Abstract
Synthetic rubber emissions from automobile tires are common in aquatic ecosystems. To assess potential impacts on exposed organisms, early life stages of the estuarine indicator species Inland Silverside (Menidia beryllina) and mysid shrimp (Americamysis bahia) were exposed to three tire particle (TP) concentrations at micro and nano size fractions (0.0038, 0.0378 and 3.778 mg/L in mass concentrations for micro size particles), and separately to leachate, across a 5-25 PSU salinity gradient. Following exposure, M. beryllina and A. bahia had significantly altered swimming behaviors, such as increased freezing, changes in positioning, and total distance moved, which could lead to an increased risk of predation and foraging challenges in the wild. Growth for both A. bahia and M. beryllina was reduced in a concentration-dependent manner when exposed to micro-TP, whereas M. beryllina also demonstrated reduced growth when exposed to nano-TP (except lowest concentration). TP internalization was dependent on the exposure salinity in both taxa. The presence of adverse effects in M. beryllina and A. bahia indicate that even at current environmental levels of tire-related pollution, which are expected to continue to increase, aquatic ecosystems may be experiencing negative impacts.
Keywords: Tires, behavior, growth, salinity
1. Introduction
An estimated 4.4 – 12.7 million metric tons of marine debris enter the ocean annually, presenting a threat to pelagic, benthic, and coastal environments (Granek et al., 2020; Jambeck et al., 2015; Rochman et al., 2016). Through photodegradation and weathering processes, these synthetic polymers fragment are dispersed throughout the ocean, often concentrating in coastal areas (Barnes et al., 2009). Synthetic rubber emissions from automobile tires, now broadly considered a common type of microplastic (CA Waterboard, 2020), are a likely threat to the health of marine ecosystems, especially in estuaries, rivers, and streams located near metropolitan areas and busy roadways (Brahney et al., 2021; Gray et al., 2018; Klöckner et al., 2019; Rochman et al., 2019; Tian et al., 2021; Wagner et al., 2018). Another potential pathway of tire particles (TP going forward) is stormwater runoff, as reported by a recent study at 12 sites within the San Francisco Bay estuary, where fibers and TP (black rubbery fragments) contributed ~85% of total particles sampled (Werbowski et al., 2021). Modern tire materials, products of fossil fuels, are composed of complex mixtures of synthetic polymers, natural rubbers, carbon black, polyester and nylon fiber, chemical additives, petroleum, and pigments (Baumann and Ismeier, 1998). These mixtures are shed as TP, characterized as airborne and road wear particles, generated by the rolling shear of tread against a surface (Kovochich et al., 2021; Rogge et al., 1993). Once produced, TP can aggregate with other auto-related particles from brake dust, pavement, and atmospheric deposition (Charters et al., 2015). The presence of these particles in aquatic environments may result in impacts to wildlife and humans. For example, changes in cell morphology and DNA damage due to inhalation of tire particles are known to occur in humans (Gualtieri et al., 2008). Additionally, tires can leach constituents known to be toxic to aquatic organisms across different taxonomic orders (Hartwell et al., 2000; Nelson et al., 1994; Tian et al., 2021). For example, TP has been recently documented to cause acute toxicity to Coho salmon due to the presence of 6PPD-quinone, a chemical commonly used as an antiozonant and antioxidant in tires. (Tian et al., 2021).
Coastal estuaries are susceptible to micro and nano plastic pollution from terrestrial sources, including automobile tires. These water bodies receive freshwater from inland rivers, which deliver nutrients and runoff that may harbor agricultural chemicals and microplastics (Le Roux, 2005). An automobile tire is designed to last for 40,000 km until it is worn down, and throughout its lifetime, about 30% of its tread erodes and enters the environment (Dannis, 1974; Piotrowska et al., 2019). It is estimated that coastal rivers in Europe transport an annual load of 1.2 kt of TP to the Atlantic Ocean (Siegfried et al., 2017). Knowledge on the distribution and concentration (mass or particle count) of TP in coastal areas is limited (Unice et al., 2019). In Charleston Harbor, TP was found in all layers (intertidal sediment, subtidal sediment, and sea surface micro layer) with a maximum concentration identified in the intertidal sediments of the Ashley River (203 mg/Kg ww) (Gray et al., 2018). Another study report predicted average coastal European surface water concentrations to contain 0.03-17.9 mg/L and measured 0.09-6.4 mg/L of TP (Wik and Dave, 2009), which is in range the mass concentration used in this study (0.0038-3.778 mg/L ww).
Once in an estuary, low-density microplastics, including TP, remain buoyant for a period of time and become available to planktonic organisms which may ingest these fragmented particles (Barnes et al., 2009). As predators consume prey organisms, those particles are susceptible to trophic transfer in estuarine food webs (Athey et al., 2020; Au et al., 2017; Stienbarger et al., 2021). Several recent studies have indicated that estuarine species such as shore crabs, oysters, shrimp, fish, and clams will internalize microplastics through ingestion and uptake through gill tissue and soft tissues (Bessa et al., 2018; Davidson and Dudas, 2016; Gray and Weinstein, 2017; Van Cauwenberghe and Janssen, 2014; Watts et al., 2014). At the same time, organisms inhabiting estuaries are exposed to a wide range of salinities, which may alter the impacts of pollutants as freshwater transitions to saltwater, and in terms of micro and nanoplastics may influence agglomeration and hence bioavailability (Shupe et al. 2021). Testing across salinities is important because as global ocean temperatures warm, salinity is evidenced to increase (Durack et al., 2012; Helm et al., 2010). This increase in salinity may alter or potentiate the effects of pollutants, including micro and nanoplastics (MNPs), on estuarine organisms (Hutton et al., 2021; Shupe et al., 2021).
Americamysis bahia and Menidia beryllina are model estuarine organisms used across a range of salinities following guidelines developed by the EPA for whole effluent toxicity testing (Brander et al., 2012; Pillard et al., 1999; Vlaming et al., 2000). Changes in organism behavior result from various cellular, biochemical, and neural processes (Døving, 1991; Little, 1990) that are critical to organism survival as well as fitness, thus a sensitive endpoint for use in toxicity testing (USEPA, 1994). Numerous studies have drawn links between the biogeochemical and ecological consequences of environmental contamination by demonstrating that subtle changes in fish behavior indicate stress (Beitinger, 1990; Little, 1990; Sprague, 1971). Swimming and feeding behavior, frequency of activity, and velocity have been established as reliable responses to measure sublethal toxicity stress in fish (Grillitsch et al., 1999; Little and Finger, 1990; Newman and Jagoe, 1996) and that also has implications for organism fitness (Weis et al., 2001). The current experiment synthesizes methods of early and recent studies to measure several of these historically documented stress responses as well as growth in M. beryllina and A. bahia using periodic light and dark cycles as introduced stimuli (Pannetier et al., 2020; Romney et al., 2019). The purposes of the light/ dark cycles are to provide a general overview of organismal behavior in the environment during these conditions, as well as a stimulus effect for fish to act on, as in the natural environment.
This study investigated the sublethal effects (behavior and growth) of micro (10-20 um) and nano (< 1um) TP exposure across a salinity gradient similar to that found in estuaries. Subtle changes in behavior and growth are essential to document because they may increase predation risk and population-level effects (Beitinger, 1990; Little and Finger, 1990; Mundy et al., 2020). We used a range of concentrations based on environmentally relevant mass concentrations of TP and its leachate on behavior in the early life stages of indicator species A. bahia and M. beryllina. We hypothesize that TP will influence both growth and behavior, that it will be readily internalized, as has been demonstrated across other microplastic types in the early life stages of aquatic organisms, and that some responses may be salinity and size dependent. As data on TP pollution and sub-lethal effects in aquatic species are currently rare, this study fills critical knowledge gaps on uptake and internalization, growth impacts, and stress responses to an emerging microplastic pollutant by species that may act as proxies for threatened or endangered species and ecosystems sensitive to anthropogenic pollution.
2. Methods
2.1. Chemicals
Suwanee River Natural organic matter (NOM) - 2R101N used to create suspensions of MNPs in exposure wells was purchased from the International Humic Substance Society, St. Paul, MN. Tissue-Clearing Reagent CUBIC-R+ [for Animals] (T3741) and Tissue-Clearing Reagent CUBIC-L [for Animals] (T3740) for visualization of particles within organisms following exposures were purchased from Tokyo Chemical Industry Co., Ltd.
2.2. Microplastics preparation
A detailed TP preparation protocol has been provided in SI. Briefly, TP from tire tread was prepared by cryomill process in a ceramic chamber (Retsch CryoMill, Haan, Germany). After milling, 3 g tire particles were combined with 300 ml of solution in a flask containing 50 mg/L Suwanee River NOM prepared in Milli-Q water then filtered through a 0.2 mm filter. The solution is then run through a coarse strainer to remove the glass beads and strained through a 20 μm standard mesh sieve, producing a resulting solution with particles <20 μm in at least one dimension. Then, using a 47 mm syringe filter holder containing a 1 μm mixed cellulose ester (Advantec) filter the solution is further filtered to produce a suspension of nanoparticles <1 μm in at least one dimension. The filter holder is then backflushed with clean NOM suspension, and the backflushed solution is collected to produce a suspension of tire particles in the range of 1-20 μm. A portion of the prepared nano-TP fraction was further filtered using a 30K MWCO centrifugal filter (Corning Spin-X #431489) ran at 7800 rpm for 5 minutes to rinse particles and to produce simulated TP leachate. The solution particle counts are determined separately for each fraction of the suspension. The micron (1-20 μm) sample particle count is determined by triplicate sampling of the suspension and the particle count analysis by flow cytometry (Acurri C6 Flow Cytometer, BD Biosciences, San Jose, CA). The nanoscale (<1 μm) sample particle count is also determined in triplicate by Nanoparticle Tracking Analysis (NTA) on a NanoSight instrument (NanoSight NS500, Malvern Instruments, Westborough, MA).
2.3. Model organisms, their sources, and experimental setup
Americamysis bahia larvae were purchased from Aquatic Biosystems in Fort Collins, Colorado and reared in three tanks at 15, 20, and 25 PSU salinities with filtered artificial seawater prepared (AFSW). For each organism, there were three biological replicates. For silversides, 2 technical replicates were averaged for each of the three biological replicates. For mysids, 3 technical replicates were averaged for each of the three biological replicates. Following EPA protocol 833-C-09-001 (USEPA, 2009), when adult A. bahia reproduced, larvae were moved to additional tanks of the same salinity and reared for seven days prior to exposures beginning. Micro and nano-TP exposures with mysids were initiated at seven days post fertilization (dpf) (n=3) under static renewal conditions for seven days. Menidia beryllina embryos were harvested from broodstock held at the Hatfield Marine Science Center into three acclimation aquaria of 5, 15, and 25 PSU salinities with filtered AFSW following modified methods from Middaugh et al. (1987) as done in previous studies in the Brander lab (e.g. DeCourten et al., 2020; Hutton et al., 2021). Larvae were placed into exposure vessels at 6 ±1 days post fertilization (dpf) (n=6 technical replicates to make n=3 biological replicates) and maintained under static renewal conditions for 96 h. All exposure vessels were covered during exposures to prevent background contamination and a blank filter water was also used.
Each model species was exposed to a total of 26 treatments (n=3): each containing a water control, NOM control with four TP concentration treatments (micro and nano with 60, 6000, and 60000 particles/mL, which is equivalent to 0.0038, 0.378 and 3.778 mg/L in mass concentration for micro-size particles; 0.014% TP leachate) across three salinities per species as described above. Nominal water concentrations with detailed QA/QC are provided in SI table 1. Water quality parameters were measured daily over the exposure period at the time of 80% water renewal. Cumulative hatching and mortality were recorded daily. A. bahia were fed concentrated brine shrimp (Artemia franciscana) ad libitum, and M. beryllina were fed Gemma Microdiet 0.2 mg/beaker/day (Skretting, Westbrook, Maine). Both organisms were fed daily and allowed to feed for at least two hours before water changed. Table SI 2 and 3 provides water quality parameters maintained throughout the experiment. A control blank filters were setup in a petri dish to measure background contamination. No particles resembling TP were observed on filter blanks.
