Abstract
Background
Harmful and/or unnecessary medication use in older adults is common. This indicates deprescribing (supervised withdrawal of inappropriate medicines) is not happening as often as it should. This study aimed to synthesize the results of the Patients’ Attitudes Towards Deprescribing (PATD) questionnaire (and revised versions).
Methods
Databases were searched from January 2013 to March 2020. Google Scholar was used for citation searching of the development and validation manuscripts to identify original research using the validated PATD, revised PATD (older adult and caregiver versions), and the version for people with cognitive impairment (rPATDcog). Two authors extracted data independently. A meta-analysis of proportions (random-effects model) was conducted with subgroup meta-analyses for setting and population. The primary outcome was the question: “If my doctor said it was possible, I would be willing to stop one or more of my medicines.” Secondary outcomes were associations between participant characteristics and primary outcome and other (r)PATD results.
Results
We included 46 articles describing 40 studies (n = 10,816 participants). The meta-analysis found the proportion of participants who agreed or strongly agreed with this statement was 84% (95% CI 81%–88%) and 80% (95% CI 74%–86%) in patients and caregivers, respectively, with significant heterogeneity (I2 = 95% and 77%).
Conclusion
Consumers reported willingness to have a medication deprescribed although results should be interpreted with caution due to heterogeneity. The findings from this study moves toward understanding attitudes toward deprescribing, which could increase the discussion and uptake of deprescribing recommendations in clinical practice.
Keywords: Caregivers, Inappropriate prescribing, Medications, Older adults, Polypharmacy
Internationally, there has been focus on the increasing prevalence and harms of multiple medication use in the older population (1). As people age, there may be changes in medical conditions and other medications, as well as a change in their preferences and treatment goals, which can shift medications toward an unfavorable benefit to risk ratio (2). A medication is considered inappropriate when potential harms outweigh potential benefits in the individual (3). An American study of older veterans (n = 462,405) found that 50% were dispensed one or more potentially inappropriate medications (4). The use of potentially inappropriate medications in older adults increases the risk of adverse drug reactions, functional impairment (5), hospitalization, and mortality (3,6–8). This places a high burden on older adults and health care systems due to associated costs (9,10). This highlights the need for deprescribing, which has been defined as the process of withdrawal of an inappropriate medication, supervised by a health care professional with the goal of managing polypharmacy and improving outcomes (11).
Systematic reviews of randomized controlled trials assessing the effectiveness of deprescribing interventions showed that deprescribing is feasible and safe to implement in a research setting (12,13). To implement deprescribing in “real life” clinical practice, it is essential to understand the barriers and enablers for deprescribing. Clinicians commonly report consumers (patients and their caregivers) as being resistant to deprescribing, and patients can have internally contradictory beliefs in that they perceive all their medications are necessary but also want to take fewer (14–16).
The most frequently used patient questionnaires for the assessment of self-reported attitudes toward deprescribing is the Patients’ Attitudes Towards Deprescribing (PATD) questionnaire (17). It was developed in 2013 as an exploratory research tool and revised with versions for older adults, caregivers, and people with cognitive impairment (rPATD (18) and rPATDcog) (19). This manuscript uses “(r)PATD” to denote all versions of the questionnaire. The original PATD underwent face, content, criterion, internal validity, and sensitivity and reliability testing. The questionnaire was then revised due to limitations of the original PATD (designed to be exploratory, no scoring ability, limited scope of potential barriers and enablers) and to simultaneously develop a version for informal caregivers. The rPATD underwent face, construct, content, criterion-related validity testing, internal consistency (Chronbach’s α > .65 for all factors), and test–retest consistency (gamma values between 0.57 and 0.89, p < .00 for factor scores). The rPATDcog was adapted from the older adult’s version of the rPATD, including shortening the questionnaire and simplifying the wording and response options, making it researcher/clinician administered (rather than self-administered), and conducting face validity. The retained questions were those with the greatest item-to-total correlation to the overall factor score. (r)PATD has been used internationally in multiple research studies with variable findings. Substantial differences exist between the published studies using the (r)PATD in terms of population, method of measurement, and associations with participant characteristics.
The aims of this systematic review were (i) to determine the willingness of adults, caregivers, and people living with cognitive impairment to have a medication deprescribed; (ii) to describe the participant characteristics associated with willingness to have a medication deprescribed; and (iii) to report the attitudes and beliefs of adults and caregivers about their medications and deprescribing as reported through use of the (r)PATD.
Method
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). The protocol was preregistered in PROSPERO (CRD42020150007).
Inclusion and Exclusion Criteria
Studies were eligible if they were original studies that enrolled adults (>18 years) with any medical condition taking at least one medication or caregivers of such adults. All study types and settings were included if one or more of the questionnaires of interest were administered and quantitative results captured. No language or other limits were applied.
Search
Medline via Ovid, EMBASE, Scopus, International Pharmaceutical Abstracts, and Web of Science core collections for conference abstracts were searched from the date of first publication of the original PATD manuscript, January 2013, to March 2020. Google Scholar was used for citation searching of the development and validation manuscripts of the (r)PATD questionnaires (17–19). We emailed anyone who had contacted the primary author of the (r)PATD (ER) for permission to use the questionnaires to identify gray literature.
Title/abstract and full text screening was conducted independently by 2 researchers. Disagreements were resolved by discussion.
Data Extraction
Data extracted independently by 2 authors using a standardized form included author, year of publication, study setting, design, participant characteristics, self-reported attitudes toward deprescribing ((r)PATD), and associations between willingness to deprescribe and participants’ characteristics. Modifications to any (r)PATD questions and details regarding translations were captured. Studies written in a language other than English were translated using a professional translation service. Corresponding authors were contacted when the primary outcome was not clearly reported.
Two authors independently assessed the quality of reporting using the SUrvey Reporting GuidelinE (SURGE) (20) (modified slightly for the purposes of this review).
Outcomes
The primary outcome of interest was self-reported willingness to have a medication deprescribed, defined as the proportion of participants who responded “agree” or “strongly agree” to “Would you be willing to have one or more of your medicines stopped if your doctor said it was possible?” A version of this question is present in all versions of the (r)PATD. Secondary outcomes were associations between the primary outcome and participant characteristics and other (r)PATD results.
Analysis
For the primary outcome, a random-effects meta-analysis of proportions using the restricted maximum likelihood method was performed in R v3.5.1 using the “meta” package. The proportion was recalculated from the relevant numerator (number who responded agree or strongly agree) and denominator (number who responded to the questionnaire). Proportions were transformed for meta-analysis via the Freeman–Tukey double arsine function to normalize distributions. Funnel plots were used to identify publication bias by plotting the proportion against the standard error and sample size.
To investigate heterogeneity, we performed analyses of predefined subgroups based on study setting, population, survey administration, and peer-reviewed status.
Secondary outcomes were synthesized and presented narratively. Caregiver and rPATDcog results are presented separately.
Results
Study Characteristics
We identified and included 40 eligible studies reported in 46 articles (Supplementary Figure 1).
