ABSTRACT
Background
Low birth weight predicts risk of infant death. However, several birth measurements may be equally predictive, for which cutoffs and associated risks are less explored.
Objectives
We assessed and optimized population cutoffs of birth length, weight, and midupper arm circumference (MUAC), head circumference (HC), and chest circumference (CC) for predicting neonatal (≤28 d) and infant (≤365 d) mortality in northwest Bangladesh.
Methods
Among 28,026 singletons born in an antenatal micronutrient supplement trial, 21,174 received anthropometry ≤72 h after birth, among whom 583 died in infancy. Optimization for predicting mortality for each measurement was guided by the Youden Index (sensitivity + specificity – 1). Relative risk ratios (RRRs) and positive predictive values (PPVs) were calculated across cutoff ranges for individual and any pair of measurements.
Results
Optimal cutoffs, harmonized to 100-g or 0.5-cm readings, for neonatal and infant mortality were 44.5 cm for length, 2200 g for weight, 9.0 cm for MUAC, 31.0 cm for HC, and 28.5 cm for CC, below which all predicted mortality. However, a CC <28.5 cm, alone and combined with HC <31.0 cm, yielded the highest RRR [9.68 (95% CI: 7.84, 11.94) and 15.74 (95% CI: 12.54, 19.75), respectively] and PPV (11.3% and 10.7%) for neonatal mortality and highest RRR [6.02 (95% CI: 5.15, 7.02) and 9.19 (95% CI: 7.72, 10.95)] and PPV (16.3% and 14.5%) for infant mortality. Pairs of measurements revealed a higher RRR for neonatal and infant mortality than individual measurements of any one pair, although the ranges of PPV remained comparable.
Conclusions
In Bangladesh, multiple birth measurements alone or in combination, particularly chest circumference, predict neonatal and infant mortality.
Keywords: birth anthropometry, infant mortality, neonatal mortality, newborn circumferential measurements, predictors
See corresponding editorial on page 1259.
Introduction
Newborn anthropometry is employed to assess the adequacy of fetal growth, evaluate nutritional status at birth, and gauge risks of subsequent poor growth, health, development, and survival throughout infancy (1). In rural health care settings of low- to middle-income countries, where births frequently occur at home, weight is most commonly measured, and low birth weight (<2500 g by convention) is the most widely reported anthropometric risk factor for neonatal and postneonatal mortality (2–4). Although less often assessed, a short birth length is also a risk factor (5–7).
Circumferential dimensions, requiring only a tape measure (8), can predict survival. For example, midupper arm circumference (MUAC), often measured in preschool children (9, 10) and occasionally in infancy (11), predicts mortality in both age groups (12, 13) but is rarely assessed at birth. Chest circumference (CC) is uncommonly measured at any age (14, 15), and its ability to predict mortality is unknown. However, when measured at birth, both arm and chest circumferences have been shown to covary with birth weight, with correlation coefficients ranging from 0.63 to 0.96 and 0.55 to 0.91, respectively (14, 16–22), suggesting predictive power.
Head circumference (HC) is a well-established birth dimension (23) that is strongly correlated with length at birth, as well as later in infancy and early childhood (24); the childhood plasma proteome (25); and cognition throughout early school aged years (26–28), but its mortality predictive potential remains unknown. Given simplicity of measurement, low cost, and logistical ease, circumferential measurements at birth may offer as yet unrealized clinical value in primary health care for assessing risks of infant morbidity and mortality.
In Bangladesh, ∼2.9 million live births occur annually (29), and neonatal and infant mortality rates are estimated to be 17 and 25 deaths per 1000 live births, respectively, reflecting 74,000 infant lives lost each year (29). Approximately half of all births in Bangladesh still occur at home (30), usually assisted by primary health care workers or traditional birth attendants who often do not measure birth size due to lack of equipment, training, or realization of its value in predicting survival throughout infancy (31). Estimating and educating primary care workers on the risk of infant mortality associated with individual and combined anthropometric measurements below distinct cutoffs at birth may help motivate their wider adoption to identify and provided extended care to infants at high risk of dying throughout the first year of life.
In this analysis, we explore in a large cohort of Bangladeshi infants the abilities of newborn weight, length, and head, chest, and arm circumferences to predict risks of neonatal (≤28 d) and infant mortality (≤365 d), individually and as any pair of 2 measurements. We identify measurement cutoffs that optimize relative risk ratios (RRRs) and predictive positive values (PPVs), harmonized for both outcomes to facilitate their adoption in primary health care settings of South Asia.
Methods
Study population
This study was carried out from 2008 to 2012 in 19 unions in the northwest Bangladesh District of Gaibandha, previously shown to exhibit many characteristics that typify rural Bangladesh (32). The study comprised part of the postnatal assessment protocol for a double-masked, cluster randomized trial (JiVitA-3), conducted from 2008 to 2012, that assessed the efficacy of antenatal multiple micronutrient supplementation compared with iron–folic acid on birth outcomes and infant mortality (33).
Field procedures
Field procedures of the JiVitA-3 trial have been previously reported (33). Briefly, the study area was divided into 596 comparably populated clusters that served as units of randomization. Married, nonpregnant women of reproductive age were enrolled at the outset while newlyweds were prospectively enrolled throughout the 4-y study. Altogether, 127,282 women were visited at home every 5 wk to detect new pregnancies via a monthly history of amenorrhea confirmed by urine testing. Newly pregnant women were consented; enrolled into the trial; interviewed about socioeconomic status, diet, morbidity, and work patterns; measured for height, weight, and arm circumference; and visited weekly to be given study supplements and monitor pregnancy status.
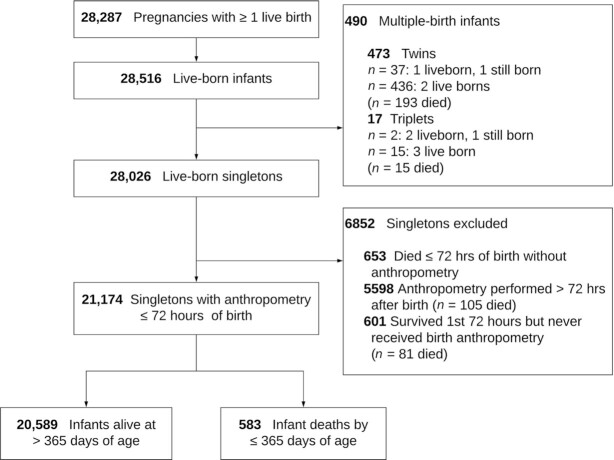
Mother–newborn dyads were assessed for vital status, infant anthropometry, and other characteristics shortly after birth and at ∼1, 3, 6, and 12 mo. At birth and subsequent postnatal visits, infant supine length was measured to the nearest 0.1 cm on locally constructed JiVitA length boards; weight was measured to the nearest 10 g (BD585 scales; Tanita Corporation), and HC, CC, and MUAC were measured to the nearest 0.1 cm using Zerfas insertion tapes (8). Of 44,567 pregnant women recruited into the trial, 28,287 delivered ≥1 live-born infants, of whom 28,026 were singletons, among whom 21,174 (75.6%) were measured within 72 h of birth. Among infants who received anthropometry, 328 (1.55%) died within 28 d, and 583 (2.75%) had died by 365 d (Figure 1).
