Abstract
Background
Prognostic factors and role of treatments are not well known in isocitrate dehydrogenase (IDH) wild-type (wt) grade 2 astrocytomas. The aim of this study was to define in these tumors clinical features, molecular characteristics, and prognostic factors, with particular focus on molecular subgroups defined by cIMPACT-NOW update 3.
Methods
We analyzed 120 patients with confirmed diagnosis of grade 2 IDHwt astrocytoma according to WHO 2016, collected from seven Italian centers between 1999 and 2017.
Results
Median PFS and OS of the whole cohort were 18.9 and 32.6 months. Patients older than 40 years and patients with modest contrast enhancement on MRI had a shorter PFS and OS. Gross total resection yielded superior PFS and OS over non-gross total resection. PFS and OS of patients with either pTERT mutation or EGRF amplification were significantly shorter. The prognostic value of age, contrast enhancement on MRI, and extent of surgery was different within the molecular subgroups. Gross total resection was associated with increased PFS (not reached versus 14 months, p = 0.023) and OS (117.9 versus 20 months, p = 0.023) in patients without EGFR amplification, and with increased OS in those without pTERT mutation (NR vs 53.7 months, p = 0.05). Conversely, for patients with EGFR amplification or pTERT mutation, gross total resection did not yield a significant survival benefit.
Conclusion
Patients without EGFR amplification and pTERT mutation could be observed after gross total resection.
Keywords: EGFR amplification, IDHwt grade 2 astrocytomas, pTERT mutation, prognostic factors, surgery
Key Points.
In IDHwt gr.2 astrocytomas the prognostic role of age, contrast enhancement, and gross total resection (GTR) differs based on EGFR/pTERT status.
Patients without EGFR amplification and pTERT mutation take advantage of GTR and observation seems a reasonable approach.
Importance of the Study.
There is lack of information on prognostic factors and role of treatments in IDHwt grade 2 astrocytomas according to WHO 2016. The cIMPACT-NOW update 3 has further subdivided these tumors by identifying a subgroup with molecular features of glioblastoma (EGFR amplification and/or 7q gain/10p loss and/or pTERT mutation) and poor outcome. We analyzed the largest series of IDHwt grade 2 astrocytomas with particular focus on molecular subgroups as defined by the cIMPACT-NOW update 3, and reports some novel findings. The prognostic value of age, contrast enhancement on MRI, and extent of surgery differs within the molecular subgroups. Patients without EGFR amplification and pTERT mutation display longer PFS and OS after gross total resection and could be observed, while patients with either EGFR amplification or pTERT mutation, in whom the advantage of gross total resection is less clear that far, could benefit from adjuvant treatments.
The 2016 WHO classification of brain tumors1 defined different subgroups of diffuse lower-grade (grade 2 and 3) gliomas based on the presence of isocitrate dehydrogenase (IDH) 1/2 mutation, with or without coexistence of 1p/19q codeletion. The IDH 1/2 mutation is strongly associated with improved survival, and the frequency is inversely related to tumor grade.2,3
IDH wild-type (IDHwt) diffuse astrocytomas are a rare group of tumors, which display heterogeneous clinical features, molecular characteristics, and poor outcome.4,5 A subgroup of patients harbors molecular abnormalities typically associated with glioblastoma (WHO grade 4), such as EGFR amplification and/or 7q gain/10p loss and/or pTERT mutation: thus, the cIMPACT-NOW Consortium for taxonomy of primary brain tumors has suggested to reclassify these IDHwt diffuse lower-grade gliomas (WHO grade 2 and 3) as diffuse astrocytic gliomas, IDHwt with molecular features of glioblastoma, WHO grade 4.6,7 A recent retrospective study has confirmed that these patients display a survival comparable to that of patients with IDHwt glioblastoma8; however, another study has reported that IDHwt grade 2 tumors with molecular features of glioblastoma have a significantly longer survival as compared to those of grade 3.9 Conversely, a smaller subgroup of IDHwt grade 2 tumors without molecular features of glioblastoma has a more indolent course and long-term survival.5
With regard to treatment of grade 2 IDHwt gliomas there is lack of information on the value of surgery and adjuvant radiotherapy and chemotherapy,10,11 as most studies have analyzed together grade 2 and 3 tumors.12
The aim of this retrospective study was twofold: to better define the clinical features, molecular characteristics, and prognostic factors of IDHwt grade 2 diffuse astrocytomas; to address the impact of extent of surgery and adjuvant treatments within the molecular subgroups as defined by cIMPACT-NOW Consortium update 3.
Patients and Methods
This national multicenter retrospective study was conducted on behalf of the Italian Association of Neuro-Oncology (AINO).
We initially searched the database of each Institution for patients ≥18 years, diagnosed with WHO grade 2 IDHwt diffuse glioma of the cerebral hemispheres from January 1999 to May 2017. All histological diagnoses were revised by dedicated neuropathologists based on WHO 2016 classification. The presence of suspected anaplastic foci in the histological specimen was an exclusion criterion. Sequencing of IDH genes was carried out in all cases in order to rule out the presence of either IDH1 or IDH2 mutations. 1p/19q codeletion was excluded by fluorescence in situ hybridization (FISH). pTERT mutations were identified by Sanger Sequencing and amplification of EGFR gene was analyzed by FISH.
We did not include patients with midline gliomas and patients with stereotactic biopsies to avoid potential biases due to limited tissue sampling. Conversely, extended open biopsies were included.
We retrieved data regarding clinico-radiological features, type of treatments, and outcome of eligible patients. Most MRI scans (113/120, 94.1%) were reviewed. Patients with an MRI pattern suggestive of high-grade glioma, such as ring-like or intense contrast enhancement, or of bilateral gliomatosis cerebri were excluded, as considered at high risk of sampling error and downgrading of the malignancy, especially in case of small biopsies.
The extent of surgical resection was estimated based on FLAIR images of an MRI performed between 2 and 3 months after surgery at the time of choice by a multidisciplinary group between observation and adjuvant treatment. Gross total resection (GTR) was defined as the absence of any signal abnormality around the surgical cavity, while all other instances were defined as non-gross total resection (non-GTR). In 86/120 (71.7%) of patients a volumetric estimation of extent of resection was performed: GTR was defined as 100% of resection and non-GTR as <100% of resection. Tumor volume data were obtained by analyzing structural imaging data routinely acquired during pre-surgical and post-surgical investigations in axial 3D FLAIR MRI slices. All tumor segmentations were realized by using the OSIRIX software tool (GNU LESSER, General Public License, Geneva, Switzerland). Specifically, the EOR was assessed as follows: (preoperative tumor volume—postoperative tumor volume)/preoperative tumor volume in axial FLAIR MRI images.
