Intact function of polymorphonuclear phagocytes (PMN) is the most important component of the nonspecific immune response against invading microorganisms. As the number of immunosuppressed patients requiring antibiotic treatment is increasing, the impact of antibacterials on the nonspecific immune system has become of increasing interest. Investigations of newer quinolones showed that these antibacterials exert heterogenous effects on the immune system (1, 3, 6, 7, 8, 11). Moxifloxacin is a new 8-methoxyquinolone that has potent antimicrobial activity against both gram-negative and gram-positive bacteria and anaerobes (2, 4). The aim of this study was to evaluate the influence of moxifloxacin on PMN function using a whole-blood flow-cytometric method. The assay, which has been described previously (5, 9, 10) is designed to investigate effects on the phagocytosis of Candida albicans and Staphylococcus aureus (the percentages of phagocytosing PMN were determined), the percentage of PMN producing respiratory burst, and the percentage of C. albicans that has been killed by PMN. All three parameters were measured at multiple time points: for phagocytosis and burst, at 0, 2, 4, 6, 8, 10, 15, 20, and 25 min; for killing, at 0, 5, 10, 15, 20, 30, and 40 min.
Blood samples of 12 healthy volunteers were incubated with three different moxifloxacin concentrations (1, 10, and 100 μg/ml) and compared to a drug-free control. Phagocytic capacity was assessed by measuring the uptake of calcein-labeled yeasts or SYTO-9-labeled bacteria. Reactive burst after phagocytosis of yeasts or bacteria was estimated by the amount of dihydroethidium converted to ethidium by PMN intracellulary. Killing of C. albicans was analyzed by the specific uptake of ethidium homodimer 1 by nonviable yeasts. Therefore, PMN lysing was necessary to count the killed and liberated yeasts as free, red particles.
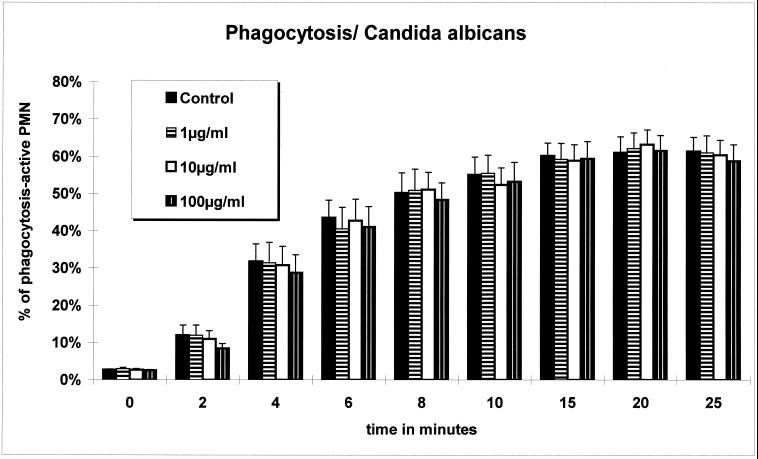
The results of investigations with the antibacterial-resistant pathogen C. albicans showed that moxifloxacin has no significant influence on PMN functions. Even the high moxifloxacin concentration of 100 μg/ml (maximum concentration in serum after oral administration of 400 mg, 1 to 10 μg/ml) did not lead to an impairment in any functional parameters studied (Fig. 1). In contrast, for the moxifloxacin-susceptible S. aureus (MIC at which 90% of the isolates are inhibited, 0.03 μg/ml) a mild dose-dependent decrease in phagocytic capability was detectable. However, respiratory burst remained unaffected. As intact viable intracellular bacteria are a prerequisite for accurate determination of the phagocytosis rate with flow cytometry, it can be suggested that the decreased phagocytosis rate is attributable to the specific interaction between the antibiotic and the bacteria and not a direct moxifloxacin effect on PMN.
FIG. 1.
Percent PMN that ingested C. albicans. No statistical difference between the three concentrations of moxifloxacin and the control was observed.
Investigating the quinolones ofloxacin, ciprofloxacin, sparfloxacin and temafloxacin, Aoki et al. (1) found that quinolones differ in their immunomodulating effects. For ofloxacin as well as ciprofloxacin they found burst-increasing attributes, but in contrast, burst-decreasing properties for were observed for sparfloxacin and temafloxacin. In the present study we demonstrated that moxifloxacin did not affect oxidative burst activity. Thus, immunmodulating properties of an individual quinolone do not represent a general class effect.
In summary, this in vitro study indicates that moxifloxacin does not impair phagocytic function with yeasts as test organisms. The results obtained with S. aureus showed only a mild reduction in phagocytic capacity with no effect on burst production.
REFERENCES
- 1.Aoki M, Ono Y, Kunii O, Goldstein E. Effect of newer quinolones on the extra- and intra-cellular chemiluminescence response of human polymorpho-nuclear leucocytes. J Antimicrob Chemother. 1994;34:383–390. doi: 10.1093/jac/34.3.383. [DOI] [PubMed] [Google Scholar]
- 2.Brueggemann A B, Kugler K C, Doern G V. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gemmell C G. Antibiotics and neutrophil function—potential immunomodulating activities. J Antimicrob Chemother. 1993;31(Suppl. B):23–33. doi: 10.1093/jac/31.suppl_b.23. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein E J, Citron D M, Hudspeth M, Gerardo S H, Merriam C V. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone, compared to the activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite would skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husfeld L. Entwicklung einer durchflusszytometrischen Methode zur Messung der Phagozytose-, Burst- und Killingfähigkeit humaner Phagozyten. Ph.D. dissertation. Munich, Germany: University of Munich; 1996. [Google Scholar]
- 6.Labro M T. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy tales”? Clin Microbiol Rev. 2000;13:615–650. doi: 10.1128/cmr.13.4.615-650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meroni P L. Immune response to antibiotics in patients with secondary immunodeficiencies. J Chemother. 1994;6(Suppl 3):16–18. [PubMed] [Google Scholar]
- 8.Rubinstein E, Shalit I. Effects of the quinolones on the immune system. In: Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. 2nd ed. Washington, D.C.: American Society for Microbiology; 1993. pp. 519–526. [Google Scholar]
- 9.Salih H, Husfeld L, Adam D. Inhibitory effect of heparin on neutrophil phagocytosis and burst production using a new whole-blood cytofluorometric method for determination. Eur J Med Res. 1997;2:507–513. [PubMed] [Google Scholar]
- 10.Salih H, Husfeld L, Adam D. Simultaneous cytofluorometric measurement of phagocytosis, burst production and killing of human phagocytes using Candida albicans and Staphylococcus aureus as target organisms. Clin Microbiol Infect. 2000;6:251–258. doi: 10.1046/j.1469-0691.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]