FIGURE 1.
Maternal multiple micronutrient compared with iron–folic acid supplementation trial (JiVitA-3), Gaibandha, Bangladesh, 2008–2012.
Verbal informed consent was obtained from mothers prior to enrollment in the trial. The study protocol was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, Baltimore, Maryland, and the Bangladesh Medical Research Council, Dhaka, Bangladesh.
Statistical analysis
The analysis was performed using Stata, version 14.0 (StataCorp LLC). Neonatal, postneonatal, and total infant mortality were defined as deaths occurring within 28 d, 29–365 d, and all deaths through 365 d, respectively, all expressed per 1000 live births. Length-for-age z score (LAZ), weight-for-age z score, and weight-for-length z score (WLZ) of newborns were derived from the WHO international growth standards (34). Baseline maternal and household characteristics and maternal and newborn size were compared for infants who survived the study period (to 365 d) and those who died as neonates (≤28 d) and postneonates (29–365 d). Differences in baseline characteristics and birth outcomes were tested with 1-factor ANOVA for continuous variables and the χ2 test for categorical variables, respectively.
Five anthropometric measurements (length, weight, and arm, head, and chest circumferences) were evaluated for their ability to predict the risk of neonatal and infant mortality across their birth measurement range to identify cutoffs optimized for each outcome by logistic regression, accounting for residential neighborhood (“sector,” a study-defined community cluster). Cutoffs were tested incrementally of 10 g for birth weight and 0.1 cm for length and each circumferential measurement. Using the STATA command of “senspec,” we estimated sensitivity and specificity at each increment of 10 g for birth weight, 0.1 cm for length, and 0.1 cm for MUAC, HC, and CC across their measurement range. Based on these estimates, we calculated PPVs and negative predictive values. A Youden Index was calculated (sensitivity + specificity – 1), representing the likelihood of measurement being below a specified cutoff in newborns who subsequently died compared with those who survived the neonatal period and first year of life (35). The cutoff for each measurement yielding the highest Youden Index was defined as optimal. Receiver operating characteristic (ROC) curves (36) for sensitivity compared with 1 – specificity were produced for each measurement.
We harmonized the optimal cutoffs by investigator consensus for both neonatal and infant mortality for each measurement as an approach to facilitating application of findings from this study in health care and community assessment settings. Sensitivity and specificity for the combinations of 2 measurements were calculated by 2 × 2 contingency tables (37). Neonatal and infant mortality rates and RRRs based on generalized linear models (38) were calculated for harmonized anthropometric cutoffs for each measurement and combinations of 2 measurements.
A Nelson–Aalen cumulative hazard function was used to draw cumulative hazard curves for neonatal and infant mortality according to each measurement's harmonized cutoff (39).
Results
This analysis examines the vital experience of 21,174 singletons measured at a median (IQR) age of 16 (8–42) h, among whom 583 died in the first year (Figure 1). A total of 6852 infants were excluded due to having died before the home visit or otherwise not having been assessed within 72 h, groups whose maternal and household characteristics are summarized in Supplemental Table 1. Within the analytic cohort, mothers of deceased compared with surviving infants were slightly less educated, literate, and wealthy, as assessed by a locally constructed Living Standards Index (40) (Table 1).
TABLE 1.
Maternal and household characteristics of singletons assessed by anthropometry ≤72 h after birth, by vital status during infancy1
| Characteristic | Total (n = 21,174) | Died | Alive (n = 20,591) | P value4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Neonatal2 (n = 328) | Postneonatal3 (n = 255) | ||||||||
| n | n (%) or mean ± SD | n | n (%) or mean ± SD | n | n (%) or mean ± SD | n | n (%) or mean ± SD | ||
| Age, y | 21,163 | 23.1 ± 5.7 | 328 | 22.6 ± 5.7 | 255 | 23.0 ± 6.0 | 20,580 | 23.1 ± 5.6 | 0.13 |
| Education, y | 21,146 | 4.7 ± 3.4 | 327 | 3.5 ± 3.3 | 253 | 4.0 ± 3.4 | 20,566 | 4.7 ± 4.0 | <0.001 |
| Literacy | 21,150 | 12,616 (59.7) | 327 | 177 (54.1) | 253 | 122 (48.2) | 20,570 | 12,317 (59.9) | < 0.001 |
| Reproductive history | |||||||||
| Parity | 21,153 | 327 | 253 | 20,573 | |||||
| 0 | 6994 (33.1) | 159 (48.6) | 90 (35.6) | 6745 (32.8) | < 0.001 | ||||
| 1–3 | 13,047 (61.7) | 145 (44.3) | 135 (53.4) | 12,767 (62.1) | |||||
| ≥4 | 1112 (5.3) | 23 (7.0) | 28 (11.1) | 1061 (5.2) | |||||
| ≥1 Previous fetal loss | 21,174 | 4239 (20.0) | 327 | 59 (18.0) | 255 | 34 (13.3) | 20,591 | 4146 (20.1) | < 0.001 |
| ≥1 Previous infant death | 21,174 | 3149 (14.9) | 328 | 55 (16.8) | 255 | 53 (20.8) | 20,591 | 3041 (14.8) | < 0.001 |
| Place of delivery | 21,160 | 327 | 255 | 20,578 | 0.012 | ||||
| Home | 19,191 (90.7) | 297 (90.8) | 245 (96.1) | 18,649 (90.6) | |||||
| Facility | 1933 (9.1) | 28 (8.6) | 10 (3.9) | 1895 (9.2) | |||||
| Elsewhere | 36 (0.2) | 2 (0.6) | 0 (0.0) | 34 (0.2) | |||||
| Gestational age, wk | 20,262 | 38.77 ± 2.93 | 307 | 36.06 ± 4.39 | 242 | 37.56 ± 3.55 | 19,713 | 38.82 ± 2.87 | < 0.001 |
| Household characteristics | |||||||||
| Electricity | 21,151 | 4143 (19.6) | 328 | 51 (15.6) | 253 | 40 (15.8) | 20,571 | 4052 (19.7) | 0.06 |
| Living standards index5 | 21,141 | –0.07 ± 0.94 | 327 | –0.34 ± 0.81 | 253 | –0.37 ± 0.84 | 20,561 | –0.07 ± 0.94 | < 0.001 |
Missing data out of possible n = 328, 255, and 20,591 in neonatal deaths, postneonatal deaths, and alive infants, respectively, are as follows: age (n = 0, n = 0, n = 11), literacy (n = 11, n = 2, n = 21), education (n = 1, n = 2, n = 25), parity (n = 1, n = 2, n = 18), previous fetal loss (n = 1, n = 0, n = 0), previous infant death (n = 0, n = 0, n = 0), electricity (n = 0, n = 2, n = 20), and living standards index (n = 1, n = 2, n = 30)
Deaths were classified as neonatal if they occurred ≤28 d after birth.
