Abstract
Background
Aging-related disease risk is exacerbated by obesity and physical inactivity. It is unclear how weight loss and increased activity improve risk in older adults. We aimed to determine the effects of diet-induced weight loss with and without exercise on insulin sensitivity, VO2peak, body composition, and physical function in older obese adults.
Methods
Physically inactive older (68.6 ± 4.5 years) obese (body mass index 37.4 ± 4.9 kg/m2) adults were randomized to health education control (HEC; n = 25); diet-induced weight loss (WL; n = 31); or weight loss and exercise (WLEX; n = 28) for 6 months. Insulin sensitivity was measured by hyperinsulinemic–euglycemic clamp, body composition by dual-energy X-ray absorptiometry and MRI, strength by isokinetic dynamometry, and VO2peak by graded exercise test.
Results
WLEX improved (p < .05) peripheral insulin sensitivity (+75 ± 103%) versus HEC (+12 ± 67%); WL (+36 ± 47%) versus HEC did not reach statistical significance. WLEX increased VO2peak (+7 ± 12%) versus WL (−2 ± 24%) and prevented reductions in strength and lean mass induced by WL (p < .05). WLEX decreased abdominal adipose tissue (−16 ± 9%) versus HEC (−3 ± 8%) and intermuscular adipose tissue (−15 ± 13%) versus both HEC (+9 ± 15%) and WL (+2 ± 11%; p < .01).
Conclusions
Exercise with weight loss improved insulin sensitivity and VO2peak, decreased ectopic fat, and preserved lean mass and strength. Weight loss alone decreased lean mass and strength. Older adults intending to lose weight should perform regular exercise to promote cardiometabolic and functional benefits, which may not occur with calorie restriction-induced weight loss alone.
Keywords: Aging, Exercise, Insulin sensitivity, Obesity, Weight loss
Aging is marked by increased risk for type 2 diabetes, reduced muscle mass and strength (ie, sarcopenia), decreased physical function and cardiorespiratory fitness, ectopic fat deposition, and insulin resistance, all of which increase risk for physical disability, morbidity, and mortality (1–3). These adverse health consequences associated with advanced age are exacerbated with obesity and physical inactivity. The prevalence of obesity in older adults has increased steadily over the last 20 years; recent data in the United States classifies 42.8% of older adults (>60 years) as obese (4), with comparable rates observed in other developed nations. Similarly, physical inactivity clearly increases with age (5). Thus, optimal intervention strategies that target obesity and/or physical inactivity in older adults are critical.
Despite the evidence that aging is associated with greater cardiometabolic risk and declining physical function, it is not clear to what extent obesity and a lack of exercise play a direct role to accentuate or accelerate these aging effects. We reported a cross-sectional observation that obesity and physical activity probably influence insulin resistance of aging (6). We posit that weight loss and exercise interventions would provide more direct evidence to support distinct roles for obesity and physical inactivity in age-associated cardiometabolic risk and physical function. Thus, the primary objective of this randomized controlled trial was to determine the efficacy of calorie restriction–induced weight loss with and without exercise in older, obese, physically inactive adults to help decipher the detrimental effects of aging, obesity, and physical inactivity on insulin resistance and other markers of cardiometabolic risk as well as physical function and cardiorespiratory fitness.
A second objective of this study was to assess the potential for diet-induced weight loss to cause unwanted loss of lean mass and muscle weakness, which could exacerbate sarcopenia in aging adults. Many older adults attempt to lose weight via calorie restriction and/or exercise. Intentional weight loss in older adults, however, remains controversial (7); though calorie restriction results in decreased fat mass and improved cardiometabolic risk (7–9), it can be accompanied by substantial loss of skeletal muscle mass (10,11). The extent to which reduction in muscle mass translates to decrements in muscle function, however, including skeletal muscle insulin sensitivity, mobility impairments, and muscle quality is unclear. Moreover, the addition of exercise to calorie restriction-induced weight loss has been reported to attenuate loss of lean body mass in both middle-aged and older adults (10–12). Few studies, however, have demonstrated additional exercise-induced benefits for other aging and obesity-specific consequences, including skeletal muscle insulin resistance, poor cardiorespiratory fitness, ectopic fat deposition (ie, intermuscular and abdominal adipose tissue [AT]), and muscle strength and physical function (8–10). This randomized controlled trial investigates the efficacy of both weight loss and exercise on cardiometabolic risk, physical function and fitness, as well as the potential risks for diet-induced weight loss to cause loss of muscle mass and weakness and to determine the extent to which exercise can mitigate or prevent these effects.
Method
Participants
We conducted a 2-site, 6-month randomized controlled trial with a parallel group design between 2012 and 2017 at the University of Pittsburgh and the AdventHealth Translational Research Institute (AH TRI). Eighty-six older (60–80 years of age), physically inactive men and women with obesity were randomized into one of the 3 treatments (1:1:1 allocation ratio): control (HEC; health education), calorie restriction-induced weight loss (WL), and weight loss with exercise (WLEX). All participants provided informed consent prior to participation, and all study protocols were approved by both University of Pittsburgh Research Ethics Board and Institutional Review Board of AdventHealth. Additionally, a Data Safety Monitoring Board comprised of external independent investigators reviewed adverse events monthly. The ClinicalTrials.gov unique identifier code is NCT02230839.
Participants 60–80 years of age were included if they met the following criteria: body mass index (BMI) ≥ 30 kg/m2; stable weight over the last 6 months; physically inactive (≤1 continuous exercise session/week); nonsmoking; resting systolic blood pressure (SBP) < 150 mmHg; and diastolic blood pressure (DBP) < 95 mmHg. Exclusion criteria included clinically significant cardiovascular disease including history of myocardial infarction within the past year; peripheral vascular disease; hepatic, renal, muscular/neuromuscular, or active hematologic/oncologic disease; the presence of bruits in the lower extremities; history of pulmonary emboli; peripheral neuropathy; anemia; and substance abuse. Medication exclusions included the following: anticoagulants, glucocorticoids, thiazolidinediones, or insulin.
Randomization
Randomization was performed electronically using a random allocation sequence designated by the statistician. A permuted-blocks approach using blocks of random sizes of 4 and/or 8 was used, with groups stratified by gender. The study coordinator was responsible for participant enrollment and group assignment. Outcome assessors were blinded to group assignment.
Intervention Groups
HEC group
Participants randomized to the HEC group received biweekly general health education seminars on medication and type 2 diabetes management. However, they were not given specific exercise or dietary education.
Calorie restriction–induced WL group
The goal of the WL intervention was to lose 10% of baseline body weight. Using the Harris–Benedict equation corrected for the activity factor, reduction of 500–1 000 kcal/d based on baseline body weight was prescribed in addition to a low-fat (< 30% of kilocalories from fat) diet. To encourage compliance, participants met individually with the Registered Dietitian and/or designated staff weekly to record body weight, review daily food logs, and receive updated dietary guidelines. To eliminate the confounding effects of acute caloric restriction on insulin sensitivity, participant weights were kept stable during the last 2 weeks of intervention.