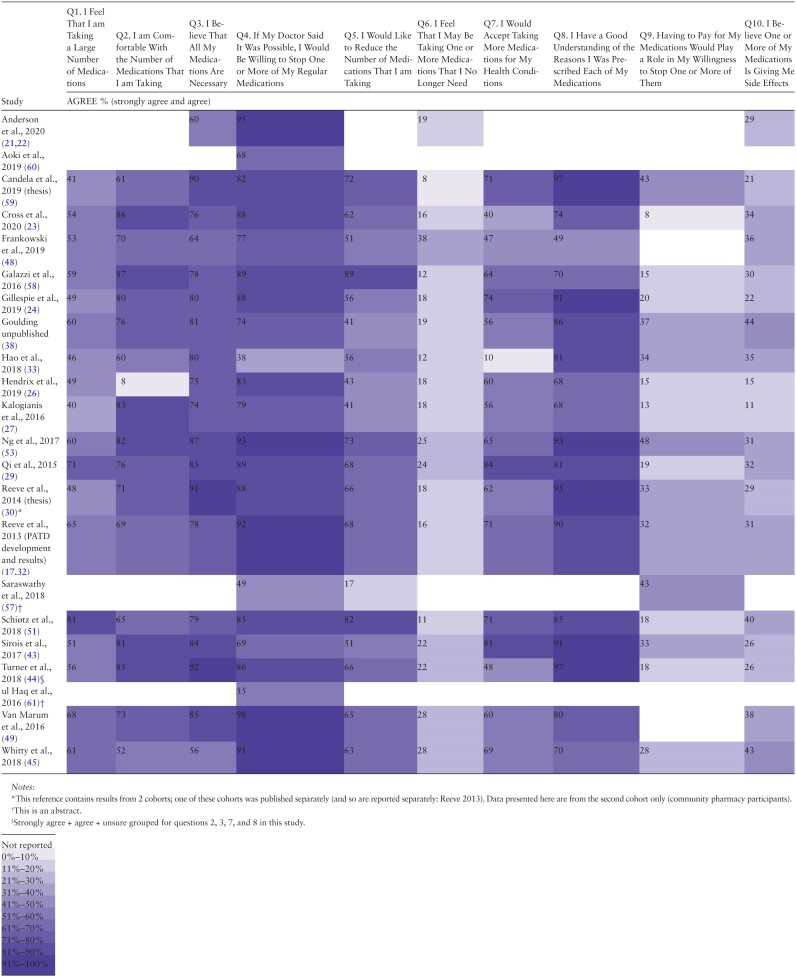
Sample sizes ranged from 18 to 1981 participants with a total of 10,816 participants (Table 1). The studies were conducted in Australia (n = 12) (17–19,21–32), Malaysia (n = 4) (33–37), the United States (n = 4) (38–42), Canada (n = 3) (43–45), the Netherlands (n = 3) (46–49), Denmark (n = 2) (50,51), Singapore (n = 2) (52,53), Jordan (54), Belgium (55), Ethiopia (56), India (57), Italy (58), Spain (59), Japan (60), Pakistan (61), Ireland (62), and the United Kingdom (63) (one each). Twenty-two studies used the original PATD (17,21–24,26,27,29,30,32,33,38,43–45,48,49,51,53,57–61), 17 used the older adults version of the rPATD (18,25,28,31,34–37,39–42,46,47,50,52,54–56,62,63), and 1 used the rPATDcog (19). Six studies that used the rPATD/rPATDcog also used the caregiver version of the rPATD (19,31,34,35,52,55,63). Most studies used the (r)PATD questionnaires specifically for measuring attitudes in a cross-sectional study. However, some studies (n = 4) (21,22,41,44,62) used the questionnaires as a baseline and/or outcome measure in a deprescribing intervention study. More than half of the 40 studies (n = 24, 60%) (18,19,21,22,24–27,29,31,33,39,40,43–45,48–52,55–58,62,63) focused on older adults. The median age of participants included in the studies ranged from 51 to 87 years old. Seventeen (43%) studies (18,21,22,24,30,31,33–35,38,41–44,46,47,49,52,53,61,62) were conducted in the community or primary care setting, 9 (23%) in the hospital setting (17,25,29,32,45,52,58,61,63), and 8 (20%) (19,23,39,40,53,54,56,59,60) in the outpatient setting. Six studies translated the PATD (43,48,49,51,58,59), and 7 studies translated the rPATD (34,35,37,46,47,50,54–56); 13 studies in total (Supplementary Table S2). Four studies used medication-specific questions in adapted (r)PATD questionnaires on statins (29), alpha-blockers (46,47), benzodiazepines (25), and proton pump inhibitors (Supplementary Table S3) (39,40).
Table 1.
Study and Participant Characteristics
| Source, Year, Country | Sample Size, Study Design | Study Population | Age, Years (Median) | Female % | Number of Medications (Median) | Translated, Language | Questionnaire Modified, How |
|---|---|---|---|---|---|---|---|
| PATD questionnaire | |||||||
| Anderson et al., 2020 (21,22), Australia | 78, pragmatic controlled, pre-post, mixed methods study | Community setting, aged 65+ y, taking ≥5 medications | 74 | 59 | 8 | N | Y, only first 10 questions reported |
| Aoki et al., 2019 (60), Japan | 1483, cross-sectional survey | Outpatient, adults aged 18+ y, taking ≥1 medication | NR | 49 | NR | N | N |
| Candela et al., 2019 (thesis) (59), Spain | 210, cross-sectional survey | Outpatient, adults aged 18+ y, HIV-positive patients on antiretroviral therapy | 51 | 23 | 5 | Y, Spanish | Y, translated |
| Cross et al., 2020 (23), Australia | 50, feasibility study, pre-post intervention study | Outpatient, patients at risk of a medication-related problem | 81 | 36 | 11 | N | Y, only first 10 questions reported |
| Frankowski et al., 2019 (48), Netherlands | 47, observational descriptive study | Geriatric psychiatry residential ward, taking ≥5 medications | 67 | 51 | 11 | Y*, Dutch | Y, deleted Q8, Q14 and Q15 |
| Galazzi et al., 2016 (58), Italy | 100, cross-sectional survey | Hospital setting, aged 65+ y | 79 | 47 | 6 | Y, Italian | Y, translated and deleted Q14 |
| Gillespie et al., 2019 (24), Australia | 137, cross-sectional survey | Community setting, aged 65+ y, taking ≥5 medications | 76 | 61 | 7 | N | Y, deleted Q14 and Q15 |
| Goulding unpublished (38), United States | 75, pre-post intervention study | Community setting, patients with serious mental illness enrolled in a medication adherence program | 60‡ | 56 | NR | N | N |
| Hao et al., 2018 (33), Malaysia | 222, cross-sectional survey | Community setting, aged 65+ y, taking ≥5 medications | 70 | 58 | 6 | NR | Y, Q11 modified |
| Hendrix et al., 2019 (26), Australia | 383, cross-sectional survey | Residential aged care facility, aged 65+ y | 88‡ | 76 | 10 | N | N |
| Kalogianis et al., 2016 (27), Australia | 232, cross-sectional survey | Residential aged care facility, aged 65+ y | 87‡ | 76 | 15†,‡ | N | Y, minor wording changes to allow for interviewer administered |
| Ng et al., 2017 (53), Singapore | 136, cross-sectional survey | Outpatient health care centers, adults aged 45+ y, taking ≥5 medications | 68 | 41 | 6 | N | NR |
| Qi et al., 2015 (29), Australia | 180, cross-sectional survey | Hospital setting, aged 65+ y, taking a statin medication | 78 | 47 | 8/10§ | N | Y, 5 statin specific questions added |
| Reeve et al., 2014 (thesis) (30),‖ Australia | 77, cross-sectional survey | Community pharmacies, adults aged 18+ y, taking ≥1 medication | 69 | 51 | 5 | N | Y, Q11 was not used |
| Reeve et al., 2013 (PATD development + results) (17,32), Australia | 100, development of a questionnaire, cross-sectional survey | Outpatients, adults aged 18+ y, taking ≥1 medication | 72 | 55 | 10 | N | N |
| Saraswathy et al., 2018 (57),¶ India | 257, observational study | Residential aged care facility | NR | 48 | NR | NR | NR |
| Schiøtz et al., 2018 (51), Denmark | 100, cross-sectional survey | Outpatient clinics, aged 65+ y, taking ≥10 medications | 75 | 63 | 12 | Y, Danish | Y, translated and Q9 modified |
| Sirois et al., 2017 (43), Canada | 129, cross-sectional survey | Community setting, aged 65+ y, taking ≥1 medication | 76 | 63 | 6 | Y, French | Y, translated. 2 questions added about nurse involvement and follow-up for deprescribing |
| Turner et al., 2018 (44), Canada | 489, secondary analysis of a randomized controlled trial | Community setting, aged 65+ y, taking ≥1 medication, taking specific medication | 75‡ | 66 | 9‡ | N | Y, only first 10 questions reported |
