Abstract
Though T-cell immunosenescence is a major risk factor for age-related diseases, susceptibility to infections, and responses to vaccines, differences in T-cell subset counts and representation by age and sex have not been determined for a large sample representative of the national population of the United States. We evaluated the counts of T-cell subsets including total, CD4+, and CD8+ T cells and their naïve (Tn), effector memory (Tem), and effector subsets, in the context of age, sex, and exposure to cytomegalovirus (CMV) infection among 8 848 Health and Retirement Study participants, a nationally representative study of adults older than 55 years. Total T cells (CD3+) and CD4+ cells declined markedly with age; CD8+ T cells declined somewhat less. While CD4+ T cell declines with age occurred for both CMV-seropositive and CMV-seronegative groups, total T cells and CD8+ cells were both substantially higher among the CMV-seropositive group. Numbers of Tn CD4+ and CD8+ cells were strongly and inversely related to age, were better conserved among women, and were independent of CMV seropositivity. By contrast, accumulation of the CD8+ and CD4+ Tem and effector subsets was CMV-associated. This is the first study to provide counts of T-cell subsets by age and sex in a national sample of US adults older than the age of 55 years. Understanding T-cell changes with age and sex is an important first step in determining strategies to reduce its impact on age-related diseases and susceptibility to infection.
Keywords: Aging, CMV seropositivity, Older adults
An accurate understanding of age-related differences in T-cell subsets and how that relates to the loss of immune function with advancing age is of great interest in assessing population responses to new viruses and vaccine effectiveness. Age-related reduction in T-cell function that results in a poorer response to newly encountered antigens and vaccines is characterized by a decrease in naïve T (Tn) cells and an increase in the numbers of highly differentiated T-effector memory cells (Tem) and effector subsets (1–3). Though several smaller studies, using heterogeneous methods to define T-cell subsets, have established population-specific reference ranges for T-cell subsets, previous studies have not evaluated counts of T-cell subsets in a large representative population of older adults in the United States, particularly not with regard to sex (4–11). Both age and cytomegalovirus (CMV) infection (12,13) play an important role in determining the distribution of T-cell subsets. Because CMV seropositivity increases with age, the small sample sizes in previous studies have made it difficult to separate the independent effects of CMV infection and aging on T-cell subsets. Understanding the independent effects of age, sex, and CMV seropositivity on the distribution of immune cells in a population-representative sample of individuals older than 55 years in the United States is an essential first step toward developing interventions to modulate age-related immunosenescence and to reduce morbidity and mortality in the rapidly increasing older population. Hence, we measured age-sensitive T-cell subsets that included the Tn cells, responsible for defense against new infections that have not been encountered before (eg, severe acute respiratory syndrome, West Nile virus disease, and coronavirus disease 2019) and Tem/effector subsets of CD4+ and CD8+ T cells, responsible for immediate defense against previously encountered infections.
While some previous studies have reported sex differences in T-cell subsets with women having higher numbers of CD4+ cells, these studies are confounded by a lack of measurement of CMV infection. Thus, characterizing differences in T-cell subsets by sex after fully accounting for age and CMV serostatus may point out why men appear less resistant to some infections, have reduced vaccine effectiveness, and suffer worse outcomes when infected (14). We performed detailed immunophenotyping on T lymphocytes and determined exposure to CMV infection in more than 8 800 individuals from the nationally representative Health and Retirement Study (HRS).
Method
The HRS is a nationally representative, prospective study that started in 1992 and currently includes more than 23 000 surviving community-dwelling adults residing in the contiguous United States with oversampling of African Americans and Hispanics. A new cohort of households is added to the sample every 6 years to adjust for aging of the cohort. Both members in coupled households are enrolled as respondents and interviews are conducted with sampled respondents every 2 years, including those who have entered nursing homes. Interviews are conducted by telephone and in-person in both English and Spanish. Additional detail about the HRS is available elsewhere (15–17). We utilized data from the 2016 HRS survey that included venous blood collected from 9 934 participants in 7 227 households during 2016–2017. All panel respondents who completed an HRS interview during the 2016 wave were asked to consent to a venous blood draw except for proxy respondents and nursing home residents. The request was made by their HRS interviewer at the end of the interview in either telephone or in-person modes. Blood was collected in the participants’ homes. At-home blood collections were managed by Hooper Holmes Health & Wellness. A vast majority of the blood samples were collected from HRS participants within 4 weeks of interview completion. Overall, 65% of eligible participants provided a blood sample. These data when weighted provide estimates for a nationally representative sample of Americans older than 55 years. Blood collected in cell preparation tubes (CPTs) was shipped at room temperature to the Advanced Research and Diagnostics Laboratory at the University of Minnesota, and samples were processed within 48 hours of collection. The samples were shipped in shipping containers that were lined with Styrofoam and had foam holders for the CPT. These containers also had 2–3 gel packs kept at room temperature on the outside of the Styrofoam layer (but within the cardboard container) to minimize temperature fluctuations during shipping. The CPT were centrifuged to obtain peripheral blood mononuclear cells (PBMCs), and the PBMCs were cryopreserved using previously published protocols and stored in liquid nitrogen freezers until further use. The immune cell subsets were identified using minor modifications to the standardized protocol published by the Human Immunology Project (18). Per these guidelines, large batch analysis of frozen and thawed PBMC was found to be superior in reproducibility, while reducing small-batch variability associated with fresh sample analysis (18). One vial of cryopreserved mononuclear cells containing ~4 million cells was thawed and cells were incubated at 37°C in Roswell Park Memorial Institute media for 1 hour. The cells were centrifuged at 1 200 rpm for 10 minutes at room temperature. The cells were resuspended in 1× phosphate buffered saline and stained as outlined previously (19). The cells were kept on ice until analysis. All flow cytometry measurements were performed on an LSRII flow cytometer or a Fortessa X20 instrument (BD Biosciences, San Diego, CA). The validity of the T-cell distributions obtained from cryopreserved PBMCs using the procedures used in HRS has been demonstrated previously (19). In addition, control samples from healthy volunteers collected and cryopreserved at the start of the study were analyzed at least twice/week using the study protocol to monitor laboratory shifts and drifts in the immunophenotyping assessments.
