Kaplan–Meier survival functions were used to confirm that the proportional hazards assumption was not violated for any variables. The log-rank test was used to calculate significant differences between the 3 survival curves, each corresponding to the different tertile of different fat types and total fat intake.
Cox proportional hazards models were used to calculate HRs and 95% CIs for the association between tertiles of different fat types and risks of all-cause mortality, HNSCC-specific mortality, and HNSCC recurrence. Subanalyses were conducted where cancer stages (I–II and III–IV) and cancer sites (oral cavity and oropharynx) were considered separately to assess interactions between the predictors and covariates on all-cause mortality, HNSCC-specific mortality, and recurrence. The significant differences between these interactions were assessed using the likelihood ratio test. Bonferroni method was used to adjust the level of α in the multiple comparisons carried out in the stratified subanalyses to avoid type I error. The first tertile of fat intake (low) was set as the referent. P-trend was tested for all HRs by setting up each participant's value to the median for their prospective tertile and then running the fat variable as a continuous variable. Two-sided statistical tests were used. Results were considered significant when the P value was <0.05. Statistics and analyses were generated using the Statistical Analyses System (SAS Institute).
Results
Participant characteristics
Participants’ clinical and epidemiologic characteristics are summarized in Table 1. The mean age at HNSCC diagnosis was 61 y, with a range of 25–95 y. About three-quarters of participants were male, one-quarter were female, and 95% were non-Hispanic whites. Approximately 66% of the cohort were overweight or obese at the time of diagnosis. The most prevalent tumor site was the oropharynx, over half presented with stage IV tumors, and almost half of the cohort had mild comorbidities. Most of the cohort reported being current or former smokers and consumers of alcohol. Among participants with known HPV status, about one-third were HPV positive.
TABLE 1.
Descriptive statistics for HNSCC participants’ clinical and epidemiologic characteristics (N = 476)1
| Characteristic | Value |
|---|---|
| Age | 61.1 (25–95) |
| Sex | |
| Female | 118 (24.7) |
| Male | 360 (75.3) |
| Race | |
| Non-Hispanic white | 451 (95.0) |
| Other | 24 (5.0) |
| Outcome of interest | |
| All-cause death events | 123 (25.8) |
| HNSCC-specific death events | 78 (16.4) |
| Recurrence events | 115 (24.2) |
| Median follow-up for survival, mo | 36.2 |
| BMI, kg/m2 | |
| Underweight (<18.5) | 18 (3.8) |
| Normal (18.5–24.9) | 146 (30.5) |
| Overweight (25–29.9) | 183 (38.3) |
| Obese (≥30) | 131 (27.4) |
| Disease site | |
| Oral cavity | 174 (36.3) |
| Oropharynx | 196 (40.9) |
| Larynx | 98 (20.5) |
| Hypopharynx | 11 (2.3) |
| Cancer stage | |
| Stage I | 88 (18.3) |
| Stage II | 59 (12.3) |
| Stage III | 63 (13.1) |
| Stage IV | 270 (56.3) |
| Treatment | |
| Surgery alone | 120 (25.1) |
| Radiation alone | 35 (7.3) |
| Chemotherapy alone | 14 (2.9) |
| Radiation + chemotherapy | 194 (40.5) |
| Surgery + adjuvant radiation or chemoradiation | 87 (18.2) |
| Unknown or palliative | 29 (6.05) |
| HPV status | |
| Positive | 81 (16.9) |
| Negative | 154 (32.2) |
| Unknown | 244 (50.9) |
| Comorbidities2 | |
| None | 125 (26.1) |
| Mild | 233 (48.6) |
| Moderate | 86 (18.0) |
| Severe | 35 (7.3) |
| Smoking status | |
| Never | 137 (28.6) |
| Current | 174 (36.3) |
| Former | 168 (35.1) |
| Alcohol use status | |
| Never | 36 (7.5) |
| Current | 331 (69.1) |
| Former | 112 (23.4) |
| Fruit and vegetable intake,3 serving/d | 4.5 ± 2.7 (0.04–16.8) |
| Education | |
| Less than college | 165 (34.6) |
| Some college and higher | 312 (65.4) |
Values are presented as mean (range), mean ± SD (range), or frequency (%). HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus.
The overall comorbidity score was defined according to the highest ranked single ailment (grade 1, mild decompensation; grade 2, moderate decompensation; or grade 3, severe decompensation), except when >2 grade 2 ailments occurred in different organ systems, where the comorbid overall score was designated as a severe.
A serving of 1 fruit is ∼150 g and a serving of 1 vegetable is ∼75 g.
