Abstract
Dicarbonyl stress describes the increased formation of 1,2-dicarbonyl compounds and is associated with age-related pathologies. The role of dicarbonyl stress in healthy aging is poorly understood. In a preliminary study, we analyzed 1,2-dicarbonyl compounds, namely 3-deoxyglucosone (3-DG), glyoxal (GO), and methylglyoxal (MGO) in plasma of older (25 months, n = 11) and younger (5 months, n = 14) male C57BL/6J (B6) mice via ultra performance liquid chromatography tandem mass spectrometry. Postprandial 3-DG was higher in younger compared to older mice, whereas no differences were found for GO and MGO. Subsequently, in the main study, we analyzed fasting serum of older women (OW, 72.4 ± 6.14 years, n = 19) and younger women (YW, 27.0 ± 4.42 years, n = 19) as well as older men (OM, 74.3 ± 5.20 years, n = 15) and younger men (YM, 27.0 ± 3.34, n = 15). Serum glucose, insulin, 1,2-dicarbonyl concentrations, and markers of oxidative stress were quantified. In a subgroup of this cohort, an oral dextrose challenge was performed, and postprandial response of 1,2-dicarbonyl compounds, glucose, and insulin were measured. In women, there were no age differences regarding fasting 1,2-dicarbonyl concentrations nor the response after the oral dextrose challenge. In men, fasting MGO was significantly higher in OM compared to YM (median: 231 vs 158 nM, p = .006), whereas no age differences in fasting 3-DG and GO concentrations were found. Glucose (310 ± 71.8 vs 70.8 ± 11.9 min·mmol/L) and insulin (7 149 ± 1 249 vs 2 827 ± 493 min·µIU/mL) response were higher in OM compared to YM, which did not translate into a higher 1,2-dicarbonyl response in older individuals. Overall, aging does not necessarily result in dicarbonyl stress, indicating that strategies to cope with 1,2-dicarbonyl formation can remain intact.
Keywords: Aging, Fasting, Glyoxal, Methylglyoxal, Postprandial, 3-Deoxyglucosone
Dicarbonyl stress is a consequence of mitochondrial dysfunction and cellular senescence, which are hallmarks of aging (1). The accumulation of reactive carbonyl compounds and oxygen species causes cellular damage, therefore contributing to the aging process (2). In vivo, the main source of 1,2-dicarbonyl compounds, such as 3-deoxyglucosone (3-DG), methylglyoxal (MGO), and glyoxal (GO), is the degradation of monosaccharides as well as triosephosphates (3). Only a minor amount is ingested with the diet (4,5). Fasting blood concentrations of 1,2-dicarbonyl compounds in healthy adults range between 100 nM and 1 µM (3,6). In normoglycemic participants, acute glucose ingestion resulted in increased plasma concentrations of 3-DG, GO, and MGO after 30 minutes due to their endogenous formation (7). Subsequently, glucose and 1,2-dicarbonyl concentrations return to fasting levels within 120 minutes after ingestion. However, participants with an impaired glucose metabolism or type 2 diabetes exhibit a delayed postprandial peak of 1,2-dicarbonyl compounds as well as a prolonged time until fasting concentrations are reached (7). The abnormal accumulation of 1,2-dicarbonyl compounds due to reduced degradation or increased formation is termed dicarbonyl stress. Naturally, the body possesses defense systems to maintain 1,2-dicarbonyl concentrations at low levels. 3-DG can be reduced to 3-deoxyfructose via aldose and aldehyde reductase or oxidized to 3-deoxy-2-ketogluconate via aldehyde or 2-oxoaldehyde dehydrogenase (3,8–10). MGO and GO are mainly metabolized via the glyoxalase system, which consists of the enzymes glyoxalase 1 and 2 (Glo1, Glo2) as well as glutathione (3). Glo1 activity is known to decrease with age (11). When 1,2-dicarbonyls are chronically elevated, the reactive carbonyl groups can irreversibly modify proteins via nonenzymatic glycation, resulting in the formation of advanced glycation end products and their pathophysiological consequences (12). Associations of dicarbonyl stress and various age-related pathologies such as diabetes (7), obesity (13), cancer (14), and cognitive decline (15) have been found. Furthermore, dicarbonyl stress is associated with oxidative stress and vice versa (3).
Because current studies analyzed 1,2-dicarbonyl compounds in samples of older participants with underlying pathologies (16,17), it is difficult to derive the role of dicarbonyl compounds in older individuals without severe health impairments. In a preliminary experiment, we examined postprandial 1,2-dicarbonyl concentrations in male mice of different ages. Subsequently, we analyzed fasting 1,2-dicarbonyl concentrations, markers of glucose metabolism, as well as oxidative stress in older and younger adults. Furthermore, a subgroup of the human cohort was subjected to a dextrose challenge to investigate postprandial 1,2-dicarbonyl response. We hypothesized that fasting concentrations and response to a dextrose challenge of 3-DG, GO, and MGO were different: (a) in older age due to the age-related activity decline of detoxification enzymes and (b) between women and men as there are sex-specific responses to oxidative stress and Glo1 activity differences described (18,19).
Method
Animal Experimental Procedure
Male C57Bl/6J (B6, Janvier’s Labs: CS 4105 Le Genest St Isle, 53941 Saint Berthevin Cedex, France) were housed in open cages of 4 to 5 animals in a controlled environment (20 ± 2°C, 12/ 12 hours light/dark cycle) with ad libitum access to a standard diet (SD; V1534-300 Ssniff, Soest, Germany) and water. At an age of 5 months (younger group) and 25 months (older group), body weight was measured and mice were subsequently sacrificed by acute isoflurane exposure. Blood samples were taken and blood glucose was immediately analyzed by using a Contour XT glucometer (Bayer, Leverkusen, Germany). Subsequently, blood was centrifuged for 5 minutes, 13 000×g, and plasma samples were stored at −80°C until analysis of 1,2-dicarbonyl compounds. Blood samples were taken in the morning between 7 and 10 am. Because mice are nocturnal animals and were not fastened before sacrifice, the morning blood drawing was considered to be postprandial. All mice were kept in agreement with the National Institutes of Health guidelines for care and use of laboratory animals and with the guidelines of the German Law on the Protection of Animals. Final organ removal and blood collection were approved by the local authorities.