| ul Haq et al., 2016 (61),¶ Pakistan | 207, cross-sectional survey | Hospitals and community pharmacies | NR | NR | NR | NR | NR |
| Van Marum et al., 2016 (49), Netherlands | 40, interview and cross-sectional survey | Community setting, older adults aged 70+ y, taking ≥7 medications | 79 | 55 | 11‡ | Y, Dutch | Y, translated. Deleted Q8, Q14 and Q15 |
| Whitty et al., 2018 (45), Canada | 53, pilot study | Hospital setting, seriously ill or frail older patients | 80‡ | 43 | 13‡ | NR | Y, Q11 response items changed to 2-point scale (Yes and No), deleted Q12 and Q13 |
| rPATD, rPATDcog questionnaires | |||||||
| Cardwell et al., 2020 (62), Ireland | 786, non-randomized pilot study | Community setting, aged 65+ y, taking ≥10 medications | 70‡ | 65 | 10‡ | N | N |
| Edelman et al., 2019 (46,47), The Netherlands | 179, cross-sectional survey | Community setting, men aged 30+ y, taking an alpha-blocker, diagnosed with lower urinary tract symptoms | 69‡ | 0 | 4 | Y, Dutch | Y, translated. Modified questions to create alpha-blocker-specific rPATD factors |
| Gnjidic et al., 2019 (25), Australia | 42, feasibility study | Hospital setting, aged 65+ y, taking a benzodiazepine | 72 | 55 | 10 | N | Y, 5 benzodiazepine-specific questions were added |
| Ikeji et al., 2019 (39,40), United States | 19, cross-sectional survey | Outpatient, aged 65+ y, taking a Proton Pump Inhibitor | NR | 60 | NR | N | Y, the questionnaire was modified to focus on proton pump inhibitors |
| Kua C-H et al., 2020 (52), Singapore | 615, cross-sectional survey | Hospitals, community pharmacies and primary care clinics, aged 65+ y, taking ≥1 medication. Caregivers | 73‡ | 44 | 5‡ | N | N |
| Kua K et al., 2019 (34,35,63), Malaysia | 502, cross-sectional survey | Community pharmacies and primary care clinics, aged 60+ y, taking ≥1 medication. Caregivers | 67 | 50 | 3 | Y, Mandarin and Malay | Y, translated |
| Lundby et al., 2019 (50),¶ Denmark | 159, validation study and cross-sectional survey | Residential aged care facility | 82 | 61 | NR | Y, Danish | Y, translated |
| Major et al., 2019 (28),# Australia | 66, intervention study and survey | Community setting | NR | NR | 12‡ | N | Y, Q7 (primary outcome) was not asked |
| Martinez et al., 2020 (41), United States | 30, pre-post intervention study | Community setting, adults aged 18+ y, with insomnia | 56‡ | 100 | 4‡ | N | NR |
| Ng et al., 2019,(36),¶ Malaysia¶ | 18, cross-sectional survey | Community setting, adults aged 18+ y, diagnosed with Parkinson’s disease | 64 | 44 | 5** | NR | NR |
| Nusair et al., 2020 (54), Jordan | 358, validation study and survey | Outpatient, adults aged 18+ y, taking ≥5 medications | 60‡ | 52 | 7‡ | Y, Arabic | Y, translated |
| Omar et al., 2019 (37), Malaysia | 182, cross-sectional survey | Primary care clinics, aged 65+ y, taking ≥1 medication | 72 | 52 | 6 | Y, Malay | Y, translated |
| Paque et al., 2019 (55), Belgium | 296, cross-sectional survey | Residential aged care facility, aged 65+ y, limited life expectancy. Caregivers | 86‡ | 74 | 7‡ | Y, Dutch | Y, translated and added a question about patients’ willingness to speak to their GP about their medications |
| Reeve et al., 2019 (rPATD development and results) (18,31), Australia | 386, cross-sectional survey | Community setting, aged 65+ y, taking ≥1 medication. Caregivers | 74 | 57 | NR | N | N |
| Reeve et al., 2018 (42),†† United States | 1981, cross-sectional survey | Community setting, aged 65+ y | NR | 55 | NR | N | Y, combined 10 questions from the PATD and rPATD (older adults’ version),modified to a 4-point Likert scale (deleted unsure) |
| Reeve et al., 2018 (rPATDcog) (19), Australia | 21, development and pilot study of the rPATDcog | Outpatient, adults aged 18+ y, taking ≥1 medication, with a diagnosis of mild cognitive impairment or dementia. Caregivers | 77‡ | 48 | 7‡ | N | Y, the rPATD questionnaire for older adults was used to develop the rPATDcog questionnaire |
| Scott et al., 2019 (63), United Kingdom | 75, cross-sectional survey | Hospital setting, aged 70+ y, with physical frailty or comorbidities. Caregivers | 87 | 45 | 8 | N | Y, Q10 minor changes to fit the UK context regarding cost of medicines |
| Tegegn et al., 2018 (56), Ethiopia | 316, cross-sectional survey | Outpatient, aged 65+ y, taking ≥1 medication | 70 | 45 | 3 | Y, Amharic | Y, translated and modified to a 4-point Likert scale (deleted unsure) |
Notes:
*Implied in the article: a translated questionnaire was based on comparative research (van Marum et al. (49)).
†Regular and medications taken as required.
‡Mean.
§Discrepancy in the manuscript text and table.
‖This reference contains results from 2 cohorts; one of these cohorts was published separately (and so are reported separately: Reeve 2013). Data presented here are from the second cohort only (community pharmacy participants).
¶This is an abstract.
#This is an editorial comment.
**Including supplements.
††Combined PATD and rPATD questions, for clarity we have classified this reference as using the rPATD questionnaire.
Regarding the quality of reporting, all studies described or partially described the questionnaire used (100%, 38/38) and most referenced the original work (95%, 36/38; see Supplementary Tables 4 and 5). Assessment of quality reporting was unable to be performed on 2 of the studies (38,59). Most studies gave a description of the desired population (89%, 34/38), 79% (30/38) reported how the survey was administered, and 74% (28/38) at least partially reported the psychometric properties of the (r)PATD. However, 26 studies (68%) did not report the format of the survey (paper, online, or both) and half (19/38) did not present a sample size calculation or justification of sample size.