Table 2 represents the means and ranges of each type of fat according to tertiles of reported intake. Total fats and SFAs were also represented as percent total calories. The first (mean: 26%) and second (mean: 33%) tertiles of total fat intake in our sample are within the daily reference intake range of total fat (20–35% of total calories), whereas the third tertile (mean: 40%) is above the recommended range. The mean percentages of SFAs from total calories were 7%, 14%, and 18% for low, medium, and high intakes, respectively, where medium and high intakes are higher than the dietary guidelines for Americans (2015–2020) of no more than 10% of total calories from saturated fat (19).
TABLE 2.
Tertiles of self-reported intakes of different fat types by patients with head and neck squamous cell carcinoma at baseline (N = 476)
| Mean [range] of intake | ||||
|---|---|---|---|---|
| Fat subtype | n | T1 (low) | T2 (medium) | T3 (high) |
| Animal fats, g/d | 476 | 21.8 [0.47–28.6] | 33.5 [28.7–38.0] | 47.3 [38.1–70.4] |
| LCFAs, g/d | 414 | 0.07 [0.01–0.11] | 0.18 [0.12–0.29] | 0.58 [0.30–2.29] |
| MUFAs, g/d | 476 | 20.13 [7.48–23.58] | 25.97 [23.59–28.60] | 33.55 [28.61–65.31] |
| ω-3 PUFAs,1 g/d | 419 | 1.04 [0.47–1.28] | 1.47 [1.29–1.69] | 2.28 [1.70–5.59] |
| ω-6 PUFAs,1 g/d | 419 | 9.06 [2.85–10.96] | 12.00 [11.00–13.02] | 15.85 [13.03–26.64] |
| ω-6/ω-3 PUFA ratio | 419 | 6.08 [1.25–7.49] | 8.17 [7.50–8.88] | 10.54 [8.89–30.27] |
| PUFAs, g/d | 476 | 10.60 [3.27–12.73] | 13.99 [12.74–15.38] | 18.24 [15.41–29.74] |
| Vegetable fats, g/d | 476 | 26.73 [6.59–33.70] | 38.05 [33.78–42.14] | 51.61 [42.18–109.47] |
| SFAs, g/d | 476 | 18.34 [7.30–22.26] | 25.11 [22.32–27.66] | 32.35 [27.75–45.54] |
| Unsaturated fats,2 g/d | 476 | 31.81 [10.74–36.96] | 40.18 [36.98–43.41] | 50.49 [43.50–92.21] |
| Trans unsaturated fats, g/d | 419 | 1.64 [0.17–2.08] | 2.41 [2.09–2.78] | 3.30 [2.79–6.69] |
| Total fats,3 g/d | 476 | 57.72 [21.84–66.70] | 72.49 [66.77–78.95] | 88.17 [79.00–128.09] |
| % calories from total fat | 476 | 26.48 [10.02–30.50] | 33.23 [30.51–36.10] | 40.37 [36.12–58.50] |
| % calories from saturated fat | 476 | 7.18 [2.36–9.55] | 13.61 [9.57–15.33] | 18.13 [15.34–70.33] |
ω-3 and ω-6 PUFAs are LCFAs. LCFA, long-chain fatty acid; SFA, saturated fatty acid; T, tertile.
Unsaturated fats include MUFAs and PUFAs.
Total fats include SFAs, MUFAs, and PUFAs.
Selected epidemiologic characteristics, according to tertiles of fat intake, are displayed in Supplemental Table 1. Current smoking, current drinking, total carbohydrate intake, higher educational level, and higher FV intakes were significantly observed among those who consumed high intakes of LCFAs, ω-3 PUFAs, PUFAs, and unsaturated FAs, whereas consuming low FV intakes, having a low educational level, and having a high BMI were observed among those who had high intakes of SFAs and trans fats.
Disease recurrence
During the longitudinal follow-up, there were 115 recurrence events. Results of Cox proportional hazard models assessing the associations between fat subtype variables and recurrence are displayed in Table 3. High intake of LCFAs (HR: 0.60; 95% CI: 0.36, .01; P-trend = 0.05) was associated with a borderline significantly reduced risk of disease recurrence. High intake of unsaturated FAs was not significantly associated with recurrence (P-trend = 0.08).
TABLE 3.