Immunophenotyping of T cells included counts of total T cells, and CD4+ T cells, CD8+ T cells, and their Tn, central memory T cells (Tcm), and Tem/effector subsets all of which have been associated with age and CMV seropositivity. The T-cell subsets were measured using previously published protocols (19,20), and the immunophenotyping data were analyzed using OpenCyto and FlowAnnotator (21). Table 1 presents the phenotypic definitions of examined T-cell subsets. Differential white blood cell count from an EDTA blood tube was collected at the same time as the CPT but shipped at 4°C and was measured using a Sysmex XE-2100 instrument (Sysmex America, Inc., Lincolnshire, IL). The absolute counts of T-cell subsets per milliliter of peripheral blood were calculated by multiplying the lymphocyte count (obtained from a differential white blood cell count) by the percentage of different T-cell subsets. We used immunophenotyping data on 8 467 respondents though the rare subsets have smaller sample sizes due to missing data. CMV seroprevalence was measured using IgG antibodies to CMV in serum using the Roche e411 immunoassay analyzer (Roche Diagnostics Corporation, Indianapolis, IN). The interassay coefficient of variation was 3.4% at a mean concentration of 1.2 COI (cutoff interval) and 2.9% at a mean concentration of 141.4 COI. Results were reported as nonreactive (<1.0 COI) or reactive (≥1.0 COI).
Table 1.
Immunophenotypic Characterization of T-Cell Subsets
| Cell Type | Markers Used in Hybrid Approach |
|---|---|
| T cells | CD3+ CD19− |
| CD8+ T cells | CD3+ CD19− CD8+ CD4− |
| CD8+ T cells: Effector | CD3+ CD19− CD8+ CD4− CD45RA+ CCR7− CD28− |
| CD8+ T cells: Effector memory (Tem) | CD3+ CD19− CD8+ CD4− CD45RA− CCR7− CD28− |
| CD8+ T cells: Central memory (Tcm) | CD3+ CD19− CD8+ CD4− CD45RA− CCR7+ CD28+ |
| CD8+ T cells: Naïve (Tn) | CD3+ CD19− CD8+ CD4− CD45RA+ CCR7+ CD28+ |
| CD4+ T cells | CD3+ CD19− CD8− |
| CD4+ T cells: Effector | CD3+ CD19– CD8– CD4+ CD45RA+ CCR7− CD28− |
| CD4+ T cells: Effector memory (Tem) | CD3+ CD19− CD8− CD4+ CD45RA− CCR7− CD28− |
| CD4+ T cells: Central memory (Tcm) | CD3+ CD19− CD8− CD4+ CD45RA− CCR7+ CD28+ |
| CD4+ T cells: Naïve (Tn) | CD3+ CD19− CD8+ CD4− CD45RA+ CCR7+ CD28+ |
Race was a 4-category variable (Whites, Blacks, Hispanics, and Others) based on the reported race and hispanicity variables in the 2016 survey. All participants who reported Hispanic ethnicity were classified as Hispanics. Participants who reported “non-Hispanic” ethnicity were classified as Whites, Blacks, or Others based on their response to the question on race. Body mass index (BMI) was calculated from the interviewer measured height and weight using the equation weight (pounds)/(height × height [inches]) × 703. Education status was a categorical variable based on the number of school years reported in the 2016 survey (“<12 grade,” “high school and some college,” and “college graduate and postcollege”), and employment status was a categorical variable reported as “working now,” “disabled,” “retired,” and “homemaker” in the 2016 survey.