Willingness to Have a Medication Deprescribed
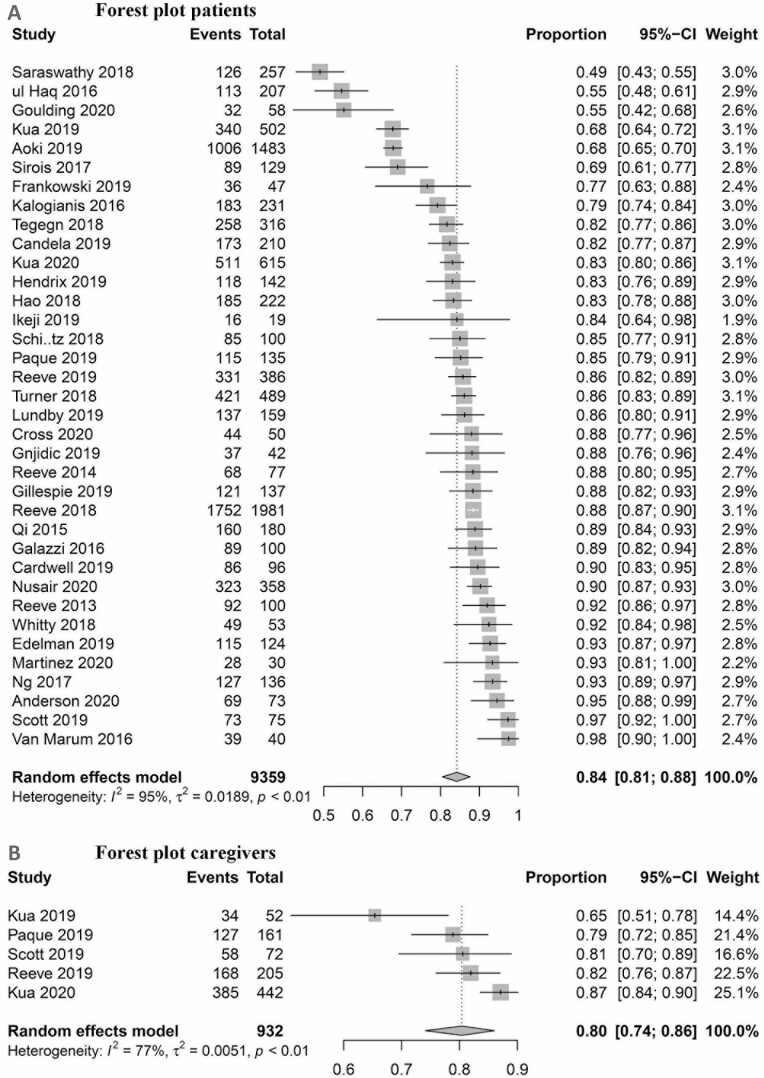
Overall, 49%–98% (n = 36 studies) of patients in the included studies were willing to stop 1 or more of their medications if their doctor said it was possible (Tables 2 and 3). Three studies did not report the results to this question as a proportion. From the rPATDcog (n = 1), 82% of patients (with cognitive impairment) were willing to have a medication deprescribed if their doctor said it was possible (19). Our meta-analysis showed the pooled proportion was 84% (95% CI 81%–88%, I2 = 95%) of patients who responded “agree” or “strongly agree” to the question: “Would you be willing to have one or more of your medicines stopped if your doctor said it was possible?” (Figure 1). There was significant heterogeneity overall and the subgroup analyses (Supplementary Figure 2) were not able to explain the heterogeneity. We found limited evidence of publication bias based on visual inspection of the funnel plots (Supplementary Figure 3).
Table 2.
PATD Questionnaire Results
Table 3.
Older Adults’ Results From the rPATD Questionnaire
Figure 1.
Forest plots of proportion of participants who agreed or strongly agreed with the question “If my doctor said it was possible, I would be willing to stop one or more of my medicines”. (A) Forest plot patients. (B) Forest plot caregivers.
The majority of caregivers (65%–87%, n = 5 studies) reported that they would be willing for one or more of their care recipient’s medications to be stopped if their care recipient’s doctor said it was possible (Supplementary Table S6) (19,31,34,35,52,55,63). The pooled effect estimate was 80% (95% CI 74%–86%, I2 = 77%).
Responses to the (r)PATD Questionnaires
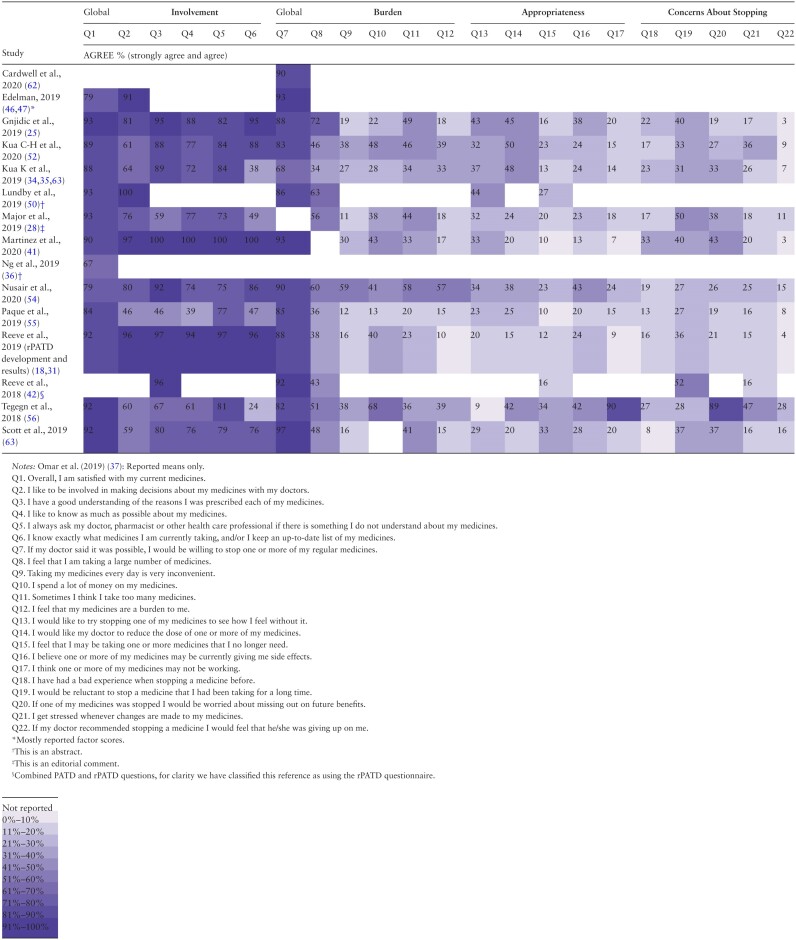
The questions from the PATD which had the smallest ranges of responses (ie, least variation in findings across studies) were “I feel that I may be taking one or more medications that I no longer need” (studies found between 8% and 38% agreement in 32/39 studies (as this question is in both the PATD and rPATD questionnaires), “I believe one or more of my medications is giving me side effects” (11%–44% over 19/22 studies), and “I believe that all my medications are necessary” (56%–92% in 18/22 studies). Although the questions with the greatest variation across studies were “I would like to reduce the number of medications that I am taking” (17%–89% over 18/22 studies) and “I would accept taking more medications for my health conditions” (10%–84% in 17/22 studies; see Table 2).
Studies that used the rPATD questionnaire (Table 3) found that 27%–52% of participants would be reluctant to stop a medicine they had taken for a long time (12/17 studies). Most participants (67%–93%) reported they were satisfied with their current medicines (12/17 studies), whereas 24%–100% of participants felt that they knew exactly which medicines they take and/or have an up-to-date list (12/17 studies) and 7%–90% of participants felt that one or more of their medicines may not be working (11/17 studies). In response to the statement: “I would like to try stopping one of my medicines to see how I feel without it,” 9%–44% of participants agreed (14/17; see Table 3).
Findings of the caregivers’ version of the rPATD are presented in Supplementary Table S6.
Associations Between Participant Characteristics and Willingness to Have a Medication Deprescribed
Fourteen studies examined relationships between participant characteristics and the primary outcome willingness to have a medication deprescribed (Table 4 and Supplementary Table S7). The most common patient characteristics examined were age (n = 12), gender (n = 6), education level (n = 6), number of medications (n = 11), and chronic health conditions (n = 4). Five of 12 studies reported a significant association between age and willingness to have a medication deprescribed, although the direction of this association varied (eg, older age compared with younger age were both found to be associated with greater willingness to have a medication deprescribed). Three studies examined relationships between caregiver characteristics and the primary outcome willingness to have a medication deprescribed (Supplementary Table S8).
Table 4.
