Abstract
Objectives
Better understanding of the lifestyle activities shared among older adult subgroups may inform further health behavioral interventions that can be deployed at the group or community level. We applied latent class analysis to characterize qualitatively distinct lifestyle engagement groups, examined their differential risk of incident dementia, and compared their predictive utility to traditional activity frequency and variety scores.
Method
Participants were from the Ginkgo Evaluation of Memory Study (N = 3,068, mean age = 78.5). Lifestyle activities were measured at baseline using the Lifestyle Activity Questionnaire. All-cause dementia was screened every 6 months and cases were clinically adjudicated. Median follow-up was 6 years. Time to dementia was assessed using discrete-time proportional hazards models, adjusted for demographic and health covariates.
Results
Latent classes provided slightly poorer case discrimination than the frequency scores but identified distinct qualitative subgroups. In the 4-class model, the Variety (22%) and Intellectual (18%) lifestyle groups had high engagement in intellectual activities, whereas the Variety and Social groups (32%) had high engagement in formal social activities. Compared to the Least Active group (28%), the Variety (hazard ratio [HR] = 0.67, 95% confidence interval [CI]: 0.48, 0.93) and Intellectual (HR = 0.65, 95% CI: 0.45, 0.93) groups had significantly lower risk of incident dementia, but only among those without prevalent mild cognitive impairment.
Discussion
Older adults highly engaged in intellectual activities, but not necessarily social activities, had the lowest risk of incident dementia. Activity frequency scores provided only slightly better case discrimination than activity variety scores and latent classes. Latent classes of older adults differed by their amount and types of activities, which may inform intervention design.
Keywords: Alzheimer’s disease, Leisure activities, Longitudinal change, Measurement, Prevention
With increasing active life expectancy postretirement (Crimmins et al., 2016), older adulthood brings about novel chances for lifestyle activity engagement. This potential for new or renewed engagement in physical, cognitive, and social activities has been suggested as one way to prevent cognitive impairments in later life (Livingston et al., 2020). To that end, several studies have found that self-reported lifestyle activities are protective against incident dementia (Bennett et al., 2014; Scarmeas et al., 2001; Verghese et al., 2003; Wilson et al., 2007). Yet, it remains unclear which aspects of activities (e.g., amount, type) are the most predictive and the most informative for interventions.
Engagement in a larger number of enriching activities in later life (i.e., activity variety) is thought to be directly related to complexity of one’s lifestyle (Carlson et al., 2012). Research using variety measures has found that the number of activities endorsed, regardless of the frequency with which one participates in them, is associated with reduced risk of incident dementia (Scarmeas et al., 2001; Verghese et al., 2003) and cognitive decline (Carlson et al., 2012; Chan et al., 2019), potentially through increased brain reserve (Bennett et al., 2014; Moored et al., 2020). To study the benefits of activities that draw on specific functions (e.g., social, cognitive, etc.), researchers typically categorize activities into subdomains, either using a priori classification (Parisi et al., 2012), cognitive intensity weighting (Carlson et al., 2012), or factor analytic (Hultsch et al., 1999) methods. These approaches are important for identifying specific activity types that are most protective against cognitive impairment and decline.
Activities that are physically demanding (e.g., aerobic exercise) have consistently been linked with greater cognitive functioning in older adults (National Academies of Sciences, Engineering, and Medicine, 2017). Although more mixed, there is also increasing evidence for intellectual and social activities contributing to cognitive health in later life. More frequent engagement in a higher number of intellectual activities has been linked with lower risk of dementia (Scarmeas et al., 2001; Verghese et al., 2003; Wilson et al., 2007) and improved cognitive functioning (Bielak, 2010; Carlson et al., 2012; Hultsch et al., 1999). Hultsch and colleagues (1999) found that intellectual activities encouraging “novel information processing” appeared to have the largest protective associations, and several studies suggest that intellectual engagement particularly benefits perceptual speed and memory domains (Bielak, 2010; Carlson et al., 2012). When accounting for level of intellectual engagement, social activities (e.g., socializing with friends) have appeared less protective against cognitive declines (Scarmeas et al., 2001; Wilson et al., 2007). Yet, social lifestyle factors (e.g., network size, loneliness) have been linked with reduced risk of dementia (Bennett et al., 2014; Fratiglioni et al., 2004; Kelly et al., 2017), suggesting that social engagement may mitigate cognitive impairment through alternative mechanisms (e.g., increased connectedness/social support, reduced loneliness).
Expanding upon prior literature, we used a novel application of latent class analysis (LCA) to characterize lifestyle engagement groups using both number and type of activities (Collins & Lanza, 2010). By grouping individuals by their response patterns, rather than activity count, LCA may better characterize lifestyle engagement variation by specifying which types of activities are shared by individuals who have similar numbers of activities. We hypothesized that individuals would group by specific activity types as defined by the prior literature (e.g., intellectual vs social activities), with those specifically active in intellectual activities being the most protected against incident dementia (Parisi et al., 2012; Wilson et al., 2007).
A further advantage of LCA is that it groups individuals rather than activities (i.e., person-centered vs item-centered), and it could further inform whether individuals group by activities within or across multiple established domains (intellectual, social, physical). Given that lifestyle activities are multidimensional, this may inform novel ways to classify activities and identify which groups are at risk by virtue of their shared lifestyles. For example, sociodemographic, health, and psychosocial factors contribute to later-life differences in life-space mobility (Allman et al., 2006), defined as the spatial area in which individuals move in daily life, which could influence settings in which they are active. Thus, we also hypothesized that individuals would group by activity setting, such as informal (e.g., playing cards) versus formal social activities (e.g., volunteering). Similar patterns were found in a recent study of social engagement in older adults with mild cognitive impairment (MCI: Amano et al., 2020), where individuals with more formal and informal (vs only informal) social engagement were more likely to have higher cognitive functioning.
The purpose of this study was twofold. First, we examined whether activity frequency and variety predicted dementia risk after adjusting for demographic and health confounders. We also examined potential effect modification by prevalent MCI, given the potential for reverse causation, where individuals may have already modified their baseline activity engagement by virtue of their cognitive status (Gow et al., 2012). Examining those without prevalent MCI separately may help address potential reverse causation by estimating a separate association for individuals whose baseline activity engagement was less likely impacted by existing cognitive impairment. Second, we examined whether using a data-driven LCA approach better predicted dementia risk or provided additional benefit in terms of understanding qualitative differences in activity patterns without sacrificing model fit. Better understanding of the types of activities driving group differences in engagement may inform which settings are most relevant for intervention and the ways in which individuals are motivated to be active.
Method
Participants
Participants were volunteers from the Ginkgo Evaluation of Memory Study, a randomized clinical trial testing the efficacy of Ginkgo biloba supplements for preventing dementia (DeKosky et al., 2006, 2008). Participants were at least 75 years old and free of dementia at baseline. Recruitment occurred at four study sites: Hagerstown, Maryland; Pittsburgh, Pennsylvania; Winston-Salem/Greensboro, North Carolina; and Sacramento, California. Details regarding eligibility, recruitment, and the intervention are found elsewhere (DeKosky et al., 2006; Fitzpatrick et al., 2006). Individuals with MCI were included (Snitz et al., 2009). Data collection occurred from September 2000 to April 2008. A total of 3,069 individuals were enrolled. One individual was missing all responses for the Lifestyle Activity Questionnaire and was removed from the analysis. Participants provided written informed consent, and the study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board and all other sites involved in the study.