Statistical Analysis
We presented weighted sample means of absolute counts of T-cell subsets across 4 age categories (56–65, 66–75, 76–85, and 86+ years) for the total population and men and women separately. We used binomial proportions to estimate the proportion of CMV seroprevalence and %CD4/CD8 ratio (<1) across various age and sex groups. We have presented weighted sample means of absolute counts to evaluate the distribution of T-cell subsets across the different age and sex groups after stratifying by CMV serostatus. We used survey regression models to evaluate the difference of mean absolute T-cell subsets counts across various age and sex groups after accounting for participant sample weights to reflect study design and strata/cluster to account for sampling error. We used NPAR1WAY to test for p for trend in sex and CMV serostatus categories to estimate the significance of changes in T-cell subsets across different age categories. All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
Results
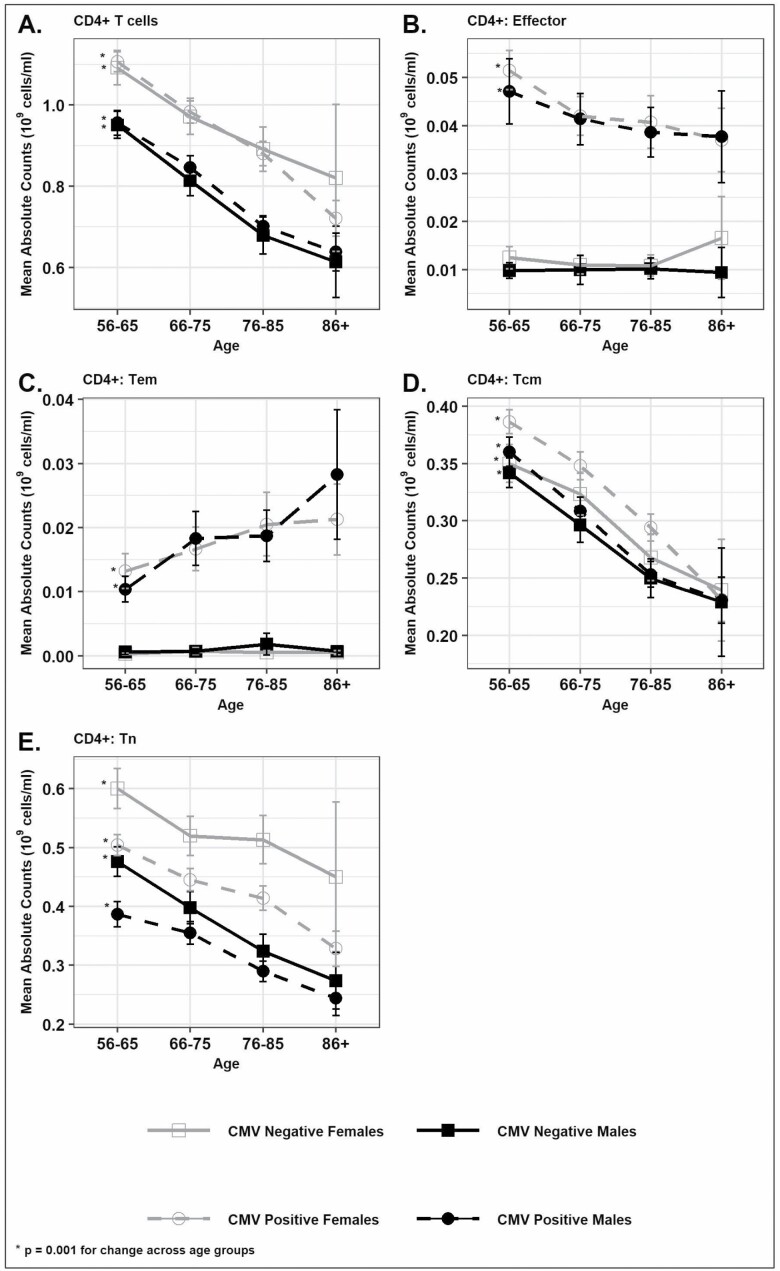
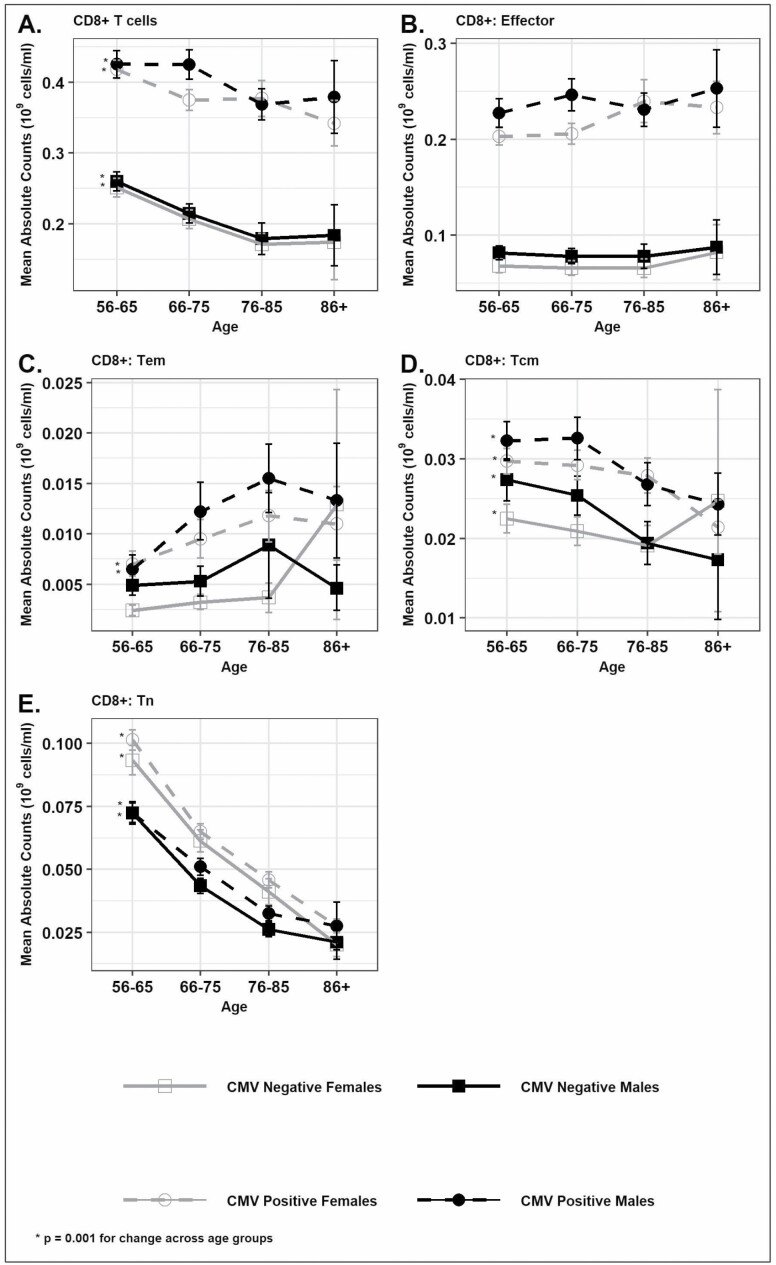
The prevalence of CMV seropositivity increased with age from 57% for ages 56–65 to 82% for those 86 years and older (Supplementary Table 1). CMV seropositivity was higher among women when compared with men (68% vs 57%; p < .0001; Supplementary Table 2). The CD4+ T cells were 16% higher among women when compared with men (p < .0001) irrespective of CMV status (Figure 1A and Supplementary Table 2) while CD8+ cells were higher among CMV-seropositive individuals than seronegative individuals, and men had a greater number of CD8+ T cells than women among CMV-seropositive individuals but not among seronegative individuals (Figure 2A and Supplementary Table 2). We found that the total T cells (CD3+) and CD4+ T cells were markedly lower with age; by 25% for total T cells (1.48 × 109 cells/mL to 1.11 × 109 cells/mL) and by 32% for CD4+ T cells (1.03 × 109 cells/mL to 0.70 × 109 cells/mL; Figure 1A and Supplementary Table 1) independent of CMV serostatus or sex. Similarly, CD8+ T cells were also lower by 9% with age independent of CMV serostatus or sex (Figure 2A and Supplementary Table 1). Both the Tem (CD45RA− CCR7− CD28−) and effector (CD45RA+ CCR7− CD28−) subsets of CD8+ and CD4+ T cells were higher among CMV-seropositive individuals when compared with CMV-seronegative individuals (Figures 1B and C and 2B and C). Among the CMV-seropositive individuals, the Tem and effector subsets of CD8+ T cells were 71% and 14% higher among those aged 86 and older than those aged 56–65; this was not true for CMV-seronegative individuals (Supplementary Table 1, Figure 2B and C). The Tem subset of CD4+ T cells was also 100% higher across age groups in CMV-seropositive individuals while the effector subset of CD4+ T cells was lower by 26% across age groups in CMV-seropositive individuals (Figure 1B and C and Supplementary Table 1). The Tn subsets (CD45RA+ CCR7+ CD28+) of CD4+ and CD8+ T cells were higher among women when compared with men irrespective of CMV serostatus (Figures 1D and 2D and Supplementary Table 2) though the absolute difference between sexes was more pronounced for the Tn subset of CD4+ T cells. Overall, the Tn subset of CD8+ T cells was 67% lower with age and the Tn subset of CD4+ T cells was 37% lower with age (Figures 1D and 2D and Supplementary Table 1) and was similar across both sexes and CMV serostatus indicating the strong effect of age in determining these Tn cell subsets and the minimal effect of CMV infection and sex on these age differences. The Tcm (CD45RA− CCR7+ CD28+) of CD4+ T cells was higher among women when compared with men (Figure 1E and Supplementary Table 2) while the Tcm subset of CD8+ T cells was higher among men (Figure 2E and Supplementary Table 2). The Tcm subset of CD8+ T cells was also higher among CMV-seropositive individuals when compared with CMV-seronegative individuals (0.030 vs 0.024; p < .0001). The Tcm subset of CD4+ and CD8+ T cells decreased with age in both sexes and independent of CMV serostatus (Figures 1E and 2E).
Figure 1.
Distribution of absolute counts (mean [±SEM]) of CD4+ T cells and subsets by age in sex and CMV serostatus categories in Health and Retirement Study 2016 survey participants: (A) Total CD4+ cells, (B) CD4+ T cells: Effector, (C) CD4+ T cells: Effector memory (Tem), (D) CD4+ T cells: Central memory (Tcm), and (E) CD4+ T cells: Naïve (Tn). *p ≤ .001 for CD4+ T-cell subsets change across age groups based on NPAR1WAY test for p for trend.
Figure 2.