WLEX group
Participants completed a progressive 6-month exercise training program, 4–5 d/wk, 45 minutes per session (180 min/wk) consisting of mostly walking (outside and on an indoor treadmill) and the option to include stationary cycling, elliptical, and rowing machines. Aerobic exercise was performed at 50%–80% HRreserve. All indoor exercise was supervised by a trained monitor; aerobic exercise performed outdoors was not supervised. Beginning at Week 8, participants were also prescribed 2 nonconsecutive resistance exercise sessions per week, 30 minutes per session, focused on major muscle groups using resistance exercise machines. Nine exercises (2–3 sets of 10–12 repetitions) alternating upper limbs, lower limbs, and trunk were performed. The resistance exercises were performed at the highest weight the participant could achieve for the given number of reps (10–12) with proper form. When the participant reached 3 × 12 reps, the weight was increased and the number of reps was decreased. Participants also met with the registered dietitian and received identical dietary instruction as the WL group.
Primary Outcomes
Insulin sensitivity
Insulin sensitivity was measured using the hyperinsulinemic–euglycemic clamp performed 36–48 hours following the last exercise bout (WLEX group) to account for the acute effect of exercise on insulin sensitivity and to prevent detraining. Participants arrived at the facility the evening prior to the clamp procedure, consumed a standard meal, and stayed overnight in the metabolic ward. After an overnight fast, an intravenous catheter was placed in the antecubital vein for subsequent insulin, glucose, and stable isotope infusions. A primed constant infusion of [6,6-2H2] glucose ran throughout the clamp procedure. An additional catheter was placed in the heated hand vein in the contralateral arm to attain arterialized blood samples for blood glucose determination and for [6,6-2H2] glucose enrichment during the insulin and glucose infusions. After a 2.5-hour baseline period, an insulin infusion was started and continued for 4 hours at 40 mU/m2 min. Glucose was measured at 5-minute intervals and maintained at 90 mg/dL. A 2-mL blood sample was collected at 0, 30, 60, 100, 110, and 120 minutes and every 10 minutes during the last 30 minutes of the clamp for gas chromatography–mass spectrometry determination of [6,6-2H2] glucose enrichment. Insulin samples were also drawn at multiple time points. Rates of glucose disposal (Rd) and endogenous glucose production (EGP) were calculated by nonsteady-state equations based on plasma [6,6-2H2] glucose enrichment (13,14). Skeletal muscle insulin sensitivity (Rd/Insulin) was assessed as the rate of glucose disposal (mg/kgFFM/min) accounting for plasma insulin during steady state. Hepatic insulin sensitivity was assessed as the suppression of EGP during steady state using the glucose enrichment data.
Secondary Outcomes
Blood analyses
Lipid profiles (total, high-density lipoprotein [HDL], low-density lipoprotein [LDL], and very-low-density lipoprotein [VLDL] cholesterol, and triglycerides) and HbA1C were measured by a fasting blood draw and analyzed in the clinical chemistry laboratory at AH TRI using standard assays.
Cardiorespiratory fitness, strength, and functional performance
A cardiopulmonary graded exercise test was conducted by an exercise physiologist on the cycle ergometer using open-circuit indirect calorimetry to measure maximal oxygen uptake (VO2peak). Following a standardized warm-up, participants exercised at a moderate intensity and resistance increased gradually until volitional fatigue.
The Short Physical Performance Battery (SPPB) was used to measure lower extremity functional capacity. The SPPB consists of 3 tasks: 5 repeated timed chair stands, timed standing balance (feet in parallel, semitandem, and tandem positions), and a 4-m walk to determine usual gait speed. Additionally, a timed step test was performed.
Muscle strength and power were assessed at baseline and 6 months using a pneumatic-driven dynamometer (Biodex 4, Biodex Medical Systems, Inc., Shirley, NY). Following a 1-minute warm-up of free pedaling on a cycle ergometer, participants were seated on the Biodex machine with the lateral condyle of the knee lined up with the axis of rotation of the machine arm. Participants performed 3 tests on each leg at each resistance of 60, 120, and 180 degrees/s with a ~2-minute rest between each adjustment and a 5-minute rest between legs.
Body composition
Weight and height were measured pre- and postintervention, and BMI was calculated. Waist circumference was measured using the Gulick II tape measure directly on the skin. Fat mass (kg) and fat-free mass (FFM; kg) were determined by dual-energy X-ray absorptiometry using a GE Lunar (GE Healthcare, UK).
Abdominal and thigh AT and muscle volume were measured by MRI at baseline and following treatment on a 3-Tesla magnet using multislice protocols (Philips Acheiva, Cambridge, MA) at AH TRI. Fifteen high-resolution axial images (5 mm thickness) were taken of the leg above and below the midpoint of the femur using a T1-weighted turbo spin echo to quantify thigh muscle volume, subcutaneous, and intermuscular AT (IMAT). For abdominal AT images, a 3D fast field echo (THRIVE) of the entire abdomen was taken to achieve 50 high-resolution axial images (8 mm thickness). Three stacks were obtained over 3 separate breath holds to ensure complete coverage from the dome of the diaphragm through the pubic symphysis. Resultant images were analyzed and segmented using Analyze 11.0 (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The density constants 0.92 and 1.04 g/cm3 were used to calculate AT and muscle mass, respectively.
Statistical Analysis
Power analyses were conducted a priori based on data from a previous study conducted in our laboratory. A sample size of 63 subjects total (~20 per group) would provide 80% power to detect a medium effect size (0.55) for statistically significant differences in Rd/Insulin. Accounting for a 20% dropout rate, we aimed to randomize 76 participants.
One-way ANOVAs were performed to evaluate baseline differences between groups. In cases where the assumption of normality (assessed using the Shapiro–Wilk test) was not met, baseline comparisons between groups for these specific variables were performed using the nonparametric Kruskal–Wallis test.
Both intention-to-treat (ITT) and completers analyses were used to explore between-group differences in the changes of the primary outcomes. The Markov chain Monte Carlo (MCMC) method was used to impute missing data. For variables wherein the MCMC approach could not be applied, ITT analysis was not completed.
All variables were log-transformed to approximate a normal distribution. An ANCOVA (1 × 3) with baseline, sex, and age (Model 1) and baseline, sex, age, and % weight change (Model 2) as covariates and pairwise comparisons using the Tukey’s HSD test for multiple comparisons were conducted to discriminate means following significant ANCOVA results. The interaction term, “group × sex,” was added to the model to assess the effect of sex. To examine the effect of type 2 diabetes status on intervention responses, Model 1 was also adjusted for type 2 diabetes status. Additionally, an ANCOVA with type 2 diabetes status as the independent variable and baseline as a covariate was applied to each group separately in addition to the WL and WLEX groups combined to assess the effect of type 2 diabetes on intervention responses. A p value of less than .05 was considered significant. Analyses were performed using JMP Version 13.2.1 (SAS Institute Inc., Cary, NC).