Deaths were classified as postneonatal if they occurred 29–365 d after birth.
P values based on χ2 test for categorical variables and 1-factor ANOVA for continuous distributions across mutually exclusive groups (neonatal, postneonatal, and alive).
Based on derived index for rural northwestern Bangladesh (40).
Birth length, weight, MUAC, HC, CC, and Ponderal Index were lowest in newborns who died as neonates, next lowest in newborns who survived their first month but died postneonatally, and highest in infants who survived their first year. Rates of preterm birth and low birth weight were 54%, 33%, and 19% and 75%, 63%, and 42% among the 3 groups, respectively. Approximately 63% of infants in each group were small for gestational age (Table 2). Proportionality across birth size measurements was evident in correlations that ranged from 0.34 (for HC-WLZ) to 0.88 (for CC-weight), a notable exception being a lack of discernable correlation between WLZ and either length or LAZ (Supplemental Table 2).
TABLE 2.
Newborn and maternal size of singletons measured ≤72 h after birth, by infant vital status at 365 d1
| Characteristic | Total (n = 21,174) | Died | Alive (n = 20 591) | P value4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Neonatal2 (n = 328) | Postneonatal3 (n = 255) | ||||||||
| n | n (%) or mean ± SD | n | n (%) or mean ± SD | n | n (%) or mean ± SD | n | n (%) or mean ± SD | ||
| Male | 21,174 | 10,798 (51.0) | 328 | 176 (53.7) | 255 | 122 (47.8) | 20, 591 | 10,500 (51.0) | 0.378 |
| Age at birth anthropometry, h | 21,174 | 15.0 ± 12.6 | 328 | 14.4 ± 12.0 | 255 | 15.1 ± 12.2 | 20, 591 | 15.1 ± 12.6 | 0.626 |
| Birth size5 | |||||||||
| Length, cm | 20,773 | 46.57 ± 2.22 | 287 | 43.25 ± 3.75 | 243 | 45.28 ± 2.80 | 20, 243 | 46.63 ± 2.14 | < 0.001 |
| Weight, g | 21,172 | 2558 ± 410 | 328 | 1996 ± 611 | 255 | 2316 ± 465 | 20, 589 | 2570 ± 399 | < 0.001 |
| MUAC, cm | 21,127 | 9.51 ± 0.84 | 309 | 8.55 ± 1.13 | 254 | 9.14 ± 0.97 | 20, 564 | 9.53 ± 0.82 | < 0.001 |
| Head circumference, cm | 21,007 | 32.59 ± 1.54 | 307 | 30.42 ± 2.75 | 249 | 31.83 ± 1.95 | 20, 451 | 32.63 ± 1.48 | < 0.001 |
| Chest circumference, cm | 21,042 | 30.82 ± 2.04 | 305 | 27.76 ± 3.21 | 249 | 29.76 ± 2.36 | 20, 488 | 30.88 ± 1.98 | < 0.001 |
| Ponderal index6 | 20,773 | 25.22 ± 2.42 | 287 | 24.17 ± 2.94 | 243 | 24.76 ± 2.38 | 20, 243 | 25.24 ± 2.40 | < 0.001 |
| Preterm (GA <37 wk) | 20,262 | 4011 (19.8) | 307 | 166 (54.1) | 242 | 80 (33.1) | 19, 713 | 3765 (19.1) | < 0.001 |
| Low birth weight (<2500 g) | 21,172 | 9084 (42.9) | 328 | 247 (75.3) | 255 | 161 (63.1) | 20, 589 | 8676 (42.1) | < 0.001 |
| Small for gestational age7 | 20,260 | 12 884 (63.6) | 307 | 192 (62.5) | 242 | 151 (62.4) | 19, 711 | 12 541 (63.6) | 0.859 |
| Maternal size8 | |||||||||
| Weight, kg | 21,092 | 43.07 ± 6.01 | 325 | 41.71 ± 5.47 | 253 | 41.83 ± 5.79 | 20, 514 | 43.11 ± 6.01 | < 0.001 |
| Height, cm | 21,083 | 149.58 ± 5.19 | 326 | 148.60 ± 5.49 | 252 | 148.49 ± 5.61 | 20, 505 | 149.61 ± 5.17 | < 0.001 |
| MUAC, cm | 21,100 | 23.39 ± 2.15 | 326 | 22.97 ± 1.93 | 253 | 23.02 ± 2.07 | 20, 521 | 23.40 ± 2.16 | < 0.001 |
GA, gestational age; MUAC, midupper-arm circumference.
Deaths were classified as neonatal if they occurred ≤28 d after birth.
Deaths were classified as postneonatal if they occurred 29–365 days after birth.
P values based on χ2 test for categorical variables and 1-factor ANOVA for continuous distributions across mutually exclusive groups (neonatal, postneonatal, and alive).
Missing values for not measurement or out of biologically acceptable range: n = 401 in length, n = 2 in weight, n = 47 in MUAC, n = 167 in head circumference, and n = 132 in chest circumference.
Ponderal index is an indicator of wasting or adequacy of weight adjusted for length calculated as weight in kilograms divided by height in meters cubed (50).
Post hoc analysis with small for gestational age defined as a birth weight <10th percentile of an Intergrowth-21st standard growth reference (51).
Maternal anthropometry collected at time of enrollment late in the first or early in the second trimester of pregnancy.
Mothers of infants who died throughout the first year were ∼1.35 kg lower in weight and ∼1 cm shorter in height than mothers of surviving infants (Table 2).
The ROC analysis revealed comparable discriminatory power to predict mortality across all anthropometric measurements for neonatal (AUC = 0.733–0.770) and infant (AUC = 0.683–0.713) mortality (Figure 2). The optimal cutoffs for the five anthropometric measures, based on the Youden Index (sensitivity + specificity – 1) for neonatal mortality, were 44.4 cm for length, 2220 g for weight, 8.8 cm for MUAC, 30.9 cm for HC, and 28.5 cm for CC (Table 3). For infant mortality through 365 days, corresponding cutoffs were similar to those for neonatal mortality: 44.9 cm for length, 2220 g for weight, 9.0 cm for MUAC, 30.9 cm for HC, and 28.5 cm for CC, representing differences of 0.5 cm, 0 g, 0.2 cm, 0 cm, and 0 cm, respectively. Thus, the cutoffs were harmonized to 44.5 cm for length, 2200 g for weight, 9.0 cm for MUAC, 31.0 cm for HC, and 28.5 cm for CC to facilitate adoption of 1 cutoff to predict either neonatal or infant mortality. Specificity and PPV for neonatal mortality were highest for CC (88.5% and 11.3%) and HC (86.8% and 8.7%), followed by length (84.6% and 7.1%), weight (82.7% and 6.8%), and lowest for MUAC (73.0% and 3.7%). The same order in measurement specificity and PPV was observed for infant mortality, with values ranging from 88.7% to 73.2% and 16.3% to 6.4%, respectively (Table 3).