The sample had an average baseline age of 78.5 (SD = 3.3), was mostly White (95%), and had an approximately even sex distribution (53% male; Table 1). Most participants were highly educated (36% with high school degree or less). About half of the participants rated their health as “very good” or “excellent,” few had significant depressive symptoms (7%), and most had few medical comorbidities (M = 1.4, SD = 1.1).
Table 1.
Ginkgo Evaluation of Memory Study Baseline Sample Characteristics (N = 3,068)
| Overall | Class 1: Variety (n = 662) | Class 2: Intellectual (n = 514) | Class 3: Social (n = 1,036) | Class 4: Least Active (n = 856) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (or M) | % (or SD) | Range | N (or M) | % (or SD) | N (or M) | % (or SD) | N (or M) | % (or SD) | N (or M) | % (or SD) | |
| Age | 78.5 | 3.3 | 72–96 | 78.1 | 3.1 | 78.2 | 3.0 | 78.7 | 3.2 | 78.9 | 3.6 |
| Sex (male) | 1,649 | 54 | 294 | 44 | 273 | 53 | 567 | 55 | 515 | 60 | |
| Race (White) | 2,929 | 95 | 637 | 96 | 500 | 97 | 985 | 95 | 807 | 94 | |
| Education | |||||||||||
| Less than or equal to HS | 1,103 | 36 | 137 | 21 | 97 | 19 | 461 | 45 | 408 | 48 | |
| Some college | 775 | 25 | 180 | 27 | 138 | 27 | 244 | 24 | 213 | 25 | |
| College graduate | 480 | 16 | 107 | 16 | 114 | 22 | 138 | 13 | 121 | 14 | |
| Professional/graduate | 710 | 23 | 238 | 36 | 165 | 32 | 193 | 19 | 114 | 13 | |
| MCI | 481 | 16 | 78 | 11.78 | 50 | 9.73 | 171 | 16.51 | 182 | 21 | |
| Medical comorbidities | 1.4 | 1.1 | 0–7 | 1.3 | 1.0 | 1.4 | 1.0 | 1.3 | 1.0 | 1.6 | 1.1 |
| Depressive symptoms (CES-D) | 3.6 | 3.5 | 0–6 | 3.2 | 3.0 | 3.7 | 3.7 | 3.5 | 3.5 | 4.0 | 3.7 |
Notes: CES-D = Center for Epidemiologic Studies Depression Scale; HS = high school; MCI = mild cognitive impairment; M = mean; N = number. Analyses of variance (continuous variables) and chi-squared tests (categorical variables) of differences between classes were all statistically significant (ps < .05).
Measures
Lifestyle Activity Questionnaire
At baseline, participants were asked the frequency with which they participated in 26 activities (e.g., reading, gardening, etc.) over the past year on a 6-point Likert scale (0 = never/less than once a month, 5 = every day). Frequencies were rescaled to be interpreted as the approximate number of days of engagement in the past month (Carlson et al., 2012). Items were also recoded as a binary (yes/no) variable indicating whether or not participants engaged in each activity at least once per month during the past year. Dichotomizing responses mitigated problems with data sparseness (Collins & Lanza, 2010).
Selecting activities
—To further mitigate data sparseness issues, we removed activities a priori based on two criteria: (a) empirical: removing activities endorsed by more than 90% or less than 10% of the sample, as these activities would not provide substantial variance for model estimation, and (b) theoretical: removing activities of daily living (ADLs) and instrumental ADLs (e.g., cooking) and activities previously identified as “passive” (e.g., listening to the radio [Parisi et al., 2015]). This resulted in a final group of 18 activities (Table 2), which included activities from the remaining “physical,” “intellectual/creative,” and “social” domains. Participants reported about nine activities, on average (SD = 3.0). “Reading books” was the most frequently reported (83%), while “drawing and painting” was the least reported (11%; Table 2).
Table 2.
Frequency of Self-Reported Lifestyle Activity Questionnaire Activities Over the Past Year
| Proportion participating at least once a month | Reason(s) removed | |||
|---|---|---|---|---|
| Activity subdomain | N | Percent | ||
| Selected activities | ||||
| Reading a book | Intellectual | 2,545 | 82.9 | |
| Walking | Physical | 2,526 | 82.3 | |
| Gardening | Physical | 2,262 | 73.7 | |
| Assist family | Social | 2,250 | 73.3 | |
| Attend church/religious service | Social | 2,248 | 73.2 | |
| Clubs/organizations | Social | 2,241 | 73.0 | |
| Sewing, mending, fixing things | Intellectual | 2,220 | 72.3 | |
| Volunteering | Social | 1,749 | 57.0 | |
| Playing cards or games | Social | 1,535 | 50.0 | |
| Using computer | Intellectual | 1,247 | 40.6 | |
| View art | Intellectual | 1,167 | 38.0 | |
| Crossword puzzles | Intellectual | 1,141 | 37.2 | |
| Going to plays/concerts | Social | 1,127 | 36.7 | |
| Singing, playing instrument | Intellectual | 1,023 | 33.3 | |
| Babysitting | Social | 943 | 30.7 | |
| Movies | Social | 867 | 28.2 | |
| Taking courses | Intellectual | 521 | 17.0 | |
| Drawing or painting | Intellectual | 344 | 11.2 | |
| Removed activities | ||||
| Watching TV | Passive | 3,021 | 98.4 | High frequency, passive |
| Shopping | Physical | 3,004 | 97.9 | High frequency, IADL |
| Reading a newspaper | Intellectual | 2,984 | 97.2 | High frequency |
| Discussing local or national issues | Social | 2,881 | 93.8 | High frequency |
| Visiting others | Social | 2,844 | 92.6 | High frequency |
| Listening to radio (music) | Passive | 2,787 | 90.8 | High frequency, passive |
| Balancing checkbook | Intellectual | 2,423 | 78.9 | IADL |
| Listening to radio (not music) | Passive | 2,416 | 78.7 | Passive |
| Cooking/preparing food | Intellectual | 2,313 | 75.3 | IADL |
| Hunting/camping | Physical | 274 | 8.9 | Low frequency |
Note: IADL = instrumental activity of daily living.
Dementia adjudication
Details regarding dementia adjudication have been described elsewhere (DeKosky et al., 2008). Screening for incident dementia took place every 6 months for up to 7.5 years. Participants were administered the full neuropsychological test battery after screening if they had a decrease in score on at least two of the three following tests: Modified Mini-Mental State Examination (Teng & Chui, 1987), Clinical Dementia Rating (CDR) Scale (Morris, 1993), or the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (Mohs, 1996). Adjudication was performed by a panel of expert clinicians using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for dementia (American Psychiatric Association, 2000), informed by results from the test battery, neurological exam, and a brain magnetic resonance imaging (MRI) scan (DeKosky et al., 2006).
Descriptive covariates
Demographics
Baseline demographic variables included age (years), race (White/non-White), education (high school, some college, college graduate, professional degree), intervention group, and study site.
Medical comorbidities
.—Participants reported their current medical conditions and risk factors at baseline. These included self-reported hypertension, current/former smoking, diabetes, acute myocardial infarction, heart failure, atrial fibrillation, stroke, and transient ischemic attack. A sum count of each binary (yes/no) response to these variables was generated to measure total medical comorbidities.