Study Population
This is a secondary analysis of a larger study which is described in detail elsewhere (20). In brief, the study was performed in community-dwelling older (65–85 years) and younger adults (18–35 years). Exclusion criteria were type 1 and 2 diabetes, a stroke or heart attack in the past 6 months, food allergies and intolerances, pregnancy, or any severe or malignant disease. The study was approved by the ethics committee of the University of Potsdam and registered at drks.de as DRKS00017090. All participants signed a written informed consent. In this study, 19 older (OW) and younger women (YW), as well as 15 older (OM) and younger men (YM), were included in the analyses. From these participants, blood samples after an oral dextrose challenge were available in n = 14 OW, n = 10 YW, n = 5 OM, and n = 5 YM.
Study Protocol
Participants were instructed to refrain from vigorous exercise and alcohol on the day prior to the study. After an overnight fast, participants arrived between 07:30 and 08:15 am at the study facility; body weight and height were measured. A cannula was then inserted into an antecubital vein for repeated blood sampling. After taking a blood sample in the fasted state, a subgroup of participants received a dextrose drink consisting of 50 g dextrose in 300 mL water that had to be consumed within 15 minutes. Blood samples were taken 15, 30, 60, 120, and 240 minutes after ingestion. During this time, participants were allowed to drink water ad libitum. Blood serum and EDTA plasma were obtained and stored at −80°C until analysis.
Measurement of Blood Parameters of the Glucose Metabolism
Serum insulin (intraassay coefficient of variance: 4.8–6.0%, interassay coefficient of variance: 8.1–9.0%; BioVendor, Brno, Czech Republic) was quantified using an immunosorbent assay. Furthermore, serum glucose was measured using a colorimetric method (ABX Pentra 400; Horiba, Ltd, Montpellier, France). Homeostasis model assessment (19) was used to estimate insulin resistance (HOMA-IR).
Analysis of 1,2-Dicarbonyl Compounds
Serum 1,2-dicarbonyl concentrations were quantified after derivatization with o-phenylenediamine (oPD) using ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) (6). In brief, 25 µL serum samples were mixed with 75 µL oPD solution (10 mg oPD in 10 mL 1.6 mol/L perchloric acid) for derivatization of 3-DG, MGO, and GO in the corresponding quinoxalines. The samples were kept in the dark for 20 hours. Optimization of derivatization procedure and information regarding the stability of 1,2-dicarbonyl compounds are described by Scheijen et al. (6). After incubation, 10 µL of an internal standard solution containing isotope-labeled quinoxalines was added. Internal standard solution was prepared according to Scheijen et al. (6). After centrifugation (10 000 rpm, 10 min), an aliquot of 80 µL was transferred to an high performance liquid chromatography (HPLC) vial and 5 µL was subjected to UPLC-MS/MS analysis. An Acquity I-class system coupled to a Xevo TQ-XS mass spectrometer (both Waters Corporation, Milford, MA) was used for analysis. For chromatographic separation, a Cortecs Solid Core C18 column (2.1 × 50 mm, 1.6 μm) at a column temperature of 40°C was used. Solvent A was 0.2% formic acid in LC-MS grade water and solvent B was LC-MS grade ACN. The solvents were pumped at a flow rate of 0.6 mL/min in gradient mode (0 min, 1% B; 1 min, 1% B; 6 min, 60% B; 6.1 min, 1% B; 8 min, 1% B). The injection volume was 5 μL. The ESI source was operated in positive mode, and nitrogen was utilized as the nebulizing gas with a gas flow of 650 L/h and a gas temperature of 350°C. The capillary voltage was set to 2.7 kV and the source temperature was 150°C. Analytes were measured in MRM mode with the following transitions used for quantification and optimized collision energies (CEs) and cone voltages (CVs). MGO: 145.1 → 77.1 (CV 24, CE 25 V), d4-MGO: 149.1 → 81.1 (CV 24, CE 20 V), 3-DG: 235.1 → 171.1 (CV 22, CE 18 V), d4-3-DG: 239.1 → 175.1 (CV 20, CE 18 V), GO: 131.1 → 77.1 (CV 20, CE 22 V), d4-GO: 135.1 → 81.1 (CV 20, CE 22 V). Data were acquired and evaluated with the MassLynx Software (Waters, version 4.2).
A 6-point calibration curve was prepared for 3-DG (2 500 – 0 nmol/L), GO (1 600 – 0 nmol/L), and MGO (1 300 – 0 nmol/L). Calibration curves were linear over the respective concentration ranges (r2 = 0.99) in water and serum. Interassay variation was determined using 2 calibration standards that were analyzed over 8 months. Interassay variation was 15.5%, 7.4%, and 12.1% for 3-DG, GO, and MGO, respectively. Intraassay variation was determined by replicate analysis of a pooled serum sample (n = 7) and was 9.0%, 3.9%, and 6.4% for 3-DG, GO, and MGO, respectively. Limit of detection (LOD) and limit of quantification (LOQ) were calculated on the basis of the signal-to-noise ratio (LOD s/N = 3 and LOQ s/N = 10). The LOD for 3-DG, GO, and MGO was 0.04, 0.41, and 0.13 nM, which corresponds to 0.2, 2.0, and 0.7 fmol on the column. The LOQ for 3-DG, GO, and MGO was 0.15, 1.36, and 0.44 nM, which corresponds to 0.7, 6.8, and 2.2 fmol on the column. For recovery analysis, 2 levels of 1,2-dicarbonyl compounds were added to a pooled serum sample and analyzed in triplicate. Concentrations 1 and 2 for addition were 1 465 and 586 nM for 3-DG, 870 and 174 nM for GO, and 692 and 138 nM for MGO. Recovery of 3-DG was 108.3% and 98.0%, for GO 89.4% and 87.3%, and for MGO 109.8% and 89.9% for concentrations 1 and 2, respectively.