2.4. Behavioral assays
Following MNP exposures of 7d (A. bahia) and 96 h (M. beryllina), behavioral assays were performed post-exposure from each treatment using a DanioVision Observation Chamber (Noldus, Wageningen, the Netherlands) for the dark: light cycle as described previously (Mundy et al., 2021; Segarra et al., 2021). Briefly, A. bahia and M. beryllina larvae were randomized and placed in individual 10 ml glass beakers within a 12-well plates tray designed and 3D-printed in Brander lab (Hutton et al., 2021), in the Ethovision Observation Chamber (EOB) to observe natural photo motor response. Larvae were acclimatized for at least 1 hour before placing into the EOB. After acclimatization outside the chamber, another 5-minute acclimatization period was provided inside the dark chamber, followed by three cycles of alternating 2-minute intervals of dark stimuli and 2-minute intervals of light stimuli. Behavior and activity were recorded and tracked by a Basler Gen 1 Camera using Ethovision XT15 software. Velocity thresholds were determined for swimming parameters between 0.5 cm/s (freezing) – 2.0 cm/s (moving) (Segarra et al., 2021). A virtual center zone (1.6 cm diameter) was established to measure the time that larvae spent in the center of the 2.2 cm diameter in the beaker. All behavioral tests were conducted between 09:00 and 18:00 h. The resolution was set at 1280 x 960, light cycles were programmed at 10,000 lux and the frame rate was set at 25/s. A total of seven variables were analyzed in this study which is included in Table 1. Following behavioral analysis, organisms were euthanized humanely, silversides per IACUC protocol #0035, and fixed in paraformaldehyde (PFA) to preserve tissues for examination of MP internalization.
Table 1.
Behavioral variables from Noldus ethovision software used in this study to analyze Mysid shrimp (A. bahia) larvae and silverside (M. beryllina) larvae behavioral response
| Variable | Unit | Description |
|---|---|---|
| Distance (Total) | cm | Total distance moved inside the well throughout the video recording time. |
| Freezing | S | The mean of the total time fish were moving for less than 2 seconds. |
| Movement | S | Duration for which the selected body point (head and tail region) was changing location with respect to the body center. |
| In Zone duration | S | The total time spent in the zone defined as the central portion of the beaker |
| In Zone Frequency | The number of times fish spent time in the zone | |
| Meander | Deg/cm | Turning in animals moving at different speed. |
| Turn Angle | degree | Difference in heading between two samples. |
2.5. Growth and TP internalization
At least three individuals from each species per treatment were collected for growth measurement. Length and width measurements were collected via dissecting scope equipped with Moticam visual software, and particle uptake was visualized on a Zeiss Axio Observer inverted microscope (Carl Zeiss, White Plains, NY). Growth data were assessed by creating a growth index with the following formula:
Where W is the width of the organism, L is the length, and d is the number of days the organism is exposed to the TP. This relationship provides the index used to plot the final growth curve. Organisms were then cleared using a protocol adapted for larval organisms with CUBIC™ clearing reagents (Ohnuma et al., 2017; Susaki et al., 2015). Briefly, to remove pigmentation and allow visualization of internalized microplastics (1-20 um), individual organisms fixed in 3% PFA were washed in 5 ml phosphate-buffered saline (PBS) for 30 minutes and incubated in 5 ml CUBIC-L at 37 ° C for seven days to encourage lipid removal. Following this step, organisms were washed again in 5 ml PBS for an additional two hours and then transferred to CUBIC-R + for an additional seven days to clear the remaining tissue.
2.6. Statistical analysis
Statistical analysis was performed using RStudio Version 1.0.153. Dose-response curves were generated to evaluate larval swimming behavior and growth effects across concentration treatments. The growth data were analyzed using a maximum likelihood estimate (MLE) approach to evaluate which of five different curves (linear regression, quadratic, sigmoidal, 5-parameter unimodal, and 6-parameter unimodal) were tested for the best fit to all three concentrations and controls. A maximum likelihood ratio test was used to examine whether each curve provided a better fit than an intercept-only null model with a significance level of α < 0.05. All calculations for the concentration–effect curves were performed using mean behavior variables, re-scaled between 0 and 1 within each cycle to facilitate comparison between salinity. R scripts used for data preparation, statistical analysis, and graphing can be found at https://github.com/branderlab/TWP-DRC-Curve.git, and examples using the same package are published in other studies (Brander et al., 2016; Frank et al., 2019; Mundy et al., 2020) Concentration dependent dose response curves for behavioral data were prepared by drm function in r using DRC package by Ritz, (2010), which does not include leachate (due to the absence of particle count). The Shapiro–Wilk test was used to test normality, and Levene’s test was used for homogeneity testing. After confirming normality and homogeneity of data, a 3-4 parameter using nonlinear regression approach was used to prepare the model at each salinity and combined using ggplot2 function in R. Analysis of Variance (ANOVA) was used to evaluate differences among treatment groups. A Tukey HSD post-hoc test was used to compare particle concentrations between treatments, and a Dunnett’s post-hoc test was used to compare leachate treatments to controls. Differences were considered statistically significant at p < 0.05.
3. Results and discussion
3.1. Behavioral responses of model species when exposed to TP in a range of salinities
3.1.1. Behavioral responses for A. bahia
Average A. bahia larvae survival for control and exposure treatments was 98 ± 2% and 90 ± 3 %, respectively, with no significant difference across the treatments (ANOVA (Normal distribution, Tukey HSD post-hoc, p < 0.05)). Out of all seven behavioral responses analyzed, ~50% of micro- TP exposures and ~33% of nano-TP (except 25 psu; ~57%) exposures were significantly different from the control group in at least one concentration in both light and dark cycle at least one salinity (SI Table 4; Fig. SI Fig. 1C &D). In both the micro and nano-sized TP treatments, A. bahia turn angle, freezing, movement and in zone duration (time spent in center of beaker) were most significantly affected at each salinity (Fig.1). In leachate-exposed A. bahia, six out of the seven variables (freezing, movement, In zone duration, frequency, meander and turn angle) were significantly different from the control group (SI Fig. 1B).
Fig. 1.
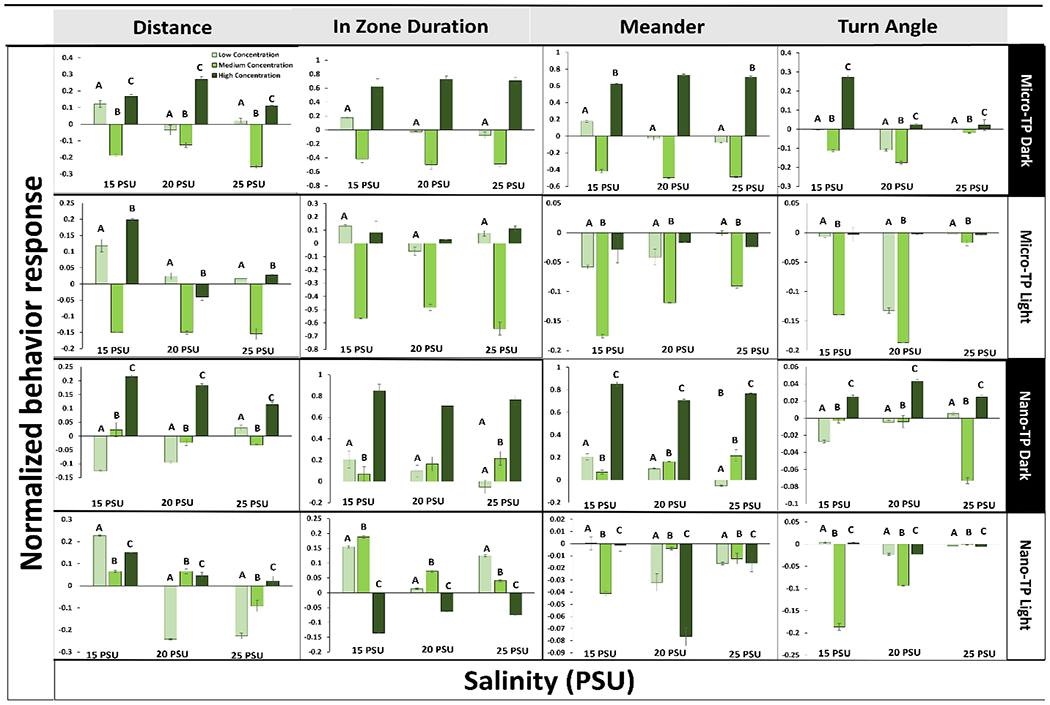
Mysid shrimp (A. bahia) behavioral responses with selective significant variables, represented after 7 days exposure to micro and nano-TP in combined average dark and light cycles 3 concentrations (60, 6000, 60000 particles/ml) (Lighter to darker color represents lowest to highest concentration) of across a salinity gradient 15PSU – 25PSU. Y-axis represents data normalized to 0-1 scale. Similar alphabets represent statistically significant difference in at least one salinity (* p < 0.05 ANOVA test followed by Dunnet’s test, comparing all concentrations to their respective salinity NOM control within each cycle per salinity (Control = 0)). Lighter to darker color represents lowest, medium and highest concentration.
When compared between dark and light cycles, A. bahia demonstrated increased distance and meander in the light cycle at highest salinity, with increasing freezing frequency and time spent in the zone at lowest salinity in micro and nano TP exposed group (Fig. 1 & 3). When compared between TP sizes, nano-TP caused hyperactivity in A. bahia, reflected by their swimming distances significantly increasing in a concentration dependent manner. Selected variables (distance, in zone duration, meander and turn angle) in the dark and light cycle micro-TP demonstrated about 70% behavioral alterations compared to control whereas in nano-TP exposure group about 80% behavioral alterations in both dark and light cycles at at least one salinity (SI Table 4; SI Fig. 1).
Fig. 3.
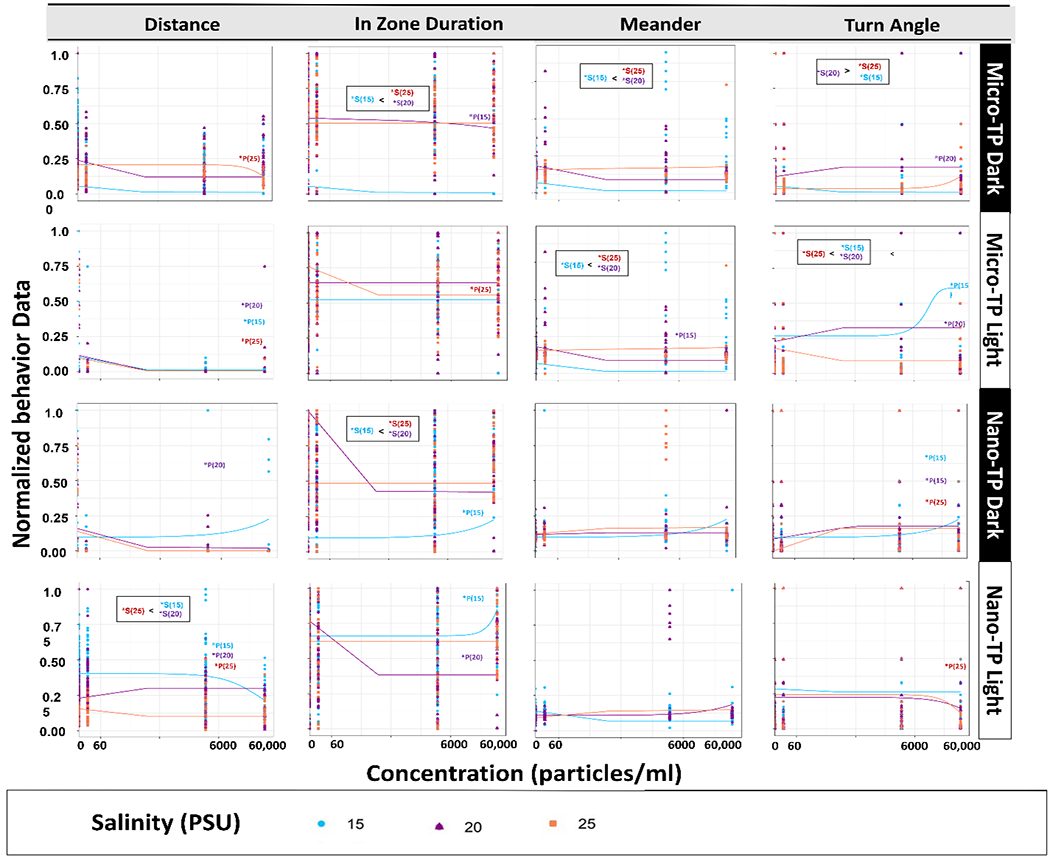
Mysid shrimp (A. bahia) behavioral concentration response curves after 7 days exposure to micro and nano-TP in combined average dark and light cycles across a salinity gradient 15PSU – 25PSU. “P” represents particle count and “S” salinity. Data normalized to 0-1 scale. * p < 0.05 ANOVA test followed by Dunnet’s test, comparing all concentrations to their respective salinity NOM control within each cycle per salinity.