FIGURE 2.
Receiver operating characteristic (ROC) for birth anthropometric measures as predictors of (A) neonatal and (B) infant mortality in singletons measured ≤72 h after birth (n = 21,174). CC, chest circumference; HC, head circumference; MUAC, midupper arm circumference.
TABLE 3.
Sensitivity, specificity, positive and negative predictive values, and AUC for predicting neonatal and infant mortality using optimal cutoffs for anthropometric measures in singletons measured ≤72 h after birth (n = 21,174)1
| Characteristic | Cutoff | Total | No. died | Alive | Rate2 | Sensitivity | Specificity | Youden Index | PPV3 | NPV4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neonatal mortality (≤8 d) | |||||||||||
| Length, cm | Optimal | (44.4) | 20,773 | 287 | 20,486 | 20.1 | 60.0 | 85.9 | 45.9 | 8.0 | 99.1 |
| Harmonized | 44.5 | 20,773 | 287 | 20,486 | 19.2 | 60.6 | 84.6 | 45.2 | 7.1 | 99.1 | |
| Weight, g | Optimal | (2220) | 21,172 | 328 | 20,844 | 19.6 | 61.3 | 81.4 | 42.7 | 6.2 | 99.1 |
| Harmonized | 2200 | 21,172 | 328 | 20,844 | 20.8 | 59.5 | 82.7 | 42.2 | 6.8 | 99.0 | |
| MUAC, cm | Optimal | (8.8) | 21,127 | 309 | 20,818 | 20.3 | 58.3 | 80.6 | 38.9 | 5.9 | 98.9 |
| Harmonized | 9.0 | 21,127 | 309 | 20,818 | 15.8 | 65.0 | 73.0 | 38.0 | 3.7 | 99.2 | |
| HC, cm | Optimal | (30.9) | 21,007 | 307 | 20,700 | 24.5 | 53.4 | 88.7 | 42.1 | 10.6 | 98.7 |
| Harmonized | 31.0 | 21,007 | 307 | 20,700 | 22.9 | 53.7 | 86.8 | 40.5 | 8.7 | 98.8 | |
| CC, cm | Optimal | (28.5) | 21,042 | 305 | 20,737 | 25.6 | 55.7 | 88.5 | 44.2 | 11.3 | 98.7 |
| Harmonized | 28.5 | 21,042 | 305 | 20,737 | 25.6 | 55.7 | 88.5 | 44.2 | 11.3 | 98.7 | |
| Infant mortality (≤365 d) | |||||||||||
| Length, cm | Optimal | (44.9) | 20,773 | 530 | 20,243 | 32.6 | 54.0 | 81.3 | 35.3 | 8.9 | 98.1 |
| Harmonized | 44.5 | 20,773 | 530 | 20,243 | 37.9 | 50.0 | 84.8 | 34.8 | 11.5 | 97.7 | |
| Weight, g | Optimal | (2220) | 21,172 | 583 | 20,589 | 38.2 | 52.3 | 81.8 | 34.1 | 10.2 | 97.8 |
| Harmonized | 2200 | 21,172 | 583 | 20,589 | 40.0 | 50.9 | 83.0 | 33.9 | 11.1 | 97.6 | |
| MUAC, cm | Optimal | (9.0) | 21,127 | 563 | 20,564 | 31.8 | 56.0 | 73.2 | 29.2 | 6.4 | 98.1 |
| Harmonized | 9.0 | 21,127 | 563 | 20,564 | 31.8 | 56.0 | 73.2 | 29.2 | 6.4 | 98.1 | |
| HC, cm | Optimal | (30.9) | 21,007 | 556 | 20,451 | 44.9 | 42.4 | 88.9 | 31.3 | 15.3 | 97.0 |
| Harmonized | 31.0 | 21,007 | 556 | 20,451 | 42.7 | 43.7 | 87.0 | 30.7 | 13.0 | 97.2 | |
| CC, cm | Optimal | (28.5) | 21,042 | 554 | 20,488 | 47.8 | 43.9 | 88.7 | 32.6 | 16.3 | 96.9 |
| Harmonized | 28.5 | 21,042 | 554 | 20,488 | 47.8 | 43.9 | 88.7 | 32.6 | 16.3 | 96.9 | |
Optimal cutoff points based off Youden Index (35). CC, chest circumference; HC, head circumference; MUAC, midupper-arm circumference; NPV, negative predictive value; PPV, positive predictive value.
Deaths per 1000 live births.
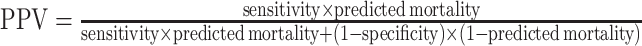
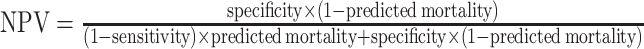
Among infants with birth measurements below the 5 harmonized cutoffs, the highest neonatal mortality risk was associated with a newborn CC <28.5 cm (RRR = 9.68; 95% CI: 7.84, 11.94), followed by HC <31.0 cm (8.49; 6.86,10.49), length < 44.5cm (8.66; 6.83, 10.97), weight <2200 g (6.57; 5.31, 8.13), and MUAC <9.0 cm (5.01; 4.03,6.23) (Table 4). Rates of mortality to 365 days were higher and RRR lower (range: 3.40–6.02) for newborns with birth measurements below the same respective cutoffs; however, RRRs were in the same order as observed for neonatal mortality. Notably, neonatal mortality rate and infant mortality rate were within very narrow ranges among newborns whose measurements were greater than or equal to harmonized cutoffs, at 6.5–7.9 and 15.3–17.3 per 1000 live births, respectively.
TABLE 4.