Multivariable time-to-event Cox proportional hazard analysis for the association between fat intake and head and neck squamous cell carcinoma recurrence risk (N = 476)
| HR (95% CI) | |||||
|---|---|---|---|---|---|
| Fat subtype1 | No. of events | Low | Medium | High | P-trend |
| Animal fats | 115 | 1.0 | 0.85 (0.53, 1.37) | 1.02 (0.65, 1.58 | 0.88 |
| LCFAs | 96 | 1.0 | 0.88 (0.54, 1.41) | 0.60 (0.36, 1.01) | 0.05 |
| MUFAs | 115 | 1.0 | 0.93 (0.59, 1.48) | 0.80 (0.51, 1.25) | 0.32 |
| ω-3 PUFAs2 | 97 | 1.0 | 1.06 (0.65, 1.75) | 0.85 (0.51, 1.40) | 0.45 |
| ω-6 PUFAs2 | 97 | 1.0 | 1.27 (0.77, 2.09) | 0.88 (0.53, 1.47) | 0.54 |
| ω-6/ω-3 PUFA ratio | 97 | 1.0 | 1.10 (0.68, 1.78) | 0.89 (0.53, 1.49) | 0.67 |
| PUFAs | 115 | 1.0 | 1.29 (0.82, 2.05) | 0.96 (0.60, 1.54) | 0.82 |
| Vegetable fats | 115 | 1.0 | 1.36 (0.86, 2.16) | 0.94 (0.58, 1.52) | 0.77 |
| SFAs | 115 | 1.0 | 1.03 (0.65, 1.64) | 0.91 (0.58, 1.44) | 0.69 |
| Unsaturated fats3 | 115 | 1.0 | 0.91 (0.58, 1.43) | 0.67 (0.42, 1.06) | 0.08 |
| Trans unsaturated fats | 97 | 1.0 | 1.15 (0.69, 1.92) | 1.31 (0.79, 2.18) | 0.30 |
| Total fats4 | 115 | 1.0 | 1.11 (0.70, 1.76) | 0.88 (0.55, 1.41) | 0.59 |
Adjusted for age, sex, smoking, tumor site, cancer stage, human papillomavirus, and caloric intake. LCFA, long-chain fatty acids; SFA, saturated fatty acid.
ω-3 and ω-6 PUFAs are LCFAs.
Unsaturated fats include MUFAs and PUFAs.
Total fats include SFAs, MUFAs, and PUFAs.
In subanalysis, LCFA was associated with a borderline significantly reduced risk for stage III–IV HNSCC (HR: 0.56; 95% CI: 0.31, 1.01; P-trend = 0.05) (Supplemental Table 2) but was insignificant when considering the oral cavity and oropharynx cancer sites (P-trend = 0.08) (Supplemental Table 3).
All-cause and HNSCC-specific mortality
During the longitudinal follow-up (36.22 mo), there were 123 death events from any cause (25.80% of all deaths), including 78 death events from HNSCC (16.40% of all deaths).
Table 4 shows the results of the Cox models for the associations between fat subtype variables and all-cause mortality. High intakes of LCFAs (HR: 0.55; 95% CI: 0.34, 0.91; P-trend = 0.02), unsaturated FAs (HR: 0.62; 95% CI: 0.40, 0.97; P-trend = 0.04), ω-3 PUFAs (HR: 0.56; 95% CI: 0.35, 0.91; P-trend = 0.02), and ω-6 PUFAs (HR: 0.57; 95% CI: 0.34, 0.94; P-trend = 0.02) were associated with a reduced risk of all-cause mortality.
TABLE 4.
Multivariable time-to-event Cox proportional hazard analysis for the association between fat intake and all-cause mortality risk (N = 476)
| HR (95% CI) | |||||
|---|---|---|---|---|---|
| Fat subtype1 | No. of events | Low | Medium | High | P-trend |
| Animal fats | 123 | 1.0 | 1.06 (0.67, 1.67) | 1.01 (0.66, 1.57) | 0.97 |
| LCFAs | 106 | 1.0 | 0.91 (0.58, 1.44) | 0.55 (0.34, 0.91) | 0.022 |
| MUFAs | 123 | 1.0 | 0.78 (0.50, 1.22) | 0.71 (0.46, 1.09) | 0.12 |
| ω-3 PUFAs3 | 107 | 1.0 | 0.76 (0.48, 1.21) | 0.56 (0.35, 0.91) | 0.022 |
| ω-6 PUFAs4 | 107 | 1.0 | 1.32 (0.84, 2.10) | 0.57 (0.34, 0.94) | 0.022 |
| ω-6/ω-3 PUFA ratio | 107 | 1.0 | 1.07 (0.67, 1.70) | 0.99 (0.61, 1.62) | 0.98 |
| PUFAs | 123 | 1.0 | 1.45 (0.94, 2.23) | 0.71 (0.45, 1.14) | 0.14 |
| Vegetable fats | 123 | 1.0 | 1.58 (1.01, 2.47) | 0.85 (0.52, 1.38) | 0.46 |
| SFAs | 123 | 1.0 | 0.84 (0.53, 1.32) | 0.95 (0.62, 1.46) | 0.83 |
| Unsaturated fats4 | 123 | 1.0 | 0.77 (0.49, 1.21) | 0.62 (0.40, 0.97) | 0.042 |
| Trans unsaturated fats | 107 | 1.0 | 1.01 (0.62, 1.65) | 1.17 (0.73, 1.88) | 0.50 |
| Total fats5 | 123 | 1.0 | 0.71 (0.44, 1.13) | 0.76 (0.49, 1.17) | 0.23 |
Adjusted for age, sex, smoking status, cancer stage, tumor site, human papillomavirus, and caloric intake. LCFA, long-chain fatty acid; SFA, saturated fatty acid.
