Abstract
Antibody affinity maturation is a critical step in development of functional antiviral immunity; however, accurate measurement of affinity maturation of polyclonal serum antibody responses to particulate antigens such as virions is challenging. We describe a novel avidity assay employing biolayer interferometry and dengue virus-like particles. After validation using anti-dengue monoclonal antibodies, the assay was used to assess avidity of antibody responses to a tetravalent dengue vaccine candidate (TAK-003) in children, adolescents, and adults during two phase 2 clinical trials conducted in dengue-endemic regions. Vaccination increased avidity index and avidity remained high through 1 year postvaccination. Neutralizing antibody titers and avidity index did not correlate overall; however, a correlation was observed between neutralizing antibody titer and avidity index in those subjects with the highest degree of antibody affinity maturation. Therefore, vaccination with TAK-003 stimulates polyclonal affinity maturation and functional antibody responses, including neutralizing antibodies.
Clinical Trials Registration
NCT01511250 and NCT02302066.
Keywords: dengue, TAK-003, vaccine, BLI, biolayer interferometry, avidity, antibody, virus-like particle, VLP
To assess antibody affinity maturation, a novel dengue virus avidity assay was developed employing biolayer interferometry and dengue virus-like particles. Vaccination with tetravalent dengue vaccine candidate (TAK-003) increased avidity index, which remained high throughout 1 year.
Antibody affinity maturation is the process whereby the immune system generates antibodies of higher affinities in response to antigen engagement through somatic hypermutation. Antibody somatic hypermutation takes place in germinal centers after exposure to antigen, either by infection or immunization [1]. B cells in the germinal centers express enzymes that insert point mutations throughout the immunoglobulin heavy and light chains. The repertoire of mutated B cells is then selected and enriched for high antibody affinity against the antigen. Iterative rounds of selection and proliferation of somatically mutated clonal variants result in a population of antibodies that are enriched for higher-affinity binders, based on successive accumulation of somatic mutations over time. Collectively, antibodies with increased affinities after antigen exposure contribute to an overall increase in polyclonal antibody avidity.
Affinity maturation leads to higher antibody affinity, which optimizes antiviral functions, including virus neutralization and antibody-dependent cell-mediated cytotoxicity [2]. Sometimes, the increase of antiviral immunity in an individual is mainly due to the activity of a single affinity-matured antibody, but in most cases antiviral immunity is mediated by the combined effect of multiple virus-specific affinity-matured antibodies that are present in polyclonal serum in the circulation [3–6]. These interactions are collectively termed avidity [7].
Serum neutralizing antibody titer is the most common measure of antibody responses to vaccination and infection [8, 9]. However, neutralizing antibody titers do not always correlate with vaccine effectiveness, and neutralization assays do not measure all antibody effector functions. The degree of affinity maturation driven by vaccination or natural infection is an important parameter to measure overall evolution of the polyclonal antibody repertoire [10, 11]. Several methods have been developed to measure affinity maturation of the antibody repertoire, including deep sequencing of B cells and whole-genome phage display libraries [12, 13]. These methods are low throughput and therefore not suitable for vaccine or natural infection history studies. Other techniques, such as enzyme-linked immunosorbent assay (ELISA) with chaotropic reagents (8 M urea or 1 M NaSCN), are higher throughput but imprecise [14]. Increased precision requires a more direct and more dynamic avidity assay. Surface plasmon resonance and biolayer interferometry (BLI) are techniques commonly used to measure monoclonal antibody affinity [15, 16]. Recently, these technologies have been applied to evaluate polyclonal antibody interaction with soluble antigens, with several studies reporting correlations between antibody avidity and other immune response parameters or protective efficacy [17–20]. Extending these methods to particulate antigens such as virions or virus-like particles (VLPs) is important because, unlike peptides and proteins, they preserve the conformational and quaternary epitopes that are the target of many potent neutralizing antibodies [21, 22].
Dengue fever is a mosquito-borne infection caused by 4 dengue virus serotypes (DENV-1, DENV-2, DENV-3, and DENV-4). With an estimated 390 million infections annually, of which approximately 96 million are symptomatic [23], dengue fever is the most rapidly spreading mosquito-borne viral disease in the world [24]. A dengue vaccine (Dengvaxia; Sanofi Pasteur) is approved in several countries for individuals 9 years of age and older with evidence of prior infection; however, the need remains for vaccines licensed for broader populations. Takeda’s tetravalent dengue vaccine candidate (TAK-003) has demonstrated efficacy in baseline seronegative and seropositive subjects in a phase 3 clinical trial.
Here we report the development of a high-throughput, low-volume avidity assay based on BLI and capable of measuring polyclonal serum antibody avidity to particulate DENV-1, -2, -3, and -4 antigens. Using sera from two phase 2 trials, we demonstrate that polyclonal antibody avidity to all 4 DENV serotypes increases over time following vaccination of seronegative volunteers with TAK-003, and high-avidity antibodies persist through 360 days.
METHODS
Ethics Statement
DEN-203 (ClinicalTrials.gov identifier NCT01511250) and DEN-204 (NCT02302066) were conducted in accordance with the Declaration of Helsinki, International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use and Good Clinical Practice guidelines, and applicable regulatory requirements. Informed consent forms and study protocols were reviewed and approved by institutional review boards, independent ethics committees, or health authorities. Written informed assent or consent was obtained from all participants or their parents or legal guardians before enrollment.
Reagents
Dengue VLPs for serotypes 1, 2, 3, and 4 were purchased from The Native Antigen Company Ltd. The streptavidin (SA) and high-precision streptavidin biosensor (SAX) were purchased from FortéBio Inc. Protein A and Protein G Sepharose were obtained from GE Healthcare. Anti-dengue monoclonal antibody clones 4G2 [25], WNV-E60 [26], 1M7 [27], DENV1-E106 [28], 2D22 [29], 5J7 [30], and DV4-75 [31] were purchased from various vendors or provided by collaborators.
Clinical Serum Samples
Sera from 56 TAK-003 vaccinated volunteers (37 baseline seronegative and 19 seropositive) from trial DEN-203 [32] and 36 vaccinated volunteers (21 baseline seronegative and 15 seropositive) from trial DEN-204 [33] were tested for antibody avidity. DEN-203 was conducted in Puerto Rico, Colombia, Singapore, and Thailand among 1.5–45 year olds and DEN-204 was conducted in the Dominican Republic, Panama, and the Philippines among 2–17 year olds. Details are described in Supplementary Materials and Methods; Supplementary text, Supplementary Tables 1 and 2.