In terms of salinity, behavioral alterations in both nano and micro-TP exposures were significantly higher at the two higher salinities (15 and 25 PSU). This suggests nano-TP affected mysids more at a higher salinity as reported by other studies (Kögel et al., 2020; Lee et al., 2013; Rist et al., 2017). This may be due to agglomeration at higher salinities (Shupe et al. 2021). When D. magna were exposed to nano (1-9 μm) and micro (>10 μm) plastics, nanoplastic was reported to decrease 21% feeding rates compared to microplastic exposure (Rist et al., 2017). Other studies reported hyperactive behavior in zebrafish (Danio rerio) exposed to micro polystyrene (PS) (0.001-20 mg/L, equals to 14.5~2.9 × 105 particles/mL) and sticklebacks (Gasterosteus aculeatus) exposed to PE (50,000 particles/ml) each, (Bour et al., 2020; Chen et al., 2020). Moreover, hyperactivity has been reported in the F1 offspring of zebrafish exposed to polyvinyl chloride (PVC) and high-density polyethylene (HDPE) (Cormier, 2020).
3.1.2. Behavioral responses for M. beryllina
Average M. beryllina larvae survival for control and exposure treatments was 97 ± 3% and 91 ± 2 %, respectively, with no significant difference across the treatments (Normal distribution, Tukey HSD post-hoc, p < 0.05). In M. beryllina, all the behavioral variables measured were significantly differently from the control group in at least one salinity (SI Table. 5; SI Fig. 2C & D). In both dark and light cycles, M. beryllina spent an increased time in the zone (the center of the beaker) compared to controls, and also had an increased turn angle (Fig. 2 & 4). Dark and light cycle behavioral observations showed a similar pattern except M. beryllina meandered more compared to the dark cycle at all the exposure concentrations, within at least one salinity condition. Following TP leachate exposure concentration, more than half of the variables (freezing, movement, In zone duration, frequency, meander and turn angle) demonstrated a significant change in behavior from the control in both dark and light cycles in at least one salinity condition (SI Table 5; SI Fig. 2B). Similar behavior changes were observed in Delta smelt (Hypomesus transpacificus) following exposure to pesticides (Mundy et al., 2021, 2020). Concentration dependent dose response curves for the selective variables demonstrated 79% behavioral alterations in micro-TP exposed silversides whereas, in nano-TP exposure group, about 75% behavioral alterations at both micro and nano exposed TP Silversides at both dark and light cycle (Fig. 4). In silversides salinity dependent behavioral changes were not significant in the micro-TP exposed group, in contrast to nano-TP where higher salinity seems to affect behavior more. This may be because of increased agglomeration of nano-TP at higher salinities (Shupe et al. 2021; Gousiadou et al., 2021), as this response to nano TP at higher salinities was also seen in mysids. Altered swimming behavior, reduced velocity and decreased feeding activity have also been observed in larval zebrafish (Danio rerio), larval rockfish (Sebastes schlegelii) and sheepshead minnow (Cyprinodon variegatus) when exposed to PS and polyethylene (PE) microplastics (Chen et al., 2017; Choi et al., 2018; Noldus et al., 2001; Yin et al., 2019, 2018).
Fig. 2.
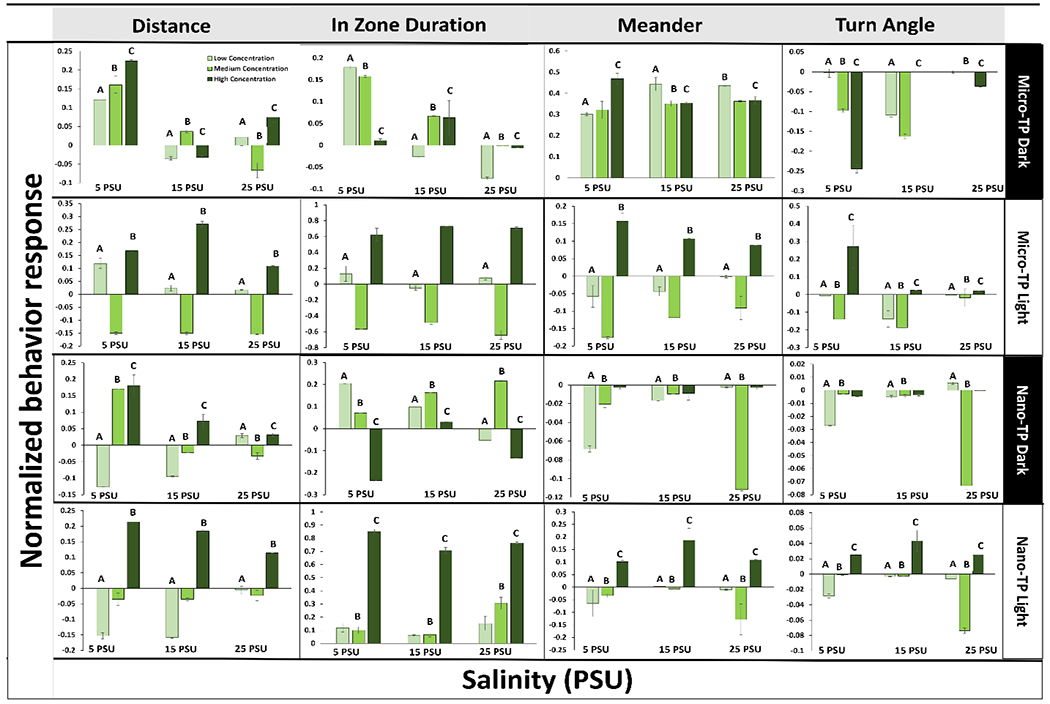
Silverside (M. beryllina) larvae behavioral responses with selective significant variables, represented after 4 days exposure to micro and nano-TP in combined average dark and light cycles 3 concentrations (60, 6000, 60000 particles/ml) (Lighter to darker color represents lowest to highest concentration) of across a salinity gradient 5PSU – 25PSU. Y-Axis data normalized to 0-1 scale. Similar alphabets represent statistically significant difference in at least one salinity (* p < 0.05 ANOVA test followed by Dunnet’s test, comparing all concentrations to their respective salinity NOM control within each cycle per salinity (Control = 0)). Lighter to darker color represents lowest, medium and highest concentration.
Fig. 4.
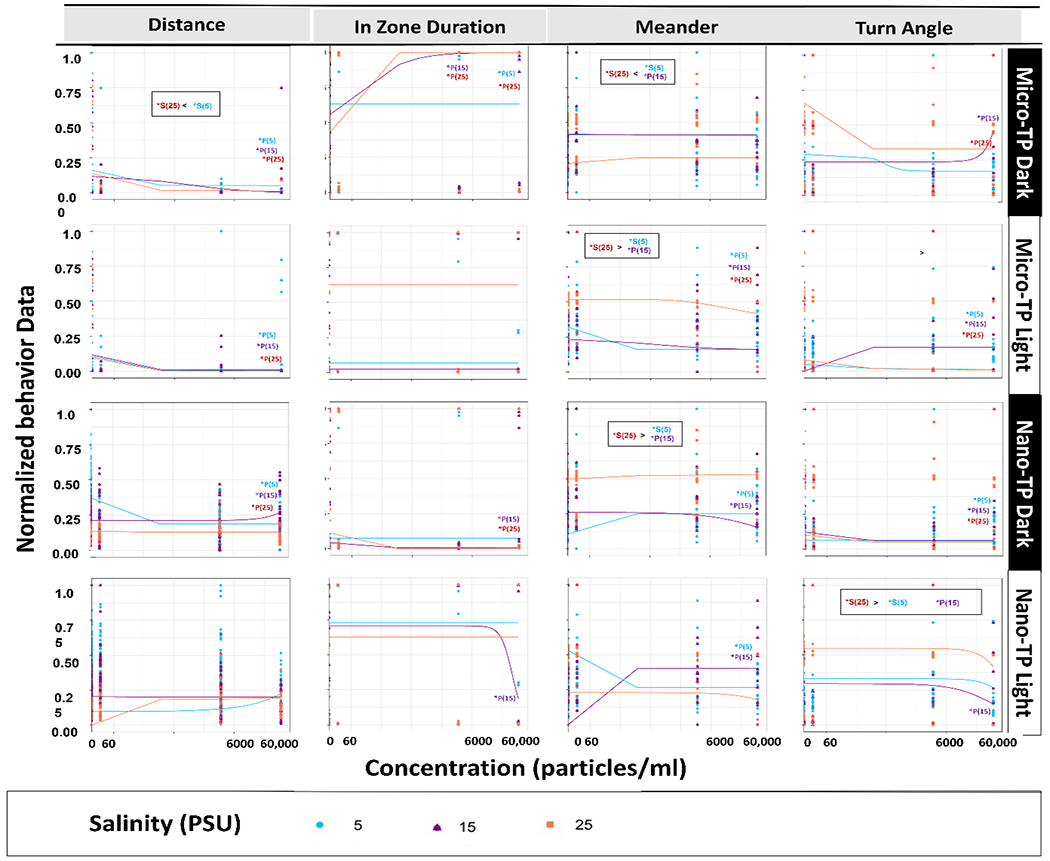
Silverside (M. beryllina) larvae behavioral concentration response curves after 7 days exposure to micro and nano-TP in combined average dark and light cycles across a salinity gradient 5PSU – 25PSU. “P” represents particle count and “S” salinity. Data normalized to 0-1 scale. * p < 0.05 ANOVA test followed by Dunnet’s test, comparing all concentrations to their respective salinity NOM control within each cycle per salinity.
3.1.3. Comparable Behavioral responses for M. beryllina and A. bahia
When comparing M. beryllina and A. bahia behavioral responses, there were some correlations (Pearson) identified between some variables (SI Table 6). There was a direct correlation observed in M. beryllina between distance related to movement, freezing (0.32-Dark, 0.16- Light) (Fig. 2). In contrast to mysid shrimp, there was an inverse relationship between movement and freezing (−0.54-Dark, −0.59-Light) (Fig. 1). Freezing demonstrated a weak inverse relationship with velocity for M. beryllina and A. bahia. Turn angle and freezing mean also showed a weak inverse relationship with the movement for M. beryllina and A. bahia. This suggests random movement that can be caused by additional stress due to the TP.
3.1.4. Salinity impacts on Behavioral responses for M. beryllina and A. bahia
Within different salinities, the lowest salinity (15 PSU) in A.bahia demonstrated the highest variation from control in combined dark and light cycles in all concentrations of micro-TP (SI Table 4; Fig.1). This was in contrast to nano-TP and leachates, where the highest salinity (25 PSU) demonstrated the most impact on behavioral variation. As mentioned above, this may be due to agglomeration behavior, and also due to some soluble chemicals becoming more bioavailable at higher salinities, and thus potentially more toxic, although this requires further research (Hutton et al., 2021; Saranjampour et al., 2017). Similar results were observed in M. beryllina, where in both the dark and light cycle the lowest salinity (5 PSU) showed most variation from control when exposed to micro-TP in contrast to nano-TP and leachate exposure group, where the most behavioral variation from control was seen in highest salinity (25 PSU) (SI Table 5; Fig. 2). These results align with recent findings on nanoplastics agglomerating more as salinity increases (Shupe et al., 2021). M. beryllina exposed to both micro and nano-TP exhibited increased duration of time spent in central habitat across all concentrations and salinities, except individuals exposed to nano-TP in the highest concentration and TP leachate, both at lowest salinity in dark cycles. Similarly, A. bahia exhibited increased in zone duration across all TP concentrations, salinities, and light-dark cycles, including individuals exposed to TP leachate. Occupancy of the boundaries of a novel environment is widely documented to indicate a stress response in fish, rodents, and humans (Kallai et al., 2007; Schnörr et al., 2012; Sharma et al., 2009; Treit and Fundytus, 1988). An increase in central habitat occupancy that is significantly different from control organisms may indicate increased exploration or indiscriminate feeding behavior. Previous studies observed impaired swimming competence and reduced exploratory behavior in N. japonica exposed to PS microbeads (Wang et al., 2020). The uninhibited exploration behavior we observed may lead to an increased risk of predation in these highly susceptible larval fish.