Results
Study Participants and Intervention Characteristics
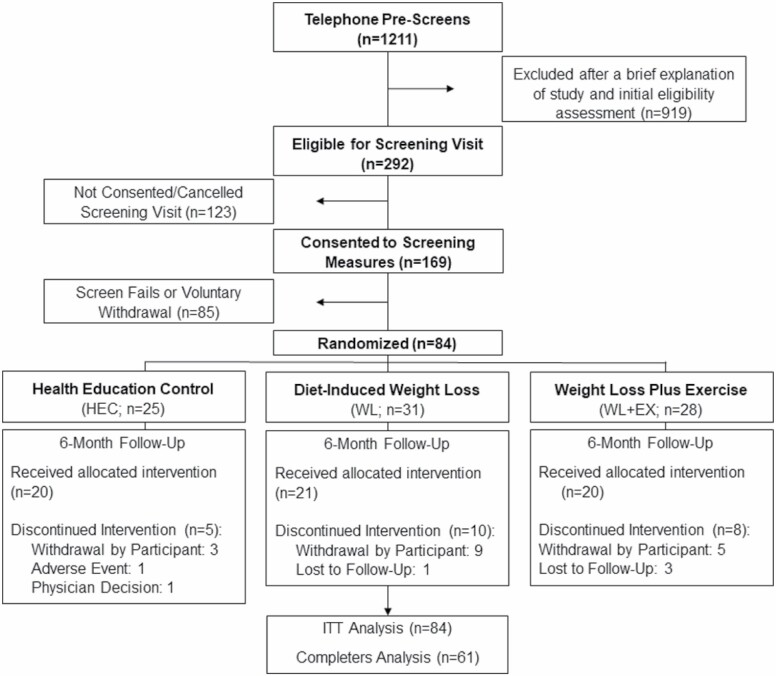
The flow of participant recruitment, screening, and randomization is illustrated in Figure 1. Of the 84 participants randomized to one of 3 intervention groups, 61 completed the follow-up assessments (HEC, n = 20; WL, n = 21; WLEX, n = 20). Twenty-three participants did not complete the intervention and were included in the ITT analyses. Four participants in the WLEX group experienced acute, minor muscle and limb discomfort.
Figure 1.
Patient flow diagram.
Participants who completed the WLEX intervention (n = 20) attended 84 ± 14% of the prescribed exercise sessions, averaging 3.3 ± 0.6 sessions per week.
Baseline characteristics of the study groups and medication use are given in Table 1. There were no differences among study groups in either completers or completers and noncompleters combined. In the HEC and WLEX groups, one participant discontinued metformin use during the trial period.
Table 1.
Participant Characteristics at Baseline
| Characteristics | HEC | WL | WLEX | |||
|---|---|---|---|---|---|---|
| Completers | All Subjects | Completers | All Subjects | Completers | All Subjects | |
| Males:females (no.) | 7:13 | 8:17 | 7:14 | 8:23 | 8:12 | 8:20 |
| Race (no.) | C: 16 | C: 19 | C: 16 | C: 24 | C: 17 | C: 21 |
| AA: 2 | AA: 3 | AA: 3 | AA: 3 | AA: 0 | AA: 2 | |
| H: 2 | H: 3 | H: 1 | H: 2 | H: 2 | H: 4 | |
| NR: 0 | NR:0 | NR: 1 | NR: 2 | NR: 1 | NR: 1 | |
| T2D (no.) | 8 | 9 | 5 | 7 | 7 | 8 |
| Age (y) | 70.1 ± 4.8 | 69.9 ± 4.5 | 70.0 ± 4.6 | 69.1 ± 5.2 | 66.8 ± 3.4 | 66.9 ± 3.9 |
| Anthropometry | ||||||
| BMI (kg/m2) | 35.7 ± 4.4 | 40.0 ± 5.0 | 36.1 ± 5.1 | 34.9 ± 4.6 | 37.3 ± 5.4 | 37.2 ± 5.0 |
| Weight (kg) | 97.8 ± 10.5 | 98.2 ± 12.4 | 101.4 ± 20.3 | 96.5 ± 18.7 | 102.9 ± 13.2 | 100.7 ± 13.6 |
| WC (cm) | 114.9 ± 9.9 | 114.8 ± 9.5 | 116.4 ± 13.9 | 113.0 ± 13.0 | 118.5 ± 14.3 | 116.7 ± 13.8 |
| Cardiometabolic risk factors | ||||||
| SBP (mmHg) | 139.8 ± 11.0 | 138.5 ± 11.8 | 135.1 ± 15.3 | 131.9 ± 15.0 | 134.5 ± 11.1 | 137.1 ± 11.4 |
| DBP (mmHg) | 73.7 ± 8.3 | 73.4 ± 8.7 | 75.2 ± 11.1 | 74.0 ± 10.2 | 72.6 ± 11.7 | 75.3 ± 11.9 |
| Insulin (mIU/L) | 14.1 ± 6.7 | 14.1 ± 6.7 | 14.9 ± 9.9 | 14.9 ± 9.9 | 15.8 ± 8.0 | 15.8 ± 8.0 |
| Glucose (mg/dL) | 108.9 ± 17.2 | 107.0 ± 16.7 | 102.3 ± 20.0 | 101.0 ± 17.9 | 109 ± 22.1 | 106.7 ± 20.2 |
| HbA1C (%) | 6.3 ± 0.8 | 6.1 ± 0.8 | 5.9 ± 0.4 | 5.8 ± 0.5 | 6.3 ± 0.9 | 6.2 ± 0.8 |
| TG (mg/dL) | 147.3 ± 55.5 | 148.2 ± 53.4 | 135.6 ± 67.5 | 128.1 ± 60.6 | 159.2 ± 66.9 | 158.8 ± 69.3 |
| Cholesterol (mg/dL) | 191 ± 38.0 | 198.0 ± 40.7 | 179.3 ± 36.6 | 186.4 ± 39.5 | 183.9 ± 38.0 | 193.0 ± 42.8 |
| LDL (mg/dL) | 108 ± 32.9 | 113.6 ± 34.4 | 101.7 ± 33.4 | 108.0 ± 37.5 | 106.6 ± 32.8 | 112.6 ± 37.1 |
| HDL (mg/dL) | 53.4 ± 17.2 | 54.6 ± 16.0 | 50.3 ± 14.8 | 54.2 ± 15.5 | 45.3 ± 7.2 | 48.5 ± 9.9 |
| VLDL (mg/dL) | 29.7 ± 11.2 | 29.8 ± 10.7 | 27.3 ± 13.6 | 25.8 ± 12.2 | 32.0 ± 13.5 | 31.9 ± 13.9 |
| Medication use, no. (%)* | ||||||
| Metformin | 6 (35%) | 3 (15%) | 3 (19%) | |||
| Other AHAs | 1 (6%) | 3 (15%) | 2 (13%) | |||
| Statin | 9 (53%) | 9 (45%) | 8 (50%) | |||
| Other lipid-lowering | 1 (6%) | 2 (10%) | 0 (0%) | |||
| β-Blocker | 3 (18%) | 4 (20%) | 5 (31%) | |||
| Other antihypertensive | 8 (47%) | 15 (75%) | 10 (63%) | |||
| Mental health | 6 (35%) | 4 (20%) | 3 (19%) | |||
| Hypothyroidism | 4 (24%) | 7 (35%) | 2 (13%) | |||
| Hormone replacement | 0 (0%) | 1 (5%) | 1 (6%) |
Notes: AA = African American; AHA = antihyperglycemic agent; BMI = body mass index; C = Caucasian; H = Hispanic; HDL = high-density lipoprotein; HEC = health education control; LDL = low-density lipoprotein; NR = not reported; SBP/DBP = systolic blood pressure/diastolic blood pressure; TG = triglycerides; VLDL = very-low-density lipoprotein; WL = diet-induced weight loss; WLEX= weight loss and exercise. Values presented are mean ± SD.