Depressive symptoms
A modified 10-item Center for Epidemiologic Studies Depression (CES-D) Scale (Radloff, 1977) was used to measure depressive symptoms at baseline (Supplementary Appendix B). Items were measured on a 4-point Likert scale (0 = rarely/none of the time to 3 = most of the time). Responses were summed to produce a composite depressive symptom score, and scores ≥10 were identified as potential clinical depression (Björgvinsson et al., 2013).
Mild cognitive impairment
—Prevalent MCI was defined as meeting two criteria (Snitz et al., 2009): (a) a CDR global score of 0.5 and (b) scoring ≤10th percentile on at least two of 10 neuropsychological test scores in memory, language, visuospatial abilities, attention, and executive function domains. The 10th percentile cutoffs were based on normative data from the Cardiovascular Health Study (Snitz et al., 2009).
Analytic Strategy
Latent class analysis
We used LCA to group individuals by both quantity and types of activity engagement. Class enumeration was determined by fitting a series of models of increasing numbers of classes. To account for the large number of indicators included, and to assess the degree to which the enumeration process was sensitive to specific activities, we first conducted repeated latent class analyses using 10 semirandom subsets of nine of the 18 activities (four intellectual, one physical, and four social; Supplementary Appendix C). We used a lower Bayesian information criterion (BIC) and significant bootstrapped likelihood ratio test (BLRT) as the selection criteria (Nylund et al., 2007).
After determining a range of best-fitting solutions using the activity subsets, we fit a full model using all 18 activities and examined the theoretical interpretability across the range of solutions (Collins & Lanza, 2010). A model with fewer classes that captured distinct activity groupings but adhering to our a priori hypotheses (e.g., social vs intellectual) was favored over a model with more classes that had significant overlap with existing classes or that appeared to be distinguished by engagement in a single activity. Individuals were assigned to classes with the highest posterior probability (Lanza, Tan, & Bray, 2013).
Comparison approaches
We compared the above LCA model with two approaches commonly used in the literature. Both approaches generated continuous composites based on item-level rather than person-level grouping. First, activity variety was defined as the sum of activities engaged in at least once per month (Chan et al., 2019). Second, we calculated average frequency scores for intellectual, social, and physical subdomains informed by prior literature (Table 2; Hultsch et al., 1999; Parisi et al., 2012; Wilson et al., 2007). Intellectual activities included those high in cognitive demand (e.g., taking courses, playing an instrument), whereas social (e.g., church, movies) and physical (e.g., gardening, walking) domains included activities previously rated as less cognitively demanding (Carlson et al., 2012).
Time-to-dementia analysis
We used activity variety scores, frequency scores, and class assignment as predictors in separate hierarchical discrete-time proportional hazards analyses of time-to-dementia onset. Complementary log–log regressions were used to estimate the hazard ratios (HRs) for each predictor. Study entry was at the baseline evaluation session. Study exit occurred at either the time of dementia onset, death, or last study contact. Time (by visits) was included as discrete indicators (visits 1–15). The proportional hazards assumption was explored using time-specific (i.e., Predictor × Time) terms for each predictor (Royston & Lambert, 2011), and any predictors in violation were examined in stratified analyses. Model 1 included only the activity variety score or activity class indicators (in separate models). Model 2 was adjusted for potential confounders, including treatment group, study site, baseline age (Buchman et al., 2014), sex (Wu et al., 2017), race (Mayeda et al., 2016), education category (Wilson et al., 1999), baseline depression (CES-D ≥ 10 [Glass et al., 2006]), and baseline medical comorbidities (Saunders et al., 2016). We included spline terms for each 5-year interval of baseline age (i.e., >80, >85, >90) to allow for a nonlinear relationship between age and dementia. Model 3 further stratified by MCI status.
To compare the predictive utility of the variety score versus the lifestyle engagement groups, we generated receiver operating characteristic curves for each model and compared their respective areas under the curves (AUCs) (Zweig & Campbell, 1993). AUCs indicated the extent to which the included variables discriminate between those who did and did not have incident dementia, with a higher AUCs representing better discrimination. Tenfold cross-validation was implemented to produce more generalizable AUC estimates using the “cvauroc” package in Stata (Luque-Fernandez et al., 2019). Mean cross-validated AUCs were reported.
Sensitivity analyses
Latent class analyses may yield classes where certain covariate ranges are not observed (i.e., “nonpositivity”), rendering simple covariate adjustment insufficient (Collins & Lanza, 2010). We therefore generated propensity scores using multinomial logistic regressions of modal class assignment on the covariates to further examine the covariate spread across classes (Lanza, Coffman, & Xu, 2013). We also tested models using an alternative three-step approach (Vermunt, 2010) to assign individuals to latent classes (Supplementary Appendix D).
Results
Latent Class Analysis
Class enumeration
Using a lower BIC and significant BLRT as the selection criteria, a three- to five-class solution was generally the best fit across the 10 semirandom subsets of activities. Notably, for some activity subsets, the five-class models had convergence issues due to sparse response patterns, in which case a four-class model was chosen (Supplementary Table C.1).
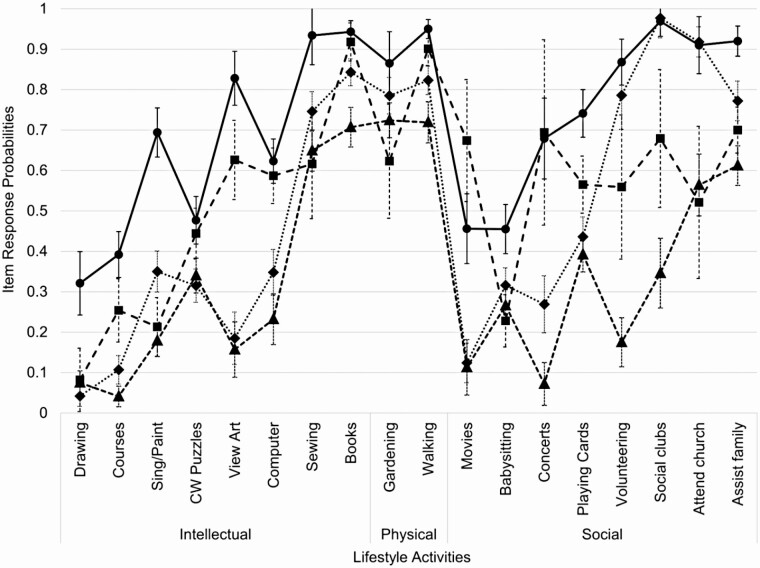
Figure 1 presents the item-response probabilities for the four-class model for the full set of 18 items. While the five-class model fit better than the four-class model when including all items (Supplementary Table C.2), the additional fifth class overlapped substantially with existing classes and the item-response estimates had wide confidence intervals (CIs; Supplementary Figure D.1). We therefore selected a four-class model for the primary analyses.
Figure 1.
Probabilities of engagement in each activity by latent class for the four-class model. CW = crossword. Error bars represent 95% confidence intervals for item-response probability estimates. Black solid/circles: Class 1: Variety; black dashed/squares: Class 2: Intellectual; gray dotted/diamonds: Class 3: Social; gray dashed/triangles: Class 4: Least Active.