Indicates significance at P < 0.05.
ω-3 and ω-6 PUFAs are LCFAs.
Unsaturated fats include MUFAs and PUFAs.
Total fats include SFAs, MUFAs, and PUFAs.
In subanalysis stratifying by cancer stage and tumor sites, the association between high intakes of LCFAs (HR: 0.48; 95% CI: 0.27, 0.86; P-trend = 0.01) and ω-3 PUFAs (HR: 0.47; 95% CI: 0.28, 0.81; P-trend = 0.01) and all-cause mortality remained significant for patients diagnosed with stage III–IV HNSCC but not for cancer stages I–II (Supplemental Table 4). High intakes of ω-6 PUFAs were associated with a borderline significantly reduced risk of all-cause mortality only for advanced stages (III–IV) (HR: 0.59; 95% CI: 0.33, 1.04; P-trend = 0.05). There was no significant association between high intakes of LCFAs (P-trend = 0.11 and P-trend = 0.26) and ω-3 PUFAs (P-trend = 0.18 and P-trend = 0.20) and all-cause mortality risk when we stratified for oral cavity and oropharynx tumor sites, respectively (Supplemental Table 5).
Table 5 shows the results of Cox proportional hazard models for the associations between fat intakes and HNSCC-specific mortality. High intakes of unsaturated FAs were significantly associated with a reduced risk of HNSCC-specific mortality (HR: 0.51; 95% CI: 0.29, 0.90; P-trend = 0.02). No significant association between ω-6 PUFAs and HNSCC-specific mortality was found (P-trend = 0.10).
TABLE 5.
Multivariable time-to-event Cox proportional hazard analysis for the association between fat intake and head and neck squamous cell carcinoma–specific mortality risk (N = 476)
| HR (95% CI) | |||||
|---|---|---|---|---|---|
| Fat subtype1 | No. of events | Low | Medium | High | P-trend |
| Animal fats | 78 | 1.0 | 0.91 (0.51, 1.61) | 0.96 (0.56, 1.64) | 0.91 |
| LCFAs | 65 | 1.0 | 1.04 (0.58, 1.88) | 0.69 (0.37, 1.28) | 0.18 |
| MUFAs | 78 | 1.0 | 0.87 (0.50, 1.51) | 0.67 (0.39, 1.17) | 0.16 |
| ω-3 PUFAs2 | 66 | 1.0 | 0.78 (0.42, 1.43) | 0.72 (0.40, 1.32) | 0.32 |
| ω-6 PUFAs2 | 66 | 1.0 | 1.41 (0.79, 2.53) | 0.60 (0.31, 1.16) | 0.10 |
| ω-6/ω-3 PUFA ratio | 66 | 1.0 | 0.88 (0.49, 1.57) | 0.74 (0.40, 1.37) | 0.34 |
| PUFAs | 78 | 1.0 | 1.64 (0.96, 2.82) | 0.69 (0.37, 1.28) | 0.21 |
| Vegetable fats | 78 | 1.0 | 1.46 (0.84, 2.53) | 0.68 (0.37, 1.25) | 0.20 |
| SFAs | 78 | 1.0 | 0.87 (0.50, 1.52) | 0.78 (0.45, 1.35) | 0.38 |
| Unsaturated fats3 | 78 | 1.0 | 0.75 (0.43, 1.30) | 0.51 (0.29, 0.90) | 0.024 |
| Trans unsaturated fats | 66 | 1.0 | 1.09 (0.60, 1.97) | 0.91 (0.49, 1.70) | 0.78 |
| Total fats5 | 78 | 1.0 | 0.68 (0.38, 1.22) | 0.67 (0.39, 1.16) | 0.16 |
Adjusted for age, sex, smoking status, cancer stage, tumor site, human papillomavirus, and caloric intake. LCFA, long-chain fatty acid; SFA, saturated fatty acid.
ω-3 and ω-6 PUFAs are LCFAs.
Unsaturated fats include MUFAs and PUFAs.
Indicates significance at P < 0.05.
Total fats include SFAs, MUFAs, and PUFAs.
In subanalysis, the association between unsaturated FAs and HNSCC-specific mortality remained significant for participants with tumors of the oral cavity (HR: 0.32; 95% CI: 0.14, 0.76; P-trend = 0.01) but not the oropharynx (Supplemental Table 6). High intakes of unsaturated FAs were associated with a borderline significantly reduced risk of HNSCC-specific mortality when considering cancer stages I–II (HR: 0.30; 95% CI: 0.08, 1.06; P-trend = 0.06) but not stages III–IV (Supplemental Table 7).