*Medication data available for 53 participants (HEC, n = 17; WL, n = 20; WLEX, n = 16).
Insulin Sensitivity
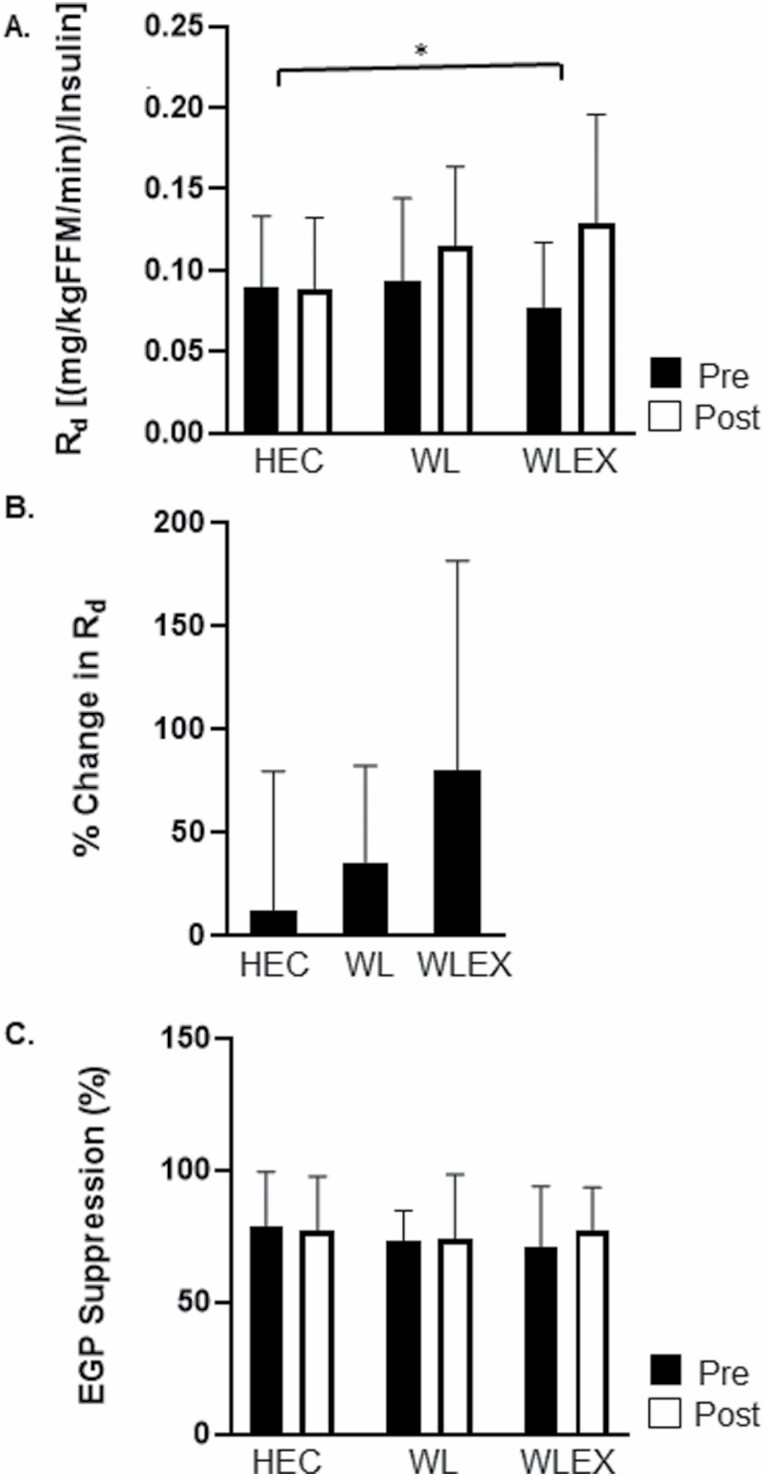
Between-group differences in peripheral and hepatic insulin sensitivity in completers are shown in Figure 2. After accounting for circulating insulin concentrations, participants in WLEX, but not WL, improved Rd/Insulin compared with HEC (WLEX, 0.05 ± 0.04 vs HEC, 0.0 ± 0.04 [mg/kgFFM/min]/Insulin ; all p < .05). After controlling for % weight loss, there were no significant between-group differences in Rd/I. There were no between-group differences in EGP suppression (p > .05).
Figure 2.
Between-group changes (mean ± SD) in peripheral and hepatic insulin sensitivity. (A) Pre- and post-values of rate of glucose disposal (Rd) adjusted for fat free mass (kg) and steady-state plasma insulin by group; *WLEX significantly different than HEC at p < .05 (ANCOVA for change in Rd followed by pairwise comparisons). (B) Relative (%) change in Rd ([mg/kgFFM/min]/insulin) by group. (C) Pre- and post-values of endogenous glucose suppression (EGP suppression, %) by group. Sample size for Rd/I and EGP suppression: HEC, n = 19; WL, n = 14; WLEX, n = 14.
Clinical Measures of Insulin Sensitivity and Cardiometabolic Risk Factors
Between-group changes in secondary outcomes including anthropometry, clinical variables, functional measurements, and body composition are given in Table 2. WLEX experienced greater improvement than HEC in fasting insulin (−5.5 ± 4.9 vs −2.0 ± 3.9 mg/dL) and HbA1C (−0.4% vs −0.1%; all p < .05). There were no differences in change in fasting glucose (p ≥ .05). SBP and DBP did not change with either intervention (p ≥ .05). Except for total cholesterol (WLEX vs WL: −22.9 ± 31.1 vs 1.5 ± 38.1 mg/dL; p < .05), no indicators of dyslipidemia (TG, LDL, HDL, VLDL) changed following WL or WLEX (all p > .05).