Reactivity of Anti-Dengue Antibodies to Dengue Virus-Like Particles
The reactivity of anti-dengue antibodies to dengue VLPs was measured using the Octet RED or Octet HTX system. Briefly, biotinylated VLP were captured to SA or SAX biosensor. Anti-dengue monoclonal antibodies were confirmed for binding to VLPs. Details are described in Supplementary Materials and Methods.
Avidity Assay
Antibody avidity was measured using the Octet RED or Octet HTX systems (Supplementary Figure 1). Briefly, anti-dengue polyclonal antibodies purified from serum by Protein G Sepharose was bound to the biotinylated VLP-captured SA or SAX biosensor for 1800 seconds and then the sensor was incubated with dissociation buffer for 1200 seconds. Optimization and assay details are described in Supplementary Materials and Methods.
Microneutralization Test
Anti-dengue neutralizing antibody titers in response to vaccination with TAK-003 were quantified by microneutralization test (MNT), as previously described [34].
Correlation Between Antibody Titers and Avidity Assay Parameters
Postvaccination avidity assay parameters of response, antibody dissociation rate constant (koff), and avidity for each serotype in 24 baseline seronegative and 19 seropositive samples from DEN-203 were analyzed for correlation with MNT titers. Further correlation analyses were performed using data sets stratified by strength of binding (koff) to dengue VLPs. Details are described in Supplementary Materials and Methods.
Statistical Analysis
All data were analyzed by GraphPad Prism software (version 8.0.0). Wilcoxon signed-rank tests were applied for comparisons between each sample collection day with day 0 for avidity parameters, response, koff, and avidity index. Correlation analysis between avidity parameters and MNT titers were used with linear regression curves for log10 converted data sets. Limit of detection was established as 3 times assay noise in accordance with ASTM E685-93(2013) [35].
RESULTS
Reactivity and Specificity of Anti-Dengue Antibody Panels
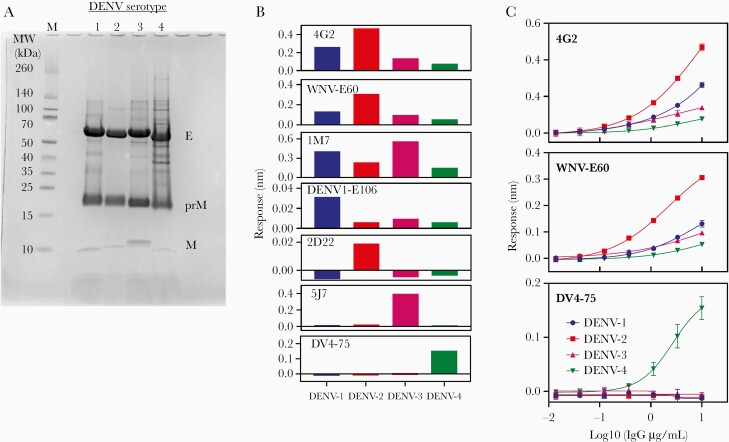
As expected, all 4 dengue VLPs contained E, prM, and M proteins, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1A). Antigenicity of dengue VLPs was evaluated using a panel of anti-dengue monoclonal antibodies (Figure 1B and 1C). Cross-reactive fusion loop-specific monoclonal antibodies 4G2, WNV-E60, and 1M7 bound to VLPs of all serotypes. Serotype-specific neutralizing antibodies DENV1-E106 for DENV-1, 2D22 for DENV-2, 5J7 for DENV-3, and DV4-75 for DENV-4 recognize quaternary epitopes [28, 31, 36]. Each serotype-specific antibody bound the corresponding dengue VLP and did not bind to VLPs of the other 3 serotypes. Therefore, we concluded that, unlike soluble E protein, dengue VLPs retain DENV quaternary epitopes.
Figure 1.
Reactivity of anti-dengue monoclonal antibodies to biotinylated dengue VLPs. A, SDS-PAGE of dengue VLPs. VLP were reduced and 20 μg of VLP was applied to 10%–20% tricine SDS-PAGE and stained by Simple Stain Blue (ThermoFisher Scientific). B, Reactivity of anti-dengue monoclonal antibodies panel. Response of 10 μg/mL of anti-dengue mAbs at 600 seconds association was measured by Octet Red or Octet HTX systems using 5 μg/mL biotinylated VLP in SA or SAX biosensor. Data of 4G2, WNV-E60, 2D22, and DV4-75 were measured 3 times and the averages are shown. 1M7, DENV-E106, and 5J7 were measured once. C, Dose dependence of anti-dengue monoclonal antibodies. 4G2, WNV-E60, and DV4-75 antibody clones were diluted from 0.01 to 10 μg/mL to measure response at 600 seconds of association. All data were measured 3 time and averages ± SD are shown. The mAb clone, specificity, and epitope of dengue E-protein were: 4G2, CR, fusion loop [25]; WNV-E60, CR, fusion loop [26]; 1M7, CR, fusion loop [27]; DENV1-E106, DENV-1 specific, QE [28]; 2D22, DENV-2 specific, QE [29]; 5J7, DENV-3 specific, QE [30]; and DV4-75, DENV-4 specific, QE [31]. Abbreviations: CR, cross-reactive; DENV, dengue virus; IgG, immunoglobulin G; mAb, monoclonal antibody; QE, quaternary E-protein; SA, streptavidin; SAX, high-precision streptavidin; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; VLP, virus-like particle.
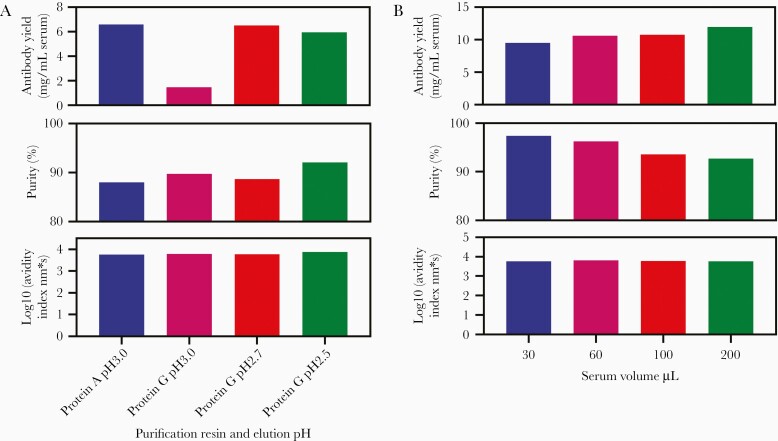
Optimization of Antibody Purification from Human Serum
First, we compared Protein A and Protein G Sepharose purification using different elution pH. Antibody yield was high with Protein A Sepharose elution at pH 3.0 and Protein G Sepharose elution at pH 2.7 or 2.5 (Figure 2A). We selected Protein G Sepharose because Protein A weakly binds IgM [37], which could contribute to nonspecific binding in the assay. Next, we determined the minimum volume of serum required. An antibody yield of 10 mg/mL and purity of >90% was obtained by purifying antibody from 30–200 µL human serum using Protein G Sepharose (Figure 2B). We defined the serum volume requirement as 50–200 µL. Next, we confirmed IgG yield between operator and occasions and established the range of antibody yield as 81%–106% when purifying IgG from 100 µL serum using Protein G Sepharose (Supplementary Table 3).