Discussion
In this longitudinal cohort study of newly diagnosed patients with HNSCC, after adjusting for age, sex, smoking status, HPV status, tumor site, cancer stage, and caloric intake, we found that high intakes of LCFAs, unsaturated FAs, ω-3 PUFAs, and ω-6 PUFAs were associated with a significantly reduced risk of all-cause mortality. High intake of unsaturated FAs was significantly associated with a reduced risk of HNSCC-specific mortality. The stratified analysis revealed that associations between fat subtypes and prognosis might differ according to the tumor site and cancer stage. As far as we know, this is the only study to consider examining the association between various fat subtypes and HNSCC mortality and recurrence.
A systematic review and meta-analysis of randomized controlled, cohort, and case-control studies looking at how adherence to the Mediterranean Diet (MedD), which is rich in MUFAs and PUFAs, influences cancer mortality and recurrence risk in cancer survivors revealed that in a total of 7 studies, cancer survivors diagnosed with HNSCC who had the highest adherence score to a MedD had a reduced risk of cancer mortality (RR: 0.49; 95% CI: 0.37, 0.66) (20). One explanation is that the high content of unsaturated FAs (MUFAs and PUFAs) from olive oil, nuts, seeds, fish, and other marine meats downregulates NF-κB via peroxisome proliferator-activated receptor and has anti-inflammatory effects (13). Oleic acid, a highly abundant MUFA in the human diet, has been shown to induce cell death and autophagy in CAL27 cells and UM1 cell lines by blocking the Akt/mechanistic target of rapamycin (mTOR) pathway in tongue cancer (21), an intracellular signaling pathway important in regulating the cell cycle.
It has been found that long-chain ω-3 PUFAs suppress the inflammatory process, stimulate apoptosis, inhibit tumor proliferation, protect against tumor progression, and compete with ω-6 PUFAs for enzymatic conversion (13, 22–24). Also, ω-3 PUFAs have essential roles in cell structure and membrane fluidity (25). These roles are due to inhibition of macrophage-elaborated tumor necrosis factor production and inactivation of the NF-κB signaling pathway, resulting in the alteration of proinflammatory cytokine transcription (13). The results of the current study were consistent with a review article by Nabavi et al. (9), which demonstrated that ω-3 PUFAs were associated with lower general infections in patients with cancer at the oral cavity and larynx compared with the control group (9, 26, 27). Collectively, these findings, together with the findings from the current analysis, suggest that fish and other marine meats, which are rich in ω-3 PUFAs, might be beneficial to patients with HNSCC.
Our findings that high intakes of ω-6 PUFAs were associated with a reduced risk of all-cause mortality were inconsistent with other studies that considered that overconsumption of ω-6 PUFAs, which are found in non–marine oil–rich diets, and an increased proportion of the ω-6/ω-3 PUFA ratio promote carcinogenesis by activating inflammation and the production of reactive oxygen species (22, 12). However, ω-6 PUFAs still have some health benefits as long as the ω-6/ω-3 PUFA ratio is low. In addition, the reasons for the inconsistency in our findings are unclear and may have been due to chance.
SFAs and trans fats are known to have harmful effects on various cancer types (28, 29). However, the mechanisms by which these types of fats promote tumorigenesis are not fully understood. Trans fats resulted from partial hydrogenation of vegetable or fish oils and were previously used in different American food products but were banned by the FDA in 2015 (30). A previous study showed that trans fats were associated with an increased risk of mouth/pharynx cancer (HR: 1.59; 95% CI: 1.08, 2.35) (31). Current knowledge indicates that high animal fat intake, which is high in SFAs, may increase tumorigenesis by decreasing apoptosis and modulating functions of the immune system (32). A prospective cohort study that included 521,120 participants, after 16 y of follow-up, found that high compared with low SFA intake was associated with higher all-cause cancer mortality (HR: 1.26; 95% CI: 1.20, 1.32) (33). In addition, a high intake of these animal fats means that the diet is low in plant-based foods, which have an antiproliferative activity due to their content of anticarcinogenic compounds (32). However, the results of our study did not find any associations between SFAs, trans fats, or animal fats and risk of disease mortality and recurrence. In addition, to our knowledge, no study has examined the effects of SFAs, trans fats, and animal fats on HNSCC recurrence and mortality in particular.
Vegetable fat, which contains various amounts of PUFAs and MUFAs, has not been examined elsewhere regarding HNSCC mortality and recurrence. However, a case-control study examining diet and the risk of oral cavity and pharyngeal cancer showed that vegetable fats were inversely associated with cancer risk (ORQ3 vs. Q1: 0.40; 95% CI: 0.27, 0.61 and ORQ4 vs. Q1: 0.54; 95% CI: 0.37, 0.78; P-trend = 0.0001) (34). Food shortenings, which are considered harmful due to their trans fats content, were included in the vegetable fat category, which might influence our results of vegetable fats, wherein it was demonstrated that medium intake of vegetable fat was associated with an increased risk of all-cause mortality.