Relative risk ratio of neonatal and infant mortality (≤365 d) for harmonized anthropometric cutoffs1
| Measurement cutoff | Neonatal mortality (≤28 d) | Infant mortality (≤365 d) | ||
|---|---|---|---|---|
| Mortality rate2 (number of deaths/number of measures) | Relative risk ratio3 (95% CI) | Mortality rate2 (number of deaths/number of measures) | Relative risk ratio3 (95% CI) | |
| Length (reference: ≥44.5 cm) | 6.5 (115/17,713) | 1.00 | 15.3 (271/17,713) | 1.00 |
| <44.5 cm | 56.2 (172/3060) | 8.66 (6.83, 10.97) | 84.6 (259/3060) | 5.53 (4.69, 6.53) |
| Weight (reference: ≥2200 g) | 7.9 (138/17,504) | 1.00 | 16.7 (293/17504) | 1.00 |
| <2200 g | 51.8 (190/3668) | 6.57 (5.31, 8.13) | 79.1 (290/3668) | 4.72 (4.06, 5.50) |
| MUAC (reference: ≥9.0 cm) | 7.6 (123/16,229) | 1.00 | 17.1 (278/16,229) | 1.00 |
| <9.0 cm | 38.0 (186/4898) | 5.01 (4.03, 6.23) | 58.2 (285/4898) | 3.40 (2.89, 3.99) |
| HC (reference: ≥31.0 cm) | 7.7 (143/18,506) | 1.00 | 17.3 (320/18,506) | 1.00 |
| <31.0 cm | 65.6 (164/2501) | 8.49 (6.86, 10.49) | 94.4 (236/2501) | 5.46 (4.63, 6.43) |
| CC (reference: ≥28.5 cm) | 7.4 (139/18,731) | 1.00 | 17.0 (318/18,731) | 1.00 |
| <28.5 cm | 71.8 (166/2311) | 9.68 (7.84, 11.94) | 102.1 (236/2311) | 6.02 (5.15, 7.02) |
Missing values for not measurement or out of biologically acceptable range: n = 401 in length, n = 2 in weight, n = 47 in MUAC, n = 167 in HC, and n = 132 in CC. CC, chest circumference; HC, head circumference; MUAC, midupper-arm circumference.
Number of deaths per 1000 live births.
Relative risk ratio was produced by general linear models.
Figure 3 presents Nelson–Aalen cumulative hazard curves depicting the mortality experience of infants by the number of days since assessment for each measurement, revealing the sharpest rise in mortality within 60 d. The highest sustained mortality rate thereafter is in infants whose CC was <28.5 cm compared with higher, relative to other measurements at their harmonized cutoffs. Figure 3 also reveals the far more uniformly favorable and comparable survival of infants whose values were above the harmonized cutoffs for all birth measurements.
FIGURE 3.
Nelson–Aalen cumulative hazard curves for infants through age 12 mo (365 d) by harmonized cutoffs at birth for length, weight, and midupper arm circumference (MUAC), head circumference (HC), and chest circumference (CC).
Given the opportunity to measure multiple anthropometric measurements, we also explored if any 2 measures at harmonized cutoffs might further improve the prediction of mortality risk (Table 5andSupplemental Table 3). Risk and predictability of neonatal mortality was highest among children with both an HC <31.0 cm and a CC <28.5 cm (RRR = 15.74; 95% CI: 12.54, 19.75; PPV = 10.7%), relative to children whose HC and CC were both at or above these respective cutoffs. A combined length <44.5 cm and CC <28.5 cm identified newborns at the next highest risk of neonatal mortality, followed by a length <44.5 cm and HC <31.0 cm. Except for a combined MUAC <9.0 cm and weight <2200 g, all other paired measurements below their harmonized cutoffs were associated with RRR ≥10 compared with having both measures above their respective cutoffs. Risk of infant mortality to 365 d associated with paired measurements largely mirrored those observed with neonatal mortality, although with higher PPVs. The highest risk was evident among newborns with an HC <31.0 cm plus CC <28.5 cm (RRR = 9.19; 95% CI: 7.72, 10.95; PPV = 14.5%), followed by a combination of length <44.5 cm plus CC <28.5 cm and length <44.5 cm plus HC <31.0 cm.
TABLE 5.
Relative risk ratio (95% CI) and positive predictive values of neonatal (≤28 d) and infant mortality (≤365 d) using harmonized cutoffs for any 2 anthropometric measurements combined1
| Characteristic | Length ≥44.5 cm | Length <44.5 cm | Weight ≥2200 g | Weight <2200 g | MUAC ≥9.0 cm | MUAC <9.0 cm | HC ≥31.0 cm | HC <31.0 cm |
|---|---|---|---|---|---|---|---|---|
| Neonatal mortality (≤28 d) | ||||||||
| Length ≥44.5 cm | — | — | ||||||
| Length < 44.5 cm | — | — | ||||||
| Weight ≥2200 g | 1.00 (reference) | 3.19 (1.93, 5.28), 2.0 | — | — | ||||
| Weight <2200 g | 1.29 (0.70, 2.41), 0.8 | 10.99 (8.60, 14.06), 7.0 | — | — | ||||
| MUAC ≥9.0 cm | 1.00 (reference) | 3.30 (2.04, 5.33), 2.1 | 1.00 (reference) | 2.58 (1.44, 4.59), 1.8 | — | — | ||
| MUAC <9.0 cm | 1.28 (0.77, 2.14), 0.8 | 11.43 (8.93, 14.63), 7.1 | 1.68 (1.04, 2.73), 1.2 | 8.00 (6.37, 10.05), 5.6 | — | — | ||
| HC ≥31.0 cm | 1.00 (reference) | 3.30 (2.15, 5.05), 2.1 | 1.00 (reference) | 1.93 (1.26, 2.97), 1.4 | 1.00 (reference) | 1.83 (1.24, 2.70), 1.24 | — | — |
| HC <31.0 cm | 1.79 (0.93, 3.44), 1.1. | 14.34 (11.24, 18.28), 9.0 | 1.61 (0.78, 3.31), 1.14 | 12.26 (9.75, 15.43), 8.7 | 2.84 (1.68, 4.82), 1.93 | 12.24 (9.68, 15.47), 8.3 | — | — |
| CC ≥28.5 cm | 1.00 (reference) | 3.20 (2.13, 4.81), 2.0 | 1.00 (reference) | 1.75 (0.57, 5.42), 1.2 | 1.00 (reference) | 1.33 (0.85, 2.10), 1.6 | 1.00 (reference) | 2.29 (1.39, 3.76), 1.56 |
| CC <28.5 cm | 2.18 (1.11, 4.28), 1.3 | 14.57 (11.41, 18.60), 9.0 | 1.54 (0.92, 2.56), 1.1 | 11.09 (8.89, 13.83), 7.9 | 2.31 (0.97, 5.51), 0.9 | 10.99 (8.79, 13.75), 7.8 | 3.35 (2.16, 5.20), 2.3 | 15.74 (12.54,19.75), 10.7 |
| Infant mortality (≤365 d) | ||||||||
| Length ≥44.5 cm | — | — | ||||||
| Length <44.5 cm | — | — | ||||||
| Weight ≥2200 g | 1.00 (reference) | 2.60 (1.79, 3.79), 3.8 | — | — | ||||
| Weight <2200 g | 1.58 (1.09, 2.30), 2.3 | 6.98 (5.88, 8.27), 10.2 | — | — | ||||
| MUAC ≥9.0 cm | 1.00 (reference) | 2.92 (2.12, 4.02), 4.3 | 1.00 (reference) | 2.61 (1.83, 3.71), 4.2 | — | — | ||
| MUAC <9.0 cm | 1.25 (0.92, 1.71), 1.9 | 6.98 (5.84, 8.35), 10.3 | 1.27 (0.90, 1.81), 2.0 | 5.34 (4.51, 6.31), 8.5 | — | — | ||
| HC ≥31.0 cm | 1.00 (reference) | 2.84 (2.15, 3.76), 4.2 | 1.00 (reference) | 2.15 (1.67, 2.77), 3.3 | 1.00 (reference) | 1.68 (1.30, 2.17), 2.6 | — | — |
| HC <31.0 cm | 1.75 (1.15, 2.65), 2.6 | 8.47 (7.08, 10.13), 12.5 | 1.92 (1.22, 3.03), 3.0 | 7.70 (6.46, 9.17), 12.0 | 2.82 (1.99, 3.99), 4.4 | 7.34 (6.13, 8.81), 11.4 | — | — |
| CC ≥28.5 cm | 1.00 (reference) | 2.89 (2.17, 3.84), 2.0 | 1.00 (reference) | 2.11 (1.06, 4.19), 3.3 | 1.00 (reference) | 1.34 (1.00, 1.80), 4.3 | 1.00 (reference) | 2.15 (1.54, 3.00), 3.4 |
| CC <28.5 cm | 2.49 (1.67, 3.70), 1.3 | 8.62 (7.23, 10.27), 9.0 | 1.96 (1.45, 2.65), 3.1 | 7.01 (5.94, 8.25), 11.0 | 2.63 (1.56, 4.45), 2.2 | 6.73 (5.70, 7.96), 10.9 | 2.77 (2.05, 3.72), 4.4 | 9.19 (7.72, 10.95), 14.5 |
Missing values for not measurement or out of biologically acceptable range: n = 401 in length, n = 2 in weight, n = 47 in MUAC, n = 167 in HC, and n = 132 in CC. Values are relative risk ratio (95% CI), positive predictive value (%). Relative risk ratio was produced by general linear models. CC, chest circumference; HC, head circumference; MUAC, midupper-arm circumference.