Figure 2.
Optimization of anti-dengue polyclonal antibody purification from human serum. A, Optimization of Protein G Sepharose elution conditions: 0.2 mL of dengue standard serum was mixed with 3 mL of Protein A MAPSII binding buffer and 0.6 mL of 50% Protein A Sepharose or 3 mL of DPBS and 0.6 mL of 50% Protein G Sepharose for 90 minutes, washed with Protein A MAPSII binding buffer or DPBS, and eluted in 0.1 M glycine buffer pH 3.0–2.5. Eluate were neutralized to pH 7.0–7.5 immediately with 1 M Tris-HCl pH 8.0 and exchanged buffer to DPBS using Amicon Ultra-4. Purity was measured by NuPAGE (ThermoFisher Scientific) and purified antibody avidity was measured using biotinylated DENV-1 VLP and Octet RED system (FortéBio). B, Optimization of serum volume: 0.03–0.2 mL of clinical trial DEN-203, volunteer No. 1044010, day 90 serum was mixed with 3 mL of DPBS and 0.6 mL of 50% Protein G Sepharose for 90 minutes and washed with DPBS and eluted in 0.1 M glycine buffer pH 2.7. Eluate was neutralized to pH 7.0–7.5 immediately with 1 M Tris-HCl pH 8.0 and exchanged buffer to DPBS using Amicon Ultra-4. Purity was measured by NuPAGE and purified antibody avidity was measured using biotinylated DENV-1 VLP and Octet RED system. Abbreviations: DENV, dengue virus; DPBS, Dulbecco’s phosphate-buffered saline; VLP, virus-like particle.
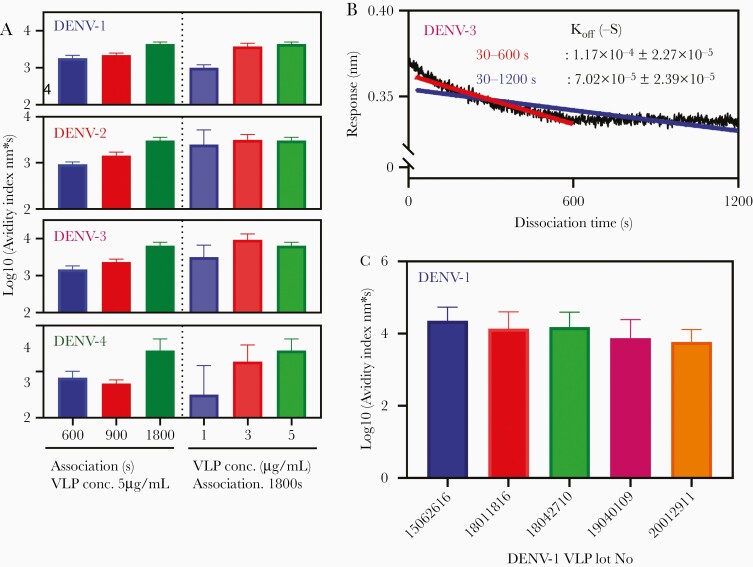
Optimization of Avidity Assay
Association was optimized using anti-dengue polyclonal antibody purified from DEN-203 serum. Avidity index, calculated by response/koff, increased when association time increased from 600 to 1800 seconds (Figure 3A) and reached a plateau using 3–5 μg/mL biotinylated dengue VLP (Figure 3A). For dissociation, some samples showed 2-phase curves, that is high dissociation rate in the early phase and lower rate in the late phase (Figure 3B). Dissociation fitting of 30–600 seconds showed good fitting and observed higher dissociation rate than 30–1200 seconds fitting (Figure 3B). Response of polyclonal dengue antibodies was at immunoglobulin G (IgG) concentration up to 250 μg/mL (Supplementary Figure 2A). Thus, avidity assay parameters were optimized as follows: antibody concentration, 125 μg/mL; biotinylated dengue VLP, 5 μg/mL; association, 1800 seconds; dissociation, 1200 seconds; dissociation rate analysis, 30–600 seconds.
Figure 3.
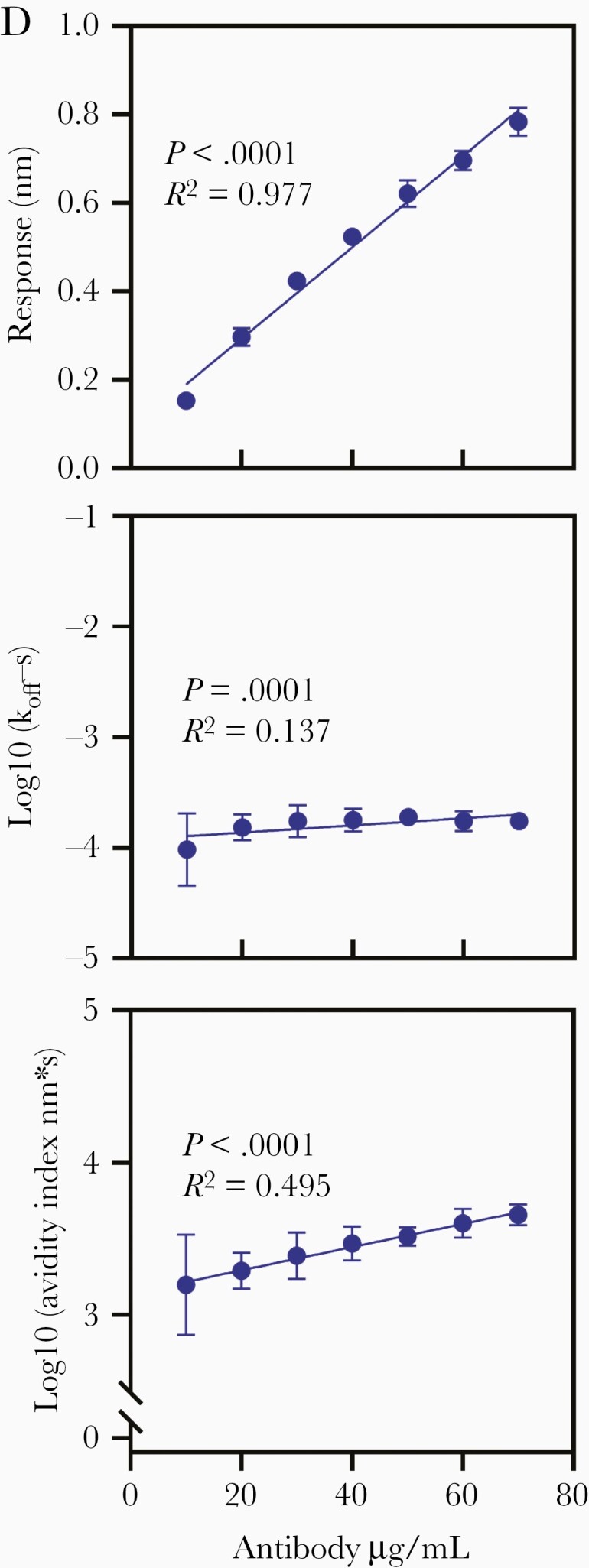
Optimization of avidity assay. A, Optimization of association and VLP concentration. Antibody avidity index was measured using Octet HTX (FortéBio) and DENV-1, 2, 3, and 4 VLP. Anti-dengue polyclonal antibody (125 μg/mL) purified from clinical trial DEN-203 volunteer serum (DENV-1, volunteer No. 1071001 day 90; DENV-2, volunteer No. 1071009 day 90; DENV-3, volunteer No. 1083005 day 0; and DENV-4, volunteer No. 1093001 day 90) was used to measure association and dissociation rates. Association times were 600, 900, and 1800 seconds at biotinylated DENV VLP 5 μg/mL, and biotinylated VLP concentrations were 1, 3, and 5 μg/mL at 1800 seconds association time. Data were measured 8 times and bar graph shows averages ± SD. B, Optimization of dissociation rate analysis. Biosensorgram of anti-dengue polyclonal antibody purified from DEN-203 volunteer serum (volunteer No. 1081001, day 0; 250 μg/mL) was generated by Otet RED systems and 5 μg/mL DENV-3 VLP. Dissociation calculation was analyzed from 30 to 600 seconds (red) and from 30 to 1200 seconds (blue). Dissociation