Table 2.
Between-Group Differences in Clinical Outcomes (Completers)
| Characteristics | HEC | WL | WLEX | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Change | % Change | Change | % Change | Change | % Change | Model 1† | Model 2‡ | |
| Anthropometry | ||||||||
| BMI (kg/m2) | −0.5 ± 1.2 | −1 ± 3 | −2.4 ± 1.6 | −7 ± 5 | −3.8 ± 1.8 | −10 ± 5 | <.0001*,#,^ | |
| Weight (kg) | −1.4 ± 3.0 | −1 ± 3 | −7.1 ± 4.7 | −7 ± 5 | −10.6 ± 4.9 | −11 ± 5 | <.0001*,#,^ | |
| WC (cm) | −2.3 ± 4.9 | −2 ± 4 | −5.6 ± 6.1 | −5 ± 5 | −8.7 ± 6.2 | −7 ± 5 | .01# | .44 |
| Clinical measures | ||||||||
| SBP (mmHg) | −4.8 ± 10.5 | −3 ± 7 | −5.6 ± 15.8 | −3 ± 11 | −1.0 ± 13.0 | −1 ± 9 | .48 | .40 |
| DBP (mmHg) | −1.3 ± 8.6 | −2 ± 12 | 0.05 ± 11.4 | 2 ± 16 | −1.3 ± 8.7 | −1 ± 12 | .41 | .55 |
| Insulin (mg/dL) | −2.0 ± 3.9 | −5 ± 35 | −4.3 ± 6.1 | −21 ± 28 | −5.5 ± 4.9 | −31 ± 27 | .02# | .59 |
| Glucose (mg/dL) | 3.0 ± 26.0 | 3 ± 20 | −1.1 ± 10.7 | −1 ± 10 | −7.7 ± 15.4 | −6 ± 12 | .29 | .23 |
| HbA1C (%) | −0.1 ± 0.6 | −0.2 ± 0.3 | −0.6 ± 0.7 | <.01# | .44 | |||
| TG (mg/dL) | −5.5 ± 49.4 | 1 ± 33 | −22.6 ± 73.0 | −6 ± 37 | −48.4 ± 73.5 | −18 ± 49 | .07 | .41 |
| Cholesterol (mg/dL) | −6.8 ± 26.9 | −2 ± 17 | 1.5 ± 38.1 | 2 ± 22 | −22.9 ± 31.1 | −12 ± 16 | .02^ | .06 |
| LDL (mg/dL) | −5.5 ± 26.4 | 0 ± 30 | 5.7 ± 35.2 | 11 ± 48 | −12.1 ± 26.5 | −9 ± 24 | .14 | .18 |
| HDL (mg/dL) | 0.05 ± 5.6 | 1 ± 12 | 0.4 ± 6.6 | 2 ± 16 | −1.1 ± 5.2 | −2 ± 11 | .34 | .48 |
| VLDL (mg/dL) | −1.3 ± 9.9 | 1 ± 33 | −4.6 ± 14.7 | −6 ± 37 | −9.7 ± 14.7 | −17 ± 50 | .07 | .43 |
| Functional measurements | ||||||||
| Single leg stance (s) | 0.5 ± 1.5 | 6 ± 23 | 0.2 ± 3.5 | 13 ± 51 | 0.6 ± 1.8 | 13 ± 49 | .79 | .94 |
| 4-m walk (s) | 0.2 ± 0.5 | 6 ± 13 | 0.3 ± 1.1 | 7 ± 25 | 0.0 ± 0.7 | 0 ± 15 | .62 | .61 |
| Chair rise (s) | −0.7 ± 1.4 | −4 ± 13 | −0.8 ± 2.0 | −6 ± 15 | −0.6 ± 2.0 | −3 ± 19 | .93 | .46 |
| Step test (s) | −1.4 ± 3.3 | −5 ± 11 | −0.2 ± 3.5 | 0 ± 12 | −2.0 ± 3.2 | −8 ± 11 | .17 | .11 |
| SPPB (total) | 0.3 ± 0.8 | 3 ± 8 | −0.1 ± 1.2 | 0 ± 14 | 0.1 ± 1.0 | 1 ± 9 | .30 | .13 |
| Body composition (DXA) | ||||||||
| Fat mass (kg) | −1.4 ± 2.6 | −3 ± 6 | −5.0 ± 3.8 | −11 ± 10 | −8.6 ± 4.3 | −19 ± 9 | <.0001*,#,^ | |
| Lean mass (kg) | 0.0 ± 1.8 | 0 ± 4 | −1.5 ± 2.1 | −3 ± 4 | −0.9 ± 1.9 | −2 ± 4 | .05* | |
| Body composition (MRI)‖ | ||||||||
| IMAT (kg) | 0.03 ± 0.05 | 9 ± 15 | 0.0 ± 0.05 | 2 ± 11 | −0.07 ± 0.07 | −15 ± 13 | <.01#,^ | .11 |
| % IMAT§ | 1.5 ± 2.5 | 0.5 ± 1.8 | −2.3 ± 2.8 | .01# | .02# | |||
| Leg SAT (kg) | −0.1 ± 0.1 | −3 ± 4 | −0.1 ± 0.2 | −6 ± 12 | −0.4 ± 0.3 | −19 ± 11 | <.01#,^ | .50 |
| Leg muscle (kg) | −0.05 ± 0.10 | −3 ± 5 | −0.04 ± 0.14 | −1 ± 7 | −0.01 ± 0.14 | −1 ± 7 | .65 | .02# |
| Abdominal AT (kg) | −0.6 ± 1.7 | −3 ± 8 | −1.9 ± 2.0 | −9 ± 9 | −3.6 ± 1.8 | −16 ± 9 | <.01# | .98 |
| Abdominal SAT (kg) | −0.5 ± 0.9 | −3 ± 6 | −1.1 ± 1.2 | −8 ± 9 | −2.2 ± 1.1 | −15 ± 7 | <.01# | .99 |
| Visceral AT (kg) | −0.1 ± 1.2 | 0 ± 23 | −0.8 ± 1.4 | −9 ± 17 | −1.4 ± 1.1 | −18 ± 13 | .03# | .95 |
Notes: BMI, body mass index; DXA = dual-energy X-ray absorptiometry; WC = waist circumference; SBP/DBP = systolic blood pressure/diastolic blood pressure; TG = triglycerides; LDL = low-density lipoprotein; HDL = high-density lipoprotein; VLDL = very-low-density lipoprotein; SPPB = Short Performance Physical Battery; IMAT = intermuscular adipose tissue; SAT = subcutaneous adipose tissue; AT = adipose tissue. Values presented are mean change ± SD and % change ± SD.