Discussion
This study assessed the discriminatory power of anthropometry taken within 72 h of birth to predict all-cause neonatal and infant mortality in a population cohort of over 21,000 singletons in rural Bangladesh. We identified best cutoffs, harmonized to predict both neonatal and infant mortality, to be 44.5 cm for length, 2200 g for weight, and 9.0 cm, 31.0 cm, and 28.5 cm for midupper arm, head, and chest circumferences, respectively.
Specificity, a metric that reflects the percentage of correctly classified infants who survived to 28 and 365 d, ranged from 83% to 88% for all birth measurements except for MUAC, for which specificity was 73% for both outcomes. RRRs for both mortality rates below their harmonized cutoffs were highest for chest circumference, followed by length and head circumference, which were comparable, weight, and lastly MUAC. Positive predictive value, which reflects the percentage of infants with measurements below a harmonized cutoff who died, was highest for chest circumference, predicting 11% and 16% of all neonatal and total infant deaths, followed by head circumference, length, weight, and MUAC. These findings emphasize the potential of all assessed birth measurements to identify infants at risk of dying during the first year of life; however, a novel finding is the superior predictive power of a chest circumference, a rarely measured dimension obtainable with an insertion tape (8). Superiority of this measurement in predicting infant mortality might be reflecting anatomic specificity to the size of the chest cavity and consequent lung size and health (41), noting the importance of acute respiratory infections as major causes of death in infancy (42).
A second novel aspect of this study was to examine the capability of 2 newborn measurements to predict infant mortality. Every index comprising both measurements lying below their respective, harmonized cutoffs was associated with a higher RRR (range: 5.34–9.19) than either single measurement in a pair (range: 3.40–6.02). The strongest predictive value emerged from an index that combined newborn chest and head circumferences at cutoffs of <31.0 cm and <28.5 cm that, when compared with infants with measured values above both cutoffs, yielded the highest RRR for neonatal mortality of 15.74. The same pair of circumferential measurements remained superior in predicting infant mortality through 12 mo of age (RRR = 9.19). Importantly, both of these measurements are taken with a single, extended insertion tape (8). Newborns with a discordant classification, whereby only 1 of 2 paired measurements were below a harmonized cutoff, were either slightly higher or comparable in risk of mortality than infants for whom both paired measurements were above their respective cutoffs. These findings are consistent with reports elsewhere showing symmetric compared with asymmetric fetal growth restriction posing a higher risk of neonatal mortality (43).
Globally, a weight <2500 g is considered the standard cutoff for classifying a low birth weight (44). Yet, based on AUC, RRR, and PPV analyses, among newborns in this large rural cohort in Bangladesh, a birth weight <2200 g (AUC = 0.67, RRR = 4.72, PPV = 11.1%) appeared superior in predicting newborn mortality than a birth weight <2500 g (AUC = 0.64, RRR = 3.10, PPV = 3.30) (data not shown), suggesting that local adjustment of a birth weight cutoff may be preferred to the conventional cutoff when screening newborns for mortality risk.
MUAC was surprisingly less predictive of mortality than either chest or head circumference, given a low MUAC (<9.0 cm) at birth has been shown to be comparable to low birth weight in predicting neonatal mortality in India (16) and Guatemala (17). Furthermore, in Nepal, MUAC measured throughout early infancy (45) and the preschool years (46) has been strongly associated with the risk of mortality. However, none of these studies compared the predictiveness of MUAC with other concurrently assessed circumferential measurements.
The data collected in this study provided an opportunity to concurrently assess the performance of multiple dimensions of birth size, obtained by highly standardized methods, in predicting infant mortality in a large, population-based birth cohort setting typical of rural Bangladesh (32). Cutoffs and associated levels of risk for neonatal and infant mortality were based on actual, unadjusted measurement distributions, facilitating their direct adoption by primary care workers to use the harmonized cutoffs for screening high-risk infants.
Our study was large, population based, highly standardized, and conducted in an area that typifies rural Bangladesh (33). However, there are limitations to note. Most important, while reaching newborns for anthropometry within 72 h is a common research practice in remote settings where most births occur at home (47), our study reveals the vital consequence of any delay in reaching newborns. Although staff reached infants ∼16 h after birth, including one-fourth within 8 h, 653 infants died prior to the home visit, comprising 63% of all neonatal deaths. Consequently, as very early neonatal deaths are most likely to occur among preterm and growth-restricted infants (48, 49), mortality risks associated with newborn size derived from this analysis are likely to be underestimates, although relevant for health care services reaching newborns the day after birth or later. On the other hand, our findings that mothers of infants who died shortly after birth, and thus not measured, were younger and shorter, were more likely to be preterm, and had obstructed labor and delivered in a health facility (Supplemental Table 1) suggests that missed birth anthropometry in the home can be partially compensated by incorporating standardized birth assessment in health facilities.
In conclusion, although weight is the most frequent and, usually, the only measurement taken at birth, we demonstrate in a rural Bangladesh setting that measurements of length and head, chest, and arm circumference can also be deployed with harmonized, optimized cutoffs to assess risk of mortality throughout infancy. Furthermore, combining information from any 2 measurements can further enhance mortality risk prediction. Among measurements tested in this study, chest circumference, alone and in combination with head circumference, best predicted neonatal and infant mortality.