rate was measured by 9 different biosensorgrams and average ± SD is shown. C, Difference in biotinylated DENV-1 VLP. Five lots of DENV-1 VLP (lot No. 15062616, 18011816, 18042710, 19040109, and 20012911) were biotinylated with 50 excess moles of EZ-Link Sulfo-NHS-Biotin. Antibody avidity index was measured using the Octet HTX system with 7 different anti-dengue polyclonal antibodies purified from DEN-203 volunteer serum (volunteer No. 1044010 day 90, 1053009 day 90, 1053011 day 120, 1071001 day 90, 1071009 day 90, 1083005 day 0, and 1093001 day 90) at 5 μg/mL of biotinylated DENV-1 VLPs. Graph shows average ± 95% confidence interval. D, Linearity of avidity assay. Antibody avidity index was measured using Octet HTX (FortéBio) and biotinylated DENV-1 VLP. Anti-dengue polyclonal antibody from DEN-203 volunteer No. 1053005, day 90, serum was used in the assay. Antibody concentrations were 10 to 70 μg/mL and measured 15 times. Data are average ± SD. Equations were DENV-1 response, y = 0.0103x + 0.087; log10[koff], y = 0.0033x − 3.927; and log10[avidity index], y = 0.0076x + 3.138). Abbreviations: Conc., concentration; DENV, dengue virus; koff, antibody dissociation rate constant; VLP, virus-like particle.
Next, reproducibility of the avidity assay was confirmed. Reproducibility was determined using 5 biotinylated DENV-1 VLP lots (Figure 3C). For intraplate reproducibility, coefficient of variation of log10[avidity index] ranged from 2.46% to 10.08% and the interplate range was 99%–108% (Supplementary Table 4). High linearity between response and IgG concentration was observed for all serotypes (DENV-1, P < .0001; R2 = 0.977). However, koff rates were not dependent on antibody concentrations (DENV-1, P = .0001; R2 = 0.137). Avidity index, calculated by response/koff, also showed positive correlation with IgG concentrations (DENV-1, P < .0001; R2 = 0.495) (Figure 3D and Supplementary Figure 2B).
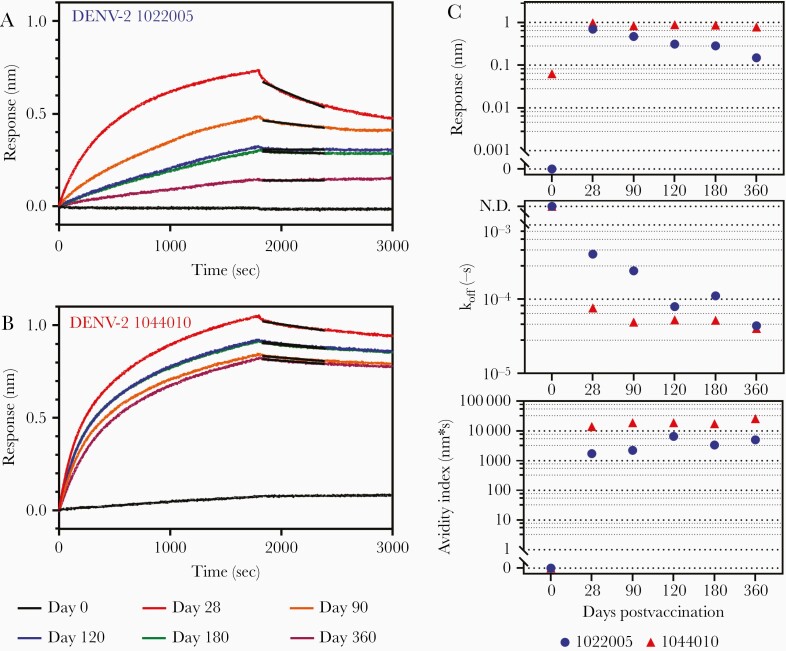
Polyclonal Serum Antibody Avidity After Vaccination
Sequential serum samples from 56 DEN-203 volunteers (day 0, 28, 90, 120, and 360) were analyzed to assess the kinetics of antibody avidity following administration of first (day 0) and second (day 90) TAK-003 doses. Kinetics of polyclonal antibody affinity maturation differed among individual DEN-203 study volunteers, with 2 different general patterns observed, each in about half of the vaccinated individuals. In some individuals, polyclonal affinity maturation gradually increased after the first vaccination and increased further following the second vaccination. For example, in volunteer number 1022005 (Figure 4A), after initial vaccination, rate of antibody dissociation (koff) gradually decreased between day 28 and day 90. The koff further decreased on day 120, following the second vaccination at day 90 and remained stable through day 180 (Figure 4C). Avidity index, calculated by response/koff, reflect these changes; the value increased from 0 to 120 days and remained stable through day 180 (Figure 4C). In other individuals, avidity index increased rapidly following the first vaccine dose and the high-avidity antibodies persisted in the serum throughout 360 days. For example, in study volunteer number 1044010 (Figure 4B), antibody responses were high and koff low at 28 days, translating to a high avidity index at the first postvaccination time point, a pattern that persisted throughout the 360-day study period (Figure 4C).