In subanalyses, our findings showed that reduction of all-cause and HNSCC-specific mortality risks was seen in advanced stages of cancer and in oral cavity cancers, respectively. Patients diagnosed with an advanced stage of HNSCC might benefit more from high intakes of LCFAs, ω-3 PUFAs, and ω-6 PUFAs compared with patients diagnosed with an early stage of the disease. Moreover, patients with cancer in the oral cavity might benefit more from high intakes of unsaturated fats than patients diagnosed with cancer of the oropharynx. An explanation for this might be that the treatment for oral cavity cancer includes surgery and CRT, whereas treatment of the oropharynx typically does not include surgery. In addition, oropharynx cancers are significantly associated with HPV status, younger age, lower smoking history, and better prognosis. The role of unsaturated fats is essential in wound healing and recovery from surgery, where they are necessary for the creation of cell membranes, and the body may need extra amounts to heal properly during recovery.
The primary strength of our study is that, to the best of our knowledge, it is the largest and only survival cohort of patients with HNSCC that has comprehensively examined the association between various fat subtypes and HNSCC prognosis. An additional strength is the ability to examine several prognostic outcomes and adjust for multiple potentially confounding variables.
This study is not without limitations. Dietary intake relied on self-report via FFQs, which are susceptible to measurement error and systematic biases (both intake-related and person-specific biases) in reporting (35). In addition, since most of our sample is non-Hispanic white men, our results may not be generalizable to other patients with HNSCC of other races or ethnicities. Smoking and drinking status were categorized as never, former, and current, which do not fully account for the intensity and duration of tobacco and alcohol exposures. Another limitation is that the results might be due to chance because this is an observational study, and we can only determine associations and not causation.
To our knowledge, this is the first epidemiologic study examining the association between various fat subtypes and prognostic outcomes in HNSCC. Our results (summarized in Figure 1) suggest that consuming a baseline diet high in LCFAs, ω-3 PUFAs, and ω-6 PUFAs may have beneficial effects on the risk of all-cause mortality, particularly among patients with advanced stages of HNSCC, and that consuming a baseline diet high in unsaturated FAs may reduce the risk of HNSCC-specific mortality at the oral cavity in particular. These findings highlight the potential significance of considering the role of both type and quantity of fats for HNSCC prognosis and not just the total fats. In order to develop specific dietary fat recommendations for patients with HNSCC, there is an urgent need for clinical intervention trials to examine these associations further.
FIGURE 1.
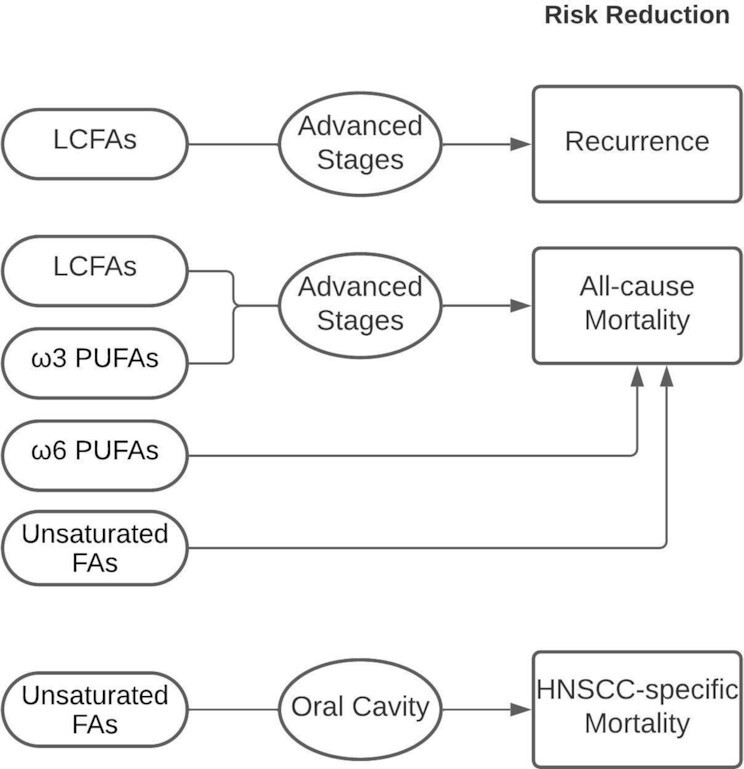
Summary of the main findings of the association between the baseline fat intake of patients with HNSCC and recurrence and mortality. Consuming a baseline diet high in LCFAs and ω-3 PUFAs may have beneficial effects on the risk of all-cause mortality, particularly in patients with advanced stages of HNSCC, and that a baseline diet high in unsaturated FAs may reduce the risk of HNSCC at the oral cavity. LCFAs may reduce the risk of recurrence in patients with advanced stages of HNSCC. Arrows indicate an association between the fat type and risk of mortality/recurrence. FA, fatty acid; HNSCC, head and neck squamous cell carcinoma; LCFA, long-chain fatty acid.