Behavioral changes can be an outcome of physiological changes like respiratory stress (Abdel-Tawwab et al., 2019; Hashemi et al., 2019) that may be caused by changes in oxygen consumption with altered ion regulation (Kolandhasamy et al., 2018; Watts et al., 2016) as observed in this study, where increasing zone duration and freezing are caused at various TP concentrations (Fig. 1& 2. ; SI Table 4&5). Similarly, ingestion of irregularly sized TP may also induce irregular behavior (Wang et al., 2016; Wright et al., 2013) and may be another reason for irregular behavior patterns in our study (Fig 1 and 2). These particles can also come in contact with the skin, gills, fins, and eyes of the organisms, when present in high concentrations, and may result in abnormal swimming behavior (Choi et al., 2018), as observed in this study with altered turn angle and meandering patterns differing from control (Fig. 1&2; SI Table 4&5). Altered turn angle and meandering patterns describe necessary behavioral patterns required by an aquatic organism for their survival, supporting actions such as predator avoidance or foraging. Some of the behaviors documented in our study (in zone, turning, and velocity) may represent hyperactive behavior of an organisms (Mundy et al., 2021). Behaviors exhibited by organisms exposed to tire particles herein may also be indicative of exploration avoidance, or an indication of anxiety-like behavior (e.g., altered in zone duration, Schnörr et al., 2012). If an organism can’t respond quickly to prey or a potential predator and gets confused due to the presence of external particles it may limit their ability to survive, causing long term population decline (Weis and Candelmo, 2012). Several studies have reported that MNPs can cause movement-related neurotoxicity in organisms (Barboza et al., 2018; Lei et al., 2018; Yin et al., 2018), as reported in this study, with changing movement and distance in model species when exposed to various TP concentrations indicating neurotoxicity as other studies have with other polymer types. Swimming behavior is crucial for predator defense and avoidance, food acquisition, and social activity (Colwill and Creton, 2011) that all require motor as well as sensory systems (Roberts et al., 2011; Wong et al., 2010) to work in concert. M. beryllina are known to occur in schools and exhibit diel migrations following zooplankton prey, often displaying high school densities during the nocturnal period, presumably to reduce predation (Wurtsbaugh and Li, 1985). The presence of high TP concentrations may alter migration or shoaling patterns and limit population ranges, although environmentally relevant TP concentrations in larger water bodies may not present significant risk at this time. A. bahia exposed to nano-TP concentrations (60 and 6,000 p/ml) at 15 PSU and 25 PSU in dark cycles exhibited further total distance moved while organisms exposed to TP leachate in light cycles exhibited shorter total distance moved at 25 PSU salinity. Increased activity from TP exposure in nocturnal periods may not present high risk to the diurnally benthic A. bahia, which becomes planktonic at night to forage for food and engage in reproductive activity (Wortham-Neal and Price, 2002). However, observations of decreased activity resulting from TP leachate exposure in A. bahia during diurnal periods may increase susceptibility to predation by fish or crustaceans who forage during the day or cause reduced food intake.
3.2. Growth and Ingestion
A. bahia growth demonstrated a significant concentration-dependent decrease in both the highest salinities (Normal distribution, Post-hoc Tukey’s test, ANOVA, p < 0.05) micro-TP exposure (Fig. 5). There were no significant differences observed in leachate concentration when compared to control (Fig. 7A). When compared between the salinities, A. bahia demonstrated comparatively better growth at the highest salinity, which was reduced significantly at the highest TP concentration (Tukey HSD post-hoc, ANOVA, p < 0.05). There was no significant growth reduction demonstrated in nano-TP-exposed A. bahia over all concentrations. However, the highest salinity demonstrated better growth compared to both lower salinities. The appearance of ingested TP was concentration-dependent in A. bahia, as shown in Fig. 8A and 9A. TP ingestion is also documented in other benthic invertebrates (Khan et al., 2019; Redondo-Hasselerharm et al., 2018). In the case of amphipod crustacean (Hyallela azteca), gut retention times of 24–48 h were observed in ingested TP with a significant impact on net growth when exposed to 500–2000 p/ml (Khan et al., 2019).
Fig. 5.
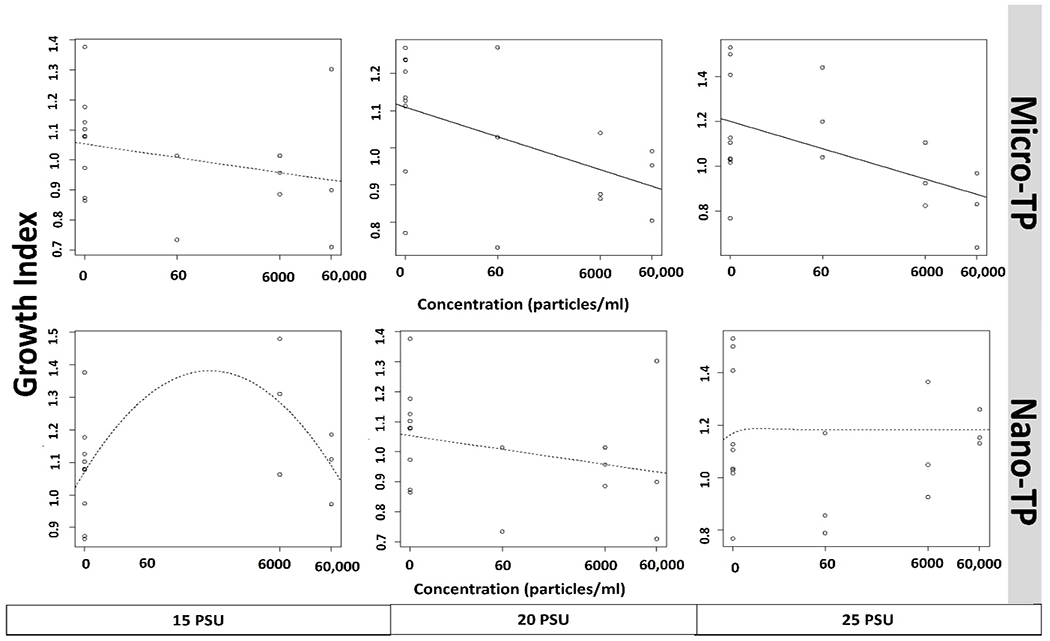
Concentration response growth curve of mysid shrimp (A. bahia) larvae exposed to micro and nano-TP across 15-25 PSU salinity gradient. Each circle represents the rescaled growth index mean of one larva (n=9). Data are presented on a log10 X+ 0.05 axis. Curves shown as a solid line are significantly better fits than a null intercept-only model (p<0.05), curves shown as a dashed line are the best-fit of the five-curve option (lowest p-value), but not significantly better than the null model.
Fig. 7.
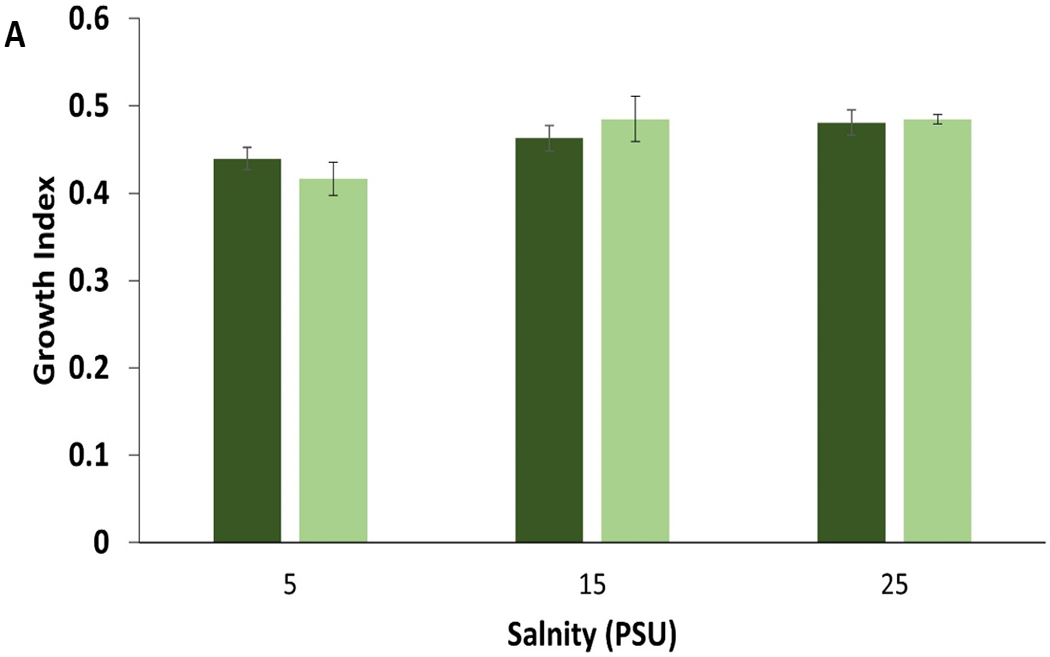
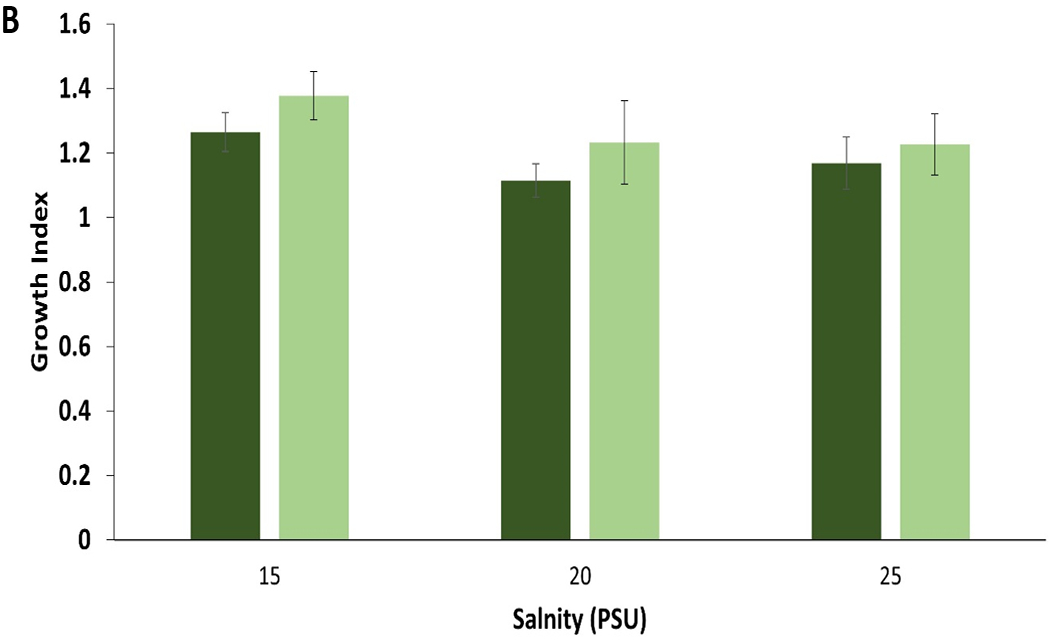
Control (dark color) compared to leachate (Light color) growth bar plot for A) mysid shrimp (A. bahia) and B) silverside (M. beryllina) yolk sac larvae from micro-TP concentration at 5-25 PSU salinity gradient range.
Fig. 8.
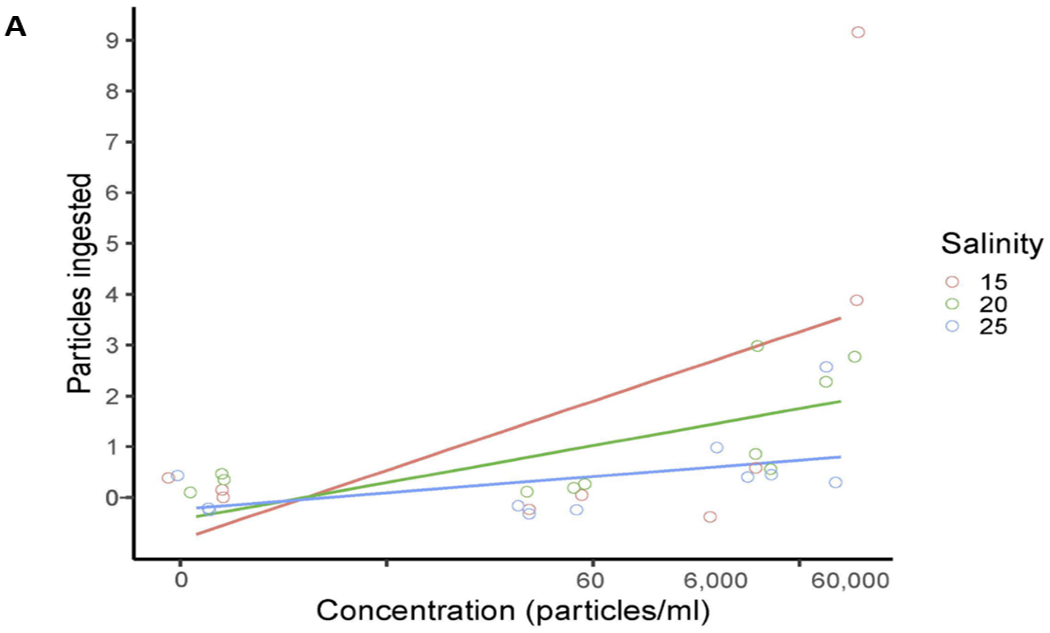
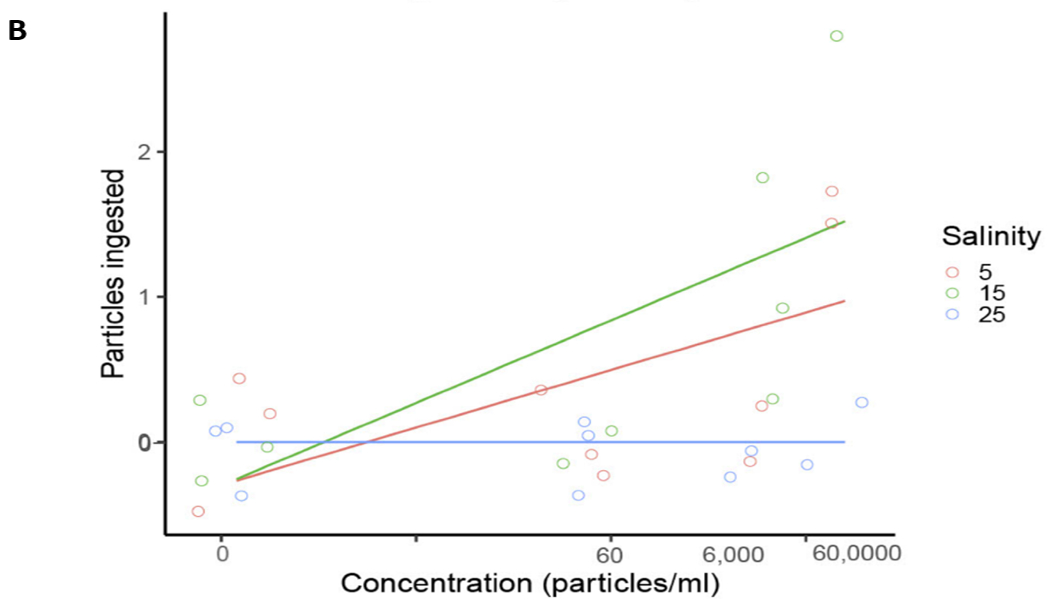
Generalized linear model of TP ingested by A) mysid shrimp (A. bahia) and B) silverside (M. beryllina) yolk sac larvae from micro-TP concentration at 5-25 PSU salinity gradient range.