Class structure
The four lifestyle groups were Variety (n = 662, 22%), Intellectual (n = 514, 18%), Social (n = 1,036, 32%), and Least Active (n = 856, 28%). There were important differences in both number and types of activities across groups (Figure 1). The Variety and Intellectual groups had high probabilities of engagement in intellectual activities (e.g., viewing art, computer use). These groups also had higher engagement in informal social activities (e.g., movies, concerts, playing cards). In contrast, the Variety and Social groups had high probabilities of engagement in formal social activities (e.g., volunteering, church). Finally, the Least Active group had lower probabilities of engagement in most activities, excluding some home-based activities (e.g., gardening).
Time-to-Dementia Analyses
There was a median of 6.0 years of follow-up (interquartile range: 4.9, 6.5). Only MCI status was in violation of the proportional hazards assumption, so we included models stratifying by MCI status (Model 3).
Lifestyle engagement classes predicting dementia risk
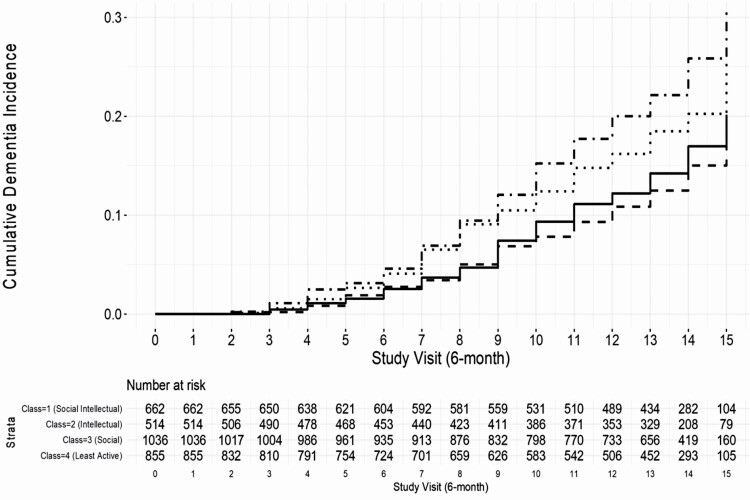
Compared to individuals in the Least Active group, the Variety group had a 40% reduced risk (95% CI: 0.47, 0.77, p < .001), the Intellectual group had a 45% reduced risk (95% CI: 0.41, 0.73, p < .001), and the Social group had a 20% reduced risk (95% CI: 0.65, 0.98, p = .033) of incident dementia in the unadjusted model (Table 3, Model 1; Figure 2). Associations for the Variety and Intellectual groups remained significant after adjusting for demographic and health confounders (ps < .05; Table 4, Model 2). Stratifying by MCI status (Model 3) revealed that the associations for the Variety (HR = 0.67, 95% CI: 0.49, 0.93, p = .017) and Intellectual (HR = 0.65, 95% CI: 0.45, 0.93, p = .018) groups remained significant for those without prevalent MCI only. There was no significant difference in risk of dementia between the Social and Least Active groups after stratifying by MCI (ps > .05).
Table 3.
Unadjusted and Adjusted Discrete-Time Proportional Hazards Models for Lifestyle Engagement Classes Predicting Time-to-Dementia Diagnosis
| Model 3 (stratified by MCI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 (unadjusted) | Model 2 (adjusted) | Non-MCI | MCI | |||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Lifestyle engagement group (ref.: Class 4: Least Active) |
||||||||||||
| Class 1: Variety | 0.601 | 0.47, 0.77 | <.001*** | 0.645 | 0.49, 0.84 | .001** | 0.669 | 0.48, 0.93 | .017* | 0.962 | 0.62, 1.49 | .862 |
| Class 2: Intellectual | 0.545 | 0.41, 0.73 | <.001*** | 0.618 | 0.46, 0.83 | .001** | 0.645 | 0.45, 0.93 | .018* | 1.029 | 0.61, 1.73 | .914 |
| Class 3: Social | 0.799 | 0.65, 0.98 | .033* | 0.808 | 0.66, 1.00 | .046* | 0.780 | 0.59, 1.03 | .075 | 1.219 | 0.87, 1.70 | .248 |
| Age | 1.214 | 1.15, 1.29 | <.001*** | 1.205 | 1.12, 1.29 | <.001*** | 1.173 | 1.06, 1.30 | .002** | |||
| >80 | 0.924 | 0.83, 1.03 | .158 | 0.922 | 0.80, 1.06 | .259 | 0.892 | 0.74, 1.07 | .224 | |||
| >85 | 0.848 | 0.70, 1.03 | .093 | 0.816 | 0.62, 1.07 | .137 | 1.063 | 0.81, 1.39 | .661 | |||
| >90 | 1.515 | 0.99, 2.31 | .054 | 1.878 | 1.03, 3.42 | .040* | 0.902 | 0.50, 1.61 | .729 | |||
| Sex (male) | 0.825 | 0.69, 0.99 | .037* | 0.823 | 0.66, 1.03 | .092 | 0.874 | 0.64, 1.19 | .400 | |||
| Race (non-White) | 1.500 | 1.04, 2.16 | .030* | 1.090 | 0.59, 2.01 | .783 | 0.940 | 0.58, 1.52 | .800 | |||
| Education (ref.: less than or equal to HS) | ||||||||||||
| Some college | 0.927 | 0.74, 1.17 | .522 | 0.947 | 0.70, 1.28 | .720 | 0.650 | 0.44, 0.95 | .028* | |||
| College graduate | 0.970 | 0.74, 1.28 | .827 | 1.028 | 0.73, 1.44 | .872 | 1.083 | 0.66, 1.79 | .755 | |||
| Professional/graduate | 1.200 | 0.95, 1.52 | .131 | 1.206 | 0.89, 1.64 | .230 | 0.764 | 0.52, 1.13 | .174 | |||
| Study site (ref: Wake Forest) | ||||||||||||
| UC Davis | 0.895 | 0.71, 1.13 | .358 | 1.023 | 0.75, 1.40 | .885 | 1.149 | 0.79, 1.67 | .469 | |||
| Johns Hopkins | 1.073 | 0.82, 1.41 | .610 | 1.205 | 0.85, 1.71 | .299 | 1.176 | 0.76, 1.83 | .473 | |||
| Pittsburgh | 0.682 | 0.53, 0.88 | .003** | 0.750 | 0.54, 1.04 | .088 | 0.737 | 0.50, 1.09 | .126 | |||
| Treatment group | 1.103 | 0.93, 1.31 | .267 | 1.032 | 0.83, 1.28 | .778 | 1.106 | 0.83, 1.48 | .497 | |||
| Medical comorbidities | 1.108 | 1.02, 1.20 | .010* | 1.166 | 1.06, 1.29 | .002** | 1.015 | 0.89, 1.16 | .829 | |||
| Depressive symptoms (CES-D ≥ 10) |
1.782 | 1.35, 2.35 | <.001*** | 1.957 | 1.37, 2.79 | <.001*** | 0.990 | 0.63, 1.55 | .963 | |||
| Mean cross-validated AUC | .673 | .728 | ||||||||||
Notes: AUC = area under the curve; CES-D = Center for Epidemiologic Studies Depression Scale; CI = confidence interval; HR = hazard ratio; HS = high school; MCI = mild cognitive impairment. Model 1 is unadjusted for covariates. Model 2 is adjusted for demographic (baseline age, study site, treatment group, sex, race, education category) and health covariates (medical comorbidities, significant depressive symptoms). Model 3 is stratified by MCI status. Individuals were assigned to the class of the highest posterior probability of membership. Mean 10-fold cross-validated area under the receiver operating characteristic curves are reported for nonstratified models.
*p < .05. **p < .01. ***p < .001, all p values are 2-sided.