†Model 1 was performed using an ANCOVA adjusted for sex, age, and baseline, followed by pairwise comparisons.
‡Model 2 was performed using an ANCOVA adjusted for sex, age, baseline, and % weight change, followed by pairwise comparisons.
§Relative to total leg volume.
‖Sample size for MRI outcomes: HEC, n = 13; WL, n = 8; WLEX, n = 13.
*Change in WL significantly different than HEC at p ≤ . 05.
#Change in WLEX significantly different than HEC at p ≤ .05.
^Change in WL significantly different than WLEX at p ≤ .05.
Aerobic Fitness, Muscle Force and Power, and Functional Performance
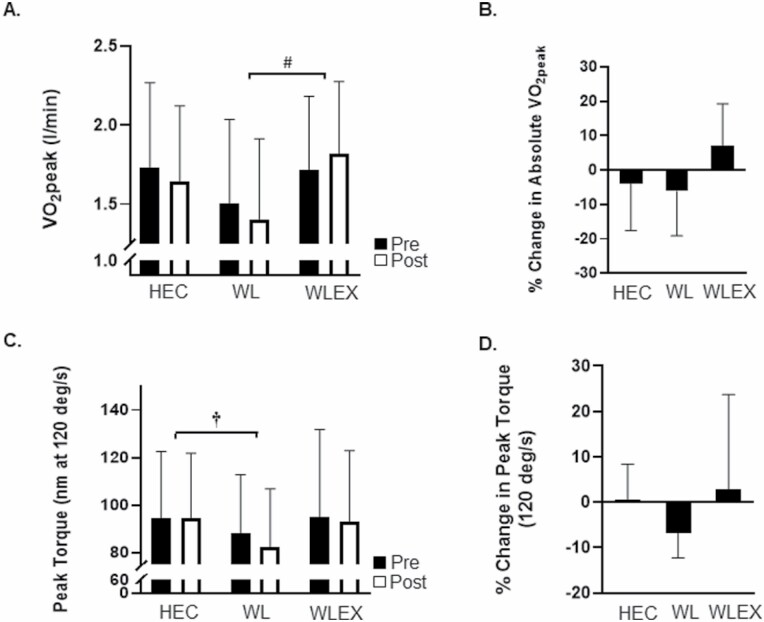
Between-group changes in VO2peak and peak torque are shown in Figure 3. Relative VO2peak (mL/kgFFM/min) increased significantly in WLEX compared with both WL and HEC (WLEX, 2.6 ± 3.2 vs WL, −1.2 ± 4.2 and HEC, −1.4 ± 4. 4 mL/kgFFM/min; p < .05). Absolute VO2peak (L/min) increased significantly in WLEX compared with WL and trended toward significance compared with HEC (WLEX, 0.1 ± 0.2 vs WL, −0.1 ± 0.2 and HEC, 0.0 ± 0.2 L/min; p < .05). Peak torque (nm at 120 degree/s) decreased in WL compared with HEC (WL, −5.8 ± 4.9 vs HEC, 0.0 ± 8.1 nm; p < .05).
Figure 3.
Between-group changes (mean ± SD) in cardiorespiratory fitness (VO2peak) and muscle strength (peak torque). (A) Pre- and post-values of VO2peak (l/min) by group; #WLEX significantly different than WL at p < .05 (ANCOVA for change in VO2peak followed by pairwise comparisons). (B) Relative (%) change in VO2peak by group. (C) Pre- and post-values of peak torque (measured at 120 degree/s) by group; †WL significantly different than HEC at p < .05 (ANCOVA for change in peak torque followed by pairwise comparisons). (D) Relative (%) change in peak torque by group.
Functional measures including the single-leg stance, 4-m walk, repeated chair rise, step test, and SPPB did not change significantly in any intervention group (all p ≥ .05).
Body Weight and Composition
Pre- and post-values and % change in body weight and composition are given in Table 2. WLEX and WL lost weight (−10.6 ± 4.9 and −7.1 ± 4.7 kg, respectively) compared with HEC (−1.4 ± 3.0%), and WLEX lost more weight than WL (all p < .05). Only WLEX reduced WC (Table 2; HEC, −2.3 ± 4.9 vs WLEX, −8.7 ± 6.2 cm) compared with HEC. WLEX lost significantly more fat mass compared with both HEC and WL (HEC, −1.4 ± 2.6 and WL, −5.0 ± 3.8 vs WLEX, −8.6 ± 4.3 kg) and WL lost more fat mass compared with HEC. WL lost significantly more lean mass compared with HEC (WL, −1.5 ± 2.1 vs HEC, 0.0 ± 1.8 kg; p = .05).
WLEX, but not WL, lost significantly more abdominal SAT (HEC vs WLEX: −0.5 ± 0.9 vs −2.2 ± 1.1 kg) and visceral AT (HEC vs WLEX: −0.1 ± 1.2 vs −1.4 ± 1.1 kg) compared with HEC (p < .05). Additionally, WLEX experienced greater reductions in leg SAT (WLEX, −0.4 ± 0.3 vs HEC, −0.1 ± 0.1 and WL, −0.1 ± 0.2 kg) and IMAT (WLEX, −0.07 ± 0.07 vs HEC, 0.03 ± 0.05 and WL, 0.0 ± 0.05 kg; p < .05) compared with both HEC and WL.
Findings from ITT analyses are shown in Supplementary Table S1. With the exception of Rd/Insulin (both WL and WLEX improved significantly compared with HEC), the ITT analysis corroborated our findings in completers.
Impact of Type 2 Diabetes Status and Sex
There was no effect of type 2 diabetes status on any outcome, both within and between groups. Additionally, there was no significant interaction of group by sex for any outcome.
Discussion
The prevalence of prediabetes and type 2 diabetes is higher in older adults (15). Obesity and physical inactivity can exacerbate the influence of aging on the development of type 2 diabetes, insulin resistance, and other cardiometabolic risk factors (6). Thus, it is paramount to understand the extent to which obesity reduction and increased exercise can prevent or mitigate aging-associated decline in health and function. The Diabetes Prevention Program reported that lifestyle intervention, including a combination of weight loss and exercise, reduced the incidence of type 2 diabetes in older men and women (16); however, the separate effects of reduced caloric intake and exercise were not systematically evaluated. Moreover, few studies have investigated the effects of calorie restriction–induced weight loss, with and without an adjunct exercise program, on insulin resistance and cardiorespiratory fitness, 2 of the primary factors involved in morbidity and mortality risk (17,18). We compared the efficacy of weight loss with or without exercise on insulin sensitivity, cardiorespiratory fitness, physical function, and body composition in obese older adults either with or at risk for developing type 2 diabetes. Our primary findings strongly support the important role for exercise to improve skeletal muscle insulin resistance and cardiorespiratory fitness and to prevent unwanted loss of lean tissue and muscular strength with intentional weight loss.