Distribution of absolute counts (mean [±SEM]) of CD8+ T cells and subsets by age in sex and CMV serostatus categories in Health and Retirement Study 2016 survey participants. (A) Total CD8+ cells, (B) CD8+ T cells: Effector, (C) CD8+ T cells: Effector memory (Tem), (D) CD8+ T cells: Central memory (Tcm), and (E) CD8+ T cells: Naïve (Tn). *p ≤ .001 for CD8+ T-cell subsets change across age groups based on NPAR1WAY test for p for trend.
CD4+/CD8+ ratio <1 has been proposed, and often used, as a measure of immunosenescence. The percent of individuals with the CD4+/CD8+ ratio <1 was almost sevenfold higher from 4.15% to 12.68% for people aged between 56 and 65 years to those older than 85 (Supplementary Figure 1 and Supplementary Table 1). When stratified for CMV infection, this measure was higher with age only among individuals positive for CMV from 6.04% to 15.16% (Supplementary Figure 1 and Supplementary Table 1). These results strongly suggest that the CD4+/CD8+ ratio <1 is not an age-sensitive measure in the absence of CMV infection. Men had a significantly higher percentage of individuals with CD4+/CD8+ ratio <1 when compared with women in both CMV-seropositive and CMV-seronegative categories (Supplementary Table 2).
Discussion
Our results analyze, for the first time, the distributions of multiple T-cell subsets in the peripheral blood of a large nationally representative sample of older adults in the United States. The large sample size of this study allows evaluation of the independent effects of age and CMV infection on T-cell distribution. We find that immunosenescence, defined as a decrease in the Tn subset of CD4+ and CD8+ cells, increases markedly with age (22,23). Furthermore, we definitively demonstrate that the inverted CD4+/CD8+ ratio <1 is seen only in CMV-seropositive individuals and is not dependent on age in CMV-seronegative individuals and that men have a higher percentage with an inverted CD4+/CD8+ ratio <1 when compared with women irrespective of CMV serostatus.
The Tn subset of CD4+ and CD8+ cells rigorously defined in this study as (CD45RA+ CCCR7+ CD28+) is generated by the thymus early in life, but the loss of thymic mass and function before or by the time of puberty reduces Tn cell production. Such cells are maintained by secondary lymphoid organs for decades, but that process also falters with aging, reducing the number of Tn cells and rendering older adults more susceptible to new infections. We confirm the results of previous studies (12,13) and show a strong effect of age on the loss of Tn subsets that is independent of CMV serostatus. This study did not show a substantial difference in CD8+ Tn cells among CMV-seropositive and CMV-seronegative individuals. However, consistent with a previous study (12), CD4+ Tn cells were substantially higher among CMV-seronegative individuals independent of the effect of sex or age. Importantly, while the decrease in Tn cells is similar in both sexes, women exhibited higher Tn cell numbers across all age groups. It will be of importance to test to what extent this trait correlates with, or perhaps causally drives, improved responses in older females to vaccination and new infections.
Though previous reports (24–27) showed that the CD8+ effector cells (CD45RA+ CCR7− CD28−) and Tem cells (CD45RA− CCR7− CD28−) increase with age, these associations did not evaluate the independent effect of age after adjustment for CMV serostatus due to limited sample sizes. These cells provide immediate protection against infections that have been encountered in the past. However, it has been hypothesized that accumulation of CD8+ effector cells, particularly those specific for persistent infections, may be detrimental to the aging host as these cells exhibit several signs of senescence such as a low proliferative response to mitogens but remain remarkably resistant to apoptosis, express higher levels of granzyme B and perforin, and exhibit elevated baseline activation and possibly of interferon-γ, commonly seen elevated in sera of older individuals (28–31). Our analysis confirmed the results of a meta-analysis that showed that the CD8+ effector T cells are strongly influenced by CMV seropositivity but not age-dependent (13). We also confirmed the results of several (24,26,27), but not all (13,32,33), previous studies that have shown an increase in Tem CD8+ cells with age. Consistent with previous studies (27,34), we also showed that CD4+ Tem cells and CD4+ effector cells are significantly higher in CMV-seropositive individuals. We showed, for the first time, that higher CD4+ Tem subsets were not observed in older age groups among the CMV-seronegative individuals among whom we found no substantial differences. This study is also the first to demonstrate that the central memory subsets of CD4+ and CD8+ T cells were lower at older ages irrespective of sex and CMV serostatus. We also showed that the central memory subsets of CD4+ T cells were higher among women when compared with men, while the central memory subsets of CD8+ T cells were higher among men irrespective of CMV serostatus. Consistent with previous studies (24,25,34,35), the central memory subset of CD4+ T cells did not differ by CMV serostatus. Our study did not confirm a previous finding that the central memory subset of CD8+ T cells was higher among CMV-seronegative older individuals (26). Our study confirmed the results of a previous study that showed the central memory CD8+ T-cell subset was higher among CMV-seropositive individuals (35). In contrast to previous studies, our study shows a significant decrease in CD4+ Tcm and, to a lesser extent, in CD8+ Tcm cells, with age. These findings are in contrast to earlier studies that showed an increase in Tcm-cell populations with age (36,37) though one study showed a decrease in CD4+ and CD8+ Tcm-cell counts in participants ≥75 years (37). The restricted age range in this study (56+ years) when compared with previous studies that had participant ages that range from 5 to 96 years may account for the observed differences between studies.