Supplementary Material
Acknowledgments
We thank the patients and their families, clinicians, and investigators for providing access to the clinical database and completing participants’ recruitment and follow-up. These investigators include Carol R. Bradford, MD, Thomas E. Carey, PhD, Douglas B. Chepeha, MD, Sonia Duffy, PhD, Avraham Eisbruch, MD, Joseph Helman, DDS, Kelly M. Malloy, MD, Jonathan McHugh, MD, Scott A. McLean, MD, Tamara H. Miller, RN, Jeff Moyer, MD, Mark E. Prince, MD, Nancy Rogers, RN, Matthew E. Spector, MD, Nancy E. Wallace, RN, Heather Walline, PhD, Brent Ward, DDS, and Francis Worden, MD.
The authors’ responsibilities were as follows—HMT, LSR, KRZ, GTW, and AEA: designed research; HMT and AEA: conducted research and wrote the paper; HMT, XC, and ZL: analyzed data; AEA: had primary responsibility for the final content. LSR, KRZ, GTW, and AEA: did the investigation and visualization; LSR and AEA: did the validation; HMT, LSR, ANS, KRZ, GTW, and AEA: reviewed and edited the paper; and all authors: have read and approved the final manuscript.
Notes
Supported by NIH/NCI P50CA097248 and USDA-NIFA Hatch Project 1011487. The funders did not interfere in the study design, data collection, analyses, interpretation of results, writing of the manuscript, nor in the decision to publish the findings.
Supported by the National Institutes of Health/National Cancer Institute P50CA097248 and the United States Department of Agriculture - National Institute of Food and Agriculture Hatch Project 1011487. The funders did not interfere in the study design, data collection, analyses, interpretation of results, writing of the manuscript, nor in the decision to publish the findings
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ACE, Adult Comorbidity Evaluation; CRT, chemoradiation; FA, fatty acid; FV, fruit and vegetable; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; LCFA, long-chain fatty acid; MedD, Mediterranean Diet; SFA, saturated fatty acid.
Contributor Information
Hania M Taha, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, USA; Augusta Victoria Hospital, The Lutheran World Federation, East Jerusalem, Palestine.
Laura S Rozek, Department of Environmental Health Sciences, University of Michigan School of Public Health, Ann Arbor, MI, USA; Department of Otolaryngology, University of Michigan Medical School of Public Health, Ann Arbor, MI, USA.
Xi Chen, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, USA.
Zonggui Li, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, USA.
Katie R Zarins, Department of Environmental Health Sciences, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Alexander N Slade, Department of Radiation Oncology, Stony Brook University School of Medicine, Stony Brook, NY, USA.
Gregory T Wolf, Department of Otolaryngology, University of Michigan Medical School of Public Health, Ann Arbor, MI, USA.
Anna E Arthur, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, USA; Department of Dietetics and Nutrition, University of Kansas Medical Center, Kansas City, KS, USA.
References
- 1. National Cancer Institute . Head and Neck Cancers [Internet]. [Reviewed: May 25, 2021]. Retrieved from: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet#what-are-cancers-of-the-head-and-neck. [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3. American Cancer Society . Survival rates for oral cavity and oropharyngeal cancer [Internet]. 2019; [cited 2021 Mar 23]. Available from: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/detection-diagnosis-staging/survival-rates.html [Google Scholar]
- 4. Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res. 2003;114:15–60. [DOI] [PubMed] [Google Scholar]
- 5. Negri E, La Vecchia C, Franceschi S, Tavani A. Attributable risk for oral cancer in northern Italy. Cancer Epidemiol Biomarkers Prev. 1993;2:189–93. [PubMed] [Google Scholar]
- 6. Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez Let al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Instit. 2007;99(10):777–89. [DOI] [PubMed] [Google Scholar]
- 7. CDC . HPV and oropharyngeal cancer. Atlanta (GA): CDC; 2018. [Google Scholar]
- 8. Jones JA, Lang WP. Nutrition during treatment of head and neck cancer. Spec Care Dentist. 1986;6(4):165–9. [DOI] [PubMed] [Google Scholar]
- 9. Nabavi S, Bilotto S, Russo GL, Erdogan Orhan I, Habtemariam S, Daglia M, Kasi PD, Loizzo M, Tundis R, Nabavi S. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34(3):359–80. [DOI] [PubMed] [Google Scholar]