Figure 2.
Cumulative incidence curves of time-to-dementia onset stratified by lifestyle engagement class. Study entry (visit 0) was at date of baseline session. Visits occurred at approximately 6-month intervals. Solid line: Class 1: Variety; dashed line: Class 2: Intellectual; dotted line: Class 3: Social; dotted/dashed line: Class 4: Least Active.
Table 4.
Unadjusted and Adjusted Discrete-Time Proportional Hazards Models for Activity Frequency Predicting Time-to-Dementia Diagnosis
| Model 1 (unadjusted) | Model 2 (adjusted) | Model 3 (stratified by MCI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-MCI (n = 2,587) | MCI (n = 481) | |||||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Activity frequency | ||||||||||||
| Intellectual | 0.937 | 0.91, 0.96 | <.001*** | 0.932 | 0.91, 0.96 | <.001*** | 0.946 | 0.91, 0.98 | .001** | 0.976 | 0.93, 1.02 | .273 |
| Physical | 1.002 | 0.99, 1.01 | .747 | 1.008 | 1.00, 1.02 | .194 | 1.008 | 0.99, 1.02 | .286 | 0.994 | 0.98, 1.01 | .514 |
| Social | 0.970 | 0.93, 1.01 | .124 | 0.975 | 0.94, 1.01 | .214 | 0.981 | 0.93, 1.03 | .430 | 0.982 | 0.92, 1.05 | .607 |
| Mean cross-validated AUC | .673 | .733 | ||||||||||
Notes: AUC = area under the curve; CI = confidence interval; HR = hazard ratio; MCI = mild cognitive impairment. Model 1 is unadjusted for covariates. Model 2 is adjusted for demographic (baseline age, study site, treatment group, sex, race, education category) and health covariates (medical comorbidities, significant depressive symptoms). Model 3 is stratified by MCI status. Activity frequency scores represent average frequency of engagement within the specified activity domain (intellectual, physical, social). Mean 10-fold cross-validated area under the receiver operating characteristic curves are reported for nonstratified models.
**p < .01. ***p < .001, all p values are 2-sided.
Sensitivity analyses using the three-step class assignment approach (Vermunt, 2010; Supplementary Table D.1) and excluding individuals with high propensity scores yielded similar results (Supplementary Table D.2). Notably, there was no significant difference in risk of dementia between the Social and Least Active groups in any model when using the three-step approach (ps > .05).
Activity frequency predicting dementia risk
Each additional day of average intellectual activity reported in the past month was associated with 6.6% reduced hazard of dementia, adjusting for average frequency of physical and social activities (Table 4, Model 1). This association remained significant after adjusting for demographic and health covariates (HR [95% CI]: 0.932 [0.91, 0.96]). Stratifying by MCI (Model 3) revealed that higher frequency of intellectual activities was associated with reduced dementia risk for those without MCI (HR: 0.946 [0.91, 0.98]), but not those with MCI (HR: 0.976 [0.93, 1.02]). Frequency of physical and social activities was not associated with dementia risk, adjusting for frequency of intellectual activity (p > .05 for all).
Activity variety predicting dementia risk
Each additional activity reported was associated with 8.4% reduced hazard of dementia (Supplementary Table E.1, Model 1). This association was slightly attenuated but still significant after adjusting for demographic and health covariates (HR [95% CI]: 0.933 [0.91, 0.96]). Stratifying by MCI (Model 3) revealed that lower activity variety was associated with higher dementia risk for those without MCI (HR: 0.936 [0.91, 0.96]), but not those with MCI (HR: 1.01 [0.96, 1.06]).
Comparing model fit indices
The mean cross-validated AUCs were slightly greater for adjusted models using average frequencies (mean AUC = 0.733) compared to the latent class indicators (mean AUC = 0.728) and activity variety scores (mean AUC = 0.729). This suggests that activity frequency scores provided slightly better predictive discrimination of dementia cases out of the three approaches.
Discussion
Our study is among the first to combine the use of traditional activity metrics with a latent class approach to characterize qualitatively distinct lifestyle engagement groups. We found that a four-class model revealed group differences in activity response patterns. As hypothesized, these patterns were distinguished by differences in amount, types (e.g., intellectual vs social), and settings (e.g., home-based vs community-based) of engagement. Using the traditional frequency scores, we found that for those without baseline MCI, lower frequency of intellectual activities was associated with higher risk of incident dementia over the 7-year study period. This association remained significant after adjusting for several relevant confounders. These findings agree with prior work suggesting that a greater frequency of intellectual activities is protective against aging-related cognitive impairments (Bielak et al., 2019; Verghese et al., 2006; Wilson et al., 2007). Frequency scores also provided slightly better predictive utility than the variety scores and latent classes.
Several potential mechanisms may explain the relationship between frequency of activities and dementia risk. Engagement in a higher frequency of activities may buffer against cognitive impairments through requiring individuals to navigate a complex environment, leading to greater utilization and maintenance of cognitive abilities (Schooler, 1984). Similarly, the enrichment hypothesis (Hertzog et al., 2008) posits that engagement in diverse activities may moderate neurocognitive impairments through maintenance or enhancement of cognitive abilities or through provision of compensatory mechanisms, for example, activation of synaptic plasticity. Greater frequency of activities may also buffer against accumulated pathologies through structural and functional brain changes (Stern, 2002).
We did not find a protective association between activity engagement and incident dementia for those with prevalent MCI. In agreement with a reverse causation account, individuals with existing cognitive impairments may engage differently in and derive less enrichment from the included activities as those without impairments, potentially due to excessive cognitive demands (Parisi et al., 2017). The Lifestyle Activity Questionnaire included a high number of intellectual activities, lending support to this interpretation. Alternatively, individuals with MCI may differ from those without MCI on unmeasured lifestyle confounders (e.g., marital status, income). Others have found that older adults with MCI were more likely to be unmarried and have lower incomes, both of which may impact both activity levels and dementia risk (Johnson et al., 2014). Importantly, despite finding no association with incident dementia in this study, prior studies have found that higher activity engagement contributes to higher quality of life regardless of cognitive status (Amano et al., 2020; Johnson et al., 2014).
For individuals without prevalent MCI, models including average activity frequency scores were better at discriminating incident dementia cases than those including activity variety or the class indicators. The predictive utility of frequency versus variety scores has been recently debated in the literature (Bielak et al., 2019; Lee et al., 2021). Bielak and colleagues (2019) reported that domain-specific frequency scores (e.g., novel, social, etc.) had larger associations with cognition than domain-specific variety scores, but both provided a similar pattern of findings, suggesting that the measures strongly overlapped. Others have found that variety of activities was predictive of cognitive outcomes above and beyond total time spent in activity (Carlson et al., 2012; Lee et al., 2021). Here, we directly compared the discriminatory capacity of frequency and variety approaches in predicting dementia using cross-validated AUCs (Zweig & Campbell, 1993). Like Bielak and colleagues (2019), our findings demonstrate substantial overlap between different operationalizations of activity engagement. Our results suggest that the quantitative difference in frequency of intellectual activities may be primarily providing the protective relationship between lifestyle engagement and dementia risk, but also that a count of activities (i.e., variety score) may be the simplest method to use to develop adequate predictive models of dementia incidence. Use of a continuous activity measure is further supported by our finding that two of the four latent classes (Variety and Least Active) were distinguished primarily by their quantity of engagement.