The project was approved by the Review Board of each participating Institutions. Informed consent to collect and analyze clinical and pathological/molecular data was obtained from all subjects who were alive at the time of start of the study or from relatives in case of death of the subjects, according to ethics regulations for retrospective studies of each local Ethics Committee.
Statistical Analysis
Baseline characteristics of patients included in the analysis are summarized using median and interquartile range (IQR), and percentages and frequencies (n, %). We adopted age at surgery as a surrogate of age at diagnosis. The observation period for progression-free survival (PFS) and overall survival (OS) started on the date of surgery until the date of recurrence or death, respectively, or until the last follow-up visit (censoring).
The distribution of characteristics between molecular subgroups was evaluated by the Mann–Whitney U test for continuous variables and the Chi-square test or Fisher’s exact test for categorical variables. Kaplan-Meier curves were drawn for PFS and OS and a Cox proportional hazard model was employed to estimate the crude and the multivariable-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) and to evaluate possible predictors of recurrence or survival. The proportional hazard assumption was also verified by graphical checks and formal tests based on Schoenfeld residuals.
The analysis was performed by IBM SPSS Statistics v.27 software.
Results
Clinical Characteristics
We initially collected a cohort of 194 patients with an IDHwt grade 2 diffuse glioma.
As the majority of patients were managed before 2016, original histological diagnosis was mostly based on 2007 (117, 96.0%) rather than 2016 (5, 4.0%) WHO classification, and in the original dataset, astrocytic, oligodendroglial, and mixed tumors were reported in 43.4, 35.2, and 21.4% of cases, respectively. Following the histologic, molecular, and clinical revision, 27 patients were excluded because IDH1 or 2 mutations were found by gene sequencing, 26 due to the presence of anaplastic foci in the histological specimen and 21 because of radiological features suggestive of glioblastoma or gliomatosis cerebri on MRI. Therefore, the original cohort was reduced to 120 eligible patients confirmed as IDHwt grade 2 diffuse astrocytomas. The clinical characteristics of the population are displayed in Table 1.
Table 1.
Patient Characteristics
| No. of patients | 120 | |
| Year of surgery | 1999–2017 | |
| Before 2010 | 30 | 25.0% |
| 2010–2012 | 33 | 27.5% |
| 2013–2014 | 34 | 28.3% |
| 2015–2017 | 23 | 19.2% |
| Sex | ||
| Male | 72 | 60.0% |
| Female | 48 | 40.0% |
| Median age, y (IQR) | 45 (18–78) | |
| Symptoms at onset | ||
| Seizures | 74 | 60.7% |
| Focal seizures with secondary generalization | 53 | 71.6% |
| Focal seizures with maintained awareness | 17 | 23.0% |
| Focal seizures with impaired awareness | 4 | 5.4% |
| Focal neurological symptoms | 25 | 20.5% |
| Headache/intracranial hypertension | 19 | 15.6% |
| Cognitive impairment/behavioral changes | 8 | 6.5% |
| Dizziness/gait disturbances | 7 | 5.7% |
| None | 5 | 4.1% |
| Contrast enhancement | ||
| No | 83 | 73.5% |
| Yes | 30 | 26.5% |
| Main tumor location | ||
| Temporal | 52 | 43.3% |
| Frontal | 42 | 35.0% |
| Parietal | 16 | 13.3% |
| Insula | 8 | 6.7% |
| Occipital | 2 | 1.7% |
| Tumor extension | ||
| 1 lobe | 79 | 65.9% |
| ≥ 2 lobes | 23 | 19.1% |
| 1 or > 1 lobes with involvement of deep structures (either corpus callosum, basal ganglia or brainstem) | 18 | 15.0% |
| Molecular data | ||
| pTERT mutation | ||
| pTERT-mutant | 30 | 50.8% |
| pTERT-intact | 29 | 49.2% |
| EGFR amplification | ||
| EGFR-amplified | 9 | 14.0% |
| EGFR-intact | 54 | 86.0% |
| pMGMT methylation | ||
| pMGMT-methylated | 31 | 40.0% |
| pMGMT-unmethylated | 46 | 60.0% |
| pTERT-mutant and EGFR-amplified | 1 | 2.0% |
| Extent of resection (EOR) | ||
| Non-gross total resection (non-GTR) | 83 | 69.2% |
| Gross total resection (GTR) | 37 | 30.8% |
| Postoperative KPS | ||
| 80–100 | 120 | 100.0% |
| Postoperative management | ||
| Observation with MRI | 57 | 47.5% |
| Radiotherapy or radio-chemotherapy | 37 | 30.8% |
| Upfront chemotherapy alone | 26 | 21.7% |
| Disease progression | ||
| Yes | 78 | 65.0% |
| No | 31 | 25.8% |
| Patients’ status | ||
| Dead | 81 | 67.5% |
| Alive | 33 | 27.5% |
| Lost to follow-up | 6 | 5.0% |
IQR: interquartile range; KPS: Karnofsky Performance Status.
Molecular Subgroups and Correlation with Clinical Characteristics
pTERT status was analyzed in 59 (49.0%) patients, and pTERT mutation was present in 30 (50.8%) and absent in 29 (49.2%). EGFR status was analyzed in 63 patients (53.0%), and EGFR amplification was present in 9 (14.0%) and absent in 54 (86.0%). MGMT promoter status was investigated in 77 patients (68.8%), and was methylated in 31 (40.0%), and unmethylated in 46 (60.0%).
Median age, frequency of seizures, presence of contrast enhancement, MGMT methylation status, and gross total resection did not significantly differ across subgroups with or without pTERT mutation and/or EGFR amplification, while adjuvant treatments were slightly more frequent among patients with TERT promoter mutation (Supplementary Table S1). Moreover, the distribution of molecular alterations (EGFR amplification, pTERT mutation, MGMTp methylation) was not significantly different between GTR and non-GTR groups including biopsies (Supplementary Table S2).