Though women exhibited higher CMV seropositivity at all age groups when compared with men, they also had higher numbers of CD4+ cells, higher naïve CD4+ cells, and a higher CD4+/CD8+ ratio. These results were consistent with results from several previous studies (23,38–40). Studies of androgen-deficient men and women after surgical menopause suggest that the observed sex differences in CD4+ and CD8+ appear to be driven, at least partly, by sex hormones (41–45). Previous studies on sex differences in effector T cells have not accounted for the effect of CMV infection and hence have shown discrepant results on the numbers of effector cells in men and women (46,47). Though a previous study that accounted for age, sex, and CMV infection did not show any statistically significant sex differences in Tn and effector cell subsets, the results were similar to this study in that the Tn subsets were higher and the effector subsets were lower among women (48).
Our study further highlights the important contribution of CMV seropositivity, in determining the CD4/CD8 ratio and points to the critical importance of measuring CMV serostatus while interpreting the T-cell distributions. Indeed, evaluation of CD4+/CD8+ ratio <1 was only informative in CMV-seropositive individuals, where it increased with aging, and more so in males. Previous studies that evaluated CMV seropositivity using several structural proteins (49,50) showed that the CD4+/CD8+ ratio had sex-specific associations with mortality where women with the inverted CD4+/CD8+ ratio had the lowest overall mortality, and there was no association between the CD4+/CD8+ ratio and mortality among men (50). However, the sex-specific association with mortality was no longer statistically significant after adjustment for CMV seropositivity (50). We conclude that this ratio is not an age-sensitive trait, at least among CMV-seronegative individuals. It remains to be determined to what extent the additional accumulation of CD8+ effector and inversion of the CD4+/CD8+ ratio may contribute to age-related immune deficiency in CMV-seropositive males in a sex-specific manner.
The major strengths of this study are the large population-representative sample using standardized immunophenotyping methods. The large sample size of this study, with more than 8 000 individuals, provides a population-representative snapshot of T-cell counts among older adults in the United States for the first time. Limitations of the study include the current availability of data on T-cell subsets for only one time point that does not allow us to evaluate longitudinal changes in the T-cell subsets with age among individuals and the lack of functional information on the T-cell subsets. In addition, this study does not evaluate the clinical significance of the observed age and sex differences in T-cell subset distribution. However, the large sample size in this study provides reference values for T-cell subsets that may be useful for future studies that evaluate alterations in T-cell subsets in various clinical conditions. We acknowledge that there is heterogeneity in accepted definitions of various T-cell subsets. We have followed recommendations from the Human Immunology Project (18) in the selection of markers and definition of various T-cell subsets. However, certain subsets such as the effector cells have utilized CD28 in the definition of this cell type, while the canonical CD4+ effector population is defined without inclusion of the CD28 marker (51,52). This difference in the definition of the CD4+ effector population may lead to underestimation of this cell subset, especially in CMV-negative individuals. The observed differences in T-cell subsets according to CMV status are not adjusted for other variables that are correlated with CMV status such as smoking and socioeconomic status. Additional adjustment for race, educational attainment, smoking, and BMI did not substantially change the observed association with age, sex, and CMV status (Supplementary Tables 3a and 3b). Of note, the CD4+ effector cell subset was no longer associated with age after adjustment for additional covariates, and the CD4+ Tcm subset was no longer associated with CMV serostatus after additional adjustment. However, these changes do not substantially affect the overall findings as the CD4+ effector cell subset was determined predominantly by CMV serostatus (not age) and the CD4+ Tcm population was determined predominantly by age (not CMV serostatus).
In summary, we provide the distribution of various T-cell subsets in a representative sample of older US adults and demonstrate the relative effect of age, sex, and CMV infection on T-cell distribution. The higher numbers of effector cells, lower numbers of Tn cells, and lower CD4+/CD8+ ratio in CMV-positive men and lower numbers of naïve T cells in men with aging may be relevant to understanding the reduced vaccine effectiveness in men when compared with women (39,53). Thus, differences in T-cell subset distribution may be useful biomarkers of T-cell immunosenescence. If the T-cell subset distributions are proven to have a mechanistically relevant, understanding, the underlying determinants of T-cell subset distribution can help reduce the impact of T-cell immunosenescence on age-related diseases and improve vaccine effectiveness.
Funding
This work was supported by the National Institute on Aging (NIA; R01 AG AG060110). The Health and Retirement Study is supported by NIA (U01 AG009740).
Conflict of Interest
None declared.
Supplementary Material
Contributor Information
Bharat Thyagarajan, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, Minnesota, USA.
Jessica Faul, Institute for Social Research, Survey Research Center, University of Michigan, Ann Arbor, Michigan, USA.
Sithara Vivek, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, Minnesota, USA.
Jung K Kim, Davis School of Gerontology, University of Southern California, Los Angeles, California, USA.
Janko Nikolich-Žugich, Department of Immunobiology and the University of Arizona Center on Aging, University of Arizona College of Medicine—Tucson, Tucson, Arizona, USA.