Fig. 9.
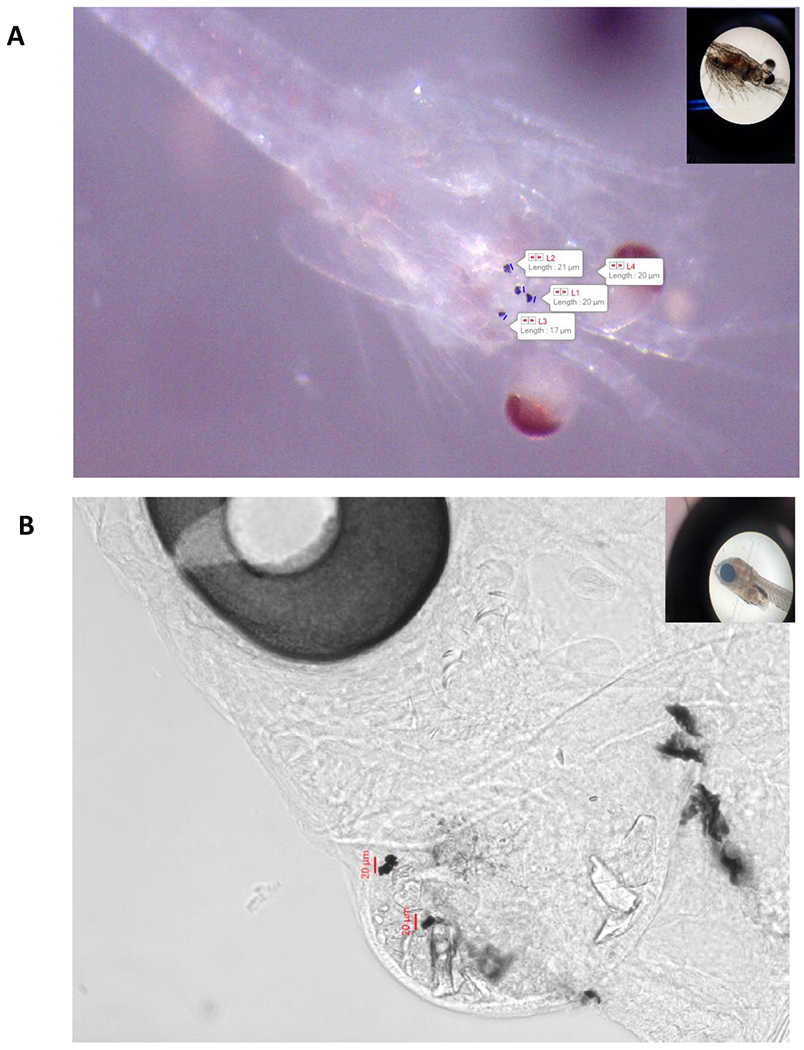
Images of A) mysid shrimp (A. bahia) at 15 PSU salinity with highest micro-TP concentrations at 35x and B) larval silverside (M. beryllina) yolk sac at 10x exposed to highest micro-TP concentration at 5 PSU salinity. Inset images in each panel are showing control organisms.
M. beryllina demonstrated significant concentration-dependent reduced growth in both micro- and nano-TP exposed groups at all salinities, except in nano-TP group at lowest salinity (Tukey HSD post-hoc ANOVA, p< 0.05; Fig. 6). There were no significant differences observed in leachate concentration when compared to control (Fig. 7B). This is true in the case of M. beryllina’s ingestion of micro-TP as well, where ingested particles were observed at the two highest concentrations with the highest number of ingested TP at middle salinity (Fig. 7B and 8B). This is consistent with a recent study that traced TP in the gut of 14% of individuals across five fish species surveyed in urbanized estuarine conditions (Parker et al., 2020).
Fig. 6.
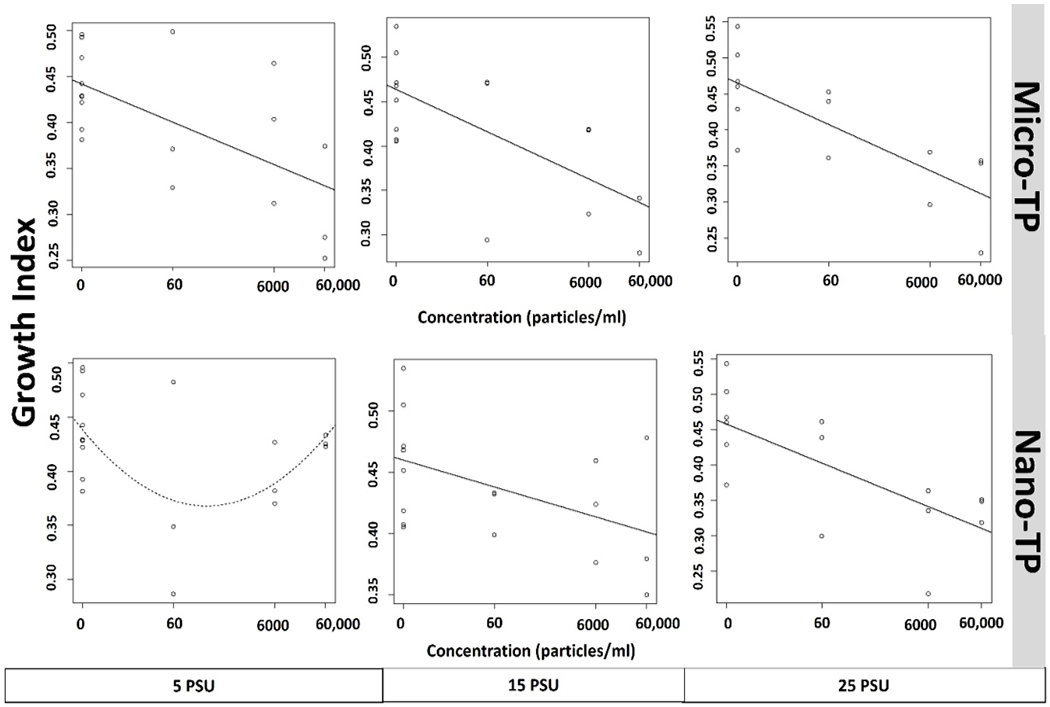
concentration response growth curve of silverside (M. beryllina) yolk-sac larvae exposed to micro and nano-TP across 5-25 PSU salinity gradient. Each circle represents the rescaled growth index mean of one larva (n=6). Data are presented on a log10 X+ 0.05 axis. Curves shown as a solid line are significantly better fits than a null intercept-only model (p<0.05), curves shown as a dashed line are the best-fit of the five-curve option (lowest p-value), but not significantly better than the null model.
Micro and nanoplastic exposures can cause adverse effects on the growth and development of larval aquatic organisms, primarily through ingestion (Athey et al., 2020; Lo and Chan, 2018). Inhibited growth may reduce the probability of attack because of inconspicuousness, but in the long term may increase failure to escape as a result of less developed sensory and locomotion abilities (Fuiman and Magurran, 1994). Further, reduced growth and stunted development increase the amount of time a larval organism spends in a specific stage or size class, impacting cumulative predation mortality rate (Shepherd and Cushing, 1980), and could also result in reduced size at reproductive maturity (e.g. DeCourten and Brander, 2017). In M. beryllina, optimal growth in laboratory conditions has been documented at 15 PSU while Mysid species was 30 PSU (Middaugh et al., 1987). Therefore, measuring growth in larval individuals following a period of salinity stress may yield unique insight into the effects of TP across different salinities on developing organisms. Particularly considering that salinity regimes are already being altered by global climate change (DeCourten et al., 2019).
Ingestion of 1 – 20 mm micro-TP was observed in A. bahia and M. beryllina at 6000 p/ml and 60,000 p/ml. A generalized linear model (GLM) was run for particle count at all three concentrations (micro-TP) at different salinities for both the model species (Fig. 8A and B). The GLM for A. bahia suggested concentration dependent ingestion at all the salinities (χ2 = 18.12, df = 3, p <0.005). Results also suggested concentration dependent ingestion at the lowest salinities (5 and 15 PSU) (χ2 = 2.55, df = 3, p<0.005). However, in M. beryllina in 15 PSU demonstrated increasing ingestion compared to the 5 and 25 PSU salinity group (χ2 = 1.04, df = 6, p<0.005). Results suggest that ingestion is likely the most common interaction that nondiscriminatory feeding fish larvae and zooplankton have with TP. Previous studies confirm that M. beryllina will ingest microplastic at high concentrations when exposed to zooplankton internalized with TP, although most particles were observed to be egested within 24 hours of internalization (Athey et al., 2020). However, the gut retention time for TP in A. bahia is unknown and may be dependent on particle size and shape. The irregularity of TP shape may contribute to the varying retention times. Egestion of 10 um polystyrene microspheres in other mysid species (N. integer) has been observed to occur within 12 hours of ingestion (Setälä et al., 2014). Microplastic can also agglomerate with increasing salinity, leading to longer retention times in estuarine species closer to marine environments (Ogonowski et al., 2016). In a study comparing the physiological toxicity of polystyrene and carboxylate polystyrene (PS-COOH) in mysid shrimp, both plastics were observed to reduce feeding efficiency in these organisms. Future studies should carefully evaluate changes in density and sinking rates for TP at different salinities.
We observed reduced growth in M. beryllina across all micro-TP and two nano-TP (6000 and 60,000 p/ml) concentrations with increasing salinity, except for individuals exposed to nano-TP at 5 PSU. Other studies have also demonstrated growth inhibition of larval fish due to ingestion and accumulation of microplastics in the gut (Athey et al., 2020; Santos et al., 2020). A. bahia appeared to be less sensitive to growth restriction by TP and exhibited a reduction in growth only in micro concentrations at 20 PSU and 25 PSU. Nano-TP did not elicit a significant response in growth reduction in A. bahia. Inhibited growth in another species, N. japonica, has been observed as a result of chronic polystyrene exposure (Lee et al., 2021). Several studies investigated the effect that microplastics have on the growth of small aquatic organisms. Though some studies found that microplastic, particularly polyethylene, exposure did not affect growth (Malinich et al., 2018; Mazurais et al., 2015), others noted detrimental effects of microplastics on growth (Athey et al., 2020; Lee et al., 2021). These contrasting reports may be attributed to the wide variety of microplastic compositions, shapes, and sizes, as well as the lengths of exposure. While no studies could be found in the current literature on the effects of TP on mysid or silverside growth, the findings of this study are in line with toxicity assessments for other microplastics. For example, exposure of mysids (N. japonica) to polystyrene (PS) and PS-COOH resulted in growth inhibition in a dose-dependent manner in which increasing concentrations resulted in decreasing growth (Wang et al., 2020). Additionally, mysids (N. awatschensis) showed impaired growth when exposed to melamine resin microparticles over four weeks (Lee et al., 2021). For amphipod (H. Azteca), chronic exposure to polyethylene microplastic particles and acute exposure to polypropylene microplastic fibers significantly decreased growth (Au et al., 2015). Similarly, growth inhibition in larval fish has been documented as an effect of exposure to micro polyvinyl chloride (Xia et al., 2020), low density polyethylene (Athey et al., 2020) and microplastic mixtures (Naidoo and Glassom, 2019; Pannetier et al., 2020). Furthermore, a meta-analysis of the literature (Foley et al., 2018) found that overall, exposure of zooplankton to microplastics decreases growth, and food dilution is thought to be one of the major mechanisms of MP toxicity to aquatic organisms in general (de Ruijter et al., 2020; Koelmans et al., 2020).