Measurement of Oxidative Stress Markers
As markers of oxidative stress, protein carbonyls (PC), 3-nitrotyrosine (3-NT), and malondialdehyde (MDA) were quantified in plasma samples. PC and 3-NT were measured using an in-house ELISA as previously described (21). Plasma MDA concentrations were determined after protein precipitation and derivatization with thiobarbituric acid by reversed-phase HPLC coupled with fluorescence detection as previously described by Weber et al. (21) with a Reprosil Pur 120 C18 AQ column (250 × 4.6 mm; 5 μm, Dr. Maisch GmbH, Ammerbuch, Germany).
Statistical Analysis
Statistical analyses were performed with SPSS (IBM version 25; SPSS Inc., Chicago, IL) and GraphPad Prism (version 8.00 for Windows; GraphPad Software, La Jolla, CA). When necessary, variables were log-transformed to achieve normal distribution. Mann–Whitney U test and Student’s t-test were used accordingly to assess age and sex differences. Fasting concentrations are shown as median (interquartile range [IQR]). Violin plots present median and the upper and lower quartile. To evaluate changes in 1,2-dicarbonyl compound concentrations over time, repeated measures analysis of variance was performed. Postprandial response was represented by the positive incremental area under the curve (iAUC), which was calculated using the trapezoid rule and considering only values above the baseline. Here data are shown as mean ± SD. Possible associations between 1,2-dicarbonyl response, glucose, and insulin are shown with Pearson’s correlations coefficients.
Results
Postprandial 1,2-Dicarbonyl Concentrations in Mice
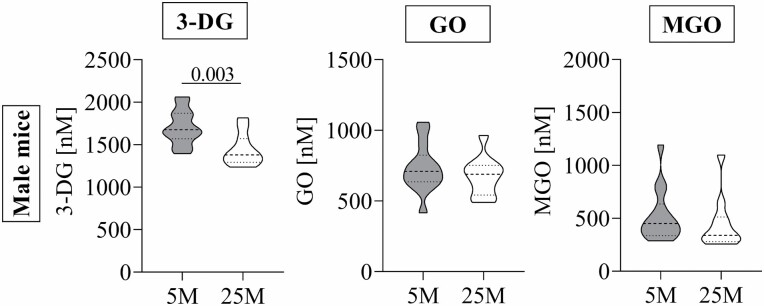
Mice were fed a standard diet for 5 and 25 months before sacrifice. Blood glucose concentrations and body weight were not significantly different between the age groups (data not shown). Postprandial 3-DG concentrations of younger mice ranged between 1 400 and 2 100 nM and were significantly higher than concentrations of older mice, which ranged between 1 200 and 1 800 nM (Figure 1). Postprandial GO and MGO concentrations were between 400–1 100 nM and 300–1 200 nM, respectively, and no age differences were found.
Figure 1.
Postprandial 1,2-dicarbonyl compound concentrations in older (25 months) and younger (5 months) male mice. Data are shown as Violin plots; dotted lines represent median and quartiles. Student’s t-test for normally distributed data and Mann–Whitney U test for nonnormally distributed data were used to assess differences between age groups. 25 months old (n = 11), 5 months old (n = 14). 3-DG = 3-deoxyglucosone; GO = glyoxal; MGO = methylglyoxal; 25 M = 25 months old; 5 M = 5 months old; p < .05.
Participants Characteristics
Younger adults were overall healthy without any apparent disease. In older adults, high blood pressure was most prevalent (n = 15), followed by dyslipidemia (n = 6) and thyroid-associated diseases (n = 5). A basic description of the study cohort is displayed in Table 1. For both sexes, older adults had significantly higher HOMA-IR values and MDA concentrations compared to younger adults. Regarding sex differences, women had a significantly lower body mass index (BMI) than men in both age groups. Fasting concentrations of oxidative stress markers were similar for older women and older men. Younger women had significantly lower PC concentrations than younger men.
Table 1.
Baseline Participants Characteristics
| YW (n = 19) | OW (n = 19) | YM (n = 15) | OM (n = 15) | p * | p † | p ‡ | p § | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 27.0 ± 4.42 | 72.4 ± 6.14 | 27.0 ± 3.34 | 74.3 ± 5.20 | .348 | .964 | ||
| BMI (kg/m²) | 22.3 ± 2.64 | 23.9 ± 2.93 | 24.9 ± 3.68 | 26.3 ± 3.78 | .089 | .325 | .041 | .019 |
| HOMA-IR | 1.92 ± 0.45 | 2.40 ± 0.88 | 2.11 ± 0.53 | 3.14 ± 1.46 | .025 | .029 | .120 | .319 |
| PC (nmol/mg)‖ | 1.03 ± 0.32 | 1.16 ± 0.29 | 1.49 ± 0.49 | 1.30 ± 0.54 | .123 | .367 | .837 | .008 |
| 3-NT (nmol/mg)‖ | 1.99 ± 1.40 | 2.15 ± 2.01 | 2.72 ± 2.49 | 2.77 ± 2.35 | .729 | .744 | .515 | .607 |
| MDA (µmol/L)‖ | 0.69 ± 0.35 | 0.98 ± 0.44 | 0.60 ± 0.25 | 1.03 ± 0.48 | .009 | .002 | .811 | .607 |
| Glucose (mmol/L) | 4.42 ± 0.37 | 5.15 ± 0.51 | 4.74 ± 0.30 | 5.67 ± 0.87 | <.001 | .001 | .039 | .012 |
| Insulin (µIU/mL)‖ | 9.71 ± 1.85 | 10.4 ± 3.29 | 10.0 ± 2.45 | 12.1 ± 4.05 | .885 | .412 | .271 | .758 |
Notes: YW = younger women; OW = older women; YM = younger men; OM = older men; BMI = body mass index; HOMA-IR = homeostasis model assessment—insulin resistance; PC = protein carbonyls; 3-NT = 3-nitrotyrosine; MDA = malondialdehyde. All data are shown as mean ± SD. Age and sex differences were calculated using Student’s t-test, unless otherwise indicated.