David Weir, Institute for Social Research, Survey Research Center, University of Michigan, Ann Arbor, Michigan, USA.
Eileen M Crimmins, Davis School of Gerontology, University of Southern California, Los Angeles, California, USA.
Author Contributions
B.T., J.F., D.W., and E.M.C. conceptualized and designed the study. S.V. and J.K.K. conducted the statistical analysis. B.T. drafted the manuscript. B.T. and E.M.C. developed the hypothesis, oversaw the statistical analysis, and manuscript preparation. J.F., J.N.-Z., and D.W. provided critical feedback to the analysis strategy and suggested specific analysis for this project and edited the manuscript.
References
- 1. Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine. 2010;28:3627–3631. doi: 10.1016/j.vaccine.2010.03.035 [DOI] [PubMed] [Google Scholar]
- 2. Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70 [DOI] [PubMed] [Google Scholar]
- 4. Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol. 2004;11:168–173. doi: 10.1128/cdli.11.1.168-173.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia-Dabrio MC, Pujol-Moix N, Martinez-Perez A, et al. Influence of age, gender and lifestyle in lymphocyte subsets: report from the Spanish Gait-2 Study. Acta Haematol. 2012;127:244–249. doi: 10.1159/000337051 [DOI] [PubMed] [Google Scholar]
- 6. Jiao Y, Qiu Z, Xie J, Li D, Li T. Reference ranges and age-related changes of peripheral blood lymphocyte subsets in Chinese healthy adults. Sci China C Life Sci. 2009;52:643–650. doi: 10.1007/s11427-009-0086-4 [DOI] [PubMed] [Google Scholar]
- 7. Melzer S, Zachariae S, Bocsi J, Engel C, Loffler M, Tarnok A. Reference intervals for leukocyte subsets in adults: results from a population-based study using 10-color flow cytometry. Cytometry B Clin Cytom. 2015;88:270–281. doi: 10.1002/cyto.b.21234 [DOI] [PubMed] [Google Scholar]
- 8. Oladepo DK, Idigbe EO, Audu RA, et al. Establishment of reference values of CD4 and CD8 lymphocyte subsets in healthy Nigerian adults. Clin Vaccine Immunol. 2009;16:1374–1377. doi: 10.1128/CVI.00378-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin L, Jing X, Qiu Z, et al. Aging of immune system: immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging (Albany NY). 2016;8:848–859. doi: 10.18632/aging.100894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rudy BJ, Wilson CM, Durako S, Moscicki AB, Muenz L, Douglas SD. Peripheral blood lymphocyte subsets in adolescents: a longitudinal analysis from the REACH project. Clin Diagn Lab Immunol. 2002;9:959–965. doi: 10.1128/cdli.9.5.959-965.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong WS, Lo AW, Siu LP, et al. Reference ranges for lymphocyte subsets among healthy Hong Kong Chinese adults by single-platform flow cytometry. Clin Vaccine Immunol. 2013;20:602–606. doi: 10.1128/CVI.00476-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wertheimer AM, Bennett MS, Park B, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weltevrede M, Eilers R, de Melker HE, van Baarle D. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: a systematic review. Exp Gerontol. 2016;77:87–95. doi: 10.1016/j.exger.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 14. Caruso C, Accardi G, Virruso C, Candore G. Sex, gender and immunosenescence: a key to understand the different lifespan between men and women? Immun Ageing. 2013;10:20. doi: 10.1186/1742-4933-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heeringa SC, Connor J. Technical Description of the Health and Retirement Study Sample Design. Institute for Social Research, University of Michigan; 1995. https://hrsisrumichedu/publications/biblio/5310 [Google Scholar]
- 16. Juster T, Suzman RM. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S7–S56. doi:10.1093/ije/dyu067 [Google Scholar]
- 17. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thyagarajan B, Barcelo H, Crimmins E, et al. Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies. J Immunol Methods. 2018;463:61–70. doi: 10.1016/j.jim.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crimmins E, Faul JD, Thyagarajan B, Weir DR. Venous Blood Collection and Assay Protocol in the 2016 Health and Retirement Study. Survey Research Center, Institute for Social Research, University of Michigan; 2017. https://hrsisrumichedu/publications/biblio/9065 [Google Scholar]
- 21. Hunter-Schlichting D, Lane J, Cole B, et al. Validation of a hybrid approach to standardize immunophenotyping analysis in large population studies: the Health and Retirement Study. Sci Rep. 2020;10:8759. doi: 10.1038/s41598-020-65016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pawelec G. Immune parameters associated with mortality in the elderly are context-dependent: lessons from Sweden, Holland and Belgium. Biogerontology. 2018;19:537–545. doi: 10.1007/s10522-017-9739-z [DOI] [PubMed] [Google Scholar]
- 23. Wikby A, Mansson IA, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9:299–308. doi: 10.1007/s10522-008-9138-6 [DOI] [PubMed] [Google Scholar]