Despite these limitations, findings from the LCA approach converged with those of the frequency approach, while also characterizing group-level differences in activity engagement. For instance, we found two groups of individuals that were more likely to participate in intellectual activities (i.e., Variety and Intellectual). These groups differed in their level of formal social engagement (e.g., church, social groups) but had a similar risk of incident dementia. Furthermore, we observed that the Social group, which had high engagement in formal social activities, did not have significantly reduced risk of incident dementia after stratifying by MCI. One potential explanation for these findings is that the formal social activities may be less cognitively demanding, in general, compared to the intellectual activities included here (e.g., taking courses). This agrees with the enrichment hypothesis (Hertzog et al., 2008), which suggests that engagement in specifically cognitively demanding activities may be especially protective against dementia. Other studies have found that more frequent engagement in specifically intellectual or cognitively demanding activities appears to have the greatest protective benefit compared to other activity types (Hultsch et al., 1999; Scarmeas et al., 2001).
Our person-centered LCA approach also did not rely on a priori specification of activity domains, allowing individuals to group both within and across established domains. This was especially important for revealing the qualitative differences in social engagement between the groups, where those in the Variety group had a high likelihood of participating in most social activities, but those in the Social group had a high likelihood of engaging primarily in formal social activities (e.g., church). This splitting of social activities into subdomains is not normally done in research using activity frequency or variety measures, which typically combine all social activities into a single index (Hultsch et al., 1999; Parisi et al., 2012; Scarmeas et al., 2001). Amano and colleagues (2020) recently used a similar latent class approach and observed a class of older adults with MCI that was characterized by their engagement in informal (e.g., meeting up), but not formal (e.g., volunteering) social activities. Our findings extend upon this by using a broader range of activities and a sample including individuals without MCI. We similarly observed a distinct group of older adults (Class 2: Intellectual) who had higher likelihood of engagement in informal social activities (e.g., movies, playing cards) compared to the other groups. Yet, this group was also characterized by higher engagement in intellectual activities and ultimately had reduced risk of incident dementia.
The difference between informal and formal social engagement is important, as it could suggest contextual differences in lifestyle between the groups that are relevant to preventing cognitive impairments. Given that the Variety and Intellectual groups were more likely to endorse going to movies and concerts; they may be of higher socioeconomic status (SES) compared to the other groups. Supporting this, the Variety and Intellectual groups were more educated than the other groups. Individuals with higher SES may have the necessary resources to maintain an active lifestyle that buffers against dementia-related pathology (Stern, 2002). Alternatively, additional informal social activities may be associated with non-SES protective factors, such as having a larger social network (Fratiglioni et al., 2004), expanded life space (James et al., 2011), or increased physical activity (Najar et al., 2019; Voss et al., 2014). Informal social activities, rather than formal social activities, may also be associated with added environmental complexity (Carlson et al., 2012) due to their less structured nature (e.g., less routine, more freedom to choose where and how to perform the activity). While we adjusted for education as a proxy of SES, future research using additional SES measures (e.g., wealth) with a similar LCA approach would help determine whether SES or social activity is driving this protective association with dementia. Past research adjusting for life-span SES measures found that activity engagement is independently associated with dementia risk (Chan et al., 2019; Wilson et al., 2007).
Finally, the current results further inform a tailored intervention approach (Gitlin et al., 2008) for dementia prevention in community-dwelling older populations. For example, given that the Social group was the most prevalent (32%) of the latent classes in the current sample, it may represent an important at-risk group relevant to future interventions. This group may be more motivated to participate in an intervention tied to social engagement or nested within a social institution, such as their church or social club. Supporting this, the Social group shared a similar activity profile to Baltimore Experience Corps Trial (BECT) participants, who generally had high volunteerism and church participation (Parisi et al., 2012). These existing behavioral factors contribute to social selection into volunteering roles (Anderson et al., 2014) and thus these individuals were likely more motivated to participate in the BECT intergenerational volunteering intervention. Integrating new interventions into existing activity contexts may ultimately promote more sustainable behavioral changes relevant to dementia prevention, by directly linking the intervention with engagement that already gives individuals purpose in life (Boyle et al., 2010).
There are limitations to the current study. First, we used a retrospective, self-reported inventory to measure activity engagement. We attempted to mitigate recall bias by conducting additional analyses using dichotomous activity responses that did not require recall of precise frequencies of engagement. Second, the activities included in the Lifestyle Activity Questionnaire are not exhaustive and contained few physical activities, which limited our ability to accurately measure the “physical” subdomain of lifestyle activities and examine class differences in this domain. We also did not include all of the Lifestyle Activity Questionnaire items in a single LCA model to prevent issues with model estimability (Collins & Lanza, 2010). Yet, we removed activities based on a clear empirical and theoretical rationale, and we examined variation in class enumeration across activity subsets. Third, our findings are sample-specific to mostly White participants in a clinical trial, and the current findings warrant further replication and measurement invariance testing in more diverse samples. Finally, despite excluding prevalent dementia cases, there may still be reverse causation wherein individuals with precursory cognitive impairments report fewer baseline activities. Nevertheless, our findings illustrate how these at-risk groups may be further restricted to specific home-based activities, potentially providing a useful preclinical marker in the absence of observable cognitive symptoms of dementia.
The current study also had several strengths. Our sample had a median follow-up of 6 years, excellent retention, and was well powered to detect differences in time-to-dementia analyses (DeKosky et al., 2006). Dementia was also adjudicated by expert clinicians using data from neuropsychological testing, neurological exams, and MRI, via a consensus conference (Snitz et al., 2009). Finally, our novel application of a person-centered LCA approach demonstrated that individuals group naturally by both amount and types of activities, and revealed qualitative differences in engagement that could imply potential motivational differences for staying active in later life. Our study is the first, to our knowledge, to use this approach to predict dementia incidence.
Conclusion
Increasing active life expectancies after retirement provides novel opportunities for encouraging lifestyle engagement in later life. The question remains how to characterize lifestyle engagement in a way that is useful both for predicting relevant health outcomes and for deploying health-related interventions. Although activity frequency had slightly better discrimination of dementia cases, we found substantial overlap in predictive utility between frequency, variety, and latent class measures. Findings from each approach converged to suggest that participating in a higher quantity of intellectual activities in later life may protect against incident dementia. Yet, the qualitative differences between latent classes indicate that older subgroups remain active in different ways, which is relevant designing sustainable interventions to promote neurocognitive health in later life.
Funding
This work was supported by the National Institute on Aging, the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, the National Heart, Lung, and Blood Institute, and the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (grants T32 AG000247, U01 AT000162) as well as the University of Pittsburgh Alzheimer’s Disease Research Center (grant P50 AG05133), Roena Kulynych Center for Memory and Cognition Research, and Wake Forest University School of Medicine.
Conflict of Interest
Dr. S. T. DeKosky is the Chair of the Medical Advisory Boards for Acumen and Cognition Therapeutics, Chair of the Data Safety Monitoring Board for aducanumab for Biogen, and the Chair of the Drug Safety Committee for Prevail Therapeutics. He is also Editor of the Section on Dementia for Up-To-Date, Associate Editor for Neurotherapeutics and Current Opinion in Neurology, and Senior Associate Editor for Alzheimer’s & Dementia: Translational Research and Clinical Interventions. The remaining authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
The authors would like to thank the Ginkgo Evaluation of Memory (GEM) Study participants for giving their time and energy to this study. We would also like to thank Dr. George Rebok, Dr. Sevil Yasar, and Dr. Jeanine Parisi for their feedback throughout the design, analysis, and drafting of this manuscript. Data used in this paper are available pending approval of the study coauthors (GEM site PIs: Drs. S. T. DeKosky, A. L. Fitzpatrick, J. D. Williamson, B. E. Snitz, and M. C. Carlson). Analytical code is available upon request of the corresponding author (K. D. Moored). This study was not preregistered.