In aquatic environments, TPs are influenced by tidal processes, currents, and waves and may disperse throughout the estuarine system. At lower salinities closer to the river mouth, TP may remain suspended or float, which may make them more available to organisms that feed in the water column. TP and other particulates will agglomerate at higher salinities and biofouling may occur, increasing the potential for higher density particles to settle out into benthic environments. As mysids are indiscriminate feeding epibenthic organisms, this settling out may increase the likelihood that mysid shrimp occurring at higher salinities will encounter and ingest TP. Additionally, mysid shrimp are confirmed to ingest MP through their prey (Setälä et al., 2014). TP is likely to follow the same fate of planktonic trophic transfer. Inland silversides typically feed in the water column on copepods, mysids, and other zooplankton, although bottom feeding has been observed (Weinstein, 1986). In this respect, inland silversides may ingest TP in both the demersal and benthic environments. Future studies should further investigate the estuarine processes that affect TP circulation and transport and how this will impact aquatic species.
4. Conclusion
Following exposure, M. beryllina and A. bahia had significantly altered swimming behaviors, such as increased freezing, changes in positioning, and total distance moved, which could lead to an increased risk of predation and foraging challenges in the wild. Growth for both A. bahia and M. beryllina was reduced in a concentration-dependent manner when exposed to micro-TP, whereas M. beryllina also demonstrated reduced growth when exposed to nano-TP (except lowest concentration). The specific effects of particles on growth in our study are notable, in comparison to the insignificant effect of leachate on growth. TP internalization was dependent on the exposure concentration and to some extent salinity in both taxa. Recently the role of behavioral ecotoxicology in environmental conservation has been discussed by various scholars (Ford et al., 2021). This includes lab-based research that can help to study more about the individual, population, and ecosystem processes. Our research demonstrated the occurrence of significant behavioral changes in response to the lowest concentration (60 particles/ml), as well as to higher potential future concentrations and leachates under various salinities found in the estuarine environment. These behaviors represent ecologically important stimulus responses in field conditions, including activity (movement, velocity, freezing), boldness (in zone duration and frequency), and exploration (meander, turn angle, distance moved). Behavioral responses connect directly to population fitness and ecosystem-level impacts, therefore carrying high relevance to be considered by policymakers. Additionally, growth and TP ingestion data represent the significant impacts of micro and nano-TP on both of these model species that may have population-level implications. Specifically, data collected in the presence of estuarine conditions over different salinity gradients, that can aid in the assessment of risk over wider environmental ranges. Although automobiles are here to stay, limiting TP from entering the environment is paramount if we wish to preserve sensitive aquatic ecosystems and fisheries. Possible actions to take in order to achieve this goal may include providing incentives for citizen awareness of and participation in waste reduction (Eriksen et al., 2014, p. 201; Rochman et al., 2021) redesigning tire constituents with biopolymers and materials for circularity (Karan et al., 2019) extending tire producer responsibility for the end of life products, (Leal Filho et al., 2019) improving wastewater treatment technology, (Edo et al., 2020; Katyal et al., 2020) and passing legislation to ban certain synthetic materials, as well as increasing use of public transportation rather than single vehicle use (Deng et al., 2020).
Supplementary Material
Highlights.
Larval mysid shrimp and silversides had significantly altered swimming behaviors.
Growth was reduced in both species in a concentration dependent manner in μTP.
TP internalization was dependent on the exposure salinity in both taxa
A. bahia & M. beryllina ingested significantly more particles at 15 PSU in μTP
Acknowledgments:
Authors would like to thank Christopher Markgraf for his assistance in experimental preparation, Amelie Segarra for advice on behavioral assay analysis, and J. Wilson White for his advice on statistical approaches.
Funding:
This research was funded by the National Science Foundation Growing Convergence Research Big Idea (to SH, SMB), 1935028 and supported by ideas and behavioral assay protocols developed under California Delta Science agreement # 18206 (to SMB). The ideas presented in this publication are those solely of the authors and do not necessarily reflect the opinions of the granting agency.
Footnotes
Institutional Review Board Statement: This study was approved by the Oregon State University Institutional Animal Care and Use Committee (IACUC) protocol #0035, approved October 19th, 2019. Adult brood stock was housed and spawned at the Oregon State University Hatfield Marine Science Center under the Animal Care and Use Program (ACUP) protocol #4999.
Contributor Information
S Siddiqui, Fisheries and Wildlife, College of Agricultural and Life Sciences, Oregon State University 97331.
JM Dickens, Marine Resources Management Program, College of Earth, Atmospheric, and Oceanic Sciences, Oregon State University Corvallis, Oregon 97331.
BE Cunningham, Environmental and Molecular Toxicology, College of Agricultural and Life Sciences, Oregon State University 97331.
SJ Hutton, Environmental and Molecular Toxicology, College of Agricultural and Life Sciences, Oregon State University 97331.
EI Pedersen, Fisheries and Wildlife, College of Agricultural and Life Sciences, Oregon State University 97331.
B Harper, Environmental and Molecular Toxicology, College of Agricultural and Life Sciences, Oregon State University 97331.
S Harper, Environmental and Molecular Toxicology, College of Agricultural and Life Sciences; Chemical, Biological and Environmental Engineering, College of Engineering, Oregon State University 97331.
SM Brander, Fisheries and Wildlife, College of Agricultural and Life Sciences, Oregon State University 97331.
References
- Abdel-Tawwab M, Monier MN, Hoseinifar SH, Faggio C, 2019. Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol Biochem 45, 997–1013. 10.1007/s10695-019-00614-9 [DOI] [PubMed] [Google Scholar]
- Athey SN, Albotra SD, Gordon CA, Monteleone B, Seaton P, Andrady AL, Taylor AR, Brander SM, 2020. Trophic transfer of microplastics in an estuarine food chain and the effects of a sorbed legacy pollutant. Limnology and Oceanography Letters 5, 154–162. 10.1002/lol2.10130 [DOI] [Google Scholar]
- Au SY, Bruce TF, Bridges WC, Klaine SJ, 2015. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environmental toxicology and chemistry 34, 2564–2572. [DOI] [PubMed] [Google Scholar]
- Au SY, Lee CM, Weinstein JE, Hurk P, van den Klaine SJ, 2017. Trophic transfer of microplastics in aquatic ecosystems: Identifying critical research needs. Integrated Environmental Assessment and Management 13, 505–509. 10.1002/ieam.1907 [DOI] [PubMed] [Google Scholar]
- Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, Guilhermino L, 2018. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquatic Toxicology 195, 49–57. 10.1016/j.aquatox.2017.12.008 [DOI] [PubMed] [Google Scholar]
- Barnes DKA, Galgani F, Thompson RC, Barlaz M, 2009. Accumulation and fragmentation of plastic debris in global environments. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 1985–1998. 10.1098/rstb.2008.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann W, Ismeier M, 1998. Emissions from tires in dedicated use. Kautsch. Gummi Kunstst 51, 182–186. [Google Scholar]
- Beitinger TL, 1990. Behavioral Reactions for the Assessment of Stress in Fishes. Journal of Great Lakes Research, Fish Community Health: Monitoring and Assessment in Large Lakes 16, 495–528. 10.1016/S0380-1330(90)71443-8 [DOI] [Google Scholar]
- Bessa F, Barría P, Neto JM, Frias JPGL, Otero V, Sobral P, Marques JC, 2018. Occurrence of microplastics in commercial fish from a natural estuarine environment. Marine Pollution Bulletin 128, 575–584. 10.1016/j.marpolbul.2018.01.044 [DOI] [PubMed] [Google Scholar]
- Bour A, Sturve J, Höjesjö J, Carney Almroth B, 2020. Microplastic Vector Effects: Are Fish at Risk When Exposed via the Trophic Chain? Front. Environ. Sci 8. 10.3389/fenvs.2020.00090 [DOI] [Google Scholar]
- Brahney J, Mahowald N, Prank M, Cornwell G, Klimont Z, Matsui H, Prather KA, 2021. Constraining the atmospheric limb of the plastic cycle. Proc Natl Acad Sci USA 118, e2020719118. 10.1073/pnas.2020719118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander SM, Cole BJ, Cherr GN, 2012. An approach to detecting estrogenic endocrine disruption via choriogenin expression in an estuarine model fish species. Ecotoxicology 21, 1272–1280. 10.1007/s10646-012-0879-2 [DOI] [PubMed] [Google Scholar]
- Brander SM, Gabler MK, Fowler NL, Connon RE, Schlenk D, 2016. Pyrethroid pesticides as endocrine disruptors: molecular mechanisms in vertebrates with a focus on fishes. Environmental science & technology 50, 8977–8992. [DOI] [PubMed] [Google Scholar]
- Waterboard CA, 2020. Proposed Definition of “Microplastics in Drinking Water” 1. [Google Scholar]
- Charters FJ, Cochrane TA, O’Sullivan AD, 2015. Particle size distribution variance in untreated urban runoff and its implication on treatment selection. Water Research 85, 337–345. 10.1016/j.watres.2015.08.029 [DOI] [PubMed] [Google Scholar]
- Chen Q, Gundlach M, Yang S, Jiang J, Velki M, Yin D, Hollert H, 2017. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Science of The Total Environment 584–585, 1022–1031. 10.1016/j.scitotenv.2017.01.156 [DOI] [PubMed] [Google Scholar]
- Chen Q, Lackmann C, Wang W, Seiler T-B, Hollert H, Shi H, 2020. Microplastics Lead to Hyperactive Swimming Behaviour in Adult Zebrafish. Aquatic Toxicology 224, 105521. 10.1016/j.aquatox.2020.105521 [DOI] [PubMed] [Google Scholar]
- Choi JS, Jung Y-J, Hong N-H, Hong SH, Park J-W, 2018. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Marine Pollution Bulletin 129, 231–240. 10.1016/j.marpolbul.2018.02.039 [DOI] [PubMed] [Google Scholar]
- Colwill RM, Creton R, 2011. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behavioural Processes 86, 222–229. 10.1016/j.beproc.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier B, 2020. Microplastic toxicity for fish : beyond simple vectors for pollutants? (phdthesis). Université de Bordeaux. [Google Scholar]
- Dannis ML, 1974. Rubber Dust from the Normal Wear of Tires. Rubber Chemistry and Technology 47, 1011–1037. 10.5254/1.3540458 [DOI] [Google Scholar]
- Davidson K, Dudas SE, 2016. Microplastic Ingestion by Wild and Cultured Manila Clams (Venerupis philippinarum) from Baynes Sound, British Columbia. Arch Environ Contam Toxicol 71, 147–156. 10.1007/s00244-016-0286-4 [DOI] [PubMed] [Google Scholar]
- de Ruijter VN, Redondo-Hasselerharm PE, Gouin T, Koelmans AA, 2020. Quality Criteria for Microplastic Effect Studies in the Context of Risk Assessment: A Critical Review. Environ. Sci. Technol 54, 11692–11705. 10.1021/acs.est.0c03057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCourten B, Romney A, Brander S, 2019. The Heat Is On: Complexities of Aquatic Endocrine Disruption in a Changing Global Climate, in: Separation Science and Technology. Elsevier, pp. 13–49. 10.1016/B978-0-12-815730-5.00002-8 [DOI] [Google Scholar]
- DeCourten BM, Brander SM, 2017. Combined effects of increased temperature and endocrine disrupting pollutants on sex determination, survival, and development across generations. Scientific Reports 7, 9310. 10.1038/s41598-017-09631-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCourten BM, Forbes JP, Roark HK, Burns NP, Major KM, White JW, Li J, Mehinto AC, Connon RE, Brander SM, 2020. Multigenerational and Transgenerational Effects of Environmentally Relevant Concentrations of Endocrine Disruptors in an Estuarine Fish Model. Environ. Sci. Technol 54, 13849–13860. 10.1021/acs.est.0c02892 [DOI] [PubMed] [Google Scholar]