Figure 4.
Biosensorgram of dengue vaccine recipients and avidity assay parameters. A and B, Biosensorgrams at 0, 28, 90, 120, 180, and 360 days of clinical trial DEN-203 (A) volunteer No. 1022005 and (B) volunteer No. 1044010. Antibody avidity index was measured using the Octet RED system (FortéBio) and DENV-2 virus-like particles. Anti-dengue polyclonal antibody was used at 250 μg/mL to measure response and dissociation rates. Black line, dissociation calculation curve by Langmuir 1:1 binding model. C, Antibody response, koff, and avidity index of dengue vaccine recipient’s serum (volunteer No. 1022005 and No. 1044010). Assays were done in duplicate and averages are shown. Abbreviations: DENV, dengue virus; koff, antibody dissociation rate constant.
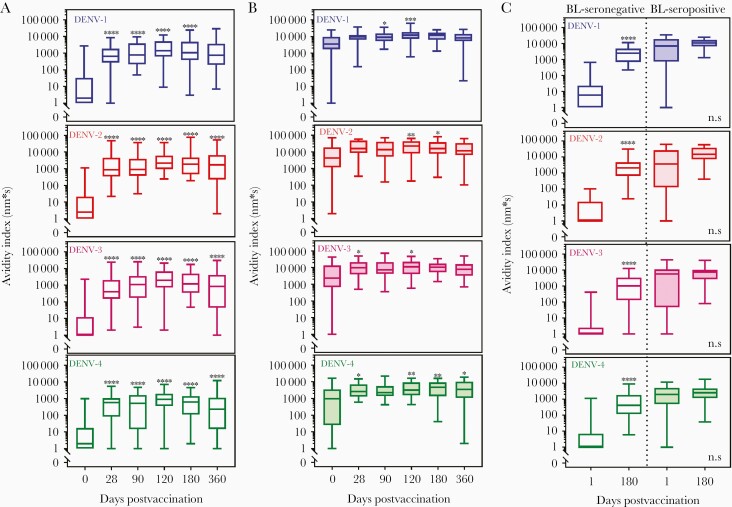
Among the 56 DEN-203 volunteers, 37 individuals were seronegative for any dengue antibodies prior to vaccination, as determined by neutralizing antibody assay [34]. In baseline seronegative volunteers, binding antibody responses were negative before vaccination and significantly increased after a single dose for all serotypes (Supplementary Figure 3A). Overall, responses increased slightly after the second vaccine dose (day 120) and declined but remained positive at day 360. Within each individual, in general, kinetics of polyclonal antibody affinity maturation was similar among the 4 DENV serotypes, although magnitude of DENV-specific binding antibodies varied among serotypes. Generally, responses were highest to DENV-2 and lowest to DENV-4. Binding koff (Supplementary Figure 4A) for all 4 dengue serotypes decreased over time, demonstrating increasing strength of antibody binding to dengue VLPs. The koff in 0% (DENV-1) to 22% (DENV-3) sera at day 120 reached less than 2×10−5-s, meaning the antibodies bound too tightly to be dissociated within 1200 seconds. Avidity increased significantly after the first vaccine dose and continued to increase or remain stable throughout the 1-year period of observation (Figure 5A). Avidity of antibodies to DENV-1, -2, and -3 were similar, while DENV-4 antibody avidity overall was lowest. In general, in seronegative study volunteers after vaccination, the strength of serum polyclonal antibody binding and avidity index to all 4 DENV serotypes increased over time, and the high-avidity antibodies persisted in the serum through 360 days.
Figure 5.
Avidity index data generated from TAK-003 recipients: (A) clinical trial DEN-203 baseline seronegative n = 37; (B) DEN-203 baseline seropositive n = 19; and (C) clinical trial DEN-204 baseline seronegative n = 21 and baseline seropositive n = 15. Data were obtained using the Octet HTX system (FortéBio) and DENV-1, DENV-2, DENV-3, and DENV-4 virus-like particles. A and B, Serum samples in trial DEN-203 were collected on study days 0 (baseline/prevaccination; first TAK-003 dose administered), 28, 90 (second TAK-003 dose administered), 120, 180, and 360. C, Serum samples in trial DEN-204 were collected on study day 0 (baseline/prevaccination; first TAK-003 dose administered) and day 180 (3 months after administration of second dose). Avidity index = response/koff. ****P < .0001, ***P < .0002, **P < .0021, *P < .0332 vs day 0 (Wilcoxon signed-rank test). Negative avidity index data were extrapolated to 1 for drawing purpose. Bars show minimum and maximum, boxes 25 and 75 percentile, and lines median. Abbreviations: DENV, dengue virus; koff, antibody dissociation rate constant; ns, not significant.
Of the 56 DEN-203 volunteers, 19 were seropositive for antibodies against at least 1 dengue serotype prior to vaccination. As expected, many seropositive vaccine recipients had positive responses prevaccination (Figure 5B). Prevaccination, binding antibodies to all 4 serotypes were present in most individuals. Responses increased overall postvaccination and persisted to day 360 in the majority of individuals (Supplementary Figure 3B). The serum of some seropositive vaccine recipients contained high-avidity antibodies prior to vaccination, as demonstrated by low koff on day 0. Postvaccination, koff decreased over time for all serotypes (Supplementary Figure 4B); 6% (DENV-1) to 50% (DENV-4) of volunteer for each serotype reached undetectable dissociation at day 120. Avidity indices for all 4 dengue serotypes increased over time (Figure 5B). In general, both pre- and postvaccination avidity indices in seropositive individuals were higher than in seronegative individuals. Overall, vaccination increased polyclonal serum antibody avidity in seropositive individuals, but the increase was less than in seronegative individuals, due to the presence of high-avidity antibodies prior to vaccination in some samples. In seropositive individuals, high-avidity antibodies persisted in serum throughout the 1-year period of observation.