*Age difference between women.
†Age differences between men.
‡Sex difference between older adults.
§Sex differences between younger adults.
‖Mann–Whitney U test.
p < .05.
Fasting 1,2-Dicarbonyl, Glucose, and Insulin Concentrations in Human Participants
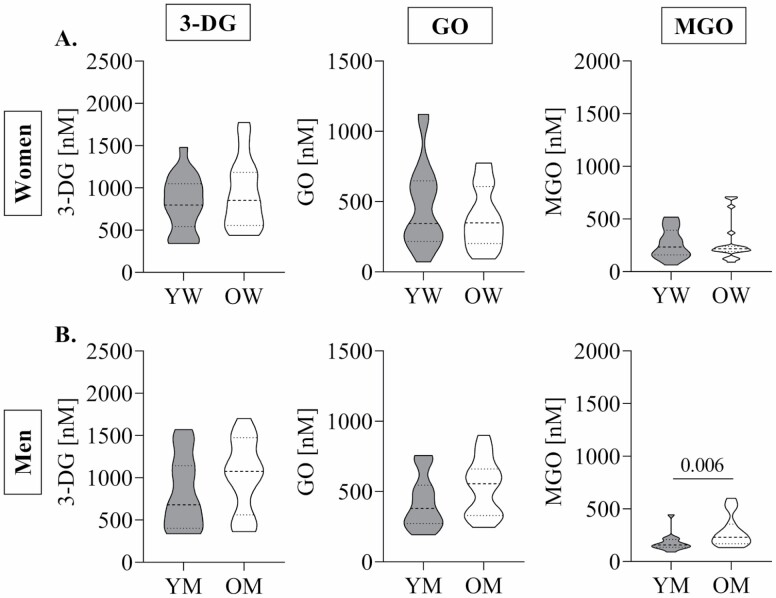
Overall, the median concentrations of 3-DG were 853 nM (IQR: 608 nM), 385 nM (IQR: 360 nM) for GO and 204 nM (IQR: 141 nM) for MGO in all participants. In men and women, fasting glucose concentrations were significantly higher in older age (Table 1). Fasting insulin concentrations were similar between age groups in both men and women. In women, 3-DG, MGO, or GO did not differ between age groups (Figure 2A). In men, only fasting MGO concentrations were significantly higher in older compared to younger individuals (Figure 2B).
Figure 2.
Fasting 1,2-dicarbonyl compound concentrations in older and younger women (A) and men (B). Data are shown as Violin plots; dotted lines represent median and quartiles. Student’s t-test for normally distributed data and Mann–Whitney U test for nonnormally distributed data were used to assess differences between age groups. YW (n = 19), OW (n = 19), YM (n = 15), OM (n = 15). 3-DG = 3-deoxyglucosone; GO = glyoxal; MGO = methylglyoxal; YW = younger women; OW = older women; YM = younger men; OM = older men; p < .05.
The evaluation of sex differences revealed that fasting glucose concentrations were significantly higher in men when compared to women (Table 1). Regarding fasting 1,2-dicarbonyl concentrations, fasting GO concentrations were significantly lower in older women compared to older men (349 nM, IQR: 406 nM vs 556 nM, IQR: 654 nM; p = .048).