First-Line Treatments
GTR was achieved in 37 patients (30.8%) and non-GTR in the remaining 85 patients (69.2%). In the 86 patients (71.7%) in whom a volumetric estimation of the EOR was obtained GTR was achieved in 32 cases (37.2%), and non-GTR in 54 (62.8%). Among non-GTR, extended open biopsies were 28 (23.3%). Supratotal resection (> 100% tumor resection in postoperative volumetric MRI) was reported in 5 patients only: due to the limited number we did not consider them separately from those with GTR for the statistical analysis.
Patients, who underwent non-GTR, had a significantly higher age (51.0 vs 41.5 y, p = 0.001) without any other significant difference at baseline.
After surgery, 57 patients (47.5%) were observed with MRI and 63 patients (52.5%) received an adjuvant treatment which consisted of radiotherapy with concurrent and adjuvant temozolomide (31 patients, 25.8%), chemotherapy with temozolomide or procarbazine, lomustine, and vincristine (PCV) (26 patients, 21.7%) or radiotherapy (6 patients, 5.0%). Radiotherapy was delivered by conformal fields with a median radiation dose of 56 Gy (40–60 Gy) in conventional fractionation.
Recurrence
Seventy-eight patients (65.0%) had a recurrence, whereas 31 (25.8%) were progression-free since first surgery. Data about clinical follow-up of 11 patients (9.2%) after first-line treatment were not available. MRI at recurrence was available in 51 patients (65.4%): 33 (64.7%) lesions developed contrast enhancement, which was absent in the original MRI in 25 (75.7%). Multifocal progression accounted for 13 (16.7%) patients, and 5 (6.4%) developed a leptomeningeal spread: 4/5 underwent a non-GTR, 5/5 received temozolomide (4 as part of Stupp regimen, one as upfront chemotherapy), and 5/5 presented a concomitant multifocal relapse in the brain. Treatments at recurrence are listed in Supplementary Table S3.
Fourteen (17.9%) patients underwent reoperation, which was the second most common option at recurrence: the diagnosis of grade 2 astrocytoma was confirmed in 5 patients (36.0%), while 6 (43.0%) and 3 (21.0%) patients showed an upgrade of histological malignancy toward GBM or grade 3 glioma, respectively. Seven of the 9 patients with increased malignancy had either EGFR amplification or pTERT mutation. Median time to second surgery was 23.4 months (2.2–132.6). A higher grade of malignancy at second surgery prevailed among patients with earlier recurrence (median time to second surgery of 8.0 and 37.3 months for patients diagnosed either with grade 3/GBM or grade 2 glioma, respectively—p < 0.001). No significant difference was seen in terms of EOR between patients who developed a higher grade of malignancy and those who remained grade 2 at second surgery, with GTR being accomplished in 4/9 (44.4%) among the former and 0/5 among the latter (p = 0.078); however, the number of reoperated patients is still limited for a statistical analysis.
Prognostic Factors and Impact of Treatments in the Whole Cohort
Median time of follow-up was 36.6 months (1–225.6 months). At the time of the analysis, 33 patients (27.5%) were alive, 81 (67.5%) were dead and 6 (5.0%) were lost to follow-up. Median progression-free survival (PFS) and overall survival (OS) were 18.9 (11.6–26.2, 95% CI) and 32.6 (19.2–46.0, 95% CI) months, respectively. Disease-free patients at 3 and 5 years were 34.9% and 29.3%. Patients alive at 3 and 5 years were 49.1% and 34.6%.
Patients older than 40 years had a shorter median PFS of 12.0 months (7.5–16.5, 95% CI) vs 25.8 months (4.3–105.4, 95% CI) (p < 0.001), and a worse median OS of 23.3 months (19.2–46.0, 95% CI) vs NR (p < 0.001). Also, patients with contrast enhancement on MRI at diagnosis had a worse median PFS of 8.7 months (1.1–16.3, 95% CI) vs 24.0 months (11.8–36.1, 95% CI) (p = 0.027), and a worse OS of 21.0 months (11.2–30.8, 95% CI) vs 46.9 months (29.0–64.7, 95% CI) (p < 0.001). The occurrence of seizures at onset was associated with a PFS of 24.0 months (13.7–34.3 95% CI) vs 11.0 months (0.9–21.0, 95% CI) (p = 0.45), and an OS of 37.0 months (21.5–52, 95% CI) vs 28.4 months (12.7–44.0, 95% CI) (p = 0.86).
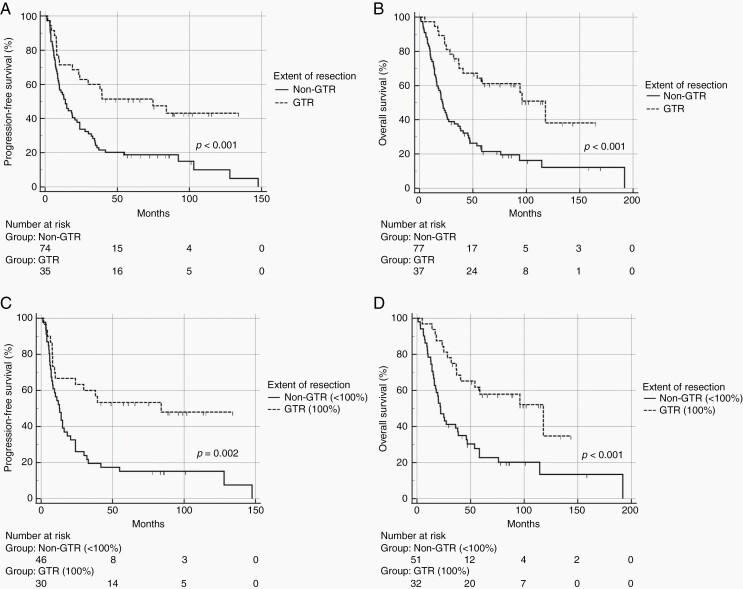
We next evaluated the impact of extent of resection (EOR) on PFS and OS (Figure 1). Median PFS was 74.7 months (18.8–130.6, 95% CI) for GTR and 14.0 months (8.6–19.4, 95% CI) for non-GTR (p < 0.001). Median OS was 117.9 months (83.6–152.2, 95% CI) for GTR and 21.6 months (17.1–26.0, 95% CI) for non-GTR (p < 0.001). When we analyzed patients with a volumetric estimation of the EOR (86, 71.7%), median PFS with GTR was 84.0 months (54.1–96.7 95% CI) vs 12.8 months (8.3–17.2 95% CI) with non-GTR (p = 0.002), and median OS with GTR was 117.9 months (39.6–196.2 95% CI) vs 22.0 months (15.8–28.2 95% CI) with non-GTR (p = 0.005).