To confirm the results of DEN-203, sera from 36 randomly selected DEN-204 volunteers collected on day 1 and day 180 were tested to assess the impact of vaccination on avidity (Figure 5C, Supplementary Figure 3C, and Supplementary Figure 4C). As in DEN-203, responses in DEN-204 seronegative volunteers were negative prevaccination and were significantly increased for all 4 dengue serotypes at day 180 after a single dose of TAK-003 (Supplementary Figure 3C). Binding antibody responses were lowest for DENV-4 and similar for DENV-1, -2, and -3. Binding koff for all 4 serotypes was decreased at day 180 (Supplementary Figure 4C). Avidity increased significantly after vaccination, with the highest avidity antibodies to DENV-1, -2, and -3, and lower DENV-4 avidity (Figure 5C).
Most baseline seropositive vaccine recipients were positive for binding antibody responses to all 4 dengue serotypes and displayed high-avidity antibodies prior to vaccination, as demonstrated by low koff on day 1 (Supplementary Figure 4C). Postvaccination increases in binding antibodies and decrease in koff was marginal for all serotypes and consequently avidity index increased marginally after vaccination (Figure 5C and Supplementary Figure 3C).
Correlation Between Antibody Titers and Avidity Index
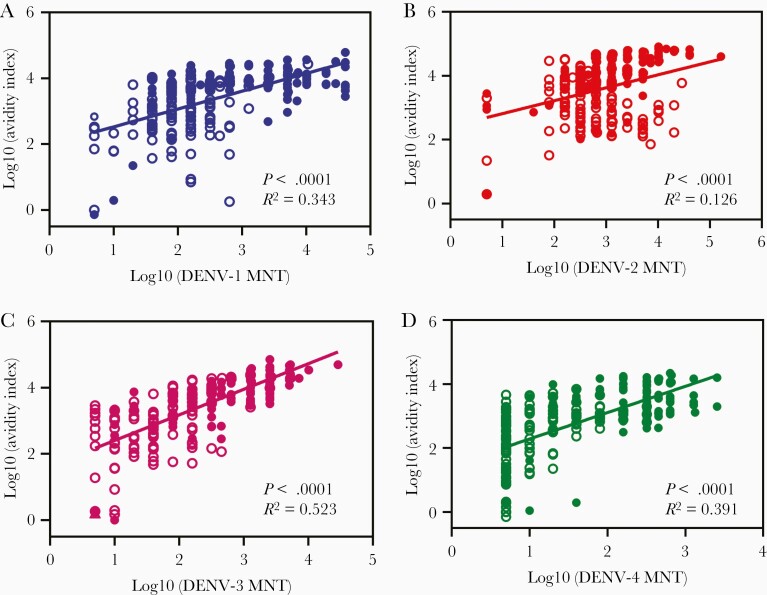
We assessed the degree of correlation between neutralizing antibody titers and avidity index parameters in DEN-203 study sera. When combining data for 43 subjects and study days for each dengue serotype, we found weak to no correlation between MNT titer and response, koff, or avidity index (Figure 6, Supplementary Figure 5, and Supplementary Table 6). Likewise, there was weak to no correlation between MNT titer and avidity index when results at each study day were analyzed separately (Supplementary Figure 6 and Supplementary Figure 7). However, there was good to weak correlation among each serotype avidity index (Supplementary Figure 8).
Figure 6.
Correlation between avidity assay parameters. Avidity index and MNT titers: (A) DENV-1, (B) DENV-2, (C) DENV-3, and (D) DENV-4. Data from clinical trial DEN-203 (24 base line seronegative and 19 seropositive volunteers) were used apart from baseline seronegative day 0 data. The number of data points is 226–228: open symbols, baseline seropositive and closed symbols, baseline seronegative. Correlation between response, koff, and MNT titer are shown in Supplementary Figure 5 and correlation analysis data in Supplementary Table 6. Abbreviations: DENV, dengue virus; koff, antibody dissociation rate constant; MNT, microneutralization test.
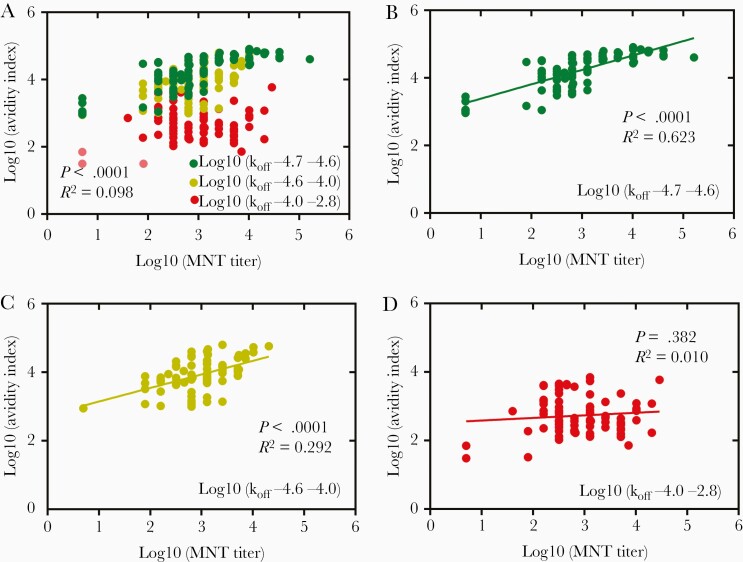
Next, we analyzed the degree of correlation between neutralizing antibody titer and avidity index in samples with high, medium, and low koff values, representing sera with a low, medium, and high degree of antibody affinity maturation. For all serotypes, the degree of correlation between MNT and avidity index was lowest among samples with high koff values (less affinity matured: DENV-1, P < .0001, R2 = 0.193; DENV-2, P = .382, R2 = 0.010; DENV-3, P < .0001, R2 = 0.341; DENV-4, P < .0001, R2 = 0.551) and highest in samples with lower koff values (more affinity matured: DENV-1, P < .0001, R2 = 0.403; DENV-2, P < .0001, R2 = 0.623; DENV-3, P < .0001, R2 = 0.814; DENV-4, P < .0001, R2 = 0.623) (Figure 7, Supplementary Figure 9, and Supplementary Table 7). These trends were also observed between MNT and responses. Thus, this finding suggests a relationship between high-affinity antibodies and neutralizing activity.
Figure 7.