Glucose, Insulin, and 1,2-Dicarbonyl Response to Dextrose Challenge in Human Participants
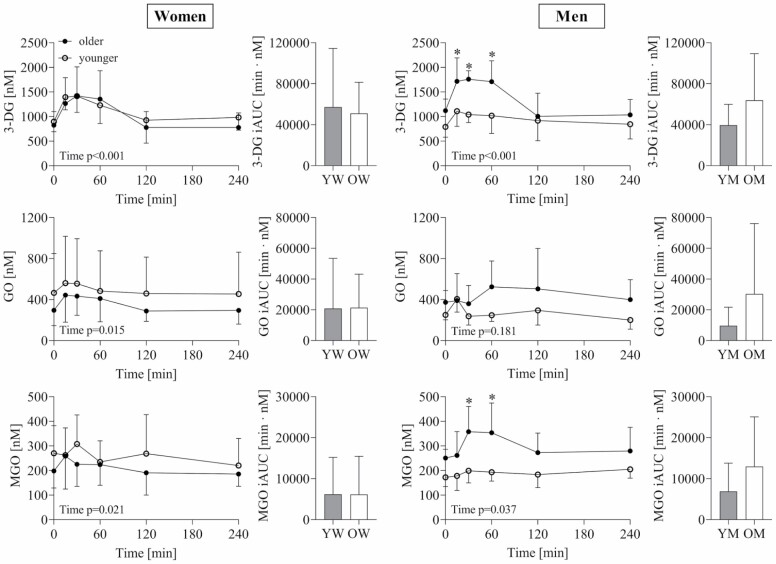
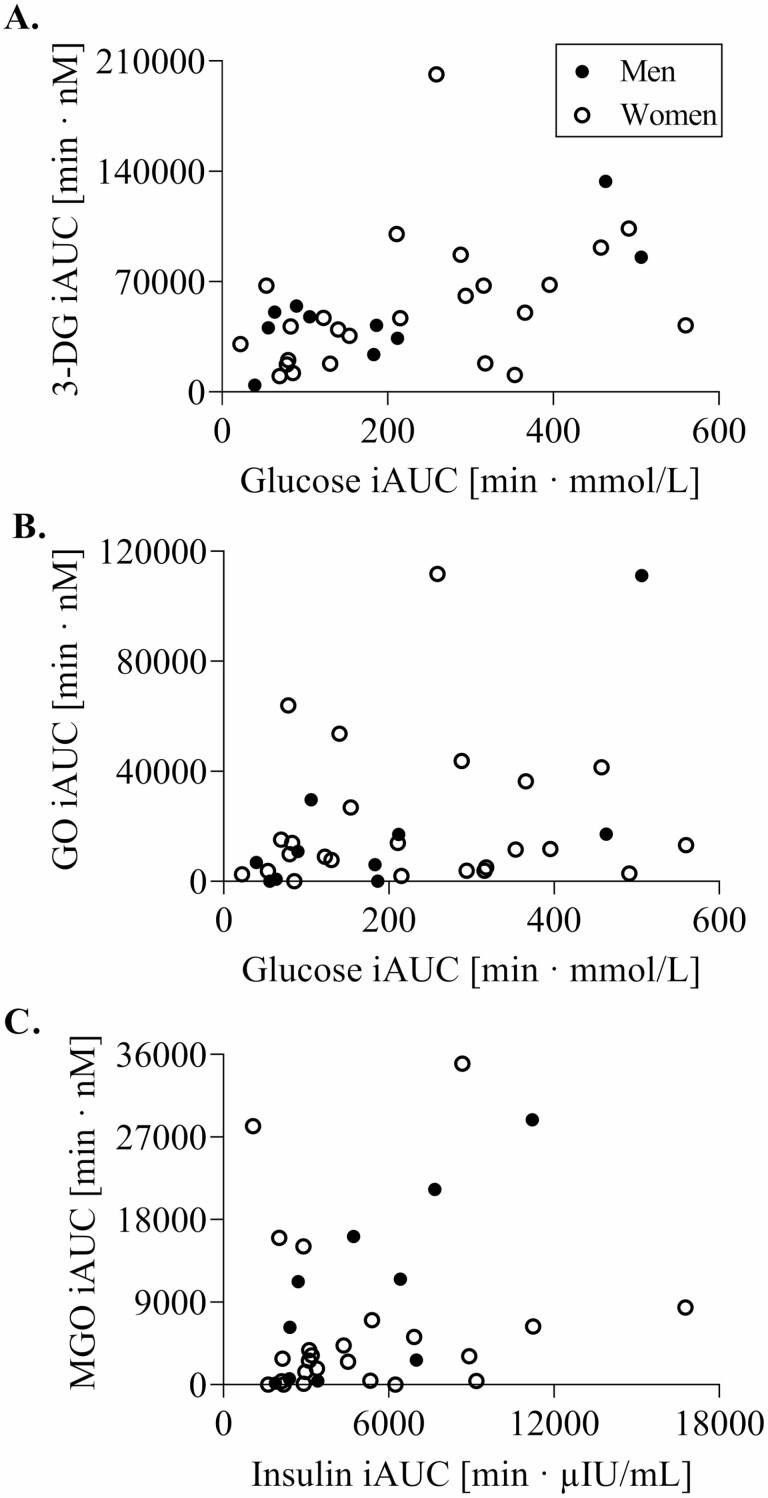
Participants’ characteristics of the oral dextrose challenge are given in Supplementary Table 1. Postprandial 1,2-dicarbonyl concentrations and response (iAUC) following dextrose ingestion are displayed in Figure 3. 1,2-dicarbonyl concentrations significantly changed over time in both sexes, with the exception of GO in men. In women, postprandial 1,2-dicarbonyl concentrations and response were not different between age groups. In men, postprandial 3-DG concentrations were higher in older compared to younger men at 15, 30, and 60 minutes. Furthermore, MGO concentrations were also higher in older compared to younger men at 30 and 60 minutes. However, this did not translate into a significantly higher response in older men. Glucose and insulin response were also not different in women, whereas, in men, older men had a significantly higher glucose iAUC (OM: 310 ± 160 min·mmol/L vs YM: 70.8 ± 26.7 min·mmol/L) as well as insulin iAUC (OM: 7 149 ± 2 792 min·µIU/mL vs YM: 2 827 ± 1 102 min·µIU/mL) compared to younger men. In addition, glucose response was positively associated with 3-DG as well as GO response (Figure 4A and B), but only in men. Moreover, higher insulin response was associated with higher MGO response (Figure 4C). Regarding age differences, glucose response was positively associated with 3-DG response (r = 0.778, p < .001) in older adults, whereas in younger adults, higher insulin response was positively correlated with 3-DG response (r = 0.639, p = .010).
Figure 3.
Postprandial 1,2-dicarbonyl concentrations and response (iAUC) to a dextrose challenge in older and younger women and men. Data are shown as mean ± SD. Repeated measures analysis of variance was used to examine changes over time and differences between age groups, and Mann–Whitney U test was used to assess age differences of the postprandial response (iAUC). *Significantly different between age groups. YW (n = 19), OW (n = 19), YM (n = 15), OM (n = 15). iAUC = incremental area under the curve; 3-DG = 3-deoxyglucosone; GO = glyoxal; MGO = methylglyoxal; YW = younger women; OW = older women; YM = younger men; OM = older men; p < .05.