Fig. 1.
Median PFS and median OS stratified for extent of resection (EOR) with qualitative estimation (panel A and B), and with volume quantification of the EOR (panel C and D). GTR: gross total resection; non-GTR: non-gross total resection.
As our series covered almost two decades, we investigated whether the evolution of surgical techniques over time could have affected the outcome. We defined four classes of patients: 30 patients (25%) underwent surgery between 1999 and 2009, 33 (28.0%) between 2010 and 2012, 34 (28.0%) between 2013 and 2014, and 23 (19.0%) between 2015 and 2017. The rate of GTR did not vary between the different periods (Supplementary Table S4), and PFS or OS were not significantly impacted by the year of surgery (Supplementary Table S5). However, the rate of GTR was significantly higher among patients who underwent an awake procedure (2 centers) as compared to those who were treated with conventional surgery (5 centers) (56.6% vs 24.7%, p = 0.003) (Supplementary Table S6).
Median PFS was 39.3 months (27.7–128.0, 95% CI) for patients who had observation with MRI, and 12.8 months (8.3–147.6, 95% CI) for patients who underwent an adjuvant treatment. Median OS was 114.5 months (58.6–192.0, 95% CI) for patients who had observation with MRI, and 22.0 months (16.1–26.1, 95% CI) for patients who underwent an adjuvant treatment. To control for the presence of selection biases in the observation and adjuvant treatment groups, we analyzed the distribution of age ≥ 40 years, presence of contrast enhancement, EOR, EGFR amplification, and pTERT mutation among observed and treated patients (Supplementary Table S7). Among patients who received adjuvant treatments there was a significant higher rate of non-GTR (54/63, 85.7% vs 29/57, 50.8%, p = 0.001) and a non-significant clustering of the other negative prognostic factors, thus partially explaining the poor prognosis of this group.
Multivariable analysis confirmed age and extent of surgery as significant prognostic factors for both PFS and OS, whereas contrast enhancement had a significant impact on OS only (Table 2).
Table 2.
Univariate and Multivariable Analysis of Clinical Prognostic Factors in the Whole Cohort
| PFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||||||
| Factor | HR | CI 95% | p-value | HR | CI 95% | p-value | HR | CI 95% | p-value | HR | CI 95% | p-value |
| Age | 2.368 | 1.420–3.949 | 0.001 | 1.855 | 1.064–3.234 | 0.029 | 3.215 | 1.795–5.759 | < 0.001 | 2.170 | 1.124–4.191 | 0.021 |
| Seizures at onset | 0.842 | 0.539–1.316 | 0.451 | 0.604 | 0.552–1.413 | 0.883 | 0.972 | 0.620–1.525 | 0.903 | 0.903 | 0.560–1.457 | 0.676 |
| Contrast enhancement | 1.774 | 1.059–2.971 | 0.029 | 1.693 | 0.994–2.882 | 0.052 | 2.331 | 1.439–3.776 | 0.001 | 2.500 | 1.536–4.067 | < 0.001 |
| GTR | 0.425 | 0.253–0.714 | 0.001 | 0.507 | 0.287–0.897 | 0.020 | 0.356 | 0.207–0.612 | 0.001 | 0.417 | 0.229–0.760 | 0.004 |
GTR: gross total resection.
Prognostic Factors and Impact of Treatments Within the Molecular Subgroups
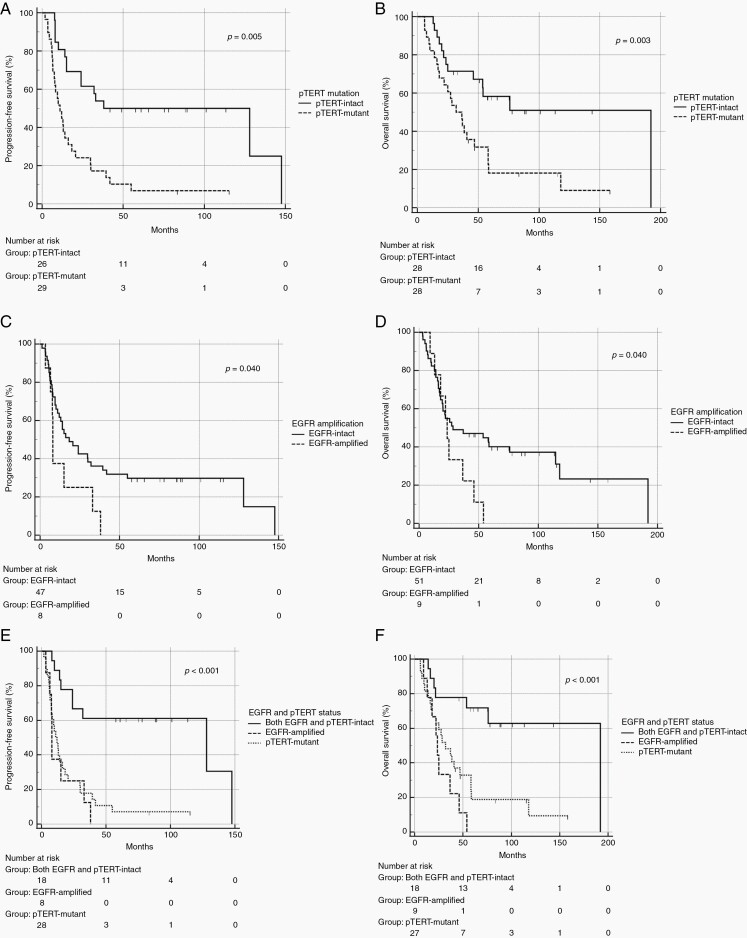
Both pTERT mutation and EGFR amplification correlated with a worse outcome (Figure 2). Median PFS and OS of patients with pTERT mutation were 11 (6.43–15.6, 95% CI) and 31.9 (19.3–44.4, 95% CI) months, as compared to 38 (1.0–94.4, 95% CI) and 192 (13–226, 95% CI) months for those without pTERT mutation (p = 0.005 and p = 0.003, respectively). Similarly, patients with EGFR amplification had a worse outcome than those without EGFR amplification, being median PFS 8 (7.6–8.4, 95% CI) months and median OS 23.5 (19.4–27.6, 95% CI) months for the EGFR-amplified subgroup versus 18.3 (7.1–29.5, 95% CI) months and 28.4 (1–64.96, 95% CI) months for the EGFR-intact subgroup (p = 0.040). PFS and OS of patients with either pTERT mutation or EGFR amplification did not differ significantly. When considered together as a unique group with poor prognosis, patients with pTERT mutation and EGFR amplification had a median PFS of 9.9 (4.5–15.3, 95% CI) months and a median OS of 27.3 (17.4–37.2, 95% CI) months, while patients with the intact copy of both genes had a significantly longer median PFS of 128 months (0.1–265.7, 95% CI) (p < 0.001) and median OS of 192 months (25.9–358.0, 95% CI) (p < 0.001). Notably, none of the patients with either EGFR amplification or pTERT mutation survived 5 years, while survival at 5 and 10 years was 72.2% and 66.7% for patients with intact copies of EGFR and pTERT genes.