References
- Allman, R. M., Sawyer, P., & Roseman, J. M. (2006). The UAB Study of Aging: Background and insights into life-space mobility among older Americans in rural and urban settings. Aging Health, 2(3), 417–429. doi: 10.2217/1745509X.2.3.417 [DOI] [Google Scholar]
- Amano, T., Morrow-Howell, N., & Park, S. (2020). Patterns of social engagement among older adults with mild cognitive impairment. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(7), 1361–1371. doi: 10.1093/geronb/gbz051 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). American Psychiatric Association. [Google Scholar]
- Anderson, N. D., Damianakis, T., Kröger, E., Wagner, L. M., Dawson, D. R., Binns, M. A., Bernstein, S., Caspi, E., & Cook, S. L.; BRAVO Team . (2014). The benefits associated with volunteering among seniors: A critical review and recommendations for future research. Psychological Bulletin, 140(6), 1505–1533. doi: 10.1037/a0037610 [DOI] [PubMed] [Google Scholar]
- Bennett, D. A., Arnold, S. E., Valenzuela, M. J., Brayne, C., & Schneider, J. A. (2014). Cognitive and social lifestyle: Links with neuropathology and cognition in late life. Acta Neuropathologica, 127(1), 137–150. doi: 10.1007/s00401-013-1226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak, A. A. (2010). How can we not “lose it” if we still don’t understand how to “use it”? Unanswered questions about the influence of activity participation on cognitive performance in older age—A mini-review. Gerontology, 56(5), 507–519. doi: 10.1159/000264918 [DOI] [PubMed] [Google Scholar]
- Bielak, A. A. M., Mogle, J. A., & Sliwinski, M. J. (2019). Two sides of the same coin? Association of variety and frequency of activity with cognition. Psychology and Aging, 34(3), 457–466. doi: 10.1037/pag0000350 [DOI] [PubMed] [Google Scholar]
- Björgvinsson, T., Kertz, S. J., Bigda-Peyton, J. S., McCoy, K. L., & Aderka, I. M. (2013). Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment, 20(4), 429–436. doi: 10.1177/1073191113481998 [DOI] [PubMed] [Google Scholar]
- Boyle, P. A., Buchman, A. S., Barnes, L. L., & Bennett, D. A. (2010). Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Archives of General Psychiatry, 67(3), 304–310. doi: 10.1001/archgenpsychiatry.2009.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman, A. S., Wilson, R. S., Yu, L., James, B. D., Boyle, P. A., & Bennett, D. A. (2014). Total daily activity declines more rapidly with increasing age in older adults. Archives of Gerontology and Geriatrics, 58(1), 74–79. doi: 10.1016/j.archger.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M. C., Parisi, J. M., Xia, J., Xue, Q. L., Rebok, G. W., Bandeen-Roche, K., & Fried, L. P. (2012). Lifestyle activities and memory: Variety may be the spice of life. The Women’s Health and Aging Study II. Journal of the International Neuropsychological Society, 18(2), 286–294. doi: 10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, T., Parisi, J. M., Moored, K. D., & Carlson, M. C. (2019). Variety of enriching early-life activities linked to late-life cognitive functioning in urban community-dwelling African Americans. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74(8), 1345–1356. doi: 10.1093/geronb/gby056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L. M., & Lanza, S. T. (2010). Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. John Wiley & Sons. [Google Scholar]
- Crimmins, E. M., Zhang, Y., & Saito, Y. (2016). Trends over 4 decades in disability-free life expectancy in the United States. American Journal of Public Health, 106(7), 1287–1293. doi: 10.2105/AJPH.2016.303120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky, S. T., Fitzpatrick, A., Ives, D. G., Saxton, J., Williamson, J., Lopez, O. L., Burke, G., Fried, L., Kuller, L. H., Robbins, J., Tracy, R., Woolard, N., Dunn, L., Kronmal, R., Nahin, R., & Furberg, C.; GEMS Investigators . (2006). The Ginkgo Evaluation of Memory (GEM) study: Design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemporary Clinical Trials, 27(3), 238–253. doi: 10.1016/j.cct.2006.02.007 [DOI] [PubMed] [Google Scholar]
- DeKosky, S. T., Williamson, J. D., Fitzpatrick, A. L., Kronmal, R. A., Ives, D. G., Saxton, J. A., Lopez, O. L., Burke, G., Carlson, M. C., Fried, L. P., Kuller, L. H., Robbins, J. A., Tracy, R. P., Woolard, N. F., Dunn, L., Snitz, B. E., Nahin, R. L., & Furberg, C. D.; Ginkgo Evaluation of Memory (GEM) Study Investigators . (2008). Ginkgo biloba for prevention of dementia: A randomized controlled trial. Journal of the American Medical Association, 300(19), 2253–2262. doi: 10.1001/jama.2008.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, A. L., Fried, L. P., Williamson, J., Crowley, P., Posey, D., Kwong, L., Bonk, J., Moyer, R., Chabot, J., Kidoguchi, L., Furberg, C. D., & DeKosky, S. T.; GEM Study Investigators . (2006). Recruitment of the elderly into a pharmacologic prevention trial: The Ginkgo Evaluation of Memory Study experience. Contemporary Clinical Trials, 27(6), 541–553. doi: 10.1016/j.cct.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Fratiglioni, L., Paillard-Borg, S., & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet. Neurology, 3(6), 343–353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Gitlin, L. N., Winter, L., Burke, J., Chernett, N., Dennis, M. P., & Hauck, W. W. (2008). Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: A randomized pilot study. The American Journal of Geriatric Psychiatry, 16(3), 229–239. doi: 10.1097/JGP.0b013e318160da72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, T. A., De Leon, C. F., Bassuk, S. S., & Berkman, L. F. (2006). Social engagement and depressive symptoms in late life: Longitudinal findings. Journal of Aging and Health, 18(4), 604–628. doi: 10.1177/0898264306291017 [DOI] [PubMed] [Google Scholar]
- Gow, A. J., Corley, J., Starr, J. M., & Deary, I. J. (2012). Reverse causation in activity–cognitive ability associations: The Lothian Birth Cohort 1936. Psychology and Aging, 27(1), 250–255. doi: 10.1037/a0024144 [DOI] [PubMed] [Google Scholar]
- Hertzog, C., Kramer, A. F., Wilson, R. S., & Lindenberger, U. (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9(1), 1–65. doi: 10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Hultsch, D. F., Hertzog, C., Small, B. J., & Dixon, R. A. (1999). Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging, 14(2), 245–263. doi: 10.1037//0882-7974.14.2.245 [DOI] [PubMed] [Google Scholar]
- James, B. D., Boyle, P. A., Buchman, A. S., Barnes, L. L., & Bennett, D. A. (2011). Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. The American Journal of Geriatric Psychiatry, 19(11), 961–969. doi: 10.1097/JGP.0b013e318211c219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. D., Whitlatch, C. J., & Menne, H. L. (2014). Activity and well-being of older adults: Does cognitive impairment play a role? Research on Aging, 36(2), 147–160. doi: 10.1177/0164027512470703 [DOI] [PubMed] [Google Scholar]