Figure 4.
Associations of glucose response with 3-DG (A) and GO (B) response as well as insulin response with MGO response (C) to a dextrose challenge. (A) No significant correlation in women, men: r = 0.782, p = .008. (B) No significant correlation in women, men: r = 0.707, p = .022. (C) No significant correlation in women, men: r = 0.784, p = .007. YW (n = 19), OW (n = 19), YM (n = 15), OM (n = 15). iAUC = incremental area under the curve; 3-DG = 3-deoxyglucosone; GO = glyoxal; MGO = methylglyoxal; YW = younger women; OW = older women; YM = younger men; OM = older men; p < .05.
Discussion
It has been postulated previously that dicarbonyl stress contributes to the aging phenotype (22). So far, little is known about the role of dicarbonyl compounds in older individuals without underlying pathologies and also whether concentrations of 1,2-dicarbonyl compounds exhibit sex differences. In a preliminary experiment, we analyzed concentrations of the reactive 1,2-dicarbonyl compounds 3-DG, MGO, and GO in male C57Bl/6J mice. Our main study included older and younger men and women. The age of the mice was comparable to the demographics of our human cohort as 25 months are equal to 70 human years and 5 months to 25 human years (23).
In contrast to our hypothesis that aging increases plasma 1,2-dicarbonyl concentrations due to cellular imbalance of formation and degradation of reactive carbonyl species, GO and MGO concentrations did not differ between older and younger mice. Moreover, plasma 3-DG concentrations were even higher in younger compared to older mice. In our human study, older women did not have higher concentrations of 1,2-dicarbonyl compounds compared to the younger control group. The same was valid for plasma concentrations of 3-DG and GO, but not MGO, in men. During an oral dextrose challenge, older women had no increased response in 1,2-dicarbonyl compounds, whereas in older men it appeared to be slightly elevated.
Recently, the influence of long-term intake of MGO on its urinary excretion and plasma concentrations was investigated in nonfasted C57BL/6N mice aged 6–24 months (24). Neither the experimental nor the control group without MGO intake showed changes in plasma MGO levels in older age. For plasma GO, a slight decrease over time was shown (24). Our results confirm that in healthy mice, 1,2-dicarbonyl compounds are not higher in older age and, as shown for 3-DG, are even lower. Lower concentrations of 1,2-dicarbonyl compounds in older mice might be due to changes in energy regulation or decreased absorption of macronutrients during aging (25). In our preliminary experiment, 1,2-dicarbonyl analysis was performed postprandially in mice. Thus, an influence of the diet on the results cannot be excluded. Mice are model organisms living in a strictly controlled environment with standardized diets, and factors affecting human metabolism and aging are more diverse. To examine whether the absence of higher 1,2-dicarbonyl compounds in older age can be confirmed in humans, we analyzed fasting serum samples of older and younger men and women in our main study.
In women, fasting glucose and MDA concentrations were higher in older adults, but no age differences for 1,2-dicarbonyl compounds were observed. For men, again higher fasting glucose and MDA concentrations, as well as higher fasting MGO, were found in older adults. The higher concentrations of glucose (26) and the oxidative stress marker MDA (27) in older adults were reported previously and indicate cellular dysfunction as well as increased glucose intolerance.
Recently, it has been shown that the activity of the glyoxalase system, which is the main detoxification route of MGO, decreases in the human eye lens in older age (28). The authors did not find sex-specific differences between the lenses of men and women. The analysis of glyoxalase activity might provide more insight into the mechanism of increased MGO concentrations in older men, but this was unfortunately not feasible in plasma nor serum samples in our study. Hence, whether the higher MGO in older men is due to decreased glyoxalase activity needs to be further elucidated. It has been shown in Glo1 knock-out cells that a compensatory detoxification can be achieved through aldose reductase (29). Whether this mechanism plays a role in the detoxification in humans has yet to be confirmed. Very recently it was postulated that protein deglycase DJ-1 can also act as a glyoxalase, thus contributing to MGO detoxification (30). Due to the various detoxification routes of MGO, there are different possibilities of how the metabolism might adjust to increased dicarbonyl concentrations during aging. Whether these enzymatic systems possess sex-specific differences is unknown. Our results of fasting concentrations in older and younger women and men indicate that older adults, despite having higher MDA and blood glucose levels, do not generally show higher plasma 1,2-dicarbonyl compounds. The nonenzymatic formation of 1,2-dicarbonyl compounds is a constant process in the presence of glucose. However, when high loads of glucose are present, for example, after food intake, higher amounts of 1,2-dicarbonyl compounds are formed. Therefore, we analyzed the 1,2-dicarbonyl compound response of older and younger human participants to an oral dextrose challenge.
Interestingly, the response of plasma glucose and insulin in older women was not significantly higher compared to younger women. Accordingly, 1,2-dicarbonyl response was not higher in older women. It has been postulated that aging itself does not solely account for glucose intolerance, but that exercise and body fat mass play an important role (31). Although our cohort was matched for BMI, we cannot exclude that some of the women participating in the dextrose challenge were particularly physically active resulting in a lower 1,2-dicarbonyl response. In men, we observed a higher glucose and insulin response after the dextrose challenge in older compared to younger men. Although this did not translate into a significantly increased dicarbonyl response in older men, concentrations of 1,2-dicarbonyl compounds tended to be higher in older compared to younger men. As the number of older men performing the dextrose challenge was low (n = 5), this has to be interpreted carefully. Furthermore, these older men exhibited higher insulin resistance, estimated by HOMA-IR, than younger men, which potentially explains the higher glucose and insulin response (Supplementary Table 1).