Fig. 2.
PFS and OS according to pTERT mutation (A, B) and EGFR amplification (C, D). Comparison between combined pTERT and EGFR-intact subgroup and either pTERT-mutant or EGFR-amplified subgroup for PFS (E) and OS (F).
In patients without EGFR amplification and pTERT mutation age ≥ 40 years was associated with shorter PFS (24 vs 147.6 months, p = 0.020) and OS (52 months vs NR, p = 0.011) while in patients with either EGFR amplification or pTERT mutation age was not significant for both PFS and OS (Supplementary Figure S1). Conversely, in patients with either EGFR amplification or pTERT mutation contrast enhancement was associated with a worse PFS (7.5 vs 11 months, p = 0.048) and OS (23.5 vs 37 months, p = 0.030), while in patients without EGFR amplification and pTERT mutation contrast enhancement was not associated with PFS and OS (Supplementary Figure S2).
Multivariable analysis on patients with molecular data showed that pTERT mutation and EGFR amplification retained a strong prognostic value in addition to age and gross total resection (defined as 100% tumor volume resection) (Table 3).
Table 3.
Multivariable Analysis of Clinical and Molecular Prognostic Factors
| Multivariable Model | ||||||
|---|---|---|---|---|---|---|
| PFS | OS | |||||
| Factor | HR | CI 95% | p-value | HR | CI 95% | p-value |
| Age | 1.032 | 0.994–1.071 | 0.101 | 1.050 | 0.999–1.103 | 0.056 |
| Contrast enhancement | 1.260 | 0.393–4.039 | 0.698 | 1.838 | 0.460–7.337 | 0.389 |
| GTR | 0.248 | 0.067–0.920 | 0.037 | 0.194 | 0.045–0.842 | 0.029 |
| EGFR amplification | 7.572 | 1.137–50.429 | 0.036 | 17.308 | 1.915–156.471 | 0.011 |
| pTERT mutation | 8.774 | 1.768–43.548 | 0.008 | 9.085 | 1.569–52.596 | 0.014 |
| MGMTp methylation | 2.024 | 0.511–8.013 | 0.315 | 0.775 | 0.166–3.621 | 0.746 |
| Adjuvant treatment | 0.703 | 0.219–2.263 | 0.555 | 0.446 | 0.074–2.679 | 0.377 |
GTR: gross total resection (for all cases with molecular alterations included in the table, a volumetric estimation of the extent of resection is provided. GTR is defined as 100% tumor volume resection).
Next, we explored the role of extent of resection within the molecular subgroups with or without EGFR amplification or pTERT mutation. For this analysis, GTR was defined as 100% resection of tumor volume on postoperative MRI.
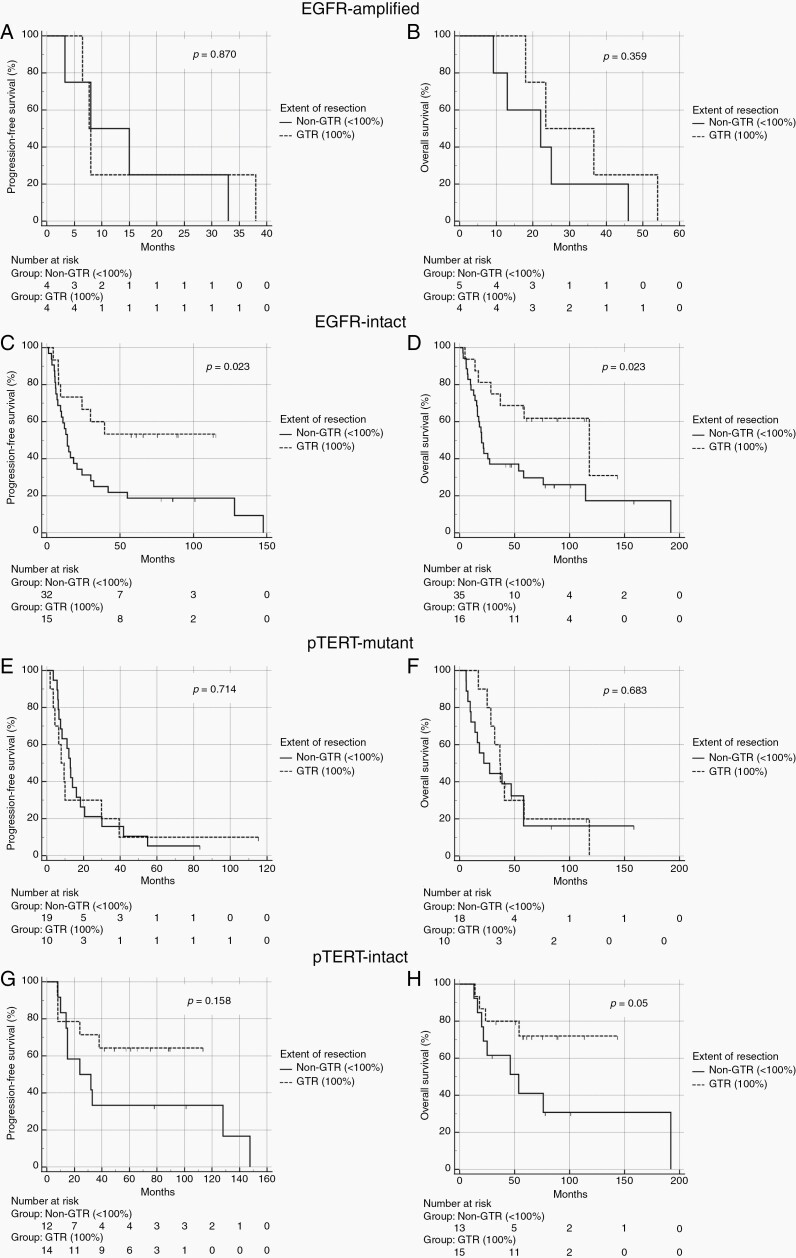
Among 9 EGFR-amplified tumors, 4 (44%) underwent GTR and 5 (56%) non-GTR. Median PFS was 7.7 (6.2–9.1, 95% CI) months in the GTR group and 8.0 (1–19.5, 95% CI) months in the non-GTR (p = 0.870). Median OS was 23.5 (5.3–41.7, 95% CI) months for the GTR group and 22.1 (2.6–41.6, 95% CI) months for the non-GTR (p = 0.359). As for the 54 EGFR-intact counterpart, 15 patients (27.8%) underwent GTR and 39 (72.2%) non-GTR. Median PFS was not reached in the GTR group and 14 months (9.8–18.1, 95% CI) in the non-GTR group, (p = 0.023). Median OS was 117.9 months (32.5–203.3, 95% CI) in the GTR group and 20 months (15.4–24.6, 95% CI) in the non-GTR group, (p = 0.023).