Associations With the Primary Outcome Question “If my doctor said it was possible, I would be willing to stop one or more of my regular medicines”
| Variables (Statistical Significance, Direction of Association) | ||||||
|---|---|---|---|---|---|---|
| Source, Year | Age | Number of Medications | Number of Chronic Health Conditions | Gender (Female) | Education Level | Access Discount Medications* (Yes) |
| PATD | ||||||
| Aoki et al., 2019 (60) | S, + | S, + | S, + | NS | NS | / |
| Gillespie et al., 2019 (24) | / | NS | / | / | / | / |
| Hao et al., 2018 (33) | S, − | / | / | / | / | / |
| Kalogianis et al., 2016 (27) | / | NS | / | / | / | / |
| Qi et al., 2015 (29) | NS | NS | / | / | / | / |
| Reeve et al., 2013 (PATD development + results) (17,32) |
NS | NS | NS | / | / | S, − |
| Reeve et al., 2014 (thesis) (30)† | NS | NS | / | / | / | / |
| ul Haq et al., 2016 (61) | S, + | NS | / | / | / | NS |
| rPATD | ||||||
| Kua C-H et al., 2020 (52) | NS | S‡ | / | NS | NS | / |
| Kua K et al., 2019 (34) | S, + | NS | / | NS | S, − | / |
| Ng et al., 2017 (53) | S, − | / | / | / | / | / |
| Reeve et al., 2019 (rPATD results) (18,31) | NS | NS | / | NS | NS | S, + |
| Reeve et al., 2018 (42) | NS | S, + | S, + | NS | NS | NS |
| Tegegn et al., 2018 (56) | NS | / | NS§ | NS | NS | / |
| Total examined | 11 | 10 | 4 | 6 | 5 | 4 |
| Total significant | 5 | 3 | 2 | 0 | 1 | 2 |
Notes: / = not examined; NS = not significant; S = significant. “+” denotes increasing/higher variable (or female gender or possession of a medication concession card) associated with increasing willingness to deprescribe. “−” denotes decreasing/lower variable (or male gender or no medication concession card) associated with increasing willingness to deprescribe.
*Participants had a medication concession card or drug cost was covered/fully subsidised.
†This reference contains results from 2 cohorts; one of these cohorts was published separately (and so are reported separately: Reeve 2013). Data presented here are from the second cohort only (community pharmacy participants).
‡Unclear if the direction of the finding is “+” or “−” ; significant difference was found between groups (1–5, 6–10, and >10), but authors report “No significant differences in sub-group analysis.”
§Charlson Comorbidity Index.
Discussion
Main Findings
We synthesized results of 40 studies that used the (r)PATD questionnaires. The included studies were diverse in study design, intended purpose, and characteristics examined. Overall, many participants were willing to have a medication deprescribed if their doctor said it was possible (84%, 95% CI 81%–88%). Caregiver data provided a similar result, 80% (95% CI 74%–86%). However, there was significant heterogeneity (I2 = 95% patients, 77% for carers) and no explanation for this was identified through the subgroup analyses. Approximately one third of the studies examined associations between participant characteristics and the primary outcome. However, there was inconsistency in whether there was statistical significance between characteristics and the primary outcome. In the studies where there was an association found, there was inconsistency in the direction of the association (ie, if the characteristic was associated with higher or lower willingness). As such, it is still unclear whether individual characteristics (such as age or number of medications) could predict participant willingness to have their medications deprescribed.
Strengths and Limitations
A strength of this review is that we included articles published in any language, conference abstracts, and gray literature. We identified unpublished (or locally published) articles/reports through contacting those who had requested permission to use the (r)PATD. Our multipronged search strategy, which included methods outside of traditional database searching, led to additional studies being included. Studies within the review were diverse in terms of setting, country, and design. Most studies in this review were from high-income countries, which may reflect missing studies, or that studies have not been done in these lower income countries. Few studies examined caregivers’ attitudes, and only a single study used the rPATDcog.
Many of the included studies were cross-sectional and as such do not allow conclusions on causality (when examining the associations between participant characteristics and willingness to deprescribe). The (r)PATD, as a self-reported measure, is susceptible to social desirability bias. Although no difference was found in the subanalysis looking at method of administration (self-report, researcher administered) and several studies collected responses anonymously. Convenience samples were used in several studies; representativeness of the sample was described as one of the limitations in many of the included articles, and how nonrespondents differed from participants was rarely described. Overall, participants may have been healthier or more involved in managing their medications, particularly in studies of self-selected participants. One U.S. study (42) was conducted in a representative population and several studies targeted disadvantaged populations (38,41,48) without any obvious differences in (r)PATD responses in these studies.
A checklist to assess the quality of reporting was used in place of a risk of bias tool (20). Such reporting checklists do not technically assess a study’s quality; however, no quality assessment tool was identified for surveys. The sections that were generally well reported included background, discussion, and ethics, whereas methods and results were less well reported. The studies within this review were somewhat heterogeneous, including how the (r)PATD was used. Although a number of translations have been published, it is unclear if they are all semantically equivalent to the English version. There was variation in the use of items and response scales, and few of the studies that modified the (r)PATD reported validation for their local context. However, most translated versions of the (r)PATD involved some piloting (5–28 patients), and modified questions were often reviewed by the research team. It is possible that cultural or country-specific differences exist in relation to patients’ attitudes toward deprescribing that may affect responses to the (r)PATD.
Comparison With Other Studies
There is an increasing understanding that medication optimization can be achieved by engaging older adults and their caregivers in deprescribing decisions and prioritizing patient-centered care. The synthesized results from this review can be interpreted in the context of findings using complementary surveys. The Patient Perceptions of Deprescribing (PPoD) survey (64,65) found that one third of participants (34%, n = 803) had experienced stopping a medication. Significant factors associated with past deprescribing experience included being told by a doctor or the patient asking to stop a medication, interest in deprescribing, shared decision making, and higher education. Alternatively, factors associated with decreased likelihood to deprescribe included polypharmacy and participants having higher trust in their doctor.
Qualitative findings of patient-related barriers to deprescribing (66,67) recognize the often coexisting positive and negative attitudes toward deprescribing that patients have, as well as the complex interplay that exists between attitudes, beliefs, and decision making. The (r)PATD results reflect these seemingly contradictory attitudes in that individuals may say they are open to deprescribing but also report high satisfaction with their medications. Indeed, qualitative findings show clinicians perceive their patients are reluctant to deprescribe medications (68,69). Additionally, in previous studies, 30%–40% of participants have refused to participate in a deprescribing intervention study (70–72), irrespective of taking potentially inappropriate medications (73). Presently, the predictive ability of the (r)PATD has not been established, and it may be difficult to discriminate patient behavior from hypothetical willingness to deprescribe. Additionally, even though participants in the included studies overwhelmingly report agreement with deprescribing if their doctor said it was possible, the factors influencing acceptance of deprescribing in clinical practice at a single point in time are complex and multifaceted (74).
Research, Clinical, and Policy Implications
We found inconsistency in the participant characteristics that were associated with willingness to deprescribe. Understanding predictors of positive attitudes toward deprescribing more generally could enable tailored deprescribing practices as singular, external, or measurable factors might not consistently predict attitudes to deprescribing. There were some participant characteristics, such as frailty and dementia, that were only captured in a few studies or were measured in different ways. This highlights the need to consistently measure characteristics to add to the evidence base, particularly for these patients that stand to benefit the most from deprescribing (75). Deprescribing is a process that should involve the patient (76), therefore, an ongoing conversation with the patient and caregiver, and consideration of the complex internal and external factors that affect the implementation of deprescribing is required (77).
Although mostly used in the research setting, the (r)PATD is increasingly used as part of a deprescribing intervention strategy, such as the Australian G-MEDSS (Goal-directed Medication Review Electronic Decision Support System) study (78,79) and the US OPTIMISE study (80,81). This highlights a shift toward implementation of self-assessment surveys in clinical practice to promote “real-time” support for deprescribing conversations. Further work is required to determine how and when the (r)PATD can be best utilized in clinical practice.