- Kelly, M. E., Duff, H., Kelly, S., McHugh Power, J. E., Brennan, S., Lawlor, B. A., & Loughrey, D. G. (2017). The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259. doi: 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, S. T., Coffman, D. L., & Xu, S. (2013). Causal inference in latent class analysis. Structural Equation Modeling, 20(3), 361–383. doi: 10.1080/10705511.2013.797816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, S. T., Tan, X., & Bray, B. C. (2013). Latent class analysis with distal outcomes: A flexible model-based approach. Structural Equation Modeling, 20(1), 1–26. doi: 10.1080/10705511.2013.742377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., Charles, S. T., & Almeida, D. M. (2021). Change is good for the brain: Activity diversity and cognitive functioning across adulthood. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(6), 1036–1048. doi: 10.1093/geronb/gbaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Fernandez, M. A., Redondo-Sánchez, D., & Maringe, C. (2019). cvauroc: Command to compute cross-validated area under the curve for ROC analysis after predictive modeling for binary outcomes. The Stata Journal, 19(3), 615–625. doi: 10.1177/1536867X19874237 [DOI] [Google Scholar]
- Mayeda, E. R., Glymour, M. M., Quesenberry, C. P., & Whitmer, R. A. (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12(3), 216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs, R. C. (1996). The Alzheimer’s Disease Assessment Scale. International Psychogeriatrics, 8(2), 195–203. doi: 10.1017/s1041610296002578 [DOI] [PubMed] [Google Scholar]
- Moored, K. D., Chan, T., Varma, V. R., Chuang, Y. F., Parisi, J. M., & Carlson, M. C. (2020). Engagement in enriching early-life activities is associated with larger hippocampal and amygdala volumes in community-dwelling older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(8), 1637–1647. doi: 10.1093/geronb/gby150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. C. (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Najar, J., Östling, S., Gudmundsson, P., Sundh, V., Johansson, L., Kern, S., Guo, X., Hällström, T., & Skoog, I. (2019). Cognitive and physical activity and dementia: A 44-year longitudinal population study of women. Neurology, 92(12), e1322–e1330. doi: 10.1212/WNL.0000000000007021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . (2017). Preventing cognitive decline and dementia: A way forward. National Academies Press. [PubMed] [Google Scholar]
- Nylund, K. L., Asparouhov, T., & Muthén, B. O. (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling, 14(4), 535–569. doi: 10.1080/10705510701575396 [DOI] [Google Scholar]
- Parisi, J. M., Kuo, J., Rebok, G. W., Xue, Q. L., Fried, L. P., Gruenewald, T. L., Huang, J., Seeman, T. E., Roth, D. L., Tanner, E. K., & Carlson, M. C. (2015). Increases in lifestyle activities as a result of experience Corps® participation. Journal of Urban Health, 92(1), 55–66. doi: 10.1007/s11524-014-9918-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi, J. M., Rebok, G. W., Seeman, T. E., Tanner, E. K., Tan, E. J., Fried, L. P., Xue, Q. L., Frick, K. D., & Carlson, M. C. (2012). Lifestyle activities in sociodemographically at-risk urban, older adults prior to participation in the Baltimore Experience Corps(®) Trial. Activities, Adaptation & Aging, 36(3), 242–260. doi: 10.1080/01924788.2012.702306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi, J. M., Roberts, L., Szanton, S. L., Hodgson, N. A., & Gitlin, L. N. (2017). Valued activities among individuals with and without cognitive impairments: Findings from the National Health and Aging Trends Study. The Gerontologist, 57(2), 309–318. doi: 10.1093/geront/gnv144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff, L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Royston, P., & Lambert, P. C. (2011). Flexible parametric survival analysis using Stata: Beyond the Cox model. Stata Press. [Google Scholar]
- Saunders, D. H., Sanderson, M., Hayes, S., Kilrane, M., Greig, C. A., Brazzelli, M., & Mead, G. E. (2016). Physical fitness training for stroke patients. The Cochrane Database of Systematic Reviews, 3, CD003316. doi: 10.1002/14651858.CD003316.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas, N., Levy, G., Tang, M. X., Manly, J., & Stern, Y. (2001). Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology, 57(12), 2236–2242. doi: 10.1212/wnl.57.12.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler, C. (1984). Psychological effects of complex environments during the life span: A review and theory. Intelligence, 8(4), 259–281. doi: 10.1016/0160-2896(84)90011-4 [DOI] [Google Scholar]
- Snitz, B. E., O’Meara, E. S., Carlson, M. C., Arnold, A. M., Ives, D. G., Rapp, S. R., Saxton, J., Lopez, O. L., Dunn, L. O., Sink, K. M., & DeKosky, S. T.; Ginkgo Evaluation of Memory (GEM) Study Investigators . (2009). Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. Journal of the American Medical Association, 302(24), 2663–2670. doi: 10.1001/jama.2009.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. doi: 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- Teng, E. L., & Chui, H. C. (1987). The Modified Mini-Mental State (3MS) examination. The Journal of Clinical Psychiatry, 48(8), 314–318. [PubMed] [Google Scholar]
- Verghese, J., LeValley, A., Derby, C., Kuslansky, G., Katz, M., Hall, C., Buschke, H., & Lipton, R. B. (2006). Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology, 66(6), 821–827. doi: 10.1212/01.wnl.0000202520.68987.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese, J., Lipton, R. B., Katz, M. J., Hall, C. B., Derby, C. A., Kuslansky, G., Ambrose, A. F., Sliwinski, M., & Buschke, H. (2003). Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine, 348(25), 2508–2516. doi: 10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- Vermunt, J. K. (2010). Latent class modeling with covariates: Two improved three-step approaches. Political Analysis, 18(04), 450–469. doi: 10.1093/pan/mpq025 [DOI] [Google Scholar]
- Voss, M. W., Carr, L. J., Clark, R., & Weng, T. (2014). Revenge of the “sit” II: Does lifestyle impact neuronal and cognitive health through distinct mechanisms associated with sedentary behavior and physical activity? Mental Health and Physical Activity, 7(1), 9–24. doi: 10.1016/j.mhpa.2014.01.001 [DOI] [Google Scholar]
- Wilson, R. S., Bennett, D. A., Beckett, L. A., Morris, M. C., Gilley, D. W., Bienias, J. L., Scherr, P. A., & Evans, D. A. (1999). Cognitive activity in older persons from a geographically defined population. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 54(3), P155–P160. doi: 10.1093/geronb/54b.3.p155 [DOI] [PubMed] [Google Scholar]
- Wilson, R. S., Scherr, P. A., Schneider, J. A., Tang, Y., & Bennett, D. A. (2007). Relation of cognitive activity to risk of developing Alzheimer disease. Neurology, 69(20), 1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- Wu, Y. T., Beiser, A. S., Breteler, M. M., Fratiglioni, L., Helmer, C., Hendrie, H. C., Honda, H., Ikram, M. A., Langa, K. M., Lobo, A., & Matthews, F. E. (2017). The changing prevalence and incidence of dementia over time—Current evidence. Nature Reviews Neurology, 13(6), 327. doi: 10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- Zweig, M. H., & Campbell, G. (1993). Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clinical Chemistry, 39(4), 561–577. doi: 10.1093/clinchem/39.4.561 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.