Among 30 pTERT-mutant tumors, 10 (33.3%) underwent GTR and 20 (66.7%) non-GTR. Median PFS was 7.8 months (3.3–12.3, 95% CI) in the GTR group and 12.8 months (9.9–15.6, 95% CI) in the non-GTR group (p = 0.714). Median OS was 36.6 months (28.7–44.5, 95% CI) in the GTR group and 22 months (2.7–41.3, 95% CI) in the non-GTR group (p = 0.683). Among 29 pTERT-intact IDH-wild-type tumors, patients with GTR were 13 (45%) and non-GTR 16 (55%). Median PFS was not reached in the GTR group and 24 months (1.0–52.8, 95% CI) in the non-GTR group (p = 0.158). Median OS was NR for the GTR group and 53.7 months (12.9–94.5, 95% CI) in the non-GTR group (p = 0.05) (Figure 3).
Fig. 3.
PFS and OS according to the extent of resection in EGFR-amplified (A, B), EGFR-intact (C, D), pTERT-mutant (E, F), and pTERT-intact (G, H) tumors. GTR: gross total resection; non-GTR: non-gross total resection.
Lastly, we evaluated the impact of adjuvant therapies on the outcome of patients according to EGFR amplification and pTERT mutation, but the numbers were too small for a meaningful statistical analysis.
Among patients with either pTERT mutation or EGFR amplification (n = 35), 21 (60.0%) received adjuvant therapies, while 14 (40.0%) did not. Median PFS was 13 (6.1–19.8, 95% CI) months for patients who received adjuvant therapies and 11 months (1–22.5, 95% CI) for those who did not. Median OS was 25 (14.6–35.3, 95% CI) months for the adjuvant therapy group and 18 (1–57.2, 95% CI) months for the observation group.
Among patients with intact copies of pTERT and EGFR genes (n = 19), 14 (73.7%) were referred to observation with MRI, while 5 only (26.3%) received adjuvant therapies. Median PFS was 14.0 months (8.0–147.6, 95% CI) for patients who received adjuvant therapies and 128 months (24.0–128.0, 95% CI) in the observation group. Median OS was 16.2 months (14.0–21.6, 95% CI) for the adjuvant therapy group and 192.0 months (76–192.0, 95% CI) for the observation group. Again, as in the whole cohort, the EOR was a significant confounding factor due to the prevalence of GTR in the observation group.
Discussion
IDHwt grade 2 diffuse astrocytomas are increasingly recognized as a molecularly heterogeneous subgroup of tumors.4,13 However, clinical characteristics at diagnosis and impact of the different treatment options in these tumors are not well known as either they have been grouped together with grade 3 tumors as a unique entity of “lower-grade gliomas” 8 or analyzed separately but with too small numbers (up to a maximum of 47–58 patients) for a meaningful statistical analysis.5,9
Here, we have investigated the largest cohort of IDHwt grade 2 diffuse astrocytomas in terms of clinico-radiological, molecular, and therapeutic aspects. In our series, these tumors showed clinical and neuroradiological features largely overlapping those of IDH-mutant astrocytomas: they prevailed among patients younger than 50, involved frequently eloquent areas, and were characterized by seizures as first manifestation in more than half of the patients (60.7%). The frequency of seizures at presentation (60.7%) was slightly lower in comparison to values reported for IDH-mutant grade 2 astrocytomas (70–80%),14 and the presence of mild or patchy contrast enhancement on MRI was seen in a minority of patients (26.5%), as reported in another study.15 Interestingly, we observed that contrast enhancement, even if modest, displayed a strong prognostic value: thus, these tumors should be carefully monitored even in case of gross total resection.
The prevalence of pTERT mutation and EGFR amplification was 51% and 14%, respectively: these values are comparable to those reported in a similar group of IDHwt grade 2 tumors (51% and 9%) in a recent study.9
Information about the clinical and radiological features of the molecular subgroups are scarce. As for symptoms and MRI characteristics at presentation, the four subgroups (pTERT-mutant/intact, EGFR-amplificated/intact) did not significantly differ. However, we observed a non-significant higher prevalence of older age at presentation for pTERT-mutant and EGFR-amplified patients: in this regard, in a recent study IDHwt lower-grade astrocytomas (grade 2 and 3 considered together) with either EGFR amplification or pTERT mutation or 7+/10– signature had a significantly older age than their counterparts with no features of glioblastoma.8
As for pTERT-mutant cases, we observed a non-significant lower frequency of seizures and higher uptake of contrast enhancement. Another study has suggested that pTERT-mutant lower-grade gliomas may have a lower incidence of preoperative seizures than pTERT-intact ones.16
In our series, pTERT mutation and EGFR amplification are independent predictors of poor prognosis in multivariable analysis, and both alterations similarly affect the outcome. These markers are now considered essential diagnostic tools in the recently updated WHO Classification 2021.17 Interestingly, Bale et al (2019)18 have proposed to employ EGFR amplification as a surrogate of a larger molecular panel for the diagnosis of IDHwt astrocytomas, being less time/cost consuming.