Different public health and policy initiatives may be implemented to increase deprescribing activities. For instance, raising public awareness and acceptance of deprescribing as a normal and positive part of patient care may alleviate concerns patients may have to trial stopping a medication (this review found between 27% and 52% of participants were reluctant to stop a long-term medication) (82). Additionally, it is important to prioritize shared decision making (76) with a focus on patients’ goals and preferences, to navigate the seemingly contradictory beliefs of willingness to deprescribe yet feeling that their medications are appropriate. Remuneration and dedicated clinical consultations for these discussions may be needed to increase widespread deprescribing in practice.
Conclusions
Overall, clinicians should be reassured of their patients’ and caregivers’ willingness to have medications deprescribed. As such, this could encourage clinicians to initiate a conversation about deprescribing with those they care for. The findings from this study moves toward understanding attitudes toward deprescribing, which could, in turn, increase the discussion and uptake of deprescribing recommendations in clinical practice.
Supplementary Material
Acknowledgments
We acknowledge Ms. Lorien Delaney, Academic Librarian at the University of South Australia for her support in refining the strategy used for the peer-review literature search. Thank you to all the researchers who responded to our requests for more information.
Contributor Information
Kristie Rebecca Weir, University of South Australia, UniSA: Clinical and Health Sciences, Quality Use of Medicines and Pharmacy Research Centre (QUMPRC), Adelaide, SA, Australia; University of Sydney, Sydney School of Public Health, Faculty of Medicine and Health, Sydney, NSW, Australia.
Nagham J Ailabouni, University of South Australia, UniSA: Clinical and Health Sciences, Quality Use of Medicines and Pharmacy Research Centre (QUMPRC), Adelaide, SA, Australia.
Carl R Schneider, University of Sydney, School of Pharmacy, Faculty of Medicine and Health, Sydney, NSW, Australia.
Sarah N Hilmer, University of Sydney, Kolling Institute of Medical Research, Royal North Shore Hospital, Sydney, NSW, Australia; Department of Clinical Pharmacology, Royal North Shore Hospital, St Leonards, NSW, Australia; Department of Aged Care, Royal North Shore Hospital, St Leonards, NSW, Australia; University of Sydney, Northern Clinical School, Faculty of Medicine and Health, Sydney, NSW, Australia.
Emily Reeve, University of South Australia, UniSA: Clinical and Health Sciences, Quality Use of Medicines and Pharmacy Research Centre (QUMPRC), Adelaide, SA, Australia; Dalhousie University and Nova Scotia Health Authority, Geriatric Medicine Research, Faculty of Medicine, and College of Pharmacy, Halifax, Canada.
Funding
E.R. is an Australian National Health and Medical Research Council (NHMRC)–Australian Research Council (ARC) Dementia Research Development Fellow and K.R.W and N.J.A.’s salaries are supported by this award.
Conflict of Interest
Dr. Reeve was the lead author of the development of the PATD, rPATD, and rPATDcog (the questionnaire of interest in this systematic review).
Author Contributions
All authors were involved in designing the study. K.R.W. and N.J.A. were involved in searching the database. K.R.W. and N.J.A. screened citations for inclusion. K.R.W., E.R., and N.J.A. were involved in extracting data and interpretation. K.R.W. and E.R. synthesized the data, and C.R.S. conducted the meta-analysis. K.R.W. drafted the manuscript, and E.R. and N.J.A. contributed to the drafting of the review. S.N.H. and C.R.S. revised the manuscript critically for important intellectual content. All authors reviewed the final manuscript and agreed to be accountable for all aspects of the work and approved the final manuscript for submission.
References
- 1. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–995. doi: 10.1016/j.jclinepi.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 2. O’Connor MN, Gallagher P, O’Mahony D. Inappropriate prescribing. Drugs Aging. 2012;29(6):437–452. doi: 10.2165/11632610-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 3. Panel AGSBCUE, Fick DM, Semla TP, et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4): 674–694. [DOI] [PubMed] [Google Scholar]
- 4. Steinman MA, Miao Y, Boscardin WJ, Komaiko KD, Schwartz JB. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29(10):1379–1386. doi: 10.1007/s11606-014-2924-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85(1):86–88. doi: 10.1038/clpt.2008.224 [DOI] [PubMed] [Google Scholar]
- 6. Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J Am Med Dir Assoc. 2020;21(2):181–187. doi: 10.1016/j.jamda.2019.10.022 [DOI] [PubMed] [Google Scholar]
- 7. Leelakanok N, Holcombe AL, Lund BC, Gu X, Schweizer ML. Association between polypharmacy and death: a systematic review and meta-analysis. J Am Pharm Assoc (2003). 2017;57(6):729–738.e10. doi: 10.1016/j.japh.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 8. Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schöttker B. The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc. 2017;18(3):211–220. doi: 10.1016/j.jamda.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 9. Hyttinen V, Jyrkkä J, Valtonen H. A systematic review of the impact of potentially inappropriate medication on health care utilization and costs among older adults. Med Care. 2016;54(10):950–964. doi: 10.1097/MLR.0000000000000587 [DOI] [PubMed] [Google Scholar]
- 10. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Internal Med. 2015:E1–E8. doi: 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 11. Reeve E, Gnjidic D, Long J, Hilmer SJBjocp. A systematic review of the emerging definition of “deprescribing” with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254–1268. doi: 10.1111/bcp.12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583–623. doi: 10.1111/bcp.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of randomised trials. Drugs Aging. 2018;35(4):303–319. doi: 10.1007/s40266-018-0536-4 [DOI] [PubMed] [Google Scholar]
- 14. Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. Challenges and enablers of deprescribing: a general practitioner perspective. PLoS One. 2016;11(4):e0151066. doi: 10.1371/journal.pone.0151066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson W, Le JV, Haastrup P, Nielsen JB, Pedersen LB, Jarbøl DE. Exploring how GPs discuss statin deprescribing with older people: a qualitative study. BJGP Open. 2020;4(1). doi: 10.3399/bjgpopen20X101022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544. doi: 10.1136/bmjopen-2014-006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Development and validation of the Patients’ Attitudes Towards Deprescribing (PATD) questionnaire. Int J Clin Pharm. 2013;35(1):51–56. doi: 10.1007/s11096-012-9704-5 [DOI] [PubMed] [Google Scholar]
- 18. Reeve E, Low LF, Shakib S, Hilmer SN. Development and validation of the revised Patients’ Attitudes Towards Deprescribing (rPATD) questionnaire: versions for older adults and caregivers. Drugs Aging. 2016;33(12):913–928. doi: 10.1007/s40266-016-0410-1 [DOI] [PubMed] [Google Scholar]
- 19. Reeve E, Anthony AC, Kouladjian O’Donnell L, et al. Development and pilot testing of the revised Patients’ Attitudes Towards Deprescribing questionnaire for people with cognitive impairment. Australas J Ageing. 2018;37(4):E150–E154. doi: 10.1111/ajag.12576 [DOI] [PubMed] [Google Scholar]
- 20. Bennett C, Khangura S, Brehaut JC, et al. Reporting guidelines for survey research: an analysis of published guidance and reporting practices. PLoS Med. 2010;8(8):e1001069. doi: 10.1371/journal.pmed.1001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson K. Minimising potentially inappropriate polypharmacy in community living older people: a multi-phase, mixed methods study to develop and pilot a general practitioner-led deprescribing intervention in primary care. 2018.