- 10. Arthur A, Goss A, Demark-Wahnefried W, Mondul A, Fontaine K, Chen YT, Carroll W, Spencer S, Rogers L, Rozek Let al. Higher carbohydrate intake is associated with increased risk of all-cause and disease-specific mortality in head and neck cancer patients: results from a prospective cohort study. Int J Cancer. 2018;143(5):1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maino Vieytes CA, Mondul AM, Li Z, Zarins KR, Wolf GT, Rozek LS, Arthur AE. Dietary fiber, whole grains, and head and neck cancer prognosis: findings from a prospective cohort study. Nutrients. 2019;11(10):2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eyre H, Kahn R, Robertson RM. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. CA Cancer J Clin. 2004;54(4):190–207. [DOI] [PubMed] [Google Scholar]
- 13. Bojková B, Winklewski PJ, Wszedybyl-Winklewska M. Dietary fat and cancer—which is good, which is bad, and the body of evidence. Int J Mol Sci. 2020;21(11):1–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harvard University . Harvard T.H. Chan School of Public Health Nutrition Department's File Download Site. Cambridge (MA): Harvard University; 2018. Available from: https://regepi.bwh.harvard.edu/health/FFQ/files. [Google Scholar]
- 15. Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–99. [DOI] [PubMed] [Google Scholar]
- 16. Rimm E, Giovannucci E, Stampfer M, Colditz G, Litin L, Willett W. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 1127. [DOI] [PubMed] [Google Scholar]
- 17. McNutt S, Zimmerman TP, Hull SG. Development of food composition databases for food frequency questionnaires (FFQ). J Food Compos Anal. 2008;21:S20–6. [Google Scholar]
- 18. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 19. Institute of Medicine . Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 20. Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9(10):1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang L, Wang W, He Q, Wu Y, Lu Z, Sun J, Liu Z, Shao Y, Wang A. Oleic acid induces apoptosis and autophagy in the treatment of tongue squamous cell carcinomas. Sci Rep. 2017;7(1):11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zanoaga O, Jurj A, Raduly L, Cojocneanu-Petric R, Fuentes-Mattei E, Wu O, Braicu C, Gherman CD, Berindan-Neagoe I. Implications of dietary ω-3 and ω-6 polyunsaturated fatty acids in breast cancer. Exp Ther Med. 2018;15:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gu Z, Shan K, Chen H, Chen YQ. n-3 polyunsaturated fatty acids and their role in cancer chemoprevention. Curr Pharmacol Rep. 2015;1(5):283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n−3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79(6):935–45. [DOI] [PubMed] [Google Scholar]
- 25. Freitas RDS, Campos MM. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients. 2019;11(5):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felekis D, Eleftheriadou A, Papadakos G, Bosinakou I, Ferekidou E, Kandiloros D, Katsaragakis S, Charalabopoulos K, Manolopoulos L. Effect of perioperative immuno-enhanced enteral nutrition on inflammatory response, nutritional status, and outcomes in head and neck cancer patients undergoing major surgery. Nutr Cancer. 2010;62(8):1105–12. [DOI] [PubMed] [Google Scholar]
- 27. Casas Rodera P, Gómez-Candela C, Benítez S, Mateo R, Armero M, Castillo R, Culebras JM. Immunoenhanced enteral nutrition formulas in head and neck cancer surgery: a prospective, randomized clinical trial. Nutr Hosp. 2008;23:105–10. [PubMed] [Google Scholar]
- 28. Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L. Dietary transfatty acids and cancer risk. Eur J Cancer Prev. 2011;20(6):530–8. [DOI] [PubMed] [Google Scholar]
- 29. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report 2018. 2018. Available from: http://dietandcancerreport.org. [Google Scholar]
- 30. US Food and Drug Administration. Trans fat. Bethesda (MD): US Food and Drug Administration; 2018. [Google Scholar]
- 31. Laake I, Carlsen MH, Pedersen JI, Weiderpass E, Selmer R, Kirkhus B, Thune I, Veierød MB. Intake of trans fatty acids from partially hydrogenated vegetable and fish oils and ruminant fat in relation to cancer risk. Int J Cancer. 2013;132(6):1389–403. [DOI] [PubMed] [Google Scholar]
- 32. Othman R. Dietary lipids and cancer. Libyan J Med. 2007;2(4):180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuang P, Zhang Y, He W, Chen X, Chen J, He L, Mao L, Wu F, Jiao J. Dietary fats in relation to total and cause-specific mortality in a prospective cohort of 521 120 individuals with 16 years of follow-up. Circ Res. 2019;124(5):757–68. [DOI] [PubMed] [Google Scholar]
- 34. Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S, Negri E, La Vecchia C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer. 2013;109:2904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kipnis V, Midthune D, Freedman L, Bingham S, Day N, Riboli E, Ferrari P, Carroll R. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002;5(6A):915–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