This is the first randomized controlled trial to investigate the effects of weight loss with or without exercise on skeletal muscle insulin sensitivity, exclusively in obese older adults. Our findings suggest that weight loss via calorie restriction alone is insufficient to significantly improve skeletal muscle insulin sensitivity and requires the addition of exercise to incur benefit, which was also true for clinical measures of insulin resistance including HbA1C and fasting insulin. The use of a stable isotope tracer technique in our study allows for the assessment of skeletal muscle insulin sensitivity directly, after accounting for hepatic endogenous glucose production. This is an important advance over prior studies given that skeletal muscle is responsible for the majority of postprandial insulin-stimulated glucose disposal and plays a critical role in the pathogenesis of type 2 diabetes in older adults (19). Although it is tempting to conclude that exercise-specific effects on skeletal muscle insulin sensitivity occur independent of weight loss, we should consider the important caveat that the WLEX group lost more weight than the WL only group, and after controlling for weight loss, there were no longer between-group differences. Studies in middle-aged adults that matched diet and exercise interventions for weight loss observed no differences in insulin sensitivity outcomes by clinical, OGTT, and clamp-derived measures (20–25). Limited evidence in older (>50 years of age) obese adults also showed similar improvements in whole-body insulin sensitivity (insulin and glucose AUC) measured indirectly (OGTT) following weight loss induced by diet, exercise, or the combination (8,20). Although the additional benefit of exercise in our study may be an extension of greater caloric deficit, it is possible that exercise has specific salutary effects on mitochondria that weight loss alone does not (22,25). Toledo et al. randomized obese adults to a diet and diet plus exercise group matched for calorie deficit and found that exercise was required for improvements in mitochondrial capacity, despite similar between-group increases in insulin sensitivity (25). Taken together, our findings suggest that exercise provides benefit by either increasing caloric deficit and/or exerting separate effects on skeletal muscle mitochondria. A larger trial is required to discern independent effects of exercise beyond weight loss in groups matched for energy balance. Regardless, our study shows that the addition of exercise to calorie restriction-induced weight loss promotes greater weight loss and improves skeletal muscle insulin sensitivity.
Our study indicates that calorie restriction-induced weight loss may exacerbate sarcopenia by simultaneously reducing lean mass and muscle strength. The addition of moderate aerobic and resistance exercise preserved lean mass and maintained muscle strength in the face of weight loss. Loss of muscle mass and strength in addition to increased adiposity leads to reduced functional independence, higher incidence of illness, and increased mortality in older adults (26–28). Furthermore, aging is associated with a decline in cardiorespiratory fitness, an important predictor of all-cause and cardiovascular mortality (2,18). We found that calorie restriction alone did not improve physical fitness and that exercise was required to elicit this change, which is consistent with others (9). Furthermore, findings in patients with type 2 diabetes who were assigned to an intensive lifestyle intervention including both physical activity and calorie restriction or a diabetes support group revealed that both weight loss and improved fitness mediated the reduced risk of mobility loss in the lifestyle intervention group (29). Contrary to others (9,30), we did not observe improvements in physical function measures following exercise; however, inclusion criteria and participants’ higher baseline physical function scores in our study may explain this discrepancy. For example, in work from Villareal et al., participants had mild–moderate frailty, which was not an inclusion requirement for our study. Taken together, our findings prompt important considerations for promoting weight loss alone in obese older adults and suggest that exercise should be a vital component of weight loss interventions for older obese adults to preserve and/or improve fitness, muscle strength, and physical function, all factors important for maintaining functional independence and reducing risk for morbidity and mortality (31).
We investigated the effects of weight loss and exercise on ectopic fat as a potential link to improved insulin sensitivity and physical function. Aging is characterized by the redistribution of AT from the periphery to ectopic fat depots including the abdomen, liver, and skeletal muscle (32). Higher levels of ectopic fat storage are associated with a host of physiological and functional consequences including insulin resistance (33), reduced skeletal muscle quality (34), and mobility limitations (35). With few exceptions (36), prior findings in middle-aged adults suggest that there is no additional benefit of exercise beyond weight loss for reducing abdominal or intermuscular AT depots (12,37,38). Although we observed that only WLEX significantly reduced abdominal subcutaneous, visceral, and intermuscular AT compared with controls, after adjusting for weight change, there were no longer significant between-group differences. However, the proportion of leg muscle composed of intermuscular AT decreased more with exercise, independent of weight change. Intermuscular AT in particular is associated with impaired skeletal muscle quality and function, such that increases with aging impairs contractility and enhances lipotoxicity, which could lead to mitochondrial dysfunction, insulin resistance, and mobility disability (39,40). Our finding that the relative proportion of intermuscular AT in muscle was reduced with combined weight loss and exercise underscores the importance of exercise training for attenuating age-associated muscle quality and mobility deficits.
There are limitations in our study that should be considered. The MRI-derived body composition measures were performed in a subset of participants; thus, the sample size may be inadequate to assess intervention differences between WL and WLEX. Our novel finding that exercise independently reduces the proportion of intermuscular AT in obese older adults warrants further investigation and confirmation. The 1-step hyperinsulinemic–euglycemic clamp protocol employed a high physiological insulin dose (40 mU/m2/min), which may abrogate our ability to assess liver and AT insulin sensitivity. Interestingly, even at this high insulin dose, endogenous glucose production was not completely suppressed in all subjects and substantial individual variability was present. This emphasizes the importance of utilizing tracer methodology to confirm the source of measured glucose and appropriately assess skeletal muscle insulin sensitivity. Finally, our participants differed in both type 2 diabetes status and medication use. Although adjusting for incidence of type 2 diabetes did not change our overall findings, possible intervention–interaction effects might have influenced participant responses to the intervention (41). Additionally, the absence of any change in select cardiometabolic risk factors (eg, blood pressure and serum lipids) may reflect prestudy medications.
In conclusion, calorie restriction–induced weight loss alone caused modest improvements in insulin sensitivity and reduced lean mass and muscle strength in older obese adults either with or at high risk for type 2 diabetes. The addition of regular exercise, however, more robustly improved skeletal muscle insulin sensitivity and cardiorespiratory fitness, maintained muscle mass and strength, and reduced ectopic fat deposition, particularly intermuscular AT. These data strongly suggest that exercise elicits important benefits for older obese at-risk adults, many of which are not observed with calorie restriction–induced weight loss alone. Regardless of whether the observed changes are due to additional weight loss or exercise per se, including regular exercise should be considered as a useful and manageable adjunct to traditional weight loss therapies for older adults with obesity to mitigate risk for chronic disease and maintain functional independence and quality of life.
Supplementary Material
Acknowledgments
The authors thank the contributions of our study participants and acknowledge the expertise and assistance of the imaging, recruitment, clinic, calorimetry, laboratory, and nutrition staff at University of Pittsburgh and AdventHealth Research Institute.