- 22. Anderson K, Freeman C, Foster M, Scott I. GP-led deprescribing in community-living older Australians: an exploratory controlled trial. J Am Geriatr Soc. 2020;68(2):403–410. doi: 10.1111/jgs.16273 [DOI] [PubMed] [Google Scholar]
- 23. Cross AJ, George J, Woodward MC, Le VJ, Elliott RA. Deprescribing potentially inappropriate medications in memory clinic patients (DePIMM): a feasibility study. Res Social Adm Pharm. 2020. [DOI] [PubMed] [Google Scholar]
- 24. Gillespie R, Mullan J, Harrison L. Attitudes towards deprescribing and the influence of health literacy among older Australians. Prim Health Care Res Dev. 2019;20:e78. doi: 10.1017/S1463423618000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gnjidic D, Ong HMM, Leung C, Jansen J, Reeve E. The impact of in hospital patient-education intervention on older people’s attitudes and intention to have their benzodiazepines deprescribed: a feasibility study. Ther Adv Drug Saf. 2019;10:2042098618816562. doi: 10.1177/2042098618816562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hendrix I, Page AT, Korhonen MJ, et al. Patterns of high-dose and long-term proton pump inhibitor use: a cross-sectional study in six south Australian residential aged care services. Drugs Real World Outcomes. 2019;6(3):105–113. doi: 10.1007/s40801-019-0157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalogianis MJ, Wimmer BC, Turner JP, et al. Are residents of aged care facilities willing to have their medications deprescribed? Res Social Adm Pharm. 2016;12(5):784–788. doi: 10.1016/j.sapharm.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 28. Major GL, Mills A, Lowthian JA. Deprescribing attitudes of older adults receiving medication management support from home-based nurses. J Am Geriatr Soc. 2019;67(8):1756–1757. doi: 10.1111/jgs.16015 [DOI] [PubMed] [Google Scholar]
- 29. Qi K, Reeve E, Hilmer SN, Pearson SA, Matthews S, Gnjidic D. Older peoples’ attitudes regarding polypharmacy, statin use and willingness to have statins deprescribed in Australia. Int J Clin Pharm. 2015;37(5):949–957. doi: 10.1007/s11096-015-0147-7 [DOI] [PubMed] [Google Scholar]
- 30. Reeve E. Development and Feasibility of a Patient-Centred Deprescribing Process [PhD Thesis]. Adelaide, Australia: University of South Australia; 2014. [Google Scholar]
- 31. Reeve E, Low LF, Hilmer SN. Attitudes of older adults and caregivers in Australia toward deprescribing. J Am Geriatr Soc. 2019;67(6):1204–1210. doi: 10.1111/jgs.15804 [DOI] [PubMed] [Google Scholar]
- 32. Reeve E, Wiese MD, Hendrix I, Roberts MS, Shakib S. People’s attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61(9):1508–1514. doi: 10.1111/jgs.12418 [DOI] [PubMed] [Google Scholar]
- 33. Hao LJ, Omar MS, Tohit N. Polypharmacy and willingness to deprescribe among elderly with chronic diseases. Int J Gerontol. 2018;12(4):340–343. [Google Scholar]
- 34. Kua KP, Saw PS, Lee SWH. Attitudes towards deprescribing among multi-ethnic community-dwelling older patients and caregivers in Malaysia: a cross-sectional questionnaire study. A comment. Int J Clin Pharm. 2019;41(5):1131–1132. doi: 10.1007/s11096-019-00891-7 [DOI] [PubMed] [Google Scholar]
- 35. Kua KP, Saw PS, Lee SWH. Attitudes towards deprescribing among multi-ethnic community-dwelling older patients and caregivers in Malaysia: a cross-sectional questionnaire study. Int J Clin Pharm. 2019;41(3):793–803. doi: 10.1007/s11096-019-00829-z [DOI] [PubMed] [Google Scholar]
- 36. Ng KY, Lee SWH. Attitude of older patients with Parkinson’s disease towards deprescribing: a pilot study. J Parkinson’s Dis. 2019;9(1):101. [Google Scholar]
- 37. Omar MS, Ariandi AH, Tohit NM. Practical problems of medication use in the elderly Malaysians and their beliefs and attitudes toward deprescribing of medications. J Res Pharm Pract. 2019;8(3):105–111. doi: 10.4103/jrpp.JRPP_19_35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goulding H. Quality Improvement Project on Optimising Medications – Serious Mental Illness. Pittsburgh, PA: University of Pittsburgh; 2018. [Google Scholar]
- 39. Ikeji C, Williams A, Hennawi G, Brandt NJ. Patient and provider perspectives on deprescribing proton pump inhibitors. J Gerontol Nurs. 2019;45(10):9–17. doi: 10.3928/00989134-20190912-03 [DOI] [PubMed] [Google Scholar]
- 40. Ikeji CA, Brandt N, Hennawi G, Williams A. Patient and prescriber perspectives on proton pump inhibitor (PPI) use and deprescribing in older adults. J Am Geriatr Soc. 2019;67(suppl 1):S287. [Google Scholar]
- 41. Martinez AI, Spencer J, Moloney M, Badour C, Reeve E, Moga DC. Attitudes toward deprescribing in a middle-aged health disparities population. Res Social Adm Pharm. 2020;16(10):1502–1507. doi: 10.1016/j.sapharm.2020.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reeve E, Wolff JL, Skehan M, Bayliss EA, Hilmer SN, Boyd CM. Assessment of attitudes toward deprescribing in older Medicare beneficiaries in the United States. JAMA Intern Med. 2018;178(12):1673–1680. doi: 10.1001/jamainternmed.2018.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sirois C, Ouellet N, Reeve E. Community-dwelling older people’s attitudes towards deprescribing in Canada. Res Social Adm Pharm. 2017;13(4):864–870. doi: 10.1016/j.sapharm.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 44. Turner JP, Martin P, Zhang YZ, Tannenbaum C. Patients beliefs and attitudes towards deprescribing: can deprescribing success be predicted? Res Social Adm Pharm. 2020;16(4):599–604. doi: 10.1016/j.sapharm.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 45. Whitty R, Porter S, Battu K, et al. A pilot study of a Medication Rationalization (MERA) intervention. CMAJ Open. 2018;6(1):E87–E94. doi: 10.9778/cmajo.20170134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edelman M, Blanker M, Jellema P, Hak E, Denig P. Patient attitudes towards deprescribing of alpha-blockers and willingness to participate in a discontinuation trial. Neurourol Urodyn. 2017;36(suppl 3):S242–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edelman M, Jellema P, Hak E, Denig P, Blanker MH. Patients’ attitudes towards deprescribing alpha-blockers and their willingness to participate in a discontinuation trial. Drugs Aging. 2019;36(12):1133–1139. doi: 10.1007/s40266-019-00712-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frankowski NEDM, Grootens KP, van der Stelt CA, Noort A, van Marum RJ. ‘You tell me, you are the doctor’; patients on long-term psychiatric residences about their medication. Tijdschr Psychiatr. 2019;61(8):527–535. [PubMed] [Google Scholar]
- 49. Van Marum RJ, Van Marum S, Van Driesten S, Verdoorn S, Boxman A, Grossklaus A. Deprescribing for the elderly: comparing opinions of patients, pharmacists and doctors. Pharm Weekbl. 2016;151(43):28–33. [Google Scholar]
- 50. Lundby C, Simonsen T, Ryg J, Sondergaard J, Pottegard A, Lauridsen HH. Translation, cultural adaptation, and psychometric properties of the Danish version of the revised patients’ attitudes towards deprescribing questionnaire. Pharmacoepidemiol Drug Saf. 2019;28(suppl 2):291–292. [Google Scholar]
- 51. Schiøtz ML, Frølich A, Jensen AK, et al. Polypharmacy and medication deprescribing: a survey among multimorbid older adults in Denmark. Pharmacol Res Perspect. 2018;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kua CH, Reeve E, Ratnasingam V, Mak VSL, Lee SWH; Pharmaceutical Society of Singapore Deprescribing Workgroup . Patients’ and caregivers’ attitudes towards deprescribing in Singapore. J Gerontol A Biol Sci Med Sci. 2020;22:22. [DOI] [PubMed] [Google Scholar]