- Deng H, Wei R, Luo W, Hu L, Li B, Di Y, Shi H, 2020. Microplastic pollution in water and sediment in a textile industrial area. Environmental Pollution 258, 113658. 10.1016/j.envpol.2019.113658 [DOI] [PubMed] [Google Scholar]
- Døving KB, 1991. Assessment of animal behaviour as a method to indicate environmental toxicity. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 100, 247–252. 10.1016/0742-8413(91)90162-M [DOI] [PubMed] [Google Scholar]
- Durack PJ, Wijffels SE, Matear RJ, 2012. Ocean Salinities Reveal Strong Global Water Cycle Intensification During 1950 to 2000. Science 336, 455–458. 10.1126/science.1212222 [DOI] [PubMed] [Google Scholar]
- Edo C, González-Pleiter M, Leganés F, Fernández-Piñas F, Rosal R, 2020. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environmental Pollution 259, 113837. 10.1016/j.envpol.2019.113837 [DOI] [PubMed] [Google Scholar]
- Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J, 2014. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLOS ONE 9, e111913. 10.1371/journal.pone.0111913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley CJ, Feiner ZS, Malinich TD, Höök TO, 2018. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Science of the Total Environment 631, 550–559. [DOI] [PubMed] [Google Scholar]
- Ford AT, Ågerstrand M, Brooks BW, Allen J, Bertram MG, Brodin T, Dang Z, Duquesne S, Sahm R, Hoffmann F, Hollert H, Jacob S, Klüver N, Lazorchak JM, Ledesma M, Melvin SD, Mohr S, Padilla S, Pyle GG, Scholz S, Saaristo M, Smit E, Steevens JA, van den Berg S, Kloas W, Wong BBM, Ziegler M, Maack G, 2021. The Role of Behavioral Ecotoxicology in Environmental Protection. Environ. Sci. Technol 10.1021/acs.est.0c06493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DF, Brander SM, Hasenbein S, Harvey DJ, Lein PJ, Geist J, Connon RE, 2019. Developmental exposure to environmentally relevant concentrations of bifenthrin alters transcription of mTOR and ryanodine receptor-dependent signaling molecules and impairs predator avoidance behavior across early life stages in inland silversides (Menidia beryllina). Aquatic Toxicology 206, 1–13. 10.1016/j.aquatox.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuiman LA, Magurran AE, 1994. Development of predator defences in fishes. Rev Fish Biol Fisheries 4, 145–183. 10.1007/BF00044127 [DOI] [Google Scholar]
- Gousiadou C, Marchese Robinson RL, Kotzabasaki M, Doganis P, Wilkins TA, Jia X, Sarimveis H, Harper SL, 2021. Machine learning predictions of concentration-specific aggregate hazard scores of inorganic nanomaterials in embryonic zebrafish. null 15, 446–476. 10.1080/17435390.2021.1872113 [DOI] [PubMed] [Google Scholar]
- Granek EF, Brander SM, Holland EB, 2020. Microplastics in aquatic organisms: Improving understanding and identifying research directions for the next decade. Limnol Oceanogr 5, 1–4. 10.1002/lol2.10145 [DOI] [Google Scholar]
- Gray AD, Weinstein JE, 2017. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environmental Toxicology and Chemistry 36, 3074–3080. 10.1002/etc.3881 [DOI] [PubMed] [Google Scholar]
- Gray AD, Wertz H, Leads RR, Weinstein JE, 2018. Microplastic in two South Carolina Estuaries: Occurrence, distribution, and composition. Marine Pollution Bulletin 128, 223–233. 10.1016/j.marpolbul.2018.01.030 [DOI] [PubMed] [Google Scholar]
- Grillitsch B, Vogl C, Wytek R, 1999. Qualification of spontaneous undirected locomotor behavior of fish for sublethal toxicity testing. Part II. Variability of measurement parameters under toxicant-induced stress. Environmental Toxicology and Chemistry 18, 2743–2750. 10.1002/etc.5620181214 [DOI] [Google Scholar]
- Gualtieri M, Mantecca P, Cetta F, Camatini M, 2008. Organic compounds in tire particle induce reactive oxygen species and heat-shock proteins in the human alveolar cell line A549. Environment International 34, 437–442. 10.1016/j.envint.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Hartwell I, Jordahl D, Dawson C, 2000. The Effect of Salinity on Tire Leachate Toxicity. Water Air and Soil Pollution 121, 119–131. 10.1023/A:1005282201554 [DOI] [Google Scholar]
- Hashemi SAR, Stara A, Faggio C, 2019. Biological Characteristics, Growth Parameters and Mortality Rate of Carassius auratus in the Shadegan Wetland (Iran). Int J Environ Res 13, 457–464. 10.1007/s41742-019-00186-9 [DOI] [Google Scholar]
- Helm KP, Bindoff NL, Church JA, 2010. Changes in the global hydrological-cycle inferred from ocean salinity. Geophysical Research Letters 37. 10.1029/2010GL044222 [DOI] [Google Scholar]
- Hutton SJ, St. Romain SJ, Pedersen EI, Siddiqui S, Chappell PE, White JW, Armbrust KL, Brander SM, 2021. Salinity Alters Toxicity of Commonly Used Pesticides in a Model Euryhaline Fish Species (Menidia beryllina). Toxics 9, 114. 10.3390/toxics9050114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL, 2015. Plastic waste inputs from land into the ocean. Science 347, 768–771. 10.1126/science.1260352 [DOI] [PubMed] [Google Scholar]
- Kallai J, Makany T, Csatho A, Karadi K, Horvath D, Kovacs-Labadi B, Jarai R, Nadel L, Jacobs JW, 2007. Cognitive and affective aspects of thigmotaxis strategy in humans. Behavioral Neuroscience 121, 21–30. 10.1037/0735-7044.121.1.21 [DOI] [PubMed] [Google Scholar]
- Karan H, Funk C, Grabert M, Oey M, Hankamer B, 2019. Green Bioplastics as Part of a Circular Bioeconomy. Trends in Plant Science 24, 237–249. 10.1016/j.tplants.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Katyal D, Kong E, Villanueva J, 2020. Microplastics in the environment: impact on human health and future mitigation strategies. Environmental Health Review. 10.5864/d2020-005 [DOI] [Google Scholar]
- Khan F, Ahmed W, Najmi A, 2019. Understanding consumers’ behavior intentions towards dealing with the plastic waste: Perspective of a developing country. Resources, Conservation and Recycling 142, 49–58. 10.1016/j.resconrec.2018.11.020 [DOI] [Google Scholar]
- Klöckner P, Reemtsma T, Eisentraut P, Braun U, Ruhl AS, Wagner S, 2019. Tire and road wear particles in road environment – Quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 222, 714–721. 10.1016/j.chemosphere.2019.01.176 [DOI] [PubMed] [Google Scholar]
- Koelmans AA, Redondo-Hasselerharm PE, Mohamed Nor NH, Kooi M, 2020. Solving the Nonalignment of Methods and Approaches Used in Microplastic Research to Consistently Characterize Risk. Environ. Sci. Technol 54, 12307–12315. 10.1021/acs.est.0c02982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögel T, Bjorøy Ø, Toto B, Bienfait AM, Sanden M, 2020. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Science of The Total Environment 709, 136050. 10.1016/j.scitotenv.2019.136050 [DOI] [PubMed] [Google Scholar]
- Kolandhasamy P, Su L, Li J, Qu X, Jabeen K, Shi H, 2018. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Science of The Total Environment 610–611, 635–640. 10.1016/j.scitotenv.2017.08.053 [DOI] [PubMed] [Google Scholar]
- Kovochich M, Parker JA, Oh SC, Lee JP, Wagner S, Reemtsma T, Unice KM, 2021. Characterization of Individual Tire and Road Wear Particles in Environmental Road Dust, Tunnel Dust, and Sediment. Environ. Sci. Technol. Lett 8, 1057–1064. 10.1021/acs.estlett.1c00811 [DOI] [Google Scholar]
- Le Roux JP, 2005. Grains in motion: A review. Sedimentary Geology 178, 285–313. 10.1016/j.sedgeo.2005.05.009 [DOI] [Google Scholar]
- Leal Filho W, Saari U, Fedoruk M, Iital A, Moora H, Klöga M, Voronova V, 2019. An overview of the problems posed by plastic products and the role of extended producer responsibility in Europe. Journal of Cleaner Production 214, 550–558. 10.1016/j.jclepro.2018.12.256 [DOI] [Google Scholar]
- Lee D-H, Lee S, Rhee J-S, 2021. Consistent exposure to microplastics induces age-specific physiological and biochemical changes in a marine mysid. Marine Pollution Bulletin 162, 111850. 10.1016/j.marpolbul.2020.111850 [DOI] [PubMed] [Google Scholar]
- Lee K-W, Shim WJ, Kwon OY, Kang J-H, 2013. Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus japonicus. Environ. Sci. Technol 47, 11278–11283. 10.1021/es401932b [DOI] [PubMed] [Google Scholar]
- Lei L, Liu M, Song Y, Lu S, Hu J, Cao C, Xie B, Shi H, He D, 2018. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci.: Nano 5, 2009–2020. 10.1039/C8EN00412A [DOI] [Google Scholar]
- Little EE, 1990. Behavioral toxicology: Stimulating challenges for a growing discipline. Environmental Toxicology and Chemistry 9, 1–2. 10.1002/etc.5620090101 [DOI] [Google Scholar]
- Little EE, Finger SE, 1990. Swimming behavior as an indicator of sublethal toxicity in fish. Environmental Toxicology and Chemistry 9, 13–19. 10.1002/etc.5620090103 [DOI] [Google Scholar]
- Lo HKA, Chan KYK, 2018. Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environmental Pollution 233, 588–595. 10.1016/j.envpol.2017.10.095 [DOI] [PubMed] [Google Scholar]
- Malinich TD, Chou N, Sepúlveda MS, Höök TO, 2018. No evidence of microplastic impacts on consumption or growth of larval Pimephales promelas. Environmental toxicology and chemistry 37, 2912–2918. [DOI] [PubMed] [Google Scholar]
- Mazurais D, Ernande B, Quazuguel P, Severe A, Huelvan C, Madec L, Mouchel O, Soudant P, Robbens J, Huvet A, Zambonino-Infante J, 2015. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Marine Environmental Research 112, 78–85. 10.1016/j.marenvres.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Middaugh DP, Hemmer MJ, Goodman LR, 1987. Methods for spawning, culturing and conducting toxicity-tests with early life stages of four atherinid fishes: the inland silverside, Menidia beryllina, Atlantic silverside, M. menidia, tidewater silverside, M. peninsulae and California grunion, Leuresthes tenuis. Office of Research and Development, U.S. Environmental Protection Agency, Washington DC: 20460. [Google Scholar]
- Mundy P, Huff Hartz K, Fulton C, Lydy M, Brander S, Hung T, Fangue N, Connon R, 2021. Exposure to permethrin or chlorpyrifos causes differential dose- and time-dependent behavioral effects at early larval stages of an endangered teleost species. Endang. Species. Res 44, 89–103. 10.3354/esr01091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy PC, Carte MF, Brander SM, Hung T-C, Fangue N, Connon RE, 2020. Bifenthrin exposure causes hyperactivity in early larval stages of an endangered fish species at concentrations that occur during their hatching season. Aquatic Toxicology 228, 105611. 10.1016/j.aquatox.2020.105611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo T, Glassom D, 2019. Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Marine Pollution Bulletin 145, 254–259. 10.1016/j.marpolbul.2019.02.037 [DOI] [PubMed] [Google Scholar]
- Nelson S, Mueller G, Hemphill D, 1994. Identification of tire leachate toxicants and a risk assessment of water quality effects using tire reefs in canals. Bulletin of Environmental Contamination and Toxicology 52, 574–581. [DOI] [PubMed] [Google Scholar]
- Newman MC, Jagoe CH, 1996. Ecotoxicology: A Hierarchical Treatment. CRC Press. [Google Scholar]
- Noldus LPJJ, Spink AJ, Tegelenbosch RAJ, 2001. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behavior Research Methods, Instruments, & Computers 33, 398–414. 10.3758/BF03195394 [DOI] [PubMed] [Google Scholar]
- Ogonowski M, Schür C, Jarsén Å, Gorokhova E, 2016. The Effects of Natural and Anthropogenic Microparticles on Individual Fitness in Daphnia magna. PLOS ONE 11, e0155063. 10.1371/journal.pone.0155063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S, Katagiri T, Maita M, Endo M, Futami K, 2017. Application of Tissue Clearing Techniques to 3D Study of Infectious Disease Pathology in Fish. Fish Pathology 52, 96–99. 10.3147/jsfp.52.96 [DOI] [Google Scholar]
- Pannetier P, Morin B, Le Bihanic F, Dubreil L, Clérandeau C, Chouvellon F, Van Arkel K, Danion M, Cachot J, 2020. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environment International 134, 105047. 10.1016/j.envint.2019.105047 [DOI] [PubMed] [Google Scholar]