Supplementary Material
Acknowledgments
We thank the ∼700 staff in the JiVitA Project. Katherine Healy conducted an early preliminary mortality analysis for the article. Sucheta Mehra provided training and oversight of field workers.
The authors’ contributions were as follows—YK: analyzed the data and wrote the paper; LSFU: prepared the database and assisted in data analysis; SS: trained and standardized anthropometrists; SS, HA, and AAS: oversaw the conduct of all field research; PC and AL: codesigned the study, secured funding, and provided critical edits; KPW: codesigned the study, secured funding, cowrote the paper, and had primary responsibility for final content; and all authors read and approved the final manuscript.
Author disclosures: The authors report no conflicts of interest.
Notes
Supported by the Bill & Melinda Gates Foundation (grants GH614 and OPP1441435) and the Sight and Life Global Nutrition Research Institute.
PC is an Associate Editor of AJCN and played no role in the journal's evaluation of the manuscript.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CC, chest circumference; HC, head circumference; LAZ, length-for-age z score; MUAC, midupper arm circumference; PPV, positive predictive values; ROC, receiver operating characteristic; RRR, relative risk ratio; WLZ, weight-for-length z score.
Contributor Information
Yunhee Kang, Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Lee Shu Fune Wu, Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Saijuddin Shaikh, The JiVitA Project, Gaibandha, Bangladesh.
Hasmot Ali, The JiVitA Project, Gaibandha, Bangladesh.
Abu Ahmed Shamim, The JiVitA Project, Gaibandha, Bangladesh; James P Grant School of Public Health, BRAC University, Dhaka, Bangladesh.
Parul Christian, Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Alain Labrique, Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Keith P West, Jr, Center for Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval by the authors.
References
- 1. Garza C. Fetal, neonatal, infant, and child international growth standards: an unprecedented opportunity for an integrated approach to assess growth and development. Adv Nutr. 2015;6(4):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vilanova CS, Hirakata VN, de Souza Buriol VC, Nunes M, Goldani MZ, da Silva CH. The relationship between the different low birth weight strata of newborns with infant mortality and the influence of the main health determinants in the extreme south of Brazil. Population Health Metrics. 2019;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yasmin S, Osrin D, Paul E, Costello A. Neonatal mortality of low-birth-weight infants in Bangladesh. Bull World Health Organ. 2001;79(7):608–14. [PMC free article] [PubMed] [Google Scholar]
- 4. Semba RD, Bloem MW, Piot Peds. Nutrition and health in developing countries nutrition and health series. Humana Press; 2008. [Google Scholar]
- 5. Mwangome M, Ngari M, Bwahere P, Kabore P, McGrath M, Kerac M, Berkley JA. Anthropometry at birth and at age of routine vaccination to predict mortality in the first year of life: a birth cohort study in Bukinafaso. PLoS One. 2019;14(3):e0213523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melve KK, Gjessing HK, Skjaerven R, Oyen N. Infants' length at birth: an independent effect on perinatal mortality. Acta Obstet Gynecol Scand. 2000;79(6):459–64. [PubMed] [Google Scholar]
- 7. Morris SS, Victora CG, Barros FC, Halpern R, Menezes AM, Cesar JA, Horta BL, Tomasi E. Length and ponderal index at birth: associations with mortality, hospitalizations, development and post-natal growth in Brazilian infants. Int J Epidemiol. 1998;27(2):242–7. [DOI] [PubMed] [Google Scholar]
- 8. Zerfas AJ. The insertion tape: a new circumference tape for use in nutritional assessment. Am J Clin Nutr. 1975;28(7):782–7. [DOI] [PubMed] [Google Scholar]
- 9. Bliss J, Lelijveld N, Briend A, Kerac M, Manary M, McGrath M, Weise Prinzo Z, Shepherd S, Marie Zagre N, Woodhead Set al. Use of mid-upper arm circumference by novel community platforms to detect, diagnose, and treat severe acute malnutrition in children: a systematic review. Global Health Sci Pract. 2018;6(3):552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tessema M, Laillou A, Tefera A, Teklu Y, Berger J, Wieringa FT. Routinely MUAC screening for severe acute malnutrition should consider the gender and age group bias in the Ethiopian non-emergency context. PLoS One. 2020;15(4):e0230502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mwangome MK, Fegan G, Fulford T, Prentice AM, Berkley JA. Mid-upper arm circumference at age of routine infant vaccination to identify infants at elevated risk of death: a retrospective cohort study in the Gambia. Bull World Health Organ. 2012;90(12):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taneja S, Rongsen-Chandola T, Mohan SB, Mazumder S, Bhandari N, Kaur J, Arya N, Chowdhury R, Martines JC, Bahl Ret al. Mid upper arm circumference as a predictor of risk of mortality in children in a low resource setting in India. PLoS One. 2018;13(6):e0197832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasmussen J, Andersen A, Fisker AB, Ravn H, Sodemann M, Rodrigues A, Benn CS, Aaby P. Mid-upper-arm-circumference and mid-upper-arm circumference z-score: the best predictor of mortality?. Eur J Clin Nutr. 2012;66(9):998–1003. [DOI] [PubMed] [Google Scholar]
- 14. Hadush MY, Berhe AH, Medhanyie AA. Foot length, chest and head circumference measurements in detection of low birth weight neonates in Mekelle, Ethiopia: a hospital based cross sectional study. BMC Pediatr. 2017;17(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thi HN, Khanh DK, Thu Hle T, Thomas EG, Lee KJ, Russell FM. Foot length, chest circumference, and mid upper arm circumference are good predictors of low birth weight and prematurity in ethnic minority newborns in Vietnam: a hospital-based observational study. PLoS One. 2015;10(11):e0142420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed FU, Karim E, Bhuiyan SN. Mid-arm circumference at birth as predictor of low birth weight and neonatal mortality. J Biosoc Sci. 2000;32(4):487–93. [DOI] [PubMed] [Google Scholar]
- 17. Bhargava SK, Ramji S, Kumar A, Mohan M, Marwah J, Sachdev HP. Mid-arm and chest circumferences at birth as predictors of low birth weight and neonatal mortality in the community. BMJ. 1985;291(6509):1617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Vaquera MV, Townsend JW, Arroyo JJ, Lechtig A. The relationship between arm circumference at birth and early mortality. J Trop Pediatr. 1983;29(3):167–74. [DOI] [PubMed] [Google Scholar]
- 19. Dhar B, Mowlah G, Nahar S, Islam N. Birth-weight status of newborns and its relationship with other anthropometric parameters in a public maternity hospital in Dhaka, Bangladesh. J Health Popul Nutr. 2002;20(1):36–41. [PubMed] [Google Scholar]