Median OS of patients of our cohort with IDHwt diffuse astrocytomas was slightly lower (32.6 vs 59 months) than that reported by Berzero et al.9 Patients with EGFR amplification had a slightly shorter OS (23.5 months) as compared to those with pTERT mutation (31.9 months), and this is in line with previous series (0.82 y, 37 months).5,9 However, in our series patients with pTERT mutation have a significantly worse OS (31 months) as compared to that reported by Berzero et al (88 months).9 Conversely, patients with the intact copy of both genes had a significantly longer median OS (192 months, p < 0.0001) than in the other series.5,9
Last, we analyzed the impact of extent of resection (EOR) and adjuvant treatments on outcome. It is well known that diffuse grade 2 gliomas of the cerebral hemispheres benefit from gross total resection19–22; however, the value of the EOR in the subgroup of IDHwt grade 2 tumors is still debated. Some authors have suggested that gross total resection confers a survival advantage when compared to partial/subtotal resection23–25; conversely, a volumetric analysis on grade 2 and 3 tumors considered together reported that pre- and post-surgical volume and percentage of resection were not associated with OS.26 Overall, patients in all these series were relatively few and methods for the estimation of EOR were heterogeneous.
In our series, the entire cohort of IDHwt grade 2 astrocytomas took a significant advantage from gross total resection, including the subgroup with volumetric estimation of extent of resection. Interestingly, a recent study described 31 IDH wild-type grade 2 astrocytomas with a median volumetric extent of resection of 94% following awake surgery, whose survival rate at 5 years was 77% without an early adjuvant treatment27; however, the long-term advantage of this advanced technique needs further confirmation.
None of the aforementioned studies have analyzed the value of EOR within the molecular subgroups of IDHwt grade 2 diffuse astrocytomas. In this regard, this is the first study addressing this issue, and reporting some novel findings.
GTR was significantly associated with an increase of PFS and OS among patients without EGFR amplification, and with an increase of OS among patients without pTERT mutation. The impact of GTR did not reach a similar statistical significance among patients with EGFR amplification or pTERT mutation. However, the survival numbers clearly favored GTR in the subgroup of the pTERT-mutant tumors, and larger series could reinforce this finding.
Overall, we recommend that all patients with IDHwt astrocytomas, regardless of the molecular subgroups, should undergo the maximal safe resection.
Due to their rarity and heterogeneity, a standard treatment outside surgical resection has not been established for IDHwt grade 2 astrocytomas. RTOG 9802 phase III trial reported the superiority of the addition of PCV chemotherapy to radiotherapy over radiotherapy alone in high-risk grade 2 gliomas28: however, a posthoc molecular analysis on a smaller sample size showed that IDHwt tumors (n = 26) did not benefit from the combined treatment and had a poorer outcome (median OS and PFS of 1.9 and 0.7 y).29 Similarly, in the EORTC 22033-26033 trial patients with IDH-wild-type grade 2 gliomas (n = 65) reported a median progression-free survival of 20 months, which did not significantly differ between radiotherapy (19.1 months) and temozolomide (23.7 months) arms (p = 0.240).30
In our retrospective study, the choice of administering an adjuvant treatment was based on the traditional distinction of high from low-risk patients.31 Thus, patients undergoing gross total resection were more often referred to observation with MRI, and those with incomplete surgery to adjuvant therapies regardless of IDH status. In this cohort, the impact of adjuvant treatments on survival was not significant in any molecular subgroup. However, in the subgroup of patients with worse prognostic factors, i.e., harboring either pTERT mutation or EGFR amplification, we observed slightly longer PFS or OS for those who underwent adjuvant therapies (chemotherapy alone, radiotherapy alone, or both), but without reaching statistical significance, maybe due to the small sample size. As for the subgroups without EGFR amplification and pTERT mutation (best prognostic subgroup) few patients only received an adjuvant treatment, and the EOR appears to be a strong confounding factor.
Overall, it is clear that patients without EGFR amplification and pTERT mutation represent a separate subgroup of IDHwt tumors with indolent course and long-term survival. They probably include tumors such as pilocytic astrocytomas or pleomorphic xanthoastrocytomas, and in the future novel diagnostic tools, such as DNA methylation techniques, will be of help in further characterization.32
Based on our data, a prognostic panel could be proposed to subdivide IDHwt astrocytomas into different subgroups, and clarify which patients should be either observed after surgical resection or undergo adjuvant treatments. This panel might include, as major factors, age, volumetric EOR, EGFR amplification, pTERT mutation, and, as a minor factor, presence of contrast enhancement at presentation. Future studies should validate the different prognostic weight of these factors.
Our study brings some relevant novelties: to our knowledge, this is the first study assessing the role of prognostic factors, extent of surgery, and adjuvant treatments in a large multicenter cohort of IDHwt grade 2 astrocytomas according to the different molecular subtypes.
We are aware of some limitations: the study is based on a retrospective series, the extent of resection has been evaluated by volumetric MRI in 71.1% of patients only, information about EGFR and pTERT status was not available in all patients, we did not look at the 7p gain/ 10q loss, and we did not further characterize molecularly the group with intact EGFR and/or pTERT. Furthermore, whether temozolomide-induced hypermutations might foster malignant transformation and/or leptomeningeal spread at recurrence in IDHwt grade 2 astrocytomas, similarly to what Yu et al. have recently demonstrated for IDH-mutant LGGs patients,33 should be investigated in larger series.
Therefore, we recognize that the impact of our findings should be taken with caution.
Conclusions
IDHwt grade 2 astrocytomas display clinical and neuroimaging features largely overlapping those of the IDH-mutant counterparts, and we confirm the strong prognostic value of EGFR amplification and pTERT mutation in association with clinical characteristics. As for treatment, in the absence of prospective data, we suggest that patients without amplification of EGFR and pTERT mutation (best prognostic subgroup) could be observed after GTR. For patients with either EGFR amplification or pTERT mutation the benefit of GTR and adjuvant radio-chemotherapy needs to be investigated in well-designed randomized trials with molecular inclusion criteria and adequate statistical power.
Supplementary Material
Contributor Information
Roberta Rudà, Department of Neuro-Oncology, University and City of Health and Science Hospital, Turin, Italy; Department of Neurology, Castelfranco Veneto and Brain Tumor Board Treviso Hospital, Italy.