- 24. Chidrawar S, Khan N, Wei W, et al. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Litjens NH, de Wit EA, Betjes MG. Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing. 2011;8:2. doi: 10.1186/1742-4933-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Derhovanessian E, Maier AB, Hahnel K, et al. Lower proportion of naive peripheral CD8+ T cells and an unopposed pro-inflammatory response to human cytomegalovirus proteins in vitro are associated with longer survival in very elderly people. Age (Dordr). 2013;35:1387–1399. doi: 10.1007/s11357-012-9425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959 [DOI] [PubMed] [Google Scholar]
- 29. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 30. Vallejo AN. Immune remodeling: lessons from repertoire alterations during chronological aging and in immune-mediated disease. Trends Mol Med. 2007;13:94–102. doi: 10.1016/j.molmed.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 31. Wang GC, Casolaro V. Immunologic changes in frail older adults. Transl Med UniSa. 2014;9:1–6. [PMC free article] [PubMed] [Google Scholar]
- 32. Alonso Arias R, Moro-Garcia MA, Echeverria A, Solano-Jaurrieta JJ, Suarez-Garcia FM, Lopez-Larrea C. Intensity of the humoral response to cytomegalovirus is associated with the phenotypic and functional status of the immune system. J Virol. 2013;87:4486–4495. doi: 10.1128/JVI.02425-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee WW, Shin MS, Kang Y, Lee N, Jeon S, Kang I. The relationship of cytomegalovirus (CMV) infection with circulatory IFN-alpha levels and IL-7 receptor alpha expression on CD8+ T cells in human aging. Cytokine. 2012;58:332–335. doi: 10.1016/j.cyto.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Libri V, Azevedo RI, Jackson SE, et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+ CD45RA+ CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132:326–339. doi: 10.1111/j.1365-2567.2010.03386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinberger B, Lazuardi L, Weiskirchner I, et al. Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol. 2007;68:86–90. doi: 10.1016/j.humimm.2006.10.019 [DOI] [PubMed] [Google Scholar]
- 36. Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673 [DOI] [PubMed] [Google Scholar]
- 37. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 38. Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279 [DOI] [PubMed] [Google Scholar]
- 39. Voigt EA, Ovsyannikova IG, Kennedy RB, et al. Sex differences in older adults’ immune responses to seasonal influenza vaccination. Front Immunol. 2019;10:180. doi: 10.3389/fimmu.2019.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103. doi: 10.1371/journal.pone.0016103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bizzarro A, Valentini G, Di Martino G, DaPonte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter’s syndrome. J Clin Endocrinol Metab. 1987;64:32–36. doi: 10.1210/jcem-64-1-32 [DOI] [PubMed] [Google Scholar]
- 42. Giglio T, Imro MA, Filaci G, et al. Immune cell circulating subsets are affected by gonadal function. Life Sci. 1994;54:1305–1312. doi: 10.1016/0024-3205(94)00508-7 [DOI] [PubMed] [Google Scholar]
- 43. Kocar IH, Yesilova Z, Ozata M, Turan M, Sengul A, Ozdemir I. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter’s syndrome. Clin Exp Immunol. 2000;121:448–452. doi: 10.1046/j.1365-2249.2000.01329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumru S, Godekmerdan A, Yilmaz B. Immune effects of surgical menopause and estrogen replacement therapy in peri-menopausal women. J Reprod Immunol. 2004;63:31–38. doi: 10.1016/j.jri.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 45. Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB. Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol. 2001;36:311–326. doi: 10.1016/s0531-5565(00)00195-9 [DOI] [PubMed] [Google Scholar]
- 46. Garcia Verdecia B, Saavedra Hernandez D, Lorenzo-Luaces P, et al. Immunosenescence and gender: a study in healthy Cubans. Immun Ageing. 2013;10:16. doi: 10.1186/1742-4933-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yan J, Greer JM, Hull R, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Di Benedetto S, Derhovanessian E, Steinhagen-Thiessen E, Goldeck D, Muller L, Pawelec G. Impact of age, sex and CMV-infection on peripheral T cell phenotypes: results from the Berlin BASE-II Study. Biogerontology. 2015;16:631–643. doi: 10.1007/s10522-015-9563-2 [DOI] [PubMed] [Google Scholar]
- 49. Adriaensen W, Derhovanessian E, Vaes B, et al. CD4:8 ratio >5 is associated with a dominant naive T-cell phenotype and impaired physical functioning in CMV-seropositive very elderly people: results from the BELFRAIL study. J Gerontol A Biol Sci Med Sci. 2015;70:143–154. doi: 10.1093/gerona/glu018 [DOI] [PubMed] [Google Scholar]
- 50. Adriaensen W, Pawelec G, Vaes B, et al. CD4:8 ratio above 5 is associated with all-cause mortality in CMV-seronegative very old women: results from the BELFRAIL study. J Gerontol A Biol Sci Med Sci. 2017;72:1155–1162. doi: 10.1093/gerona/glw215 [DOI] [PubMed] [Google Scholar]
- 51. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]
- 52. Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643 [DOI] [PubMed] [Google Scholar]
- 53. Chambers C, Skowronski DM, Rose C, et al. Should sex be considered an effect modifier in the evaluation of influenza vaccine effectiveness? Open Forum Infect Dis. 2018;5:ofy211. doi: 10.1093/ofid/ofy211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.