Correlation between DENV-2 avidity index and MNT antibody titer for subjects divided by low, medium and high koff ranges. A, Correlation analysis using all data sets, n = 230. B–D, Correlation analysis data divided into 3 ranges by log10 koff values: (B) log10[koff] −4.7 to −4.6, n = 77; (C) log10[koff] −4.6 to −4.0, n = 77; (D) log10[koff] −4.0 to −2.8, n = 76. Data were from 24 baseline seronegative and 19 seropositive volunteers and data under response LoD (0.015 nm; Supplementary Table 5) were eliminated from the analysis. Correlation of DENV-1, DENV-3, and DENV-4 are shown in Supplementary Figure 9. Correlation analysis date parameters are shown in Supplementary Table 7. Abbreviations: DENV, dengue virus; koff, antibody dissociation rate constant; LoD, limit of detection; MNT, microneutralization test.
DISCUSSION
The process of antibody affinity maturation forms the basis for evolution of effective antibody responses to specific pathogens from the diverse B-cell repertoire. The importance of antibody affinity maturation to effective antiviral responses is well established. Influenza antibody affinity correlates with neutralization potency and breadth [3]. Affinity maturation of B cells specific for conserved epitopes after sequential exposure to infection is required for protection from reinfection by diverse influenza viruses [38]. A common theme of successful antiviral immunity is induction of high-affinity functional antibodies to conserved epitopes, in the context of abundant ineffective immune responses to variable viral epitopes.
Information on antibody affinity maturation following dengue infection is limited; dengue disease severity was associated with lower antibody avidity index, as assessed by ELISA [39]. Similarly, antibody avidity to Zika E-protein negatively correlated with disease severity [4]. Repeated dengue infections have been shown to increase monoclonal and polyclonal antibody avidity and increased neutralization potency [40].
Collectively, antibodies with increased affinities after antigen exposure contribute to an overall increase in polyclonal antibody avidity. The measurement of avidity index, response divided by dissociation rate, was proposed by Lynch et al [19] and reflects both antibody concentration and affinity maturation. Methods to measure polyclonal antibody avidity to peptides or soluble proteins have been reported [4, 17–20]; however, characterization of polyclonal antibody avidity to viral particles or other particulate antigens has proven challenging. We have developed a novel assay to assess the avidity of serum polyclonal antibodies specific for DENV-1, -2, -3, and -4 particles by BLI. Antigen selection and characterization is a key factor in development of dengue antibody assays. Limitations to the use of VLPs as an antigen include differences in arrangement of E-dimers on VLPs versus virions and potential differences in maturation state of VLPs and virions [21]. Nevertheless, VLPs are noninfections, more stable than live virions, can be purified, and retain many antigenic features of dengue virions [21].
We found that reproducible avidity measurement required purification of IgG from serum. Several methods are available for rapid and simple purification of serum IgG [17, 20]; however, some nonspecific binding remained. We selected Protein G Sepharose to further reduce nonspecific binding activity. Even after IgG purification, the 384-well plate format of the Octet HTX makes it possible to test avidity to all 4 dengue serotypes using less than 50 µL serum.
As antibody avidity increased rapidly in serum of some volunteers following TAK-003 vaccination, it was necessary to use a high ionic strength buffer to accelerate antibody dissociation. In some volunteers, 2-phase dissociation was observed, that is higher dissociation rate in the early phase and lower rate in the later phase. Similar kinetics of dissociation rate have been reported previously [19]. These changes may be caused by antibody rebinding to VLP. Therefore, to prevent overestimation, 50% dissociation curves (600 seconds) were applied to measure dissociation rate.
BLI was used to study kinetics of polyclonal serum avidity to DENV-1, -2, -3, and -4 following vaccination of dengue seropositive and seronegative volunteers with TAK-003. In seronegative volunteers, antibody affinity maturation, as evidenced by decline of antibody dissociation rate, was observed beginning after the first vaccination. Further decline of antibody dissociation rate was also observed in some individuals following the second TAK-003 dose. In general, high-avidity antibodies persisted in the serum through the 360-day follow-up period. Prior dengue exposure, determined by seropositivity, increased antibody avidity. In general, if high-avidity antibodies were present to a dengue serotype, vaccination did not increase avidity, but postvaccination antibody avidity was increased in seropositive volunteers with lower-avidity serum antibodies prevaccination. The volunteers in DEN-203 and DEN-204 represented a range of ages (1.5 to 45 years) and geographical locations (Puerto Rico, Colombia, Singapore, Thailand, Dominican Republic, Panama, and Philippines). Avidity results were consistent between the 2 studies, and avidity testing was conducted on sera that were broadly representative of the studies as a whole in terms of tetravalent seroconversion rate. Overall, the results demonstrate that vaccination with TAK-003 can drive polyclonal antibody affinity maturation in seronegative and seropositive adults and children across dengue-endemic regions.
Surprisingly, correlation between MNT titer and avidity assay parameters was weak. Correlation between MNT titer and avidity index was highest in samples with higher affinity maturation. These data suggest that neutralizing antibodies are affinity matured, but the affinity-matured antibody population also includes nonneutralizing antibodies. Correlation between neutralizing antibodies and affinity maturation has been described for human immunodeficiency virus (HIV) and influenza antibodies [41, 42]. Different viruses may elicit high-avidity antibodies that vary in functional properties. The functional antiviral activities of other dengue-specific affinity matured antibodies will be assessed in future studies.
In conclusion, we have developed a novel, high-throughput avidity assay using dengue VLPs and applied it to evaluate evolution of polyclonal serum antibody avidity following vaccination with a tetravalent DENV vaccine candidate, TAK-003, in two phase 2 trials. Vaccination drove antibody affinity maturation against all 4 dengue serotypes, with high-affinity antibodies detectable in serum through 1 year postvaccination. Development of this method will facilitate a deeper understanding of the mechanisms of induction of immunity to dengue infection and vaccination, including the relationship between affinity-matured antibodies and protection against dengue infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all clinical trial volunteers and their parents/legal guardians, and the investigators and site staff for their contributions; Kelley Moss, Astrid Borkowski, Derek Wallace, and the Takeda Development team; and Ankita Sanjali, Jonathan Hernandez, Nicole Messere, Lovkesh Karwal, Fue Vang, Sunil Palani, Christina DeMaso, Swati Mukherjee, Ralph Braun, Hetal Patel, Steph Sonnberg, and Mayuri Sharma for technical assistance and suggestions. The authors thank Dr James Crowe, Dr Ralph Baric, and Dr Aravinda de Silva for providing monoclonal antibodies. The authors appreciate technical support from ForteBio and are grateful to Dr J Stirling (OLC Bioscience Ltd, London, UK) for editorial assistance in the preparation of this manuscript.
Author contributions . I. T., M. E., and H. D. carried out conception and design of the study. I. T. and H. D. developed the statistical design. I. T., D. D., and H. D. performed acquisition, analysis, and interpretation of data. All authors were involved in the decision to submit for publication.