Francesco Bruno, Department of Neuro-Oncology, University and City of Health and Science Hospital, Turin, Italy.
Tamara Ius, Neurosurgery Unit, Department of Neurosciences, Santa Maria della Misericordia University Hospital, Udine, Italy.
Antonio Silvani, Department of Neuro-Oncology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Giuseppe Minniti, Radiation Oncology Unit, Department of Medicine, Surgery and Neurosciences, University Hospital, Siena, Italy.
Andrea Pace, Department of Neuro-Oncology, University and City of Health and Science Hospital, Turin, Italy.
Giuseppe Lombardi, Department of Oncology, Veneto Institute of Oncology, Padua, Italy.
Luca Bertero, Pathology Unit, Department of Medical Sciences, University of Turin, Italy.
Stefano Pizzolitto, Department of Pathology, Santa Maria della Misericordia University Hospital, Udine, Italy.
Bianca Pollo, Neuropathology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Marco Conti Nibali, Neurosurgical Oncology Division, Department of Oncology and Hemato-Oncology, University of Milan, Italy.
Alessia Pellerino, Neuro-Oncology Unit, Regina Elena National Cancer Institute, Rome, Italy.
Enrica Migliore, Unit of Cancer Epidemiology (CPO Piemonte), University of Turin, Turin, Italy.
Miran Skrap, Neurosurgery Unit, Department of Neurosciences, Santa Maria della Misericordia University Hospital, Udine, Italy.
Lorenzo Bello, Department of Pathology, Santa Maria della Misericordia University Hospital, Udine, Italy; Neurosurgical Oncology Division, Department of Oncology and Hemato-Oncology, University of Milan, Italy.
Riccardo Soffietti, Department of Neuro-Oncology, University and City of Health and Science Hospital, Turin, Italy.
Funding
No financial support was provided.
Conflict of interest statement. None.
Authorship statement. Experimental design was created by R.R., F.B., L.B., R.S. Data analysis was performed by F.B., A.P. Analysis interpretation was performed by R.R., F.B., T.I., A.S., G.M., A.P., G.L., L.B., S.P., B.P., M.C.N., A.P., E.M., M.S., L.B., R.S.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. [DOI] [PubMed] [Google Scholar]
- 4. Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130(3):407–417. [DOI] [PubMed] [Google Scholar]
- 5. Aibaidula A, Chan AK, Shi Z, et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017;19(10):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol. 2020;22(4):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berzero G, Di Stefano AL, Ronchi S, et al. IDH-wildtype lower grade diffuse gliomas: the importance of histological grade and molecular assessment for prognostic stratification. Neuro Oncol. 2020;23(6):955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120(6):719–729. [DOI] [PubMed] [Google Scholar]
- 11. Di Carlo DT, Duffau H, Cagnazzo F, Benedetto N, Morganti R, Perrini P. IDH wild-type WHO grade II diffuse low-grade gliomas. A heterogeneous family with different outcomes. Systematic review and meta-analysis. Neurosurg Rev. 2020;43(2):383–395. [DOI] [PubMed] [Google Scholar]
- 12. Wijnenga MMJ, Dubbink HJ, French PJ, et al. Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol. 2017;134(6):957–959. [DOI] [PubMed] [Google Scholar]
- 13. Hasselblatt M, Jaber M, Reuss D, et al. Diffuse astrocytoma, IDH-Wildtype: a dissolving diagnosis. J Neuropathol Exp Neurol. 2018;77(6):422–425. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Mao Q, Wang X, et al. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J Clin Neurosci. 2016;31:56–62. [DOI] [PubMed] [Google Scholar]
- 15. Michiwaki Y, Hata N, Mizoguchi M, et al. Relevance of calcification and contrast enhancement pattern for molecular diagnosis and survival prediction of gliomas based on the 2016 World Health Organization Classification. Clin Neurol Neurosurg. 2019;187:105556. [DOI] [PubMed] [Google Scholar]
- 16. Shen S, Bai Y, Zhang B, Liu T, Yu X, Feng S. Correlation of preoperative seizures with a wide range of tumor molecular markers in gliomas: an analysis of 442 glioma patients from China. Epilepsy Res. 2020;166:106430. [DOI] [PubMed] [Google Scholar]
- 17. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bale TA, Jordan JT, Rapalino O, et al. Financially effective test algorithm to identify an aggressive, EGFR-amplified variant of IDH-wildtype, lower-grade diffuse glioma. Neuro Oncol. 2019;21(5):596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125(3):503–530. [DOI] [PubMed] [Google Scholar]
- 20. Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117(6):1039–1052. [DOI] [PubMed] [Google Scholar]
- 21. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossi M, Gay L, Ambrogi F, et al. Association of supratotal resection with progression-free survival, malignant transformation, and overall survival in lower-grade gliomas. Neuro Oncol. 2021;23(5):812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel T, Bander ED, Venn RA, et al. The role of extent of resection in IDH1 wild-type or mutant low-grade gliomas. Neurosurgery. 2018;82(6):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi J, Kim SH, Ahn SS, et al. Extent of resection and molecular pathologic subtype are potent prognostic factors of adult WHO grade II glioma. Sci Rep. 2020;10(1):2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherer M, Ahmeti H, Roder C, et al. Surgery for diffuse WHO grade II gliomas: volumetric analysis of a multicenter retrospective cohort from the German Study Group for intraoperative magnetic resonance imaging. Neurosurgery. 2020;86(1):E64–E74. [DOI] [PubMed] [Google Scholar]
- 26. Patel SH, Bansal AG, Young EB, et al. Extent of surgical resection in lower-grade gliomas: differential impact based on molecular subtype. AJNR Am J Neuroradiol. 2019;40(7):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poulen G, Gozé C, Rigau V, Duffau H. Huge heterogeneity in survival in a subset of adult patients with resected, wild-type isocitrate dehydrogenase status, WHO grade II astrocytomas. J Neurosurg. 2018;130(4):1289–1298. [DOI] [PubMed] [Google Scholar]
- 28. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: a phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020;38(29):3407–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group . Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 32. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network . Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu Y, Villanueva-Meyer J, Grimmer MR, et al. Temozolomide-induced hypermutation is associated with distant recurrence and reduced survival after high-grade transformation of low-grade IDH-mutant gliomas. Neuro-Oncology. 2021;noab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.