Contributor Information
Andrea M Brennan, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Robert A Standley, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Steven J Anthony, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
Kory E Grench, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Nicole L Helbling, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
James P DeLany, AdventHealth, Translational Research Institute, Orlando, Florida, USA; Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
Heather H Cornnell, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Fanchao Yi, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Maja Stefanovic-Racic, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
Frederico G S Toledo, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
Paul M Coen, AdventHealth, Translational Research Institute, Orlando, Florida, USA; Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
Elvis A Carnero, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Bret H Goodpaster, AdventHealth, Translational Research Institute, Orlando, Florida, USA; Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pennsylvania, USA.
Funding
This work was supported by the National Institutes of Health, National Institute on Aging (RO1 AG021961-09) awarded to B.H.G.
Conflict of Interest
None declared.
Author Contributions
B.H.G. concepted and designed the primary trial. R.A.S., K.E.G., and E.A.C. coordinated the primary trial and organized all data collection and analysis. H.H.C. provided support and expertise for all body composition analysis with MRI and dual-energy X-ray absorptiometry. J.P.D. provided support and expertise for use and analysis of glucose tracer during the clamp. S.J.A. and K.E.G. provided intervention expertise and support. M.S.-R. and F.G.S.T. provided medical oversight and contributed to the study design. F.Y. provided statistical support for the manuscript. A.M.B. completed statistical analysis and data interpretation. A.M.B. and B.H.G. were responsible for drafting the manuscript. All authors assisted with manuscript revision. B.H.G. is the guarantor of the data and takes responsibility for the contents of this article.
References
- 1. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–537. doi: 10.1038/s41574-018-0062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson AS, Sui X, Hébert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169(19):1781–1787. doi: 10.1001/archinternmed.2009.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30(5):327–346. doi: 10.2165/00007256-200030050-00002 [DOI] [PubMed] [Google Scholar]
- 4. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 5. Bauman A, Merom D, Bull FC, Buchner DM, Fiatarone Singh MA. Updating the evidence for physical activity: summative reviews of the epidemiological evidence, prevalence, and interventions to promote “active aging”. Gerontologist. 2016;56(suppl 2):S268–S280. doi: 10.1093/geront/gnw031 [DOI] [PubMed] [Google Scholar]
- 6. Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32(8):1547–1549. doi: 10.2337/dc09-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol. 2013;48(10):1054–1061. doi: 10.1016/j.exger.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond). 2014;38(3):423–431. doi: 10.1038/ijo.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40(7):1213–1219. doi: 10.1249/MSS.0b013e31816a85ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chomentowski P, Dubé JJ, Amati F, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci. 2009;64(5):575–580. doi: 10.1093/gerona/glp007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimura E, Kumahara H, Tobina T, et al. Aerobic exercise attenuates the loss of skeletal muscle during energy restriction in adults with visceral adiposity. Obes Facts. 2014;7(1):26–35. doi: 10.1159/000358576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56(8):2142–2147. doi: 10.2337/db07-0141 [DOI] [PubMed] [Google Scholar]
- 14. Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. Wiley; 1992. [Google Scholar]
- 15. Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42(2):333–347. doi: 10.1016/j.ecl.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eschwège E. The dysmetabolic syndrome, insulin resistance and increased cardiovascular (CV) morbidity and mortality in type 2 diabetes: aetiological factors in the development of CV complications. Diabetes Metab. 2003;29(4 Pt 2):6S19–6S27. doi: 10.1016/s1262-3636(03)72784-0 [DOI] [PubMed] [Google Scholar]
- 18. Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. doi: 10.1001/jama.1996.03540030039029 [DOI] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S157–S163. doi: 10.2337/dc09-S302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss EP, Racette SB, Villareal DT, et al. ; Washington University School of Medicine CALERIE Group. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prior SJ, Joseph LJ, Brandauer J, Katzel LI, Hagberg JM, Ryan AS. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92(3):880–886. doi: 10.1210/jc.2006-2113 [DOI] [PubMed] [Google Scholar]
- 22. Menshikova EV, Ritov VB, Dube JJ, et al. Calorie restriction-induced weight loss and exercise have differential effects on skeletal muscle mitochondria despite similar effects on insulin sensitivity. J Gerontol A Biol Sci Med Sci. 2017;73(1):81–87. doi: 10.1093/gerona/glw328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi: 10.2337/diabetes.48.4.839 [DOI] [PubMed] [Google Scholar]
- 24. Dubé JJ, Amati F, Toledo FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147–1156. doi: 10.1007/s00125-011-2065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57(4):987–994. doi: 10.2337/db07-1429 [DOI] [PubMed] [Google Scholar]
- 26. Levine ME, Crimmins EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring). 2012;20(10):2101–2106. doi: 10.1038/oby.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang CI, Huang KC, Chan DC, et al. The impacts of sarcopenia and obesity on physical performance in the elderly. Obes Res Clin Pract. 2015;9(3):256–265. doi: 10.1016/j.orcp.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 28. Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond). 2005;29(9):1011–1029. doi: 10.1038/sj.ijo.0803005 [DOI] [PubMed] [Google Scholar]
- 29. Rejeski WJ, Ip EH, Bertoni AG, et al. ; Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366(13):1209–1217. doi: 10.1056/NEJMoa1110294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943–1955. doi: 10.1056/NEJMoa1616338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13(11):1849–1863. doi: 10.1038/oby.2005.228 [DOI] [PubMed] [Google Scholar]
- 32. Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 33. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–1141. doi: 10.1056/NEJMra1011035 [DOI] [PubMed] [Google Scholar]
- 34. De Carvalho FG, Justice JN, Freitas EC, et al. Adipose tissue quality in aging: how structural and functional aspects of adipose tissue impact skeletal muscle quality. Nutrients. 2019;11(11):2553. doi: 10.3390/nu11112553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koster A, Patel KV, Visser M, et al. ; Health, Aging and Body Composition Study. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc. 2008;56(4):636–643. doi: 10.1111/j.1532-5415.2007.01632.x [DOI] [PubMed] [Google Scholar]
- 36. Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol (1985). 2012;112(1):79–85. doi: 10.1152/japplphysiol.00355.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E; Pennington CALERIE Team. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–872. doi: 10.1210/jc.2006-2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25(3):431–438. doi: 10.2337/diacare.25.3.431 [DOI] [PubMed] [Google Scholar]
- 39. Tumova J, Andel M, Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res. 2016;65(2):193–207. doi: 10.33549/physiolres.932993 [DOI] [PubMed] [Google Scholar]
- 40. Delmonico MJ, Harris TB, Visser M, et al. ; Health, Aging, and Body. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boulé NG, Kenny GP, Larose J, Khandwala F, Kuzik N, Sigal RJ. Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Diabetologia. 2013;56(11):2378–2382. doi: 10.1007/s00125-013-3026-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.