Financial support. This work was supported by Takeda Vaccines, Inc.
Potential conflicts of interest. I. T., D. D., and H. D. are employees of Takeda Vaccines, Inc and M. E. is a former employee of Takeda Vaccines, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Tropical Medicine and Hygiene 68th Annual Meeting, 20–24 November 2019, National Harbor, MD.
References
- 1. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol 2012; 30:429–57. [DOI] [PubMed] [Google Scholar]
- 2. Doria-Rose NA, Joyce MG. Strategies to guide the antibody affinity maturation process. Curr Opin Virol 2015; 11:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pappas L, Foglierini M, Piccoli L, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 2014; 516:418–22. [DOI] [PubMed] [Google Scholar]
- 4. Ravichandran S, Hahn M, Belaunzarán-Zamudio PF, et al. Differential human antibody repertoires following Zika infection and the implications for serodiagnostics and disease outcome. Nat Commun 2019; 10:1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker LM, Phogat SK, Chan-Hui PY, et al. ; Protocol G Principal Investigators . Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009; 326:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008; 453:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klasse PJ. How to assess the binding strength of antibodies elicited by vaccination against HIV and other viruses. Expert Rev Vaccines 2016; 15:295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol 2002; 83:2091–108. [DOI] [PubMed] [Google Scholar]
- 9. Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol 2001; 77:195–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Negl Trop Dis 2013; 7:e2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slon Campos JL, Mongkolsapaya J, Screaton GR. The immune response against flaviviruses. Nat Immunol 2018; 19:1189–98. [DOI] [PubMed] [Google Scholar]
- 12. Chen Z, Ren X, Yang J, et al. An elaborate landscape of the human antibody repertoire against enterovirus 71 infection is revealed by phage display screening and deep sequencing. MAbs 2017; 9:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sundling C, Zhang Z, Phad GE, et al. Single-cell and deep sequencing of IgG-switched macaque B cells reveal a diverse Ig repertoire following immunization. J Immunol 2014; 192:3637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boxus M, Lockman L, Fochesato M, Lorin C, Thomas F, Giannini SL. Antibody avidity measurements in recipients of Cervarix vaccine following a two-dose schedule or a three-dose schedule. Vaccine 2014; 32:3232–6. [DOI] [PubMed] [Google Scholar]
- 15. Dysinger M, King LE. Practical quantitative and kinetic applications of bio-layer interferometry for toxicokinetic analysis of a monoclonal antibody therapeutic. J Immunol Methods 2012; 379:30–41. [DOI] [PubMed] [Google Scholar]
- 16. O’Shannessy DJ, Brigham-Burke M, Soneson KK, Hensley P, Brooks I. Determination of rate and equilibrium binding constants for macromolecular interactions using surface plasmon resonance: use of nonlinear least squares analysis methods. Anal Biochem 1993; 212:457–68. [DOI] [PubMed] [Google Scholar]
- 17. Canelle Q, Dewé W, Innis BL, van der Most R. Evaluation of potential immunogenicity differences between Pandemrix™ and Arepanrix™. Hum Vaccin Immunother 2016; 12:2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dennison SM, Reichartz M, Seaton KE, et al. Qualified biolayer interferometry avidity measurements distinguish the heterogeneity of antibody interactions with Plasmodium falciparum circumsporozoite protein antigens. J Immunol 2018; 201:1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lynch HE, Stewart SM, Kepler TB, Sempowski GD, Alam SM. Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen. J Immunol Methods 2014; 404:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Persson J, Zhang Y, Olafsdottir TA, et al. Nasal immunization confers high avidity neutralizing antibody response and immunity to primary and recurrent genital herpes in guinea pigs. Front Immunol 2016; 7:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Metz SW, Thomas A, White L, et al. Dengue virus-like particles mimic the antigenic properties of the infectious dengue virus envelope. Virol J 2018; 15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teoh EP, Kukkaro P, Teo EW, et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 2012; 4:139ra183. [DOI] [PubMed] [Google Scholar]
- 23. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization (WHO). Dengue: guidelines for diagnosis, treatment, prevention and control: new edition. Geneva, Switzerland: WHO, 2009. [PubMed] [Google Scholar]
- 25. Halstead SB, Venkateshan CN, Gentry MK, Larsen LK. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J Immunol 1984; 132:1529–32. [PubMed] [Google Scholar]
- 26. Oliphant T, Engle M, Nybakken GE, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 2005; 11:522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith SA, de Alwis AR, Kose N, et al. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 2013; 4:e00873–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shrestha B, Brien JD, Sukupolvi-Petty S, et al. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog 2010; 6:e1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Alwis R, Smith SA, Olivarez NP, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 2012; 109:7439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fibriansah G, Tan JL, Smith SA, et al. Highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 2015; 6:e6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sukupolvi-Petty S, Brien JD, Austin SK, et al. Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection. J Virol 2013; 87:8826–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis 2016; 213:1562–72. [DOI] [PubMed] [Google Scholar]
- 33. Sáez-Llorens X, Tricou V, Yu D, et al. Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2017; 17:615–25. [DOI] [PubMed] [Google Scholar]
- 34. Osorio JE, Velez ID, Thomson C, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. ASTM International. ASTM E685-93(2013). Standard practice for testing fixed-wavelength photometric detectors used in liquid chromatography. West Conshohocken, PA: ASTM International, 2013. [Google Scholar]
- 36. Fibriansah G, Lok SM. The development of therapeutic antibodies against dengue virus. Antiviral Res 2016; 128:7–19. [DOI] [PubMed] [Google Scholar]
- 37. GE Healthcare. Affinity chromatography. Vol. 1. Antibodies. Uppsala, Sweden: GE Healthcare, 2016:47–9. [Google Scholar]
- 38. Leach S, Shinnakasu R, Adachi Y, et al. Requirement for memory B-cell activation in protection from heterologous influenza virus reinfection. Int Immunol 2019; 31:771–9. [DOI] [PubMed] [Google Scholar]
- 39. Lau L, Green AM, Balmaseda A, Harris E. Antibody avidity following secondary dengue virus type 2 infection across a range of disease severity. J Clin Virol 2015; 69:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsai WY, Lai CY, Wu YC, et al. High-avidity and potently neutralizing cross-reactive human monoclonal antibodies derived from secondary dengue virus infection. J Virol 2013; 87:12562–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang R, Verkoczy L, Wiehe K, et al. Initiation of immune tolerance-controlled HIV gp41 neutralizing B cell lineages. Sci Transl Med 2016; 8:336ra362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khurana S, Hahn M, Coyle EM, et al. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat Commun 2019; 10:3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.