Glucose response of older adults was positively associated with 3-DG response but not with GO and MGO response. This indicates that 3-DG formation and degradation in older individuals might not be as well-regulated as for MGO and GO. In contrast to 3-DG, GO and MGO are degraded by multiple detoxification systems as mentioned above. This could be due to the lower reactivity of 3-DG toward proteins and thus lower risk of glycation reactions compared to MGO (32,33). Moreover, in men, glucose response is positively correlated with 3-DG and GO response, which was not found in women. Potentially, the male detoxification system is more vulnerable to the aging process, which is seen in a more pronounced reduction of Glo1 activity in older men compared with older women (18). For aldose and aldehyde reductase, the main 3-DG degrading enzymes, no such studies are available. Overall, we showed that although it is known that glucose tolerance decreases with age (34), this does not necessarily affect 1,2-dicarbonyl compound levels.
In the present study, the sample size with n < 20 per group is small, especially regarding the dextrose challenge. This limits the possibilities of statistical analyses. We are aware of the limitations of this study; nevertheless, our results indicate that 1,2-dicarbonyl formation and degradation routes might be differently affected in older men and women. The sex-specific differences in plasma 1,2-dicarbonyl concentrations in older age have not been reported before and provide a basis for further studies of glycation and aging research.
Overall, our results suggest that aging in the absence of severe health impairments does not necessarily result in dicarbonyl stress, indicating that endogenous strategies to cope with 1,2-dicarbonyl formation may be unaffected by aging. The results help understand the impact of glycation during aging and present new perspectives regarding the process of healthy aging.
Supplementary Material
Acknowledgments
The authors would like to thank Andrea Katschak (German Institute of Human Nutrition, Potsdam-Rehbrücke, Department of Nutrition and Gerontology) for supporting the human study and analytical work, as well as Stefanie Deubel (German Institute of Human Nutrition, Potsdam-Rehbrücke, Department of Molecular Toxicology) for supporting the animal work.
Contributor Information
Catrin Herpich, Department of Nutrition and Gerontology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany; University of Potsdam, Institute of Nutritional Science, Nuthetal, Germany.
Bastian Kochlik, Department of Nutrition and Gerontology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany.
Daniela Weber, Department of Molecular Toxicology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany.
Christiane Ott, Department of Molecular Toxicology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Berlin, Berlin, Germany.
Tilman Grune, University of Potsdam, Institute of Nutritional Science, Nuthetal, Germany; Department of Molecular Toxicology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany.
Kristina Norman, Department of Nutrition and Gerontology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany; Department of Geriatrics and Medical Gerontology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Jana Raupbach, Department of Molecular Toxicology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Nuthetal, Germany.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (RA 3524/2-1 to J.R.).
Conflict of Interest
None declared.
Author Contributions
Concept and design of the study: C.H., K.N., and J.R.; recruitment and measurements of all data: C.H., D.W., and J.R.; first draft: C.H. and J.R.; critical advice: B.K., D.W., C.O., K.N., and T.G. All authors commented on previous versions of the manuscript and approved the final manuscript.
References
- 1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rabbani N, Thornalley PJ. Glyoxalase Centennial Conference: introduction, history of research on the glyoxalase system and future prospects. Biochem Soc Trans. 2014;42(2):413–418. doi: 10.1042/BST20140014 [DOI] [PubMed] [Google Scholar]
- 3. Rabbani N, Thornalley PJ. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem Biophys Res Commun. 2015;458(2):221–226. doi: 10.1016/j.bbrc.2015.01.140 [DOI] [PubMed] [Google Scholar]
- 4. Degen J, Beyer H, Heymann B, Hellwig M, Henle T. Dietary influence on urinary excretion of 3-deoxyglucosone and its metabolite 3-deoxyfructose. J Agric Food Chem. 2014;62(11):2449–2456. doi: 10.1021/jf405546q [DOI] [PubMed] [Google Scholar]
- 5. Degen J, Vogel M, Richter D, Hellwig M, Henle T. Metabolic transit of dietary methylglyoxal. J Agric Food Chem. 2013;61(43):10253–10260. doi: 10.1021/jf304946p [DOI] [PubMed] [Google Scholar]
- 6. Scheijen JL, Schalkwijk CG. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: evaluation of blood specimen. Clin Chem Lab Med. 2014;52(1):85–91. doi: 10.1515/cclm-2012-0878 [DOI] [PubMed] [Google Scholar]
- 7. Maessen DE, Hanssen NM, Scheijen JL, et al. Post-glucose load plasma α-dicarbonyl concentrations are increased in individuals with impaired glucose metabolism and type 2 diabetes: the CODAM Study. Diabetes Care. 2015;38(5):913–920. doi: 10.2337/dc14-2605 [DOI] [PubMed] [Google Scholar]
- 8. Fujii E, Iwase H, Ishii-Karakasa I, Yajima Y, Hotta K. The presence of 2-keto-3-deoxygluconic acid and oxoaldehyde dehydrogenase activity in human erythrocytes. Biochem Biophys Res Commun. 1995;210(3):852–857. doi: 10.1006/bbrc.1995.1736 [DOI] [PubMed] [Google Scholar]