- 20. Das JC, Afroze A, Khanam ST, Paul N. Mid-arm circumference: an alternative measure for screening low birth weight babies. Bangladesh Med Res Counc Bull. 2005;31(1):1–6. [PubMed] [Google Scholar]
- 21. Taksande A, Vilhekar KY, Chaturvedi P, Gupta S, Deshmukh P. Predictor of low birth weight babies by anthropometry. J Trop Pediatr. 2007;53(6):420–3. [DOI] [PubMed] [Google Scholar]
- 22. Sreeramareddy CT, Chuni N, Patil R, Singh D, Shakya B. Anthropometric surrogates to identify low birth weight Nepalese newborns: a hospital-based study. BMC Pediatr. 2008;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO . WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva (Switzerland): WHO; 2007. [Google Scholar]
- 24. Sindhu KN, Ramamurthy P, Ramanujam K, Henry A, Bondu JD, John SM, Babji S, Koshy B, Bose A, Kang Get al. Low head circumference during early childhood and its predictors in a semi-urban settlement of Vellore, southern India. BMC Pediatr. 2019;19(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SE, West KP Jr, Cole RN, Schulze KJ, Wu LS, Yager JD, Groopman J, Christian P. Novel plasma proteins in Nepalese school-aged children are associated with a small head size at birth. Sci Rep. 2018;8(1):6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gampel SB, Nomura Y. Short and long-term effects of compromised birth weight, head circumference, and Apgar scores on neuropsychological development. J Psychol Abnorm Child. 2014;3(3):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egashira T, Hashimoto M, Shiraishi TA, Shichijo A, Egashira M, Mizukami T, Takayanagi T. A longer body length and larger head circumference at term significantly influences a better subsequent psychomotor development in very-low-birth-weight infants. Brain Dev. 2019;41(4):313–9. [DOI] [PubMed] [Google Scholar]
- 28. Bach CC, Henriksen TB, Larsen RT, Aagaard K, Matthiesen NB. Head circumference at birth and school performance: a nationwide cohort study of 536,921 children. Pediatr Res. 2020;87(6):1112–8. [DOI] [PubMed] [Google Scholar]
- 29. United Nations Inter-agency Group for Child Mortality Estimation (UNIGME) . Levels & trends in child mortality: report 2019, estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York: United Nations Children's Fund; 2019. [Google Scholar]
- 30. National Institute of Population Research and Training (NIPORT), Mitra and Associates, ICF International . Bangladesh Demographic and Health Survey 2017-18: key indicators. Dhaka (Bangladesh): NIPORT, Mitra and Associates, and ICF International; 2016. [Google Scholar]
- 31. Zaman RU, Khaled A, Sabur MA, Islam S, Ahmed S, Varghese J, Sherratt D, Witter S. Experiences of a new cadre of midwives in Bangladesh: findings from a mixed method study. Hum Resources Health. 2020;18(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Labrique AB, Christian P, Klemm RD, Rashid M, Shamim AA, Massie A, Schulze K, Hackman A, West KP Jr. A cluster-randomized, placebo-controlled, maternal vitamin a or beta-carotene supplementation trial in Bangladesh: design and methods. Trials. 2011;12(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. West KP Jr, Shamim AA, Mehra S, Labrique AB, Ali H, Shaikh S, Klemm RD, Wu LS, Mitra M, Haque Ret al. Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA. 2014;312(24):2649–58. [DOI] [PubMed] [Google Scholar]
- 34. WHO . WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland): WHO; 2006. [Google Scholar]
- 35. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. [DOI] [PubMed] [Google Scholar]
- 36. Metz CE, Herman BA, Shen JH. Maximum likelihood estimation of receiver operating characteristic (ROC) curves from continuously-distributed data. Stat Med. 1998;17(9):1033–53. [DOI] [PubMed] [Google Scholar]
- 37. Altman DG, Bland JM. Diagnostic tests. 1: sensitivity and specificity. BMJ. 1994;308(6943):1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hardin J, Hilbe J. Generalized linear models and extensions. 3rd ed. Stata Press. College Station, Texas; 2018. [Google Scholar]
- 39. Aalen O. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6(4):701–26. [Google Scholar]
- 40. Gunnsteinsson S, Labrique AB, West KP Jr, Christian P, Mehra S, Shamim AA, Rashid M, Katz J, Klemm RD. Constructing indices of rural living standards in northwestern Bangladesh. J Health Popul Nutr. 2010;28(5):509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng X, Xu X, Zhang Y, Li W, Huo X. Chest circumference and birth weight are good predictors of lung function in preschool children from an e-waste recycling area. Environ Sci Pollut Res. 2017;24(28):22613–21. [DOI] [PubMed] [Google Scholar]
- 42. McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, Adeloye D, Rudan I, Black RE, Campbell Het al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Global Health. 2019;7(1):e47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction—part 1. J Matern Fetal Neonatal Med. 2016;29(24):3977–87. [DOI] [PubMed] [Google Scholar]
- 44. WHO . [NUTRITION LANDSCAPE INFORMATION SYSTEM (NLiS)]. Low birth weight. Available from: https://www.who.int/data/nutrition/nlis/info/low-birth-weight.
- 45. West KP Jr, Katz J, Shrestha SR, LeClerq SC, Khatry SK, Pradhan EK, Adhikari R, Wu LS, Pokhrel RP, Sommer A. Mortality of infants < 6 mo of age supplemented with vitamin A: a randomized, double-masked trial in Nepal. Am J Clin Nutr. 1995;62(1):143–8. [DOI] [PubMed] [Google Scholar]
- 46. West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. Efficacy of vitamin a in reducing preschool child mortality in Nepal. Lancet North Am Ed. 1991;338(8759):67–71. [DOI] [PubMed] [Google Scholar]
- 47. Saville NM, Shrestha BP, Style S, Harris-Fry H, Beard BJ, Sengupta A, Jha S, Rai A, Paudel V, Pulkki-Brannstrom AMet al. Protocol of the Low Birth Weight South Asia Trial (LBWSAT), a cluster-randomised controlled trial testing impact on birth weight and infant nutrition of participatory learning and action through women's groups, with and without unconditional transfers of fortified food or cash during pregnancy in Nepal. BMC Pregnancy Childbirth. 2016;16(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, Ezzati M, Bhutta ZA, Marchant T, Willey BAet al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382(9890):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, Sania A, Rosen HE, Schmiegelow C, Adair LSet al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ. 2017;358:j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Florey Cdu V. The use and interpretation of ponderal index and other weight-height ratios in epidemiological studies. J Chronic Dis. 1970;23(2):93–103. [DOI] [PubMed] [Google Scholar]
- 51. Villar J, Giuliani F, Bhutta ZA, Bertino E, Ohuma EO, Ismail LC, Barros FC, Altman DG, Victora C, Noble JAet al. Postnatal growth standards for preterm infants: the preterm postnatal follow-up study of the INTERGROWTH-21(st) project. Lancet Global Health. 2015;3(11):e681–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval by the authors.