- 53. Ng WL, Tan MZW, Koh EYL, Tan NC. Deprescribing: What are the views and factors influencing this concept among patients with chronic diseases in a developed Asian community? Proc Singapore Healthcare. 2017;26(3):172–179. [Google Scholar]
- 54. Nusair MB, Arabyat R, Al-Azzam S, El-Hajji FD, Nusir AT, Al-Batineh M. Translation and psychometric properties of the Arabic version of the revised Patients’ Attitudes Towards Deprescribing questionnaire. J Pharm Health Serv Res. 2020. [Google Scholar]
- 55. Paque K, Elseviers M, Vander Stichele R, et al. Balancing medication use in nursing home residents with life-limiting disease. Eur J Clin Pharmacol. 2019;75(7):969–977. doi: 10.1007/s00228-019-02649-6 [DOI] [PubMed] [Google Scholar]
- 56. Tegegn HG, Tefera YG, Erku DA, et al. Older patients’ perception of deprescribing in resource-limited settings: a cross-sectional study in an Ethiopia university hospital. BMJ Open. 2018;8(4):e020590. doi: 10.1136/bmjopen-2017-020590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saraswathy GR, Pratheeksha M, Maheswari E. Analysing the attitude of old age home residents on polypharmacy and deprescribing of medications in Mangalore, Karnataka. Value Health. 2018;21(suppl 3):S220. [Google Scholar]
- 58. Galazzi A, Lusignani M, Chiarelli MT, et al. Attitudes towards polypharmacy and medication withdrawal among older inpatients in Italy. Int J Clin Pharm. 2016;38(2):454–461. doi: 10.1007/s11096-016-0279-4 [DOI] [PubMed] [Google Scholar]
- 59. Candela M. Ánalisis de la complejidad farmacoterapéutica y la necesidad potencial de desprescripción en pacientes con infección por el VIH. Proyecto Invest. 2019. [Google Scholar]
- 60. Aoki T, Yamamoto Y, Ikenoue T, Fukuhara S. Factors associated with patient preferences towards deprescribing: a survey of adult patients on prescribed medications. Int J Clin Pharm. 2019;41(2):531–537. doi: 10.1007/s11096-019-00797-4 [DOI] [PubMed] [Google Scholar]
- 61. ul Haq N, Mohaib M, Nasim A, Razaque G, Riaz S. Chronic patients attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe in Quetta. Value Health. 2016;19(7):A823. [Google Scholar]
- 62. Cardwell K, Smith SM, Clyne B, et al. ; General Practice Pharmacist (GPP) Study Group . Evaluation of the General Practice Pharmacist (GPP) intervention to optimise prescribing in Irish primary care: a non-randomised pilot study. BMJ Open. 2020;10(6):e035087. doi: 10.1136/bmjopen-2019-035087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scott S, Clark A, Farrow C, et al. Attitudinal predictors of older peoples’ and caregivers’ desire to deprescribe in hospital. BMC Geriatr. 2019;19(1):108. doi: 10.1186/s12877-019-1127-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Linsky A, Simon SR, Stolzmann K, Lippin-Foster RA, Meterko M. Patient Perceptions of Deprescribing (PPoD): survey development and validation. J Gen Intern Med. 2016;31(2):S327. [Google Scholar]
- 65. Linsky A, Simon SR, Stolzmann K, Meterko M. Patient attitudes and experiences that predict medication discontinuation in the Veterans Health Administration. J Am Pharm Assoc (2003). 2018;58(1):13–20. doi: 10.1016/j.japh.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807. doi: 10.1007/s40266-013-0106-8 [DOI] [PubMed] [Google Scholar]
- 67. Weir K, Nickel B, Naganathan V, et al. Decision-making preferences and deprescribing: perspectives of older adults and companions about their medicines. J Gerontol B Psychol Sci Soc Sci. 2018;73(7):e98–e107. doi: 10.1093/geronb/gbx138 [DOI] [PubMed] [Google Scholar]
- 68. Weir KR, Naganathan V, Carter SM, et al. The role of older patients’ goals in GP decision-making about medicines: a qualitative study. BMC Fam Pract. 2021;22(1):13. doi: 10.1186/s12875-020-01347-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. General practitioners’ insight into deprescribing for the multimorbid older individual: a qualitative study. Int J Clin Pract. 2016;70(3):261–276. doi: 10.1111/ijcp.12780 [DOI] [PubMed] [Google Scholar]
- 70. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–898. doi: 10.1001/jamainternmed.2014.949 [DOI] [PubMed] [Google Scholar]
- 71. Beer C, Loh PK, Peng YG, Potter K, Millar A. A pilot randomized controlled trial of deprescribing. Ther Adv Drug Saf. 2011;2(2):37–43. doi: 10.1177/2042098611400332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS One. 2016;11(3):e0149984. doi: 10.1371/journal.pone.0149984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Komagamine J, Sugawara K, Hagane K. Characteristics of elderly patients with polypharmacy who refuse to participate in an in-hospital deprescribing intervention: a retrospective cross-sectional study. BMC Geriatr. 2018;18(1):96. doi: 10.1186/s12877-018-0788-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ailabouni NJ, Reeve E. Can existing tools predict older adults’ willingness to deprescribe? Considerations for D-PRESCRIBE’s post-hoc secondary analysis. Res Social Adm Pharm. 2019;15(11):1379–1380. doi: 10.1016/j.sapharm.2019.08.025 [DOI] [PubMed] [Google Scholar]
- 75. Maher D, Ailabouni N, Mangoni AA, Wiese MD, Reeve E. Alterations in drug disposition in older adults: a focus on geriatric syndromes. Expert Opin Drug Metab Toxicol. 2021;17(1):41–52. doi: 10.1080/17425255.2021.1839413 [DOI] [PubMed] [Google Scholar]
- 76. Jansen J, Naganathan V, Carter SM, et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ. 2016;353:i2893. doi: 10.1136/bmj.i2893 [DOI] [PubMed] [Google Scholar]
- 77. Linsky A, Gellad WF, Linder JA, Friedberg MW. Advancing the science of deprescribing: a novel comprehensive conceptual framework. J Am Geriatr Soc. 2019;67(10):2018–2022. doi: 10.1111/jgs.16136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sawan M, O’Donnell LK, Reeve E, et al. The utility of a computerised clinical decision support system intervention in home medicines review: a mixed-methods process evaluation. Res Social Adm Pharm. 2021;17(4):715–722. doi: 10.1016/j.sapharm.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 79. Kouladjian O’Donnell L, Gnjidic D, Sawan M, et al. Impact of the goal-directed medication review electronic decision support system on drug burden index: a cluster-randomised clinical trial in primary care. Br J Clin Pharmacol. 2021;87(3):1499–1511. doi: 10.1111/bcp.14557 [DOI] [PubMed] [Google Scholar]
- 80. Bayliss EA, Shetterly SM, Drace ML, et al. The OPTIMIZE patient- and family-centered, primary care-based deprescribing intervention for older adults with dementia or mild cognitive impairment and multiple chronic conditions: study protocol for a pragmatic cluster randomized controlled trial. Trials. 2020;21(1):542. doi: 10.1186/s13063-020-04482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Green AR, Boyd CM, Gleason KS, et al. Designing a primary care–based deprescribing intervention for patients with dementia and multiple chronic conditions: a qualitative study. J Gen Intern Med. 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thompson W, Reeve E, Moriarty F, et al. Deprescribing: future directions for research. Res Social Adm Pharm. 2019;15(6):801–805. doi: 10.1016/j.sapharm.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.