- Parker BW, Beckingham BA, Ingram BC, Ballenger JC, Weinstein JE, Sancho G, 2020. Microplastic and tire wear particle occurrence in fishes from an urban estuary: Influence of feeding characteristics on exposure risk. Marine Pollution Bulletin 160, 111539. 10.1016/j.marpolbul.2020.111539 [DOI] [PubMed] [Google Scholar]
- Pillard DA, Dufresne DL, Tietge JE, Evans JM, 1999. Response of mysid shrimp (Mysidopsis bahia), sheepshead minnow (Cyprinodon variegatus), and inland silverside minnow (Menidia beryllina) to changes in artificial seawater salinity. Environmental Toxicology and Chemistry 18, 430–435. 10.1002/etc.5620180310 [DOI] [Google Scholar]
- Piotrowska K, Kruszelnicka W, Bałdowska-Witos P, Kasner R, Rudnicki J, Tomporowski A, Flizikowski J, Opielak M, 2019. Assessment of the Environmental Impact of a Car Tire throughout Its Lifecycle Using the LCA Method. Materials 12, 4177. 10.3390/ma12244177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Hasselerharm PE, de Ruijter VN, Mintenig SM, Verschoor A, Koelmans AA, 2018. Ingestion and Chronic Effects of Car Tire Tread Particles on Freshwater Benthic Macroinvertebrates. Environ. Sci. Technol 52, 13986–13994. 10.1021/acs.est.8b05035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rist S, Baun A, Hartmann NB, 2017. Ingestion of micro- and nanoplastics in Daphnia magna – Quantification of body burdens and assessment of feeding rates and reproduction. Environmental Pollution 228, 398–407. 10.1016/j.envpol.2017.05.048 [DOI] [PubMed] [Google Scholar]
- Ritz C, 2010. Toward a unified approach to dose-response modeling in ecotoxicology. Environmental Toxicology and Chemistry 29, 220–229. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Reichl J, Song MY, Dearinger AD, Moridzadeh N, Lu ED, Pearce K, Esdin J, Glanzman DL, 2011. Habituation of the C-Start Response in Larval Zebrafish Exhibits Several Distinct Phases and Sensitivity to NMDA Receptor Blockade. PLoS ONE 6, e29132. 10.1371/journal.pone.0029132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman CM, Brookson C, Bikker J, Djuric N, Earn A, Bucci K, Athey S, Huntington A, McIlwraith H, Munno K, Frond HD, Kolomijeca A, Erdle L, Grbic J, Bayoumi M, Borrelle SB, Wu T, Santoro S, Werbowski LM, Zhu X, Giles RK, Hamilton BM, Thaysen C, Kaura A, Klasios N, Ead L, Kim J, Sherlock C, Ho A, Hung C, 2019. Rethinking microplastics as a diverse contaminant suite. Environmental Toxicology and Chemistry 38, 703–711. 10.1002/etc.4371 [DOI] [PubMed] [Google Scholar]
- Rochman CM, Browne MA, Underwood AJ, van Franeker JA, Thompson RC, Amaral-Zettler LA, 2016. The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived. Ecology 97, 302–312. 10.1890/14-2070.1 [DOI] [PubMed] [Google Scholar]
- Rochman CM, Munno K, Box C, Cummins A, Zhu X, Sutton R, 2021. Think Global, Act Local: Local Knowledge Is Critical to Inform Positive Change When It Comes to Microplastics. Environ. Sci. Technol 55, 4–6. 10.1021/acs.est.0c05746 [DOI] [PubMed] [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT, 1993. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: roads as sources and sinks. Environ. Sci. Technol 27, 1892–1904. 10.1021/es00046a019 [DOI] [Google Scholar]
- Romney ALT, Yanagitsuru YR, Mundy PC, Fangue NA, Hung T-C, Brander SM, Connon RE, 2019. Developmental staging and salinity tolerance in embryos of the delta smelt, Hypomesus transpacificus. Aquaculture 511, 634191. 10.1016/j.aquaculture.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, Félix L, Luzio A, Parra S, Cabecinha E, Bellas J, Monteiro SM, 2020. Toxicological effects induced on early life stages of zebrafish (Danio rerio) after an acute exposure to microplastics alone or co-exposed with copper. Chemosphere 261, 127748. 10.1016/j.chemosphere.2020.127748 [DOI] [PubMed] [Google Scholar]
- Saranjampour P, Vebrosky EN, Armbrust KL, 2017. Salinity impacts on water solubility and n-octanol/water partition coefficients of selected pesticides and oil constituents. Environmental toxicology and chemistry 36, 2274–2280. [DOI] [PubMed] [Google Scholar]
- Schnörr SJ, Steenbergen PJ, Richardson MK, Champagne DL, 2012. Measuring thigmotaxis in larval zebrafish. Behavioural Brain Research 228, 367–374. 10.1016/j.bbr.2011.12.016 [DOI] [PubMed] [Google Scholar]
- Segarra A, Mauduit F, Amer NR, Biefel F, Hladik ML, Connon RE, Brander SM, 2021. Salinity Changes the Dynamics of Pyrethroid Toxicity in Terms of Behavioral Effects on Newly Hatched Delta Smelt Larvae. Toxics 9, 40. 10.3390/toxics9020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setälä O, Fleming-Lehtinen V, Lehtiniemi M, 2014. Ingestion and transfer of microplastics in the planktonic food web. Environmental Pollution 185, 77–83. 10.1016/j.envpol.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Sharma S, Coombs S, Patton P, de Perera TB, 2009. The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax). J Comp Physiol A 195, 225–240. 10.1007/s00359-008-0400-9 [DOI] [PubMed] [Google Scholar]
- Shepherd JG, Cushing DH, 1980. A mechanism for density-dependent survival of larval fish as the basis of a stock-recruitment relationship. ICES Journal of Marine Science 39, 160–167. 10.1093/icesjms/39.2.160 [DOI] [Google Scholar]
- Shupe HJ, Boenisch KM, Harper BJ, Brander SM, Harper SL, 2021. Effect of Nanoplastic Type and Surface Chemistry on Particle Agglomeration over a Salinity Gradient. Environ Toxicol Chem etc.5030. 10.1002/etc.5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried M, Koelmans AA, Besseling E, Kroeze C, 2017. Export of microplastics from land to sea. A modelling approach. Water Research 127, 249–257. 10.1016/j.watres.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Sprague JB, 1971. Measurement of pollutant toxicity to fish—III: Sublethal effects and “safe” concentrations. Water Research 5, 245–266. 10.1016/0043-1354(71)90171-0 [DOI] [Google Scholar]
- Stienbarger CD, Joseph J, Athey SN, Monteleone B, Andrady AL, Watanabe WO, Seaton P, Taylor AR, Brander SM, 2021. Direct ingestion, trophic transfer, and physiological effects of microplastics in the early life stages of Centropristis striata, a commercially and recreationally valuable fishery species. Environmental Pollution 285, 117653. 10.1016/j.envpol.2021.117653 [DOI] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR, 2015. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc 10, 1709–1727. 10.1038/nprot.2015.085 [DOI] [PubMed] [Google Scholar]
- Tian Z, Zhao H, Peter KT, Gonzalez M, Wetzel J, Wu C, Hu X, Prat J, Mudrock E, Hettinger R, Cortina AE, Biswas RG, Kock FVC, Soong R, Jenne A, Du B, Hou F, He H, Lundeen R, Gilbreath A, Sutton R, Scholz NL, Davis JW, Dodd MC, Simpson A, McIntyre JK, Kolodziej EP, 2021. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 371, 185–189. 10.1126/science.abd6951 [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M, 1988. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacology Biochemistry and Behavior 31, 959–962. 10.1016/0091-3057(88)90413-3 [DOI] [PubMed] [Google Scholar]
- Unice KM, Weeber MP, Abramson MM, Reid RCD, van Gils JAG, Markus AA, Vethaak AD, Panko JM, 2019. Characterizing export of land-based microplastics to the estuary - Part I: Application of integrated geospatial microplastic transport models to assess tire and road wear particles in the Seine watershed. Science of The Total Environment 646, 1639–1649. 10.1016/j.scitotenv.2018.07.368 [DOI] [PubMed] [Google Scholar]
- USEPA, 2009. Culturing Americamysis bahia Supplement to Training Video- EPA 833-C-09-001. [Google Scholar]
- USEPA, 1994. Risk Assessment Guidance for Superfund, Volume II: Environmental Evaluation Manual (EPA/540-1-89/001). [Google Scholar]
- Van Cauwenberghe L, Janssen CR, 2014. Microplastics in bivalves cultured for human consumption. Environmental Pollution 193, 65–70. 10.1016/j.envpol.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Vlaming V. de, Connor V, DiGiorgio C, Bailey HC, Deanovic LA, Hinton DE, 2000. Application of whole effluent toxicity test procedures to ambient water quality assessment. Environmental Toxicology and Chemistry 19, 42–62. 10.1002/etc.5620190106 [DOI] [Google Scholar]
- Wagner S, Hüffer T, Klöckner P, Wehrhahn M, Hofmann T, Reemtsma T, 2018. Tire wear particles in the aquatic environment - A review on generation, analysis, occurrence, fate and effects. Water Research 139, 83–100. 10.1016/j.watres.2018.03.051 [DOI] [PubMed] [Google Scholar]
- Wang X, Liu L, Zheng H, Wang M, Fu Y, Luo X, Li F, Wang Z, 2020. Polystyrene microplastics impaired the feeding and swimming behavior of mysid shrimp Neomysis japonica. Marine Pollution Bulletin 150, 110660. 10.1016/j.marpolbul.2019.110660 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yin L, Zhao J, Xing B, 2016. Trophic transfer and accumulation of TiO2 nanoparticles from clamworm (Perinereis aibuhitensis) to juvenile turbot (Scophthalmus maximus) along a marine benthic food chain. Water Research 95, 250–259. 10.1016/j.watres.2016.03.027 [DOI] [PubMed] [Google Scholar]
- Watts AJ, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS, 2014. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environmental science & technology 48, 8823–8830. [DOI] [PubMed] [Google Scholar]
- Watts AJR, Urbina MA, Goodhead R, Moger J, Lewis C, Galloway TS, 2016. Effect of Microplastic on the Gills of the Shore Crab Carcinus maenas. Environ. Sci. Technol 50, 5364–5369. 10.1021/acs.est.6b01187 [DOI] [PubMed] [Google Scholar]
- Weinstein MP, 1986. Habitat Suitability Index Models: Inland silverside. National Wetlands Research Center. [Google Scholar]
- Weis JS, Candelmo A, 2012. Pollutants and fish predator/prey behavior: A review of laboratory and field approaches. Current Zoology 58, 9–20. 10.1093/czoolo/58.1.9 [DOI] [Google Scholar]
- Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P, 2001. Effects of Contaminants on Behavior: Biochemical Mechanisms and Ecological Consequences. BioScience 51, 209. https://doi.org/10.1641/0006-3568(2001)051[0209:EOCOBB]2.0.CO;2 [Google Scholar]
- Werbowski LM, Gilbreath AN, Munno K, Zhu X, Grbic J, Wu T, Sutton R, Sedlak MD, Deshpande AD, Rochman CM, 2021. Urban Stormwater Runoff: A Major Pathway for Anthropogenic Particles, Black Rubbery Fragments, and Other Types of Microplastics to Urban Receiving Waters. ACS EST Water acsestwater.1c00017. 10.1021/acsestwater.1c00017 [DOI] [Google Scholar]
- Wik A, Dave G, 2009. Occurrence and effects of tire wear particles in the environment – A critical review and an initial risk assessment. Environmental Pollution 157, 1–11. 10.1016/j.envpol.2008.09.028 [DOI] [PubMed] [Google Scholar]
- Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV, 2010. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behavioural Brain Research 208, 450–457. 10.1016/j.bbr.2009.12.023 [DOI] [PubMed] [Google Scholar]
- Wortham-Neal JL, Price WW, 2002. Marsupial Developmental Stages in Americamysis Bahia (Mysida: Mysidae). Journal of Crustacean Biology 22, 98–112. 10.1163/20021975-99990213 [DOI] [Google Scholar]
- Wright SL, Rowe D, Thompson RC, Galloway TS, 2013. Microplastic ingestion decreases energy reserves in marine worms. Current Biology 23, R1031–R1033. 10.1016/j.cub.2013.10.068 [DOI] [PubMed] [Google Scholar]
- Wurtsbaugh W, Li H, 1985. Diel migrations of a zooplanktivorous fish (Menidia beryllina) in relation to the distribution of its prey in a large eutrophic lake1. Limnology and Oceanography 30, 565–576. 10.4319/lo.1985.30.3.0565 [DOI] [Google Scholar]
- Xia X, Sun M, Zhou M, Chang Z, Li L, 2020. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Science of The Total Environment 716, 136479. [DOI] [PubMed] [Google Scholar]
- Yin L, Chen B, Xia B, Shi X, Qu K, 2018. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). Journal of Hazardous Materials 360, 97–105. 10.1016/j.jhazmat.2018.07.110 [DOI] [PubMed] [Google Scholar]
- Yin L, Liu H, Cui H, Chen B, Li L, Wu F, 2019. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). Journal of Hazardous Materials 380, 120861. 10.1016/j.jhazmat.2019.120861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