- 9. Kato H, van Chuyen N, Shinoda T, Sekiya F, Hayase F. Metabolism of 3-deoxyglucosone, an intermediate compound in the Maillard reaction, administered orally or intravenously to rats. Biochim Biophys Acta. 1990;1035(1):71–76. doi: 10.1016/0304-4165(90)90175-v [DOI] [PubMed] [Google Scholar]
- 10. Knecht KJ, Feather MS, Baynes JW. Detection of 3-deoxyfructose and 3-deoxyglucosone in human urine and plasma: evidence for intermediate stages of the Maillard reaction in vivo. Arch Biochem Biophys. 1992;294(1):130–137. doi: 10.1016/0003-9861(92)90146-n [DOI] [PubMed] [Google Scholar]
- 11. Xue M, Rabbani N, Thornalley PJ. Glyoxalase in ageing. Semin Cell Dev Biol. 2011;22(3):293–301. doi: 10.1016/j.semcdb.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 12. Thornalley PJ. Dicarbonyl intermediates in the Maillard reaction. Ann N Y Acad Sci. 2005;1043:111–117. doi: 10.1196/annals.1333.014 [DOI] [PubMed] [Google Scholar]
- 13. Masania J, Malczewska-Malec M, Razny U, et al. Dicarbonyl stress in clinical obesity. Glycoconj J. 2016;33(4):581–589. doi: 10.1007/s10719-016-9692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin JA, Wu CH, Lu CC, Hsia SM, Yen GC. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: an emerging biological factor in cancer onset and progression. Mol Nutr Food Res. 2016;60(8):1850–1864. doi: 10.1002/mnfr.201500759 [DOI] [PubMed] [Google Scholar]
- 15. Srikanth V, Westcott B, Forbes J, et al. Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci. 2013;68(1):68–73. doi: 10.1093/gerona/gls100 [DOI] [PubMed] [Google Scholar]
- 16. Rabbani N, Thornalley PJ. Protein glycation—biomarkers of metabolic dysfunction and early-stage decline in health in the era of precision medicine. Redox Biol. 2021;42:101920. doi: 10.1016/j.redox.2021.101920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sibbersen C, Johannsen M. Dicarbonyl derived post-translational modifications: chemistry bridging biology and aging-related disease. Essays Biochem. 2020;64(1):97–110. doi: 10.1042/EBC20190057 [DOI] [PubMed] [Google Scholar]
- 18. Peters AS, Hakimi M, Vittas S, et al. Gender difference in glyoxalase 1 activity of atherosclerotic carotid artery lesions. J Vasc Surg. 2015;62(2):471–476. doi: 10.1016/j.jvs.2014.02.058 [DOI] [PubMed] [Google Scholar]
- 19. Tower J, Pomatto LCD, Davies KJA. Sex differences in the response to oxidative and proteolytic stress. Redox Biol. 2020;31:101488. doi: 10.1016/j.redox.2020.101488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herpich CH, Haß U, Kochlik B, et al. Postprandial dynamics and response of fibroblast growth factor 21 in older adults. Clin Nutr. 2021;40(6):3765–3771. doi: 10.1016/j.clnu.2021.04.037 [DOI] [PubMed] [Google Scholar]
- 21. Weber D, Stuetz W, Bernhard W, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2014;68(2):215–222. doi: 10.1038/ejcn.2013.263 [DOI] [PubMed] [Google Scholar]
- 22. Nigro C, Leone A, Fiory F, et al. Dicarbonyl stress at the crossroads of healthy and unhealthy aging. Cells. 2019;8(7):749–776. doi: 10.3390/cells8070749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fox, J.G., Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The Mouse in Biomedical Research—History, Wild Mice, and Genetics. Academic Press; 2006. [Google Scholar]
- 24. Zunkel K, Simm A, Bartling B. Long-term intake of the reactive metabolite methylglyoxal is not toxic in mice. Food Chem Toxicol. 2020;141:111333. doi: 10.1016/j.fct.2020.111333 [DOI] [PubMed] [Google Scholar]
- 25. Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86(2):651–667. doi: 10.1152/physrev.00019.2005 [DOI] [PubMed] [Google Scholar]
- 26. Shimokata H, Muller DC, Fleg JL, Sorkin J, Ziemba AW, Andres R. Age as independent determinant of glucose tolerance. Diabetes. 1991;40(1):44–51. doi: 10.2337/diab.40.1.44 [DOI] [PubMed] [Google Scholar]
- 27. Pinchuk I, Weber D, Kochlik B, et al. Gender- and age-dependencies of oxidative stress, as detected based on the steady state concentrations of different biomarkers in the MARK-AGE study. Redox Biol. 2019;24:101204. doi: 10.1016/j.redox.2019.101204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haik GM Jr, Lo TW, Thornalley PJ. Methylglyoxal concentration and glyoxalase activities in the human lens. Exp Eye Res. 1994;59(4):497–500. doi: 10.1006/exer.1994.1135 [DOI] [PubMed] [Google Scholar]
- 29. Morgenstern J, Fleming T, Schumacher D, et al. Loss of glyoxalase 1 induces compensatory mechanism to achieve dicarbonyl detoxification in mammalian Schwann cells. J Biol Chem. 2017;292(8):3224–3238. doi: 10.1074/jbc.M116.760132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andreeva A, Bekkhozhin Z, Omertassova N, et al. The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals. J Biol Chem. 2019;294(49):18863–18872. doi: 10.1074/jbc.RA119.011237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reaven G. Age and glucose intolerance: effect of fitness and fatness. Diabetes Care. 2003;26(2):539–540. doi: 10.2337/diacare.26.2.539 [DOI] [PubMed] [Google Scholar]
- 32. Gobert J, Glomb MA. Degradation of glucose: reinvestigation of reactive alpha-dicarbonyl compounds. J Agric Food Chem. 2009;57(18):8591–8597. doi: 10.1021/jf9019085 [DOI] [PubMed] [Google Scholar]
- 33. Henning C, Liehr K, Girndt M, Ulrich C, Glomb MA. Extending the spectrum of α-dicarbonyl compounds in vivo. J Biol Chem. 2014;289(41):28676–28688. doi: 10.1074/jbc.M114.563593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. doi: 10.2337/diabetes